Abstract
The etiology of bacterial vaginosis is unknown, and there are no long-term therapies for preventing this frequently recurring condition. Vaginal douching has been reported to be associated with bacterial vaginosis in observational studies. However, this association may be due to confounding by indication—that is, confounding by women douching in response to vaginal symptoms associated with bacterial vaginosis. The authors used marginal structural modeling to estimate the causal effect of douching on bacterial vaginosis risk while controlling for this confounding effect. In 1999–2002, nonpregnant women (n = 3,620) were recruited into a prospective study when they visited one of 12 public health clinics in Birmingham, Alabama, for routine care. Participants were assessed quarterly for 1 year. Bacterial vaginosis was based on a Nugent's Gram stain score of 7 or higher. Thirty-two percent of participants douched in every study interval, and 43.0% never douched. Of the 12,349 study visits, 40.2% were classified as involving bacterial vaginosis. The relative risk for regular douching as compared with no douching was 1.21 (95% confidence interval: 1.08, 1.38). These findings indicate that douching confers increased risk of disruption of vaginal flora. In the absence of a large randomized trial, these findings provide the best evidence to date for a risk of bacterial vaginosis associated with douching.
Keywords: confounding factors (epidemiology); epidemiologic methods; longitudinal studies; models, structural; vaginal douching; vaginosis, bacterial
Bacterial vaginosis is an ecologic disease of the vaginal microflora which is traditionally characterized by a shift in bacterial ecology from one dominated by lactic-acid-producing lactobacilli to a predominance of Gardnerella and anaerobic flora (1, 2). Bacterial vaginosis is the most common vaginal infection of reproductive-age women and is the most frequently cited cause of vaginal discharge and malodor (3). Epidemiologically, bacterial vaginosis has been associated with adverse reproductive tract outcomes, including increased risk of pelvic inflammatory disease (4), preterm delivery and low birth weight (5–9), and acquisition of human immunodeficiency virus type 1 (10–12). Prevalences of bacterial vaginosis are 9 percent (13) to 18 percent (14) in United Kingdom clinic-based studies, 29 percent in US population-based surveys (15), and more than 50 percent in rural Ugandan villages (16, 17).
The etiology of bacterial vaginosis is unknown. Sexual transmission has been implicated, since bacterial vaginosis is rarely found in sexually inexperienced persons (18) and greater than 95 percent concordance on bacterial vaginosis status is found in monogamous lesbian couples (19, 20). Bacterial vaginosis has been associated with having a new sexual partner, receptive oral sex, and lack of condom use (20–26).
Observational studies also suggest a strong association between vaginal douching and bacterial vaginosis (27–31). However, the reported association may be due to confounding by indication—that is, existing study designs cannot determine whether douching increases the risk of bacterial vaginosis or bacterial vaginosis symptoms lead women to douche (28, 32). Proponents of the douching hypothesis propose that douching causes disequilibrium in vaginal microflora or induces inflammation through physical or chemical irritation, which predisposes women to bacterial vaginosis. While most commercial douche products consist primarily of fragrance, acetic acid, and water, some also contain surfactants, such as oxtoxynol-9 or cetyl pyridinium chloride (33). Surfactant detergents can disrupt lipid membranes and thus have antimicrobial and viricidal activities. Additionally, these detergents may wash away antibacterial agents or disturb cell membranes, causing irritation to mucosal surfaces, which in turn can increase susceptibility to genital tract infection (34).
Douching is common, so any adverse health consequences linked to douching would have a major public health impact. In 2001, the National Health and Nutrition Examination Survey found that 22 percent of reproductive-age women had used vaginal douches in the 6 months prior to the survey, with rates approaching 50 percent in non-Hispanic Black women (15, 35).
There are few long-term clinical trials that have evaluated the effect of douching on vaginal microflora (36, 37). Microbiologic studies have reported observations of less than 4 days (33, 38, 39). Our purpose in this study was to assess the risk of bacterial vaginosis conferred by douching in a cohort of 3,620 women followed longitudinally for 12 months. We utilized a specialized method (40) to control for the confounding effect of douching in response to bacterial vaginosis symptoms.
MATERIALS AND METHODS
Participants
Cohort participants were enrolled in the Longitudinal Study of Vaginal Flora, which has been previously described (41). Briefly, the purpose of the Longitudinal Study of Vaginal Flora was to evaluate the natural history of bacterial vaginosis. Nonpregnant women aged 15–44 years were recruited between August 1999 and February 2002 when they visited one of 12 clinics in the Birmingham, Alabama, area for routine health care. Participants were assessed at a baseline visit and at four quarterly follow-up visits for up to 1 year of observation. Women with significant medical or gynecologic conditions—those who were immunocompromised, postmenopausal, posthysterectomy, or post-pelvic radiotherapy or were using antibiotics long term (daily for at least 30 days)—were ineligible. Other exclusions included having a condition hindering informed consent or planning to move from the area in the following 12 months. All participants provided written informed consent. The protocol was approved by the institutional review boards of the Jefferson County Department of Health, the University of Alabama at Birmingham, and the National Institute of Child Health and Human Development.
Interview
In a standardized 60-minute interview conducted by trained female research staff, participants reported demographic characteristics, use of feminine hygiene products, lifestyle, sexual risk behaviors, and vaginal symptoms. Time-varying factors were ascertained at each study visit and pertained to the 6 months before the baseline visit and the 3 months preceding each follow-up visit.
A douche product was defined as “a fluid to flush out your vagina.” At every visit, participants were asked about the frequency with which they used douche products; possible answers were “never,” “less than once a week,” “about once a week,” or “several times a week.” A binary variable for douching was defined as the use of any douche product or no douche products in the preceding study interval. The majority of women who reported douching did so less than once per week. Only 10 percent of women who douched reported douching more frequently. Additionally, over 90 percent of those who douched did so using commercial products; therefore, all types of douche products, including commercial, water, and homemade solutions, were evaluated together.
Women were questioned about the presence of specific vaginal symptoms, indicators which may prompt the use of a vaginal douche product. Specifically, each participant was asked “In the previous [3 months or 6 months], have you had [symptom]?” The symptoms were “vaginal odor,” “vaginal discharge,” “vaginal itch that does not easily go away,” and “vaginal irritation.”
Genital tract infections
Participants underwent a standardized clinical assessment and pelvic examination at each visit. Neisseria gonorrhoeae was evaluated by culture. Testing for Chlamydia trachomatis was performed by ligase chain reaction (Abbott Laboratories, Abbott Park, Illinois). Vaginal smears were obtained and assessed using the Gram stain criteria of Nugent et al. (42). The Nugent score evaluates the numbers of Lactobacillus (large, Gram-positive rods), Gardnerella (Gram-negative coccobacillary organisms), and Mobiluncus (thin, curved Gram-variable rods) morphotypes per oil-immersion microscope field. Nugent scores reflect the range of vaginal flora disruption; by convention, a score of 0–3 is normal, 4–6 is considered intermediate disruption, and 7–10 is regarded as bacterial vaginosis. Our primary outcome measure was bacterial vaginosis, defined as a Nugent score of 7 or higher. The presence of yeast was determined by potassium hydroxide smear and abnormal vaginal discharge and/or vulvovaginal erythema/edema. Trichomoniasis was determined by means of a positive finding upon culture or microscopic evaluation for trichomonads.
Statistical model
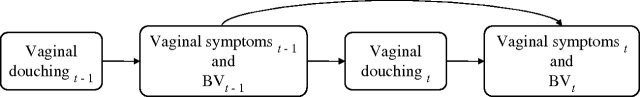
Because douching is often practiced by women who have vaginal symptoms and/or disrupted vaginal flora, characteristics which are indicative of bacterial vaginosis (43, 44), adjustment for this confounding by indication is necessary in observational studies. A conventional analysis would adjust for vaginal symptoms before initiation of douching; however, because vaginal symptoms are simultaneously a confounder and an intermediate variable on the causal pathway (figure 1), conditioning on symptoms in a regression model would produce a biased estimate of the total effect of douching on bacterial vaginosis.
FIGURE 1.
Vaginal symptoms and bacterial vaginosis (BV) represented as both confounding factors and intermediaries in relation to douching. t, time of current measurement; t − 1, time of previous measurement.
Since douching is a time-varying, nonrandomized “treatment,” we used the marginal structural modeling approach of Robins et al. (40) to estimate the causal effect of douching patterns (0, 1, 2, 3, 4, or 5 intervals of douching) on bacterial vaginosis at 12 months. This technique accounts for the potential confounding and intermediary role of time-varying vaginal symptoms. The marginal structural approach seeks to mimic the results that would be obtained had the douching patterns been randomized (45, 46).
In the marginal structural approach, one defines for each individual and for each of the 32 possible douching patterns the vaginal flora outcome at 0, 3, 6, 9, and 12 months had the individual, possibly contrary to fact, followed the pattern. In our analysis, we assumed an unconditional logistic regression model in which the log odds of bacterial vaginosis at month t for each pattern were linear in the number of douching periods at or prior to that month. Month-specific intercepts were included in the model.
Nonrandomized douching
To account for nonrandomized douching status, we assumed that the decision to douche at time t was random, with the probability depending on demographic and socioeconomic factors and past histories of sexual behavior, vaginal symptoms, douching, and genital tract infections. We also assumed that these factors perfectly explained douching behavior (“no unmeasured confounding”). Under this assumption, we fitted, for t = 1, a logistic regression model for the probability of douching at baseline given factors reported for the 6 months prior to baseline; for t > 1, this was a pooled logistic regression model for douching at time t given factors reported for the 3 months prior to time t, douching status at t − 1, and genital tract infections measured at time t. Data on all individuals were used to estimate the parameters of these models. For each woman with data, at month t, a predicted probability of following her observed douching pattern through that month was computed. The inverse of these predictions was then used to reweight the observed data. The inverse-probability-of-treatment weighting approach served to rebalance the complete data in such a way that relations between confounders and douching were eliminated at each time point.
Censored data
During the course of follow-up, there were missed clinic visits. To use the marginal structural method, we censored any information that was collected on participants after their first missed clinic visit. This resulted in the deletion of 9.1 percent of the 13,591 visits. The deletion was performed before model-fitting but after imputation. In this restricted data set, 24 percent, 14 percent, 8 percent, 4 percent, and 50 percent of the 3,620 women had 1, 2, 3, 4, and 5 study visits, respectively. To account for selection bias caused by censoring (i.e., loss to follow-up and the artificial deletion of visits), we assumed that censoring at visit t among those still in the study was random, with the probability depending on demographic and socioeconomic factors and past histories of douching, vaginal symptoms, and genital tract infections (i.e., “coarsening at random”). Under this assumption, we fitted a pooled discrete logistic regression hazard model for attending visit t (t > 1) given factors reported for the 3 months prior to time t, douching status at t − 1, and genital tract infections measured at time t − 1. Data on all participants were used to estimate the parameters of this model. For each woman with data, at month t, a predicted probability of attending the month t assessment was computed. The inverse of these predictions was used to further reweight the observed data. The inverse-probability-of-completion weighting approach served to rebalance the data in such a way that relations between confounders and visit attendance were eliminated at each time point.
Item missingness and imputation
Of the 12,349 study visits, there was scattered missingness with regard to key variables used in the analysis. All variables other than menstrual regularity (1.02 percent), condom use (1.30 percent), income (2.36 percent), and days since last menstrual period (16.79 percent) had less than 0.63 percent missing observations. Multivariate imputation by chained equations (MICE) was implemented (47) in STATA/SE 9.0 if a participant attended a follow-up visit but had incomplete information. The MICE method employs several multivariate imputation models that support categorical data, including binary, nominal, and ordinal distributions. Constitutional variables such as ethnicity, age, and marital status were imputed using baseline information, and missing data for follow-up visits were imputed using data from contemporaneously collected variables and the given variable's value at the previous visit. Five copies of the data set were produced, with missing values being filled in by maximum likelihood imputation.
Estimation
For each of these data sets, we fitted the marginal structural model by reweighting the observed data at each month by the product of the inverse-probability-of-treatment weights and the inverse-probability-of-attendance weights at that month. Because large variation in weights leads to wide confidence intervals, the treatment and censoring weights were stabilized. For each imputed data set, the marginal structural model coefficients were estimated using weighted, pooled logistic regression. In addition, the covariance matrix of these estimators was estimated by nonparametric bootstrap. The five imputed data sets were combined to produce an overall estimate of the regression parameters and their covariance matrix using Rubin's combining rules (48, 49). The relative risk of bacterial vaginosis at 12 months for regular douching versus no douching was estimated, with the standard error being estimated by the delta method. Programming was performed in R (R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org).
Conventional model
For comparison with the inferences obtained from fitting the marginal structural model, we utilized a conventional model to describe the probability of bacterial vaginosis at month t (t = 3, 6, 9, 12) as a function of the number of douching intervals at or prior to that month, confounders recorded at that month, bacterial vaginosis status at the previous visit, and month-specific intercepts. A modified Poisson model (50) was used to estimate the relative risk. For each imputed data set, standard errors of the regression coefficients were estimated using a nonparametric bootstrap. The overall results were computed using Rubin's combining rules.
Potential confounders
Potentially confounding characteristics, including demographic factors and time-dependent factors identified as possible confounders on the basis of previous literature, biologic plausibility, and preliminary univariable analyses, were included in the multivariable models.
RESULTS
The participants had a median age of 23.6 years (interquartile range, 20.1–29.4); 79.9 percent reported their race/ethnicity as African-American. At study entry, participants who used vaginal douches were older, reported lower incomes, had less education, and had a higher prevalence of trichomoniasis and worse vaginal flora scores than those who did not use douche products (table 1).
TABLE 1.
Characteristics of 3,620 women according to vaginal douching status at study entry, Longitudinal Study of Vaginal Flora, Birmingham, Alabama, 1999–2003†
| Douching in the 6 months prior to baseline visit | ||||
| None | Ever | |||
| No. | % | No. | % | |
| Median age (years) | 25.40 (6.63)‡ | 26.40 (7.13) | ||
| Race/ethnicity* | ||||
| Non-Black | 422 | 22.77 | 304 | 17.31 |
| Black | 1,431 | 77.23 | 1,452 | 82.69 |
| Education completed (years)* | ||||
| ≤12 | 1,321 | 71.41 | 1,354 | 77.24 |
| >12 | 529 | 28.59 | 399 | 22.76 |
| Monthly income* | ||||
| <$500 | 329 | 19.49 | 364 | 22.28 |
| $500–<$800 | 472 | 27.96 | 515 | 31.52 |
| $800–$3,000 | 727 | 43.07 | 672 | 41.13 |
| >$3,000 | 160 | 9.48 | 83 | 5.08 |
| Vaginal Gram stain score* | ||||
| 0–3 (normal flora) | 765 | 41.5 | 505 | 28.9 |
| 4–6 (intermediate flora) | 461 | 25.0 | 411 | 23.5 |
| 7–10 (bacterial vaginosis flora) | 618 | 33.5 | 830 | 47.5 |
| Sexually transmitted infection§ | ||||
| None* | 1,558 | 84.1 | 1,324 | 75.4 |
| Gonorrhea | 35 | 1.9 | 41 | 2.3 |
| Chlamydia | 185 | 10.0 | 198 | 11.3 |
| Trichomonas* | 18 | 6.4 | 248 | 14.2 |
p < 0.001.
In the analysis, data were imputed for missing values: douching status, n = 11; race/ethnicity, n = 5; education, n = 11; income, n = 292; and bacterial vaginosis, n = 19.
Numbers in parentheses, standard deviation.
Sample collection at clinic visit.
Thirty-two percent of participants (n = 1,180) douched during every interval for which they made a study visit, and 43 percent (n = 1,555) never douched. Fifty percent (n = 1,793) attended all five study visits. Of the 12,349 visits included in the analysis, bacterial vaginosis was classified as being present in 40.2 percent, while intermediate disruption of vaginal flora was classified as being present in 23.1 percent.
The marginal structural models (table 2) reflected the factors associated with douching and censoring. Douching at baseline was associated with age greater than 26 years, Black race/ethnicity, history of tubal ligation, having more than a single sexual partner, alcohol consumption, and cigarette smoking (model A). Douching at follow-up visits was associated with the factors described in model A and, from the prior visit, both a finding of bacterial vaginosis and a report of douching (model B). The censoring model demonstrates that loss to follow-up was associated with increasing age, Black race/ethnicity, alcohol drinking, self-reported vaginal malodor, and contraceptive use (model C).
TABLE 2.
Factors associated with vaginal douching and loss to follow-up, Longitudinal Study of Vaginal Flora (n = 3,620), Birmingham, Alabama, 1999–2003
| Model A: douching at study entry* | Model B: douching at follow-up visits† | Model C: censoring‡ | ||||
| aOR§ | 95% CI§ | aOR | 95% CI | aOR | 95% CI | |
| Demographic factors | ||||||
| Age (years) | ||||||
| 15–25 | Referent | |||||
| 26–35 | 1.37 | 1.15, 1.64 | 1.24 | 1.08, 1.43 | 1.58 | 1.38, 1.82 |
| 36–46 | 1.57 | 1.21, 2.04 | 1.21 | 1.00, 1.46 | 2.12 | 1.72, 2.61 |
| Race/ethnicity | ||||||
| Non-Black | Referent | |||||
| Black | 1.76 | 1.46, 2.13 | 1.36 | 1.15, 1.62 | 1.49 | 1.29, 1.72 |
| Education completed (years) | ||||||
| ≤12 | Referent | |||||
| >12 | 0.72 | 0.61, 0.86 | 0.69 | 0.60, 0.80 | 1.10 | 0.96, 1.26 |
| Time-dependent factors | ||||||
| Vaginal douching | 30.67 | 26.12, 36.01 | 0.89 | 0.80, 1.00 | ||
| Alcohol drinking | ||||||
| Never | Referent | |||||
| Monthly or less | 1.17 | 0.98, 1.38 | 1.21 | 1.04, 1.41 | 1.23 | 1.08, 1.40 |
| Several times per month or more | 1.59 | 1.33, 1.90 | 1.19 | 1.03, 1.38 | 1.21 | 1.06, 1.38 |
| Cigarette smoking | 1.67 | 1.41, 1.97 | 1.16 | 1.00, 1.33 | 0.79 | 0.70, 0.89 |
| Use of oral contraceptives | 0.96 | 0.81, 1.16 | 1.04 | 0.89, 1.22 | 1.15 | 1.00, 1.33 |
| Tubal ligation | 1.46 | 1.16, 1.82 | 1.42 | 1.19, 1.69 | 1.45 | 1.21, 1.75 |
| Use of depo-medroxyprogesterone acetate | 1.13 | 0.91, 1.40 | 0.89 | 0.74, 1.08 | 1.40 | 1.21, 1.62 |
| Regularity of menstrual cycle | ||||||
| Regular, could predict within 1 week | Referent | |||||
| Not regular, hard to predict | 0.97 | 0.81, 1.16 | 1.05 | 0.88, 1.25 | ||
| Absent | 0.55 | 0.42, 0.71 | 1.28 | 1.04, 1.58 | ||
| Days since last menstrual period | ||||||
| 0–10 | Referent | |||||
| 11–20 | 1.00 | 0.83, 1.20 | 1.02 | 0.89, 1.18 | 0.95 | 0.83, 1.10 |
| 21–30 | 1.21 | 0.99, 1.48 | 0.97 | 0.82, 1.14 | 0.95 | 0.82, 1.11 |
| 31–40 | 1.18 | 0.83, 1.67 | 1.03 | 0.75, 1.41 | 0.77 | 0.61, 0.97 |
| ≥41 | 1.04 | 0.76, 1.42 | 0.62 | 0.48, 0.79 | 0.60 | 0.50, 0.73 |
| No. of recent¶ sexual partners | ||||||
| 0 | Referent | |||||
| 1 | 1.62 | 1.29, 2.03 | 0.87 | 0.74, 1.03 | 0.91 | 0.78, 1.07 |
| ≥2 | 1.75 | 1.31, 2.35 | 0.83 | 0.63, 1.08 | 1.01 | 0.80, 1.29 |
| Consistency of condom use | ||||||
| Never | Referent | |||||
| Sometimes | 1.18 | 0.99, 1.42 | 1.03 | 0.87, 1.23 | 1.19 | 1.02, 1.39 |
| Always | 1.04 | 0.86, 1.26 | 1.02 | 0.86, 1.21 | 1.08 | 0.93, 1.25 |
| Rectal sex | 1.14 | 0.82, 1.58 | 0.99 | 0.72, 1.37 | 1.42 | 1.04, 1.93 |
| Use of insertive sex toy | 1.18 | 0.87, 1.60 | 1.23 | 0.91, 1.65 | 1.01 | 0.75, 1.34 |
| Self-reported vaginal symptoms | ||||||
| Malodor | 0.81 | 0.68, 0.97 | 1.19 | 1.03, 1.39 | ||
| Discharge | 0.95 | 0.82, 1.10 | 0.85 | 0.75, 0.96 | ||
| Irritation | 0.76 | 0.59, 0.96 | 1.06 | 0.86, 1.31 | ||
| Itching | 1.00 | 0.78, 1.27 | 0.91 | 0.75, 1.11 | ||
| Use of over-the-counter antifungal medication or terazol | 0.83 | 0.64, 1.07 | 1.04 | 0.82, 1.32 | ||
| Metronidazole or clindamycin | 1.11 | 0.92, 1.34 | 0.96 | 0.82, 1.12 | ||
| Vaginal Gram stain score | ||||||
| 0–3 (normal flora) | Referent | |||||
| 4–6 (intermediate flora) | 1.14 | 0.97, 1.33 | 0.95 | 0.82, 1.10 | ||
| 7–10 (bacterial vaginosis flora) | 1.21 | 1.05, 1.40 | 0.90 | 0.80, 1.03 | ||
| Sexually transmitted infection | ||||||
| Gonorrhea | 0.78 | 0.57, 1.07 | ||||
| Chlamydia | 1.10 | 0.93, 1.32 | ||||
| Trichomonas | 0.96 | 0.76, 1.21 | 0.70 | 0.59, 0.84 | ||
A logistic regression model for the probability of douching at baseline given factors reported for the 6 months prior to baseline (t = 1).
A pooled logistic regression model for douching at t (t > 1) given factors at t − 1, including douching status, and genital tract infections.
A pooled discrete logistic regression hazard model for attending study visit t (t > 1) given factors reported for the 3 months prior to time t, douching status at t − 1, and genital tract infections measured at time t − 1.
aOR, adjusted odds ratio; CI, confidence interval.
Number of sex partners in the most recent study interval.
Our marginal structural model (table 3) indicated that the relative risk of bacterial vaginosis at 12 months, comparing women who douched regularly with women who did not douche, was 1.21 (95 percent confidence interval (CI): 1.08, 1.38). Results were consistent when compared with an outcome defined by Nugent scores of 4–10 (relative risk = 1.20, 95 percent CI: 1.11, 1.30).
TABLE 3.
Relative risk of bacterial vaginosis* among women who regularly practiced vaginal douching as compared with women who never douched, Longitudinal Study of Vaginal Flora (n = 3,620), Birmingham, Alabama, 1999–2003
| Adjusted relative risk | 95% confidence interval | |
| Marginal structural model† | ||
| Never douching | Referent | |
| Douching in all study intervals | 1.21 | 1.08, 1.38 |
| Conventional model (univariable)‡ | ||
| Never douching | Referent | |
| Douching in all study intervals | 1.81 | 1.60, 2.04 |
| Conventional model (adjusted)§ | ||
| Never douching | Referent | |
| Douching in all study intervals | 1.13 | 1.05, 1.22 |
Bacterial vaginosis was defined as a Nugent Gram stain criteria score of 7 or higher.
Estimated from weighted logistic regression models, with adjustment for age, race/ethnicity, high school education, and exposures reported at the previous visit: douching, bacterial vaginosis, cigarette smoking, alcohol drinking, season, sexual behavior (number of sex partners, a new sex partner, receptive oral sex, condom use, use of an insertive sex toy), menstrual regularity, time since last menstrual period, vaginal symptoms (odor, discharge, itching, irritation), vaginitis treatment (metronidazole, clindamycin, antifungal medication), contraception (oral contraceptives, tubal ligation, depo-medroxyprogesterone acetate), sexually transmitted infections (Trichomonas vaginalis, Chlamydia trachomatis, Neisseria gonorrhoeae), and vulvovaginal candidiasis.
Univariable model from modified Poisson regression.
Multivariable model from modified Poisson regression, with adjustment for age, race/ethnicity, high school education, and history of confounding factors as listed above for the marginal structural model.
A multivariable conventional model demonstrated a relative risk of bacterial vaginosis which was attenuated in comparison (adjusted relative risk = 1.13, 95 percent CI: 1.05, 1.22). The relative risk derived from a crude conventional model was 1.81 (95 percent CI: 1.60, 2.04).
DISCUSSION
Our results indicate that douching was practiced more often by women who had bacterial vaginosis. After applying methods that adjusted for douching behavior prompted by bacterial vaginosis symptoms—a problem of time-varying confounding by indication (28, 32)—we found a significant 21 percent increase in risk of bacterial vaginosis for participants who used vaginal douches.
The marginal structural model allows for the control of prognostic factors, which may be on the casual pathway between douching and bacterial vaginosis and therefore cannot be adjusted for by standard methods (40). Indeed, the conventional analysis that adjusted for previous bacterial vaginosis and prior sexual risk factors resulted in an attenuated estimate and demonstrated the importance of proper adjustment of time-varying confounding. Further, in the absence of control for sexual risk factors and other prognostic factors, our univariable conventional model demonstrated that douching conferred an 80 percent increased risk of bacterial vaginosis (table 3). Interpretation of the relative risk estimate derived from the marginal structural model is analogous to findings that would be expected from a randomized trial (45) and represent the best causal estimates to date on the role of douching in the development of bacterial vaginosis.
A pilot trial of douching cessation demonstrated that cessation of douching reduced the odds of bacterial vaginosis by 77 percent in women who douched for the purpose of menstrual hygiene (37). Douching has also been shown to alter the composition of vaginal flora in microbiologic studies, but there have been few reports and they were limited to very short observation periods. Onderdonk et al. (38) found that douching with saline or acetic acid resulted in changes in vaginal microflora within 10 minutes; additionally, normal flora took up to 72 hours to return to predouching levels. When women douched with the bactericidal agent providone-iodine, more dramatic short-term and prolonged changes in microflora resulted, which allowed an overgrowth of pathogenic organisms that have a faster growth rate than Lactobacillus species. In contrast, Monif et al. (39) found that normal levels of vaginal flora were restored 4 hours after an iodine douche. Pavlova and Tao (33) noted that in broth cultures, three vinegar-containing douche products selectively inhibited vaginal pathogens associated with bacterial vaginosis, group B streptococcal vaginitis, and candidiasis while simultaneously leaving Lactobacillus unaffected. Juliano et al. (51) demonstrated that vaginal antiseptic douche solutions showed marked antibacterial activity in vitro against lactobacilli.
Longitudinal observational epidemiologic studies of vaginal douching (28, 52, 53) have used varying methods to account for confounding by indication in conventional cohort analyses. In a 2-year prospective study of 182 women assessing 1) douching in response to symptoms and 2) douching for hygiene (53), douching for either cleanliness or hygiene was independently associated with a twofold risk of acquiring bacterial vaginosis (hazard ratio = 2.1, 95 percent CI: 1.0, 4.3). In a cohort of 1,193 women who provided vaginal samples at baseline and at 6, 12, 24, and 36 months of follow-up, investigators approached the problem of reverse causality by establishing a temporal order of events, analyzing bacterial vaginosis status by reported douching behavior 6 months prior (28). Although the study was limited by relatively long intervals between assessments, the investigators reported that douching monthly or more often was associated with onset of bacterial vaginosis among women with previously imbalanced flora (hazard ratio = 1.51, 95 percent CI: 1.08, 2.10; p for interaction = 0.23) (28).
Vaginal douching represents one modifiable risk behavior in a lengthy list of suspected putative factors affecting vaginal microflora. In short, the factors involved with acquisition, maintenance, and recurrence of bacterial vaginosis are largely unknown; as such, the topic remains an active area of research. Suspected causative factors include failure to eliminate bacterial vaginosis-associated organisms, reinoculation with organisms from an exogenous source (sex partner or fomite), persistence of risk factors (intrauterine device, douching, etc.), failure to recolonize lactobacilli, failure of lactobacilli to fully acidify the vagina, and the presence of bacteriophages that destroy lactobacilli (20, 54–56).
To our knowledge, this was the first prospective cohort study to evaluate the relation between douching and bacterial vaginosis utilizing a specialized model to address the significant epidemiologic problem of confounding by indication. Other strengths of the study included the large population size (n = 3,620), the use of qualitative research to develop the data collection instruments (57), and the quarterly assessments. A complication of research on vaginal flora is the common recurrence of bacterial vaginosis (58, 59) and the occurrence of bacterial vaginosis more frequently after menstruation (60, 61). A major strength of our study is that we were able to adjust for important time-varying factors, including prior bacterial vaginosis status, menstrual cycle phase, and medications used for the treatment of bacterial vaginosis, trichomoniasis, and vulvovaginal candidiasis. Menstrual cycle phase was also randomly distributed in that women were not asked to return to the clinic in any specific phase of their cycle.
The study had a number of limitations. First, the results may not be readily applicable to other populations with different feminine hygiene practices (62). Our study enrolled 80 percent African-American women; however, their bacterial vaginosis and douching prevalence rates were similar to those reported in a nationally representative survey (35, 63). Loss to follow-up may be an additional limitation, since only 50 percent of participants provided data in all five intervals; however, we used statistical techniques to adjust for potentially informative dropout. Recurrence of bacterial vaginosis may represent a prior infection that was unresponsive to treatment (64). The distinction between bacterial vaginosis reinfection and relapse remains unclear; however, it is well documented that bacterial vaginosis fluctuates over the course of a menstrual cycle (25, 61, 65). Rapid fluctuations in vaginal flora have also been documented by Priestley et al. (66) and Hay et al. (67) in their prospective studies of daily vaginal flora sampling, and the 3-month intervals which separated assessments in our study may necessarily represent a gross assessment over 12 months of the effect of douching on bacterial vaginosis. In addition, we did not address participants' varied frequencies of douching. Although there were relatively few women who douched weekly, these women were categorized with women who douched monthly or less frequently. It is also not known whether specific ingredients (33) in douche products might cause a differential effect. The marginal structural model assumes the absence of unmeasured confounding, but in spite of adjustment for a comprehensive survey of time-varying sexual and hygiene behaviors, vaginal symptoms, and other relevant covariates, we cannot rule this out (68). Another report from the Longitudinal Study of Vaginal Flora found that vaginal discharge and malodor, the two classic symptoms of bacterial vaginosis, are only slightly more frequently reported by women with bacterial vaginosis, and reporting of any vaginal symptoms upon direct questioning was common in all women (41). Lastly, the Longitudinal Study of Vaginal Flora was conducted among reproductive-age women, and thus we cannot address the effect of douching in girls, adolescents, and women over age 45 years.
In summary, douching was common in this cohort, and there was a spectrum of douching patterns. Our findings indicate that vaginal douching may confer increased risk for disruption of vaginal flora. In the absence of a randomized trial, these findings provide the best evidence to date of a risk of bacterial vaginosis associated with douching.
Acknowledgments
Conflict of interest: none declared.
Glossary
Abbreviations
- CI
confidence interval
- MICE
multivariate imputation by chained equations
References
- 1.Klebanoff SJ, Hillier SL, Eschenbach DA, et al. Control of the microbial flora of the vagina by H2O2-generating lactobacilli. J Infect Dis. 1991;164:94–100. doi: 10.1093/infdis/164.1.94. [DOI] [PubMed] [Google Scholar]
- 2.Pybus V, Onderdonk AB. Evidence for a commensal, symbiotic relationship between Gardnerella vaginalis and Prevotella bivia involving ammonia: potential significance for bacterial vaginosis. J Infect Dis. 1997;175:406–13. doi: 10.1093/infdis/175.2.406. [DOI] [PubMed] [Google Scholar]
- 3.Amsel R, Totten PA, Spiegel CA, et al. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74:14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 4.Ness RB, Kip KE, Hillier SL, et al. A cluster analysis of bacterial vaginosis-associated microflora and pelvic inflammatory disease. Am J Epidemiol. 2005;162:585–90. doi: 10.1093/aje/kwi243. [DOI] [PubMed] [Google Scholar]
- 5.Goldenberg RL, Andrews WW, Yuan AC, et al. Sexually transmitted diseases and adverse outcomes of pregnancy. Clin Perinatol. 1997;24:23–41. [PubMed] [Google Scholar]
- 6.Gravett MG, Nelson HP, DeRouen T, et al. Independent associations of bacterial vaginosis and Chlamydia trachomatis infection with adverse pregnancy outcome. JAMA. 1986;256:1899–903. [PubMed] [Google Scholar]
- 7.Hillier SL, Nugent RP, Eschenbach DA, et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. N Engl J Med. 1995;333:1737–42. doi: 10.1056/NEJM199512283332604. [DOI] [PubMed] [Google Scholar]
- 8.McDonald HM, O'Loughlin JA, Jolley P, et al. Prenatal microbiological risk factors associated with preterm birth. Br J Obstet Gynaecol. 1992;99:190–6. doi: 10.1111/j.1471-0528.1992.tb14497.x. [DOI] [PubMed] [Google Scholar]
- 9.Meis PJ, Goldenberg RL, Mercer B, et al. The preterm prediction study: significance of vaginal infections. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Obstet Gynecol. 1995;173:1231–5. doi: 10.1016/0002-9378(95)91360-2. [DOI] [PubMed] [Google Scholar]
- 10.Martin HL, Richardson BA, Nyange PM, et al. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis. 1999;180:1863–8. doi: 10.1086/315127. [DOI] [PubMed] [Google Scholar]
- 11.Martin HL, Jr, Nyange PM, Richardson BA, et al. Hormonal contraception, sexually transmitted diseases, and risk of heterosexual transmission of human immunodeficiency virus type 1. J Infect Dis. 1998;178:1053–9. doi: 10.1086/515654. [DOI] [PubMed] [Google Scholar]
- 12.Taha TE, Hoover DR, Dallabetta GA, et al. Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. AIDS. 1998;12:1699–706. doi: 10.1097/00002030-199813000-00019. [DOI] [PubMed] [Google Scholar]
- 13.Lamont RF, Morgan DJ, Wilden SD, et al. Prevalence of bacterial vaginosis in women attending one of three general practices for routine cervical cytology. Int J STD AIDS. 2000;11:495–8. doi: 10.1258/0956462001916371. [DOI] [PubMed] [Google Scholar]
- 14.Morris M, Nicoll A, Simms I, et al. Bacterial vaginosis: a public health review. BJOG. 2001;108:439–50. doi: 10.1111/j.1471-0528.2001.00124.x. [DOI] [PubMed] [Google Scholar]
- 15.Koumans EH, Sternberg M, Bruce C, et al. The prevalence of bacterial vaginosis in the United States, 2001 –2004; associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis. 2007;34:864–9. doi: 10.1097/OLQ.0b013e318074e565. [DOI] [PubMed] [Google Scholar]
- 16.Sewankambo N, Gray RH, Wawer MJ, et al. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet. 1997;350:546–50. doi: 10.1016/s0140-6736(97)01063-5. [DOI] [PubMed] [Google Scholar]
- 17.Wawer MJ, Sewankambo NK, Serwadda D, et al. Control of sexually transmitted diseases for AIDS prevention in Uganda: a randomised community trial. Rakai Project Study Group. Lancet. 1999;353:525–35. doi: 10.1016/s0140-6736(98)06439-3. [DOI] [PubMed] [Google Scholar]
- 18.Bump RC, Buesching WJ., 3rd Bacterial vaginosis in virginal and sexually active adolescent females: evidence against exclusive sexual transmission. Am J Obstet Gynecol. 1988;158:935–9. doi: 10.1016/0002-9378(88)90097-x. [DOI] [PubMed] [Google Scholar]
- 19.Berger BJ, Kolton S, Zenilman JM, et al. Bacterial vaginosis in lesbians: a sexually transmitted disease. Clin Infect Dis. 1995;21:1402–5. doi: 10.1093/clinids/21.6.1402. [DOI] [PubMed] [Google Scholar]
- 20.Marrazzo JM, Koutsky LA, Eschenbach DA, et al. Characterization of vaginal flora and bacterial vaginosis in women who have sex with women. J Infect Dis. 2002;185:1307–13. doi: 10.1086/339884. [DOI] [PubMed] [Google Scholar]
- 21.Beigi RH, Wiesenfeld HC, Hillier SL, et al. Factors associated with absence of H2O2-producing Lactobacillus among women with bacterial vaginosis. J Infect Dis. 2005;191:924–9. doi: 10.1086/428288. [DOI] [PubMed] [Google Scholar]
- 22.Koumans EH, Markowitz LE, Berman SM, et al. A public health approach to adverse outcomes of pregnancy associated with bacterial vaginosis. Int J Gynaecol Obstet. 1999;67(suppl 1):S29–33. doi: 10.1016/s0020-7292(99)00136-8. [DOI] [PubMed] [Google Scholar]
- 23.Ness RB, Hillier S, Richter HE, et al. Can known risk factors explain racial differences in the occurrence of bacterial vaginosis? J Natl Med Assoc. 2003;95:201–12. [PMC free article] [PubMed] [Google Scholar]
- 24.Schmid GP. The epidemiology of bacterial vaginosis. Int J Gynaecol Obstet. 1999;67(suppl 1):S17–20. doi: 10.1016/s0020-7292(99)00133-2. [DOI] [PubMed] [Google Scholar]
- 25.Schwebke JR, Richey CM, Weiss HL. Correlation of behaviors with microbiological changes in vaginal flora. J Infect Dis. 1999;180:1632–6. doi: 10.1086/315065. [DOI] [PubMed] [Google Scholar]
- 26.Tchamouroff SE, Panja SK. The association between receptive cunnilingus and bacterial vaginosis. Sex Transm Infect. 2000;76:144–5. doi: 10.1136/sti.76.2.144-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holzman C, Leventhal JM, Qiu H, et al. Factors linked to bacterial vaginosis in nonpregnant women. Am J Public Health. 2001;91:1664–70. doi: 10.2105/ajph.91.10.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hutchinson KB, Kip KE, Ness RB, et al. Vaginal douching and development of bacterial vaginosis among women with normal and abnormal vaginal microflora. Sex Transm Dis. 2007;34:671–5. doi: 10.1097/01.olq.0000258435.34879.da. [DOI] [PubMed] [Google Scholar]
- 29.Newton ER, Piper JM, Shain RN, et al. Predictors of the vaginal microflora. Obstet Gynecol. 2001;184:845–53. doi: 10.1067/mob.2001.113848. [DOI] [PubMed] [Google Scholar]
- 30.Rajamanoharan S, Low N, Jones SB, et al. Bacterial vaginosis, ethnicity, and the use of genital cleaning agents: a case control study. Sex Transm Dis. 1999;26:404–9. doi: 10.1097/00007435-199908000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Schwebke JR, Desmond RA, Oh MK. Predictors of bacterial vaginosis in adolescent women who douche. Sex Transm Dis. 2004;31:433–6. doi: 10.1097/01.olq.0000129948.91055.9f. [DOI] [PubMed] [Google Scholar]
- 32.Monif GR. The great douching debate: to douche, or not to douche. Obstet Gynecol. 1999;94:630–1. [PubMed] [Google Scholar]
- 33.Pavlova SI, Tao L. In vitro inhibition of commercial douche products against vaginal microflora. Infect Dis Obstet Gynecol. 2000;8:99–104. doi: 10.1002/(SICI)1098-0997(2000)8:2<99::AID-IDOG7>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cone RA, Hoen T, Wong X, et al. Vaginal microbicides: detecting toxicities in vivo that paradoxically increase pathogen transmission. BMC Infect Dis. 2006;6:90. doi: 10.1186/1471-2334-6-90. (Electronic article) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sutton MY, Bruce C, Sternberg MR, et al. Prevalence and correlates of vaginal douching among women in the United States, 2001 –2002. Presented at the 2006 National STD Prevention Conference, Jacksonville, Florida, May 8–11, 2006. ( http://cdc.confex.com/cdc/std2006/techprogram/P11190.HTM) [Google Scholar]
- 36.Klebanoff MA, Andrews WW, Yu KF, et al. A pilot study of vaginal flora changes with randomization to cessation of douching. Sex Transm Dis. 2006;33:610–13. doi: 10.1097/01.olq.0000216050.41305.c1. [DOI] [PubMed] [Google Scholar]
- 37.Brotman RM, Ghanem KG, Klebanoff MA, et al. The effect of vaginal douching cessation on bacterial vaginosis: a pilot study. Am J Obstet Gynecol. 2008 doi: 10.1016/j.ajog.2007.11.043. Feb 21 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Onderdonk AB, Delaney ML, Hinkson PL, et al. Quantitative and qualitative effects of douche preparations on vaginal microflora. Obstet Gynecol. 1992;80:333–8. [PubMed] [Google Scholar]
- 39.Monif GR, Thompson JL, Stephens HD, et al. Quantitative and qualitative effects of povidone-iodine liquid and gel on the aerobic and anaerobic flora of the female genital tract. Am J Obstet Gynecol. 1980;137:432–8. doi: 10.1016/0002-9378(80)91123-0. [DOI] [PubMed] [Google Scholar]
- 40.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–60. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 41.Klebanoff MA, Schwebke JR, Zhang J, et al. Vulvovaginal symptoms in women with bacterial vaginosis. Obstet Gynecol. 2004;104:267–72. doi: 10.1097/01.AOG.0000134783.98382.b0. [DOI] [PubMed] [Google Scholar]
- 42.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of Gram stain interpretation. J Clin Microbiol. 1991;29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ness RB, Hillier SL, Richter HE, et al. Why women douche and why they may or may not stop. Sex Transm Dis. 2003;30:71–4. doi: 10.1097/00007435-200301000-00014. [DOI] [PubMed] [Google Scholar]
- 44.Brotman RM, Klebanoff MA, Nansel T, et al. Why do women douche? A longitudinal study with two analytic approaches. Ann Epidemiol. 2008;18:65–73. doi: 10.1016/j.annepidem.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 45.Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the joint causal effect of nonrandomized treatments. J Am Stat Assoc. 2001;96:440–8. [Google Scholar]
- 46.Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11:561–70. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 47.Royston P. Multiple imputation of missing values: update. Stata J. 2005;5:188–201. [Google Scholar]
- 48.Rubin DB. New York, NY: John Wiley and Sons, Inc; 1987. Multiple imputation for nonresponse in surveys. [Google Scholar]
- 49.Little RJA, Rubin DB. New York, NY: John Wiley and Sons, Inc; 1987. Statistical analysis with missing data. [Google Scholar]
- 50.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 51.Juliano C, Piu L, Gavini E, et al. In vitro antibacterial activity of antiseptics against vaginal lactobacilli. Eur J Clin Microbiol Infect Dis. 1992;11:1166–9. doi: 10.1007/BF01961138. [DOI] [PubMed] [Google Scholar]
- 52.Ness RB, Kip KE, Soper DE, et al. Variability of bacterial vaginosis over 6- to 12-month intervals. Sex Transm Dis. 2006;33:381–5. doi: 10.1097/01.olq.0000204748.89222.33. [DOI] [PubMed] [Google Scholar]
- 53.Hawes SE, Hillier SL, Benedetti J, et al. Hydrogen peroxide-producing lactobacilli and acquisition of vaginal infections. J Infect Dis. 1996;174:1058–63. doi: 10.1093/infdis/174.5.1058. [DOI] [PubMed] [Google Scholar]
- 54.Marrazzo JM. A persistent(ly) enigmatic ecological mystery: bacterial vaginosis. J Infect Dis. 2006;193:1475–7. doi: 10.1086/503783. [DOI] [PubMed] [Google Scholar]
- 55.Ferris MJ, Masztal A, Aldridge KE, et al. Association of Atopobium vaginae, a recently described metronidazole resistant anaerobe, with bacterial vaginosis. BMC Infect Dis. 2004;4:5. doi: 10.1186/1471-2334-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med. 2005;353:1899–911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- 57.Lichtenstein B, Nansel TR. Women's douching practices and related attitudes: findings from four focus groups. Women Health. 2000;31:117–31. doi: 10.1300/j013v31n02_06. [DOI] [PubMed] [Google Scholar]
- 58.Larsson PG. Treatment of bacterial vaginosis. Int J STD AIDS. 1992;3:239–47. doi: 10.1177/095646249200300402. [DOI] [PubMed] [Google Scholar]
- 59.Sobel JD, Schmitt C, Meriwether C. Long-term follow-up of patients with bacterial vaginosis treated with oral metronidazole and topical clindamycin. J Infect Dis. 1993;167:783–4. doi: 10.1093/infdis/167.3.783. [DOI] [PubMed] [Google Scholar]
- 60.Schwebke JR, Morgan SC, Weiss HL. The use of sequential self-obtained vaginal smears for detecting changes in the vaginal flora. Sex Transm Dis. 1997;24:236–9. doi: 10.1097/00007435-199704000-00009. [DOI] [PubMed] [Google Scholar]
- 61.Eschenbach DA, Thwin SS, Patton DL, et al. Influence of the normal menstrual cycle on vaginal tissue, discharge, and microflora. Clin Infect Dis. 2000;30:901–7. doi: 10.1086/313818. [DOI] [PubMed] [Google Scholar]
- 62.Hassan WM, Lavreys L, Chohan V, et al. Associations between intravaginal practices and bacterial vaginosis in Kenyan female sex workers without symptoms of vaginal infections. Sex Transm Dis. 2007;34:384–8. doi: 10.1097/01.olq.0000243624.74573.63. [DOI] [PubMed] [Google Scholar]
- 63.Allsworth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001 –2004 National Health and Nutrition Examination Survey data. Obstet Gynecol. 2007;109:114–20. doi: 10.1097/01.AOG.0000247627.84791.91. [DOI] [PubMed] [Google Scholar]
- 64.Sobel JD. Vaginitis. N Engl J Med. 1997;337:1896–903. doi: 10.1056/NEJM199712253372607. [DOI] [PubMed] [Google Scholar]
- 65.Keane FE, Ison CA, Taylor-Robinson D. A longitudinal study of the vaginal flora over a menstrual cycle. Int J STD AIDS. 1997;8:489–94. doi: 10.1258/0956462971920631. [DOI] [PubMed] [Google Scholar]
- 66.Priestley CJ, Jones BM, Dhar J, et al. What is normal vaginal flora? Genitourin Med. 1997;73:23–8. doi: 10.1136/sti.73.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hay PE, Ugwumadu A, Chowns J. Sex, thrush and bacterial vaginosis. Int J STD AIDS. 1997;8:603–8. doi: 10.1258/0956462971918850. [DOI] [PubMed] [Google Scholar]
- 68.Eschenbach DA. Bacterial vaginosis and anaerobes in obstetric-gynecologic infection. Clin Infect Dis. 1993;16(suppl 4):S282–7. doi: 10.1093/clinids/16.supplement_4.s282. [DOI] [PubMed] [Google Scholar]