Abstract
In animal studies of nociception, females are often more sensitive to painful stimuli, whereas males are often more sensitive to analgesia induced by μ agonists. Sex differences are found even at birth, and in adulthood are likely caused, at least in part, by differences in levels of gonadal hormones. Here we investigate nociception and analgesia in neonatal mice, and assess the contribution of the direct action of sex chromosome genes in hotplate and tail withdrawal tests. We used the four core genotypes mouse model, in which gonadal sex is independent of the complement of sex chromosomes (XX vs. XY). Mice were tested at baseline and then injected with μ-opioid agonist morphine (10mg/kg), or with the κ-opioid agonist U50,488H (U50, 12.5mg/Kg) with or without the N-methyl-D-aspartate (NMDA) receptor antagonist, MK-801 (0.1mg/kg). On the day of birth, XX mice showed faster baseline latencies than XY in tail withdrawal, irrespective of their gonadal type. Gonadal males showed greater effects of morphine than gonadal females in the hotplate test, irrespective of their sex chromosome complement. U50 and morphine were both effective analgesics in both tests, but MK-801 did not block the U50 effect. The results suggest that sex chromosome complement and gonadal secretions both contribute to sex differences in nociception and analgesia by the day of birth.
Perspective: Sex differences in pain may stem not only from the action of gonadal hormones on pain circuits, but from the sex-specific action of X and Y genes. Identification of sex chromosome genes causing sex differences could contribute to better pain therapy in females and males.
Keywords: pain, sex difference, hotplate, tail withdrawal, sex chromosomes, neonate
Introduction
In various species including humans, pain perception and sensitivity to analgesic drugs can differ in two sexes. Females are generally more sensitive to pain and/or differ from males in neural circuits mediating nociception.5, 18, 30 In mice, a variety of NMDA antagonists block the acute analgesic effects of μ- or k-agonists or development of tolerance to morphine.6, 12, 33, 34, 37, 41 However, specific NMDA antagonists block the morphine analgesia only in females, whereas other NMDA antagonists block analgesia in both sexes.37 Estradiol activates a female-specific mechanism, insensitive to NMDA antagonists and mediated by the melanocortin-1 receptor, that appears to be absent in males.33, 34
In animals including mice, μ-agonists such as morphine are more effective in males than females,11, 12, 27, 32, 35 although the literature is complex and contradictory.12 Activational and organizational effects of steroids contribute to sex differences in opioid analgesia.9, 10, 12, 25, 32 For example, estradiol can reduce morphine analgesia12 and testosterone can increase opioid analgesia, because of activational effects.33, 42 In addition, treating neonatal female mice with testosterone permanently masculinizes their response to morphine, and castrating males at birth demasculinizes them (organizational effects).9, 25 Analgesia produced by the selective k-opioid receptor agonist U50,488H (U50) shows sex differences in the same direction as the μ-opioid receptor agonist morphine.34, 40 U50 can be more potent in males than in females, and the U50 effects are blocked specifically in males by MK-801.34, 40 However, organizational and activational effects of gonadal steroids do not account for all of the sex differences in nociception and drug-induced analgesia.
Sex differences are also caused by “sex chromosome effects” which are direct actions of X and Y genes on non-gonadal cells.1–3 Here we used the “four core genotypes” (FCG) model to study the biological origins of sex differences in nociception and analgesia in neonatal mice. In this mouse model the complement of sex chromosomes (XX vs. XY) is independent of the gonadal sex (testes vs. ovaries) of the mouse.3, 7, 8, 16, 20, 21, 38
We recently observed that neonatal XX mice of either gonadal sex have higher expression in brain of prodynorphin mRNA than XY mice (Chen et al., in preparation). That finding suggested that dynorphin levels might be higher in XX than XY mice, which might alter k-opioid nociception. μ- and k-receptors are found in neonatal rodent spinal cord and brain.39 We therefore asked whether neonatal mice show hormonal and sex chromosome effects on nociception and μ-and k-mediated analgesia, and whether NMDA antagonist MK801 could block any U50 effect. We found that morphine and U50 were both effective analgesics at this age, but that the NMDA antagonist MK-801 did not block the effects of U50 as it does in adults.40 Groups differing in sex chromosome complement and in gonadal sex showed differences in the two assays of nociception.
Materials and Methods
Mice
In FCG mice, the testis-determining Sry gene is deleted from the Y chromosome, producing the “Y minus” chromosome, Y−, so that the Y− chromosome no longer determines gonadal sex.3, 26, 28 An Sry transgene is inserted onto an autosome, producing XY−Sry males. Breeding these males with XX females produces four types of progeny. XX females, XY− females, XXSry males and XY−Sry males. Here we define “male” and “female” according to gonadal sex. Comparing the phenotype of males (XXSry and XY−Sry) and females (XX and XY−) tests for the effects of presence or absence of Sry. The effects of Sry are thought to be mediated primarily by sex differences in gonadal secretions, although direct effects of Sry on the brain also occur.17 On the other hand, comparing XX mice (XX females and XXSry males) with XY− mice (XY− females and XY−Sry males) tests for the differential effects of sex chromosome complement (XX vs. XY). The FCG model tests simultaneously for the effects of gonadal steroids, for sex chromosome effects, and their interaction (e.g., hormonal effects that occur only in XY but not XX mice). In the present experiment, we used random bred MF1 mice kindly provided to us by Paul Burgoyne (National Institute for Medical Research, London UK) in which the MF1 X chromosome of all mice was invariant (i.e., had no allelic variation across animals, a substrain produced by breeding male mice to their XO mothers). The Y− chromosome derives from strain 129.26
Mice were quasi-randomly assigned to groups, attempting to maximize the number of groups represented within each litter. The sex and sex chromosome complement of each mouse was determined by PCR-amplifying from genomic DNA a Y chromosome sequence (to detect the presence / absence of the Y− chromosome), Sry (to detect the Sry transgene), and a control gene.
Experimental subjects and procedures
Experimental subjects were male (n=175, 91 XXSry and 84 XY−Sry) and female (n=146, 67 XX and 79 XY−) FCG MF1 mice, bred in our colony. Animals were tested on the day of birth (P1) between 8AM and 12PM. Animals were housed in a light controlled (L:D 12:12, lights on at 7AM) and temperature controlled (21±1°C) environment. XY−Sry males were each housed with pairs of XX females for two weeks. Dams were then separated from males and singly housed for the duration of the pregnancy with no disruption except for the routine cage maintenance. Dams were given free access to standard laboratory food and tap water. As parturition approached, the cages were observed daily for the presence of pups. On the day of birth, pups were removed from the dam, weighed, and placed in individual cardboard containers. The containers were kept on a warming pad for the duration of the testing session. Two tests of nociception were performed as described below. Pups within each litter were randomly assigned to one of the drug conditions. Immediately after hot plate and tail withdrawal baseline assessment, the pup was injected with a drug (see below), and returned to its container. Pups were retested on both pain assays 15, 45 and 90 minutes after injection by an experimenter who was blind to the drug condition and sex / sex chromosome complement. All procedures were approved by the UCLA Chancellor’s Animal Research Committee and conformed to applicable national and international guidelines.
Tests of nociception
Mice were tested on two different behavioral tests of thermal nociception as described by Sternberg et al.:41 the hotplate and tail withdrawal assays, which show diverse sensitivity to drugs and sex. Both the hotplate and tail withdrawal assays have been used extensively in adult rodents and have also been adapted for use in neonates previously.41
In the hotplate test, the experimenter held the pup between the thumb and forefinger in an upright position and gently placed one hindfoot of the mouse on the surface of the hot plate (52.5°C, AccuScan Instruments). Latency to remove the foot from the surface was recorded, with a 15 second cutoff time for non-responsive animals. The procedure was repeated on the opposite foot after a 10 second interval, and an average of the latencies for both feet was used in the analysis. Foot withdrawal was measured at time T=0 (before injection), and 15, 45 and 90 minutes later.
In the tail withdrawal test, the pup was held in the same manner, and the distal tip of the tail was lowered into a water bath maintained at 50°C. The latency to vigorous tail withdrawal was recorded, with a 15 second cutoff time. The test was repeated after a 10-second interval and the latencies averaged. The tail withdrawal test was performed one minute after the hotplate test, at each of the time points (0, 15, 45, and 90).
Drugs
To study the acute effects of morphine, pups were injected subcutaneously (s.c.) with morphine at one of three doses, 0, 1 or 10 mg/kg, immediately after the baseline tests of hotplate and tail withdrawal at time T=0. Pups were tested again on both assays at 15 and 45 minutes after injection. Group sizes were 10–15 for each group (i.e., one sex chromosome complement in one sex at one drug treatment dose).
To study the effect of a k-opiate agonist and interactions with the NMDA system, pups were injected s.c. with the NMDA antagonist MK-801 (MK; 0.1 mg/kg; Sigma, Indianapolis, IN) or saline vehicle (SAL) immediately prior to injection of the k agonist U50,488H (12.5mg/kg Sigma) or saline vehicle (SAL). Pups were tested again on both assays at 15, 45 and 90 minutes after injection. Although NMDA systems that mediate opioid analgesia are reported not to have matured at birth in rats,23, 45, 46 we rationalized that testing for NMDA system involvement in U50 analgesia in neonatal mice was warranted because of the robust sex differences reported in the effects of NMDA antagonists on U50 analgesia in adult mice.40
Morphine, MK and U50 were dissolved in saline in a volume of 10ml/kg. Group sizes were 7–20 (usually 10–15) for each combination of sex, sex chromosome complement, and drug regime.
Statistical analysis of data
The experimental design included three between factors (sex chromosome, gonadal sex and drug) and one within factor (time). Baseline hot plate and tail withdrawal latencies were analyzed with a 2-way ANOVA with factors of sex (male vs. female) and sex chromosome (XX vs. XY). The response to analgesic drugs was analyzed using a mixed factorial four-way ANOVA on nociceptive latencies with one within factor (time) and three between factors of sex chromosome, sex, and drug. The initial analysis of data showed that the effect of 1mg/kg morphine did not differ from the effect of saline. Therefore for all subsequent statistical tests we combined the data for the 0 and 1 mg/kg morphine doses to increase statistical power. Thus the drug groups in the morphine study were morphine 0 and 1 vs. morphine 10. In the U50 study, the four drug groups were saline-saline (sal), saline-U50 (U50), MK-saline (MK-sal), and MK-U50. In the morphine study and in the study of U50 effects on tail withdrawal test, because some groups differed in their baseline latencies, we used the dependent variable of the percentage of maximum possible effect of morphine (%MPE = 100 * (latency – baseline) / (15-baseline)). In the U50 study, however, there were no group differences in hotplate baseline latencies, so that we used response latency in seconds as the dependent variable. The significance level was set at α = 0.05. Post-hoc tests were Tukey’s test.
Results
Hotplate and tail withdrawal baselines
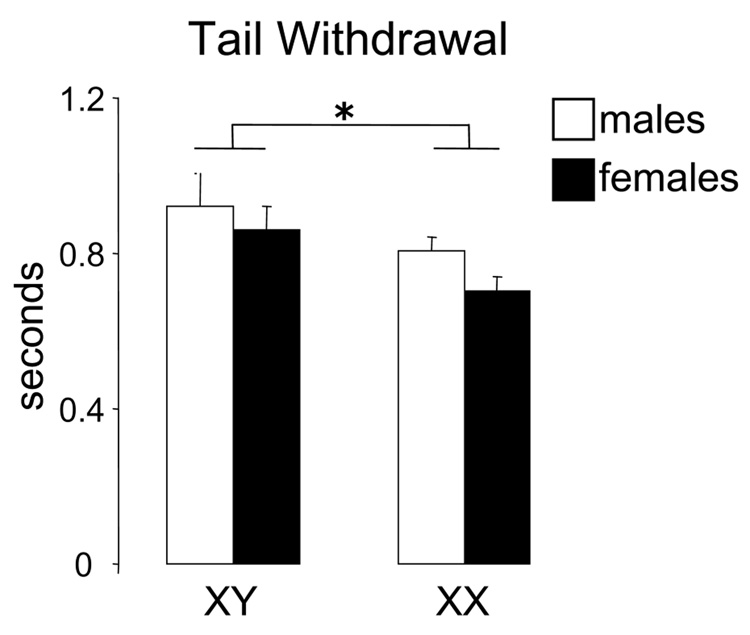
In the baseline measurements of the hotplate test, there were no group differences of experimental subjects due to gonadal sex or sex chromosome complement, in the morphine or the U50 experiments (p>0.05). In the tail withdrawal assay, however, there was an overall main effect of sex chromosomes on tail withdrawal baseline latencies (XY > XX, F(1,317)=5.14, p=0.02, Fig. 1, XY n=163 and XX n=158).
Figure 1.
In the baseline tail withdrawal tests for all experiments, XX mice showed faster latencies than XY mice, irrespective of their gonadal sex (*p=0.02). Mean latencies were XY male 0.92, XY female 0.86, XX male 0.80, XX female 0.70 seconds.
Morphine study: analgesia in hotplate and tail withdrawal assays
Hotplate
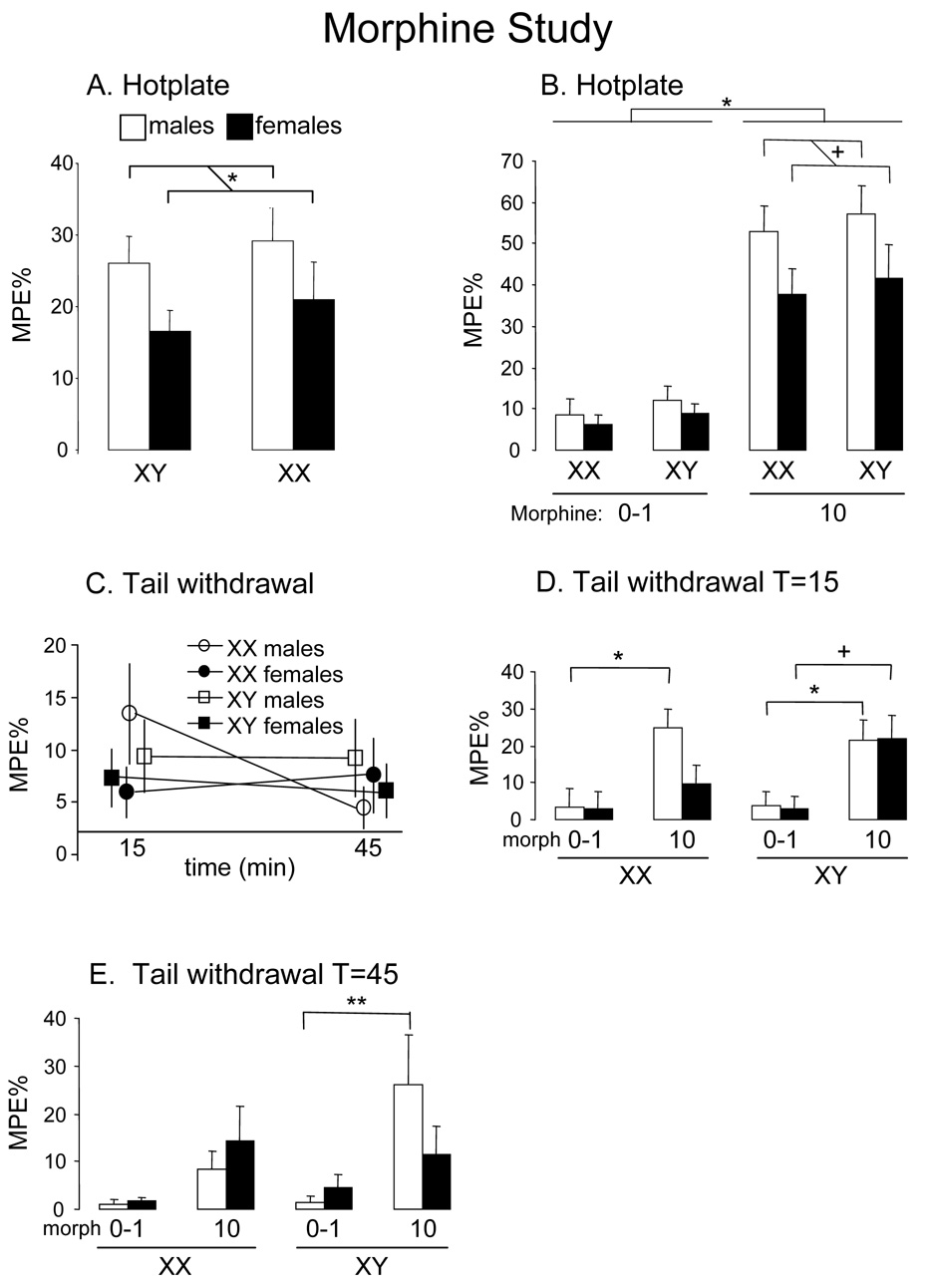
Hotplate tests showed effects of sex but not sex chromosome. As expected, morphine significantly increased %MPE for foot withdrawal on the hotplate (main effect of drug: (F(1, 131)=130; p=0.000001). In the 4-way ANOVA, there was a significant main effect of sex (F(1, 131)=7.06; p=0.009): males had higher %MPE than females (Fig. 2a). Interestingly, there was a trend towards significance in the sex by drug interaction (F(1, 131)=3.55; p=0.06), because males had greater analgesia (higher %MPE) than females in response to morphine (p=0.02, Tukey test comparing 10mg/kg males and females) (Fig. 2b).
Figure 2.
Results of the hotplate and tail withdrawal assays in the morphine study. A. Averaging over all drug (including saline) and time conditions in the hotplate test, males had a higher %Maximum Possible Effect (%MPE) than females (*p=0.009). B. The graphs shows %MPE averaged across time. In the hotplate test, morphine at 10mg/kg had significant analgesic effect (*p=0.000001) relative to the 0–1 mg/kg morphine group. The response to morphine tended to be higher in males than females irrespective of their sex chromosome complement (p=0.06 sex by drug interaction; +, p=0.02 comparison of males vs. females at 10mg/kg). C. Graphs show results averaged across all drug doses, including saline, so that the main effects of sex and sex chromosome complement can be seen. In the tail-withdrawal test, the %MPE over time differed as a function of both sex and sex chromosome complement because XX males had a more variable response than the other three groups (sex by sex chromosome by time interaction, p=0.035). D and E. In the tail withdrawal test, there was a statistically significant interaction of time, sex chromosome, sex, and drug (p=0.015), meaning that the effects of sex chromosome, sex, and drug were different at different times. To illustrate those differences, the %MPE are graphed separately for T=15 and T=45 minutes. At T=15 minutes after morphine injection, there was a differential effect of morphine on XX vs. XY females (+, p=0.07 in XY females and not significant for XX females) but not between XX and XY males (* both p<0.05). At 45 minutes after injection (T=45), the effect of morphine was significant only in XY males (**p =0.0002).
Tail withdrawal
Tail withdrawal showed effects of sex and sex chromosome complement. Morphine significantly increased %MPE in the tail withdrawal test (main effect of drug: F(1, 131)=30.06; p<0.000001) (Fig. 2c). In the four-way ANOVA, XX and XY mice, and male and female mice, responded differently over time (significant interaction between time, sex chromosome and sex, F(1, 131)=4.54; p=0.035), which can be seen in Fig. 2c as a lower %MPE for XX males at T=45 than at T15. There was also a significant interaction of time by sex chromosome by sex by drug (F(1, 131)=6.03; p=0.015). That significant effect is illustrated by group differences in Figs. 2d and 2e. Several factors appear to explain the statistically significant interaction. At T=15, morphine nearly increased %MPE in XY females but not XX females (XY females treated with morphine vs. controls, Tukey test p=0.07). In males, at T=15 (Fig. 2d) and T=45 (Fig. 2e), morphine increased %MPE in XY males (vs. controls: p=0.045 at T=15 and p=0.0002 at T=45), whereas morphine increased latency in XX males only at T=15 (vs. controls: p=0.014).
U50 study: Effects of acute U50 and MK on hotplate and tail withdrawal assays
Hotplate
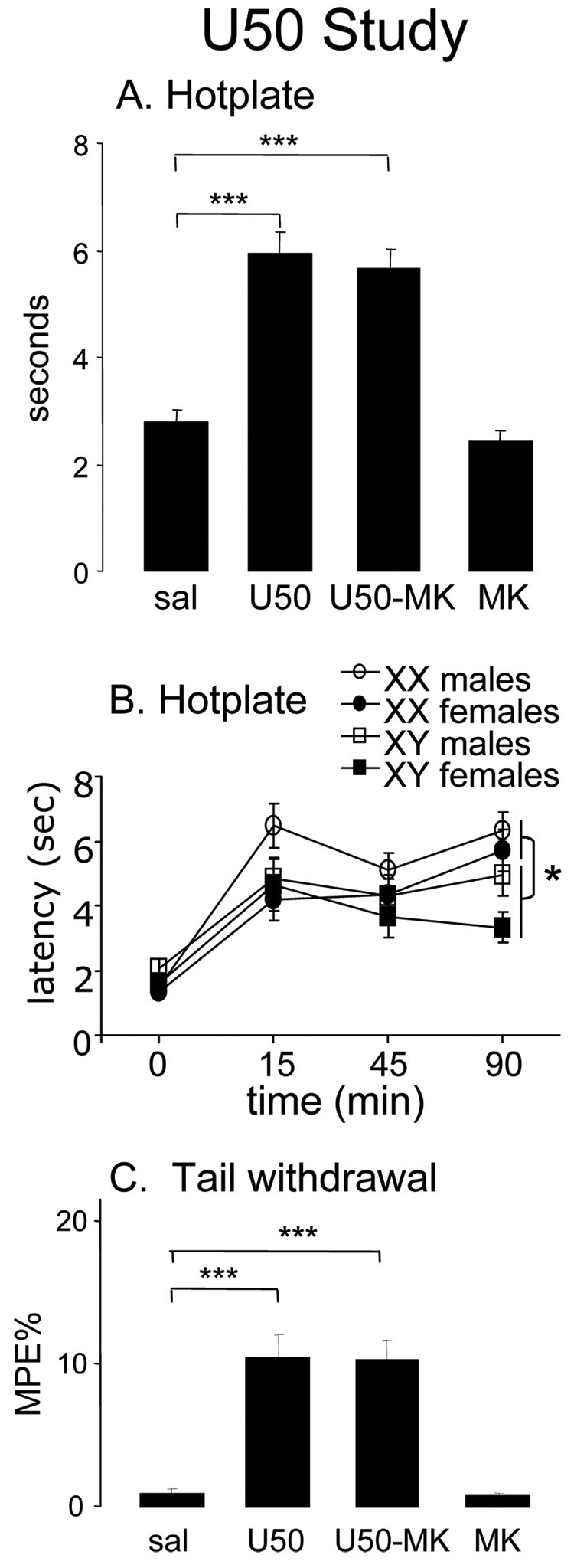
Hotplate tests showed effects of sex chromosome complement. As expected, the drugs significantly influenced latencies for foot withdrawal from the hotplate (main effect of drug: (F(3, 166)=31.3; p=0.000001): U50 significantly increased hotplate latencies, but MK did not block U50 analgesia (U50 vs. sal, p<0.0001; U50-MK vs. sal, p<0.0001) (Fig. 3a). In the overall 4-way ANOVA, there was a trend in the main effect of sex (F(1, 166)=3.49; p=0.06): males tended to have higher hot plate latencies than females (males, mean ± standard error=4.52 ± 0.207; females, 3.60 ± 0.215). Interestingly, the time by sex chromosome interaction was significant (F(3, 496)=3.42; p=0.02), because XY mice differed from XX only at T=90 when XX mice had longer latencies than XY regardless of their treatment (p<0.05, Tukey) (Fig. 3b). (In other words, at T=90 XX mice of all treatment groups considered together had longer latencies than XY mice of all treatment groups considered together.)
Figure 3.
Hotplate and tail withdrawal assays in the U50 study. A, Averaging across all groups before and after injection in the hotplate test, the U50 group had longer latencies than controls (U50 vs. sal, ***p<0.0001), but MK did not block the effect of U50 (U50-MK vs. sal, ***p<0.0001). B, Hotplate latencies as a function of time and sex and sex chromosome complement. There was a significant interaction of time and sex chromosome complement -- XX and XY mice had longer latencies than XX mice only at T=90 (*p<0.05). To show this interaction, which did not depend on drug treatment, data from all drug treatment groups, including saline, are combined for each of the FCG groups. C, Averaging across time and sex and sex chromosome complement, U50 with or without MK significantly increased latencies in the tail withdrawal test (U50 vs. sal, and U50-MK vs. sal, both ***p<0.00001).
Tail withdrawal
There were no significant main effects or interactions involving sex or sex chromosome complement. Drugs significantly affected %MPE in the tail withdrawal test (main effect of drug: F(3, 166)=16.4; p<0.000001) (Fig. 3c). U50 with or without MK was effective in increasing tail withdrawal %MPE when compared to saline animals (U50 vs. sal and U50-MK vs. sal: p<0.00001, Tukey test); and MK alone was no different than saline.
Discussion
We report here that sex chromosome complement (XX vs. XY) and gonadal sex (male vs. female) both influence the development of nociception and/or response to analgesic drugs by the day of birth in mice. In the FCG model using MF1 mice, neonatal XX mice showed greater basal nociception than XY mice in a test-dependent fashion (faster tail withdrawal but no difference in hotplate latencies), irrespective of their gonadal sex. Moreover, morphine was an effective analgesic at this age, but had a greater effect on gonadal males than females in the hotplate test, suggesting that differences in gonadal secretions prior to birth or on P1 caused these sex differences. The tail withdrawal test in the morphine study showed complex interactions of sex, sex chromosome complement, and time. We found that U50 had analgesic effects at this age on hotplate and tail withdrawal, which were not blocked by MK-801, and mostly did not differ according to gonadal sex or sex chromosome complement. In the study on the effects of U50 on the hotplate test, XX and XY mice differed only at one time point. In general, the results suggest that both gonadal hormones and sex chromosome complement contribute to sex differences in nociception and analgesia in newborn mice, although the effects of sex chromosome complement are modest and often limited to specific subsets of conditions tested. In some cases the two effects interact, suggesting that gonadal hormones have different effects in XX and XY mice. This is the first demonstration of sex chromosome effects on nociception and morphine analgesia in neonatal mice.
The differences between XX and XY groups are attributable to differences in the action of X or Y genes. Such differences include the effects of Y genes, and differences in the expression of X genes resulting from XX-XY differences in gene dose44 or genomic imprinting of the X chromosome.2, 13 The sex chromosome effects could be exerted directly on the brain to induce changes in nociception, or elsewhere in the body. It is possible, for example, that XX and XY males, or XX and XY females, differ from each other in the level of prenatal sex steroid secretions coming from the gonads or from other organs such as the adrenals or the brain itself. However, the pattern of the present and previous results argues against the idea that the sex chromosome effect on baseline tail withdrawal latencies are mediated by differences in gonadal hormones. XX and XY mice differed in their baseline response in the tail withdrawal test, irrespective of gonadal sex. Because FCG gonadal males are similarly masculinized in numerous brain and behavioral traits16, 20, 29, 43 including the response to morphine in hotplate tests of the present study, it is likely that both types of males experience similar male-typical levels of testicular hormones and that both female groups do not. In the tail withdrawal tests reported here, gonadal males and gonadal females did not differ in baseline latencies, suggesting that their large differences in gonadal secretions did not cause sexual differences of that response by P1 under the present conditions. In contrast, sex chromosome complement did cause a significant difference, irrespective of gonadal sex, which is therefore not likely a result of XX vs. XY differences in gonadal secretions in mice with either type of gonad. It is more likely that the effects of X or Y genes are exerted on non-gonadal tissues such as the brain or other components of the nociception pathways.
Sternberg et al.41 found sex differences in basal nociception and analgesic effects of morphine in neonatal CD-1 mice. Female neonates had longer latencies in a tail withdrawal test using parameters similar to those used here. In the same study, females tended to have shorter latencies than males in the hotplate test. These results contrast with the present results in which no effect of gonadal sex was found in either test. Sternberg et al.41 also found that analgesic effects of morphine lasted longer in males than in females when tested in the tail withdrawal paradigm. In the present study, males were more sensitive than females to the analgesic effects of morphine in the hotplate test. These two findings, although not equivalent, agree with the general finding that the analgesic effects of morphine are greater and last longer in males rather than in females.32, 41 When we tested the FCG neonates in the tail withdrawal test, morphine lasted longer in XY males than in XX males (Fig. 2E), whereas it did not have a significant analgesic effect in females (only a trend in XY females, Fig. 2D). The differences between the results of Sternberg et al.41 and ours could be attributed to several factors. The two studies used different strains (CD-1 vs. MF1). Moreover, many of the statistical tests and conclusions of Sternberg et al. 41 are based on combined analysis of P1 and P8 pups, in contrast to our focus only on P1. Moreover, we cannot exclude the idea that there are differences between normal XY males with an endogenous Sry gene encoded on the Y chromosome, and FCG mice in which gonadal males have an Sry transgene encoded on an autosome.16
At birth the neural circuits underlying nociception are incompletely developed. Studies in rats show that by the time of birth, nociceptive afferent C fibers express TRPV1 receptors mediating thermal pain, project into the dorsal horn, and show responses to nociceptive stimuli similar to adult C fibers.19 Central connections of polymodal pain receptors are established but immature on the day of birth. Neonatal rats have thresholds for withdrawal from heat that are lower than adults, and the dorsal horn neurons are more excitable. Especially immature at the time of birth are the descending inputs to the dorsal horn from the brainstem. For example, stimulation of periaqueductal gray (PAG), which induces analgesia in adults, does not have this effect until three weeks after birth.19 Similarly, the biphasic response to formalin injection into the paw, which is thought to reflect the function of the descending pathways, does not mature until P15.19 Thus, the nociceptive responses, and sex or sex chromosome effects, that we have observed here are likely mediated predominantly spinally or peripherally, assuming that the maturation of circuits is similar in rats and mice. Mu receptors are expressed in the rat dorsal horn and dorsal root ganglia by P1, and thus represent likely sites of action of morphine which has an analgesic effect in this and previous studies.36 Moreover, κ receptors are expressed in the rat spinal cord by the time of birth,39 thus the analgesic actions of U50 found here may be spinal, although peripheral analgesic effects of U50 have been reported and are also a possibility.4 Extensive studies in rats also demonstrate sensitivity of neonatal rodents to κ and μ agonists.22, 24, 31 The lack of effect of MK801 found here is probably explained by the lack of maturity of descending NMDA circuits in the brain (e.g., PAG) or at their termination onto spinal nociception circuits. The absence of an MK801 effect found here in mice is compatible with evidence in rats that NMDA receptor systems, and their involvement in nociception, mature in the first few weeks after birth.23, 45, 46
Sex differences have been reported in the analgesic effects of U50 and MK blockade. Intact adult male mice have been found to be more sensitive than females to analgesia produced by U50.40 Moreover, aged females had a male-like response to U50. We did not observe a differential effect of U50 or MK on males and females (no significant effect of gonadal sex or interaction of gonadal sex with other variables). Age and strain of animals might have contributed to the difference in results. Sternberg et al.40 used as experimental subjects male and female young adult mice and old female mice from the C57BL/6 strain, in contrast to our use of neonatal MF-1 FCG mice.
The biological origins of sex differences in pain and analgesia are likely to be complex. On the one hand, it is abundantly clear from previous studies that sex differences are widespread, that specific gonadal steroids alter the response to pain and analgesics in adulthood (activational effects), and that perinatal sex differences in levels of testosterone produce long-lasting organizational differences.12, 32 We have recently also found that gonadectomized adult XX mice respond faster than XY mice at baseline in hotplate tests on C57BL/6 FCG mice.21 That finding, together with the present evidence, suggests that the complement of sex chromosomes is also a factor contributing to sex differences in pain and analgesia. It is likely that the gonadal steroid effects interact in complex ways with the sex chromosome effects. The current results offer evidence for such complex interactions, for example because the sex chromosome effect in the tail withdrawal paradigm depended on gonadal sex. In XY males the effects of morphine were evident at both time points, whereas morphine decreased tail withdrawal latency in XX males only at T=15 (Fig. 2de). This result suggests that testosterone, secreted in males before or after birth, might have a different effect in XX than in XY mice. Alternatively, the levels of testosterone present in XX and XY males might differ. Among the females, the effects of morphine were not significant at both time points in this test. Such interaction between sex chromosome and gonadal sex is consistent with previous literature showing that males are usually more sensitive than females to morphine.32, 41 The effects of sex chromosome complement could be to enhance or counteract the effects of gonadal steroids.14, 15, 38 It will be important in future studies to manipulate the level of gonadal hormones in FCG mice directly, at different times of life, to determine how hormonal and sex chromosomal influences interact and the role they play in the maturation of pain circuits.
Acknowledgements
MF1 breeder mice were the kind gift of Paul Burgoyne. Thanks to Carolyn Lew-Karon for assistance. Supported by NIH grants NS045966 and NS043196.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Arnold AP. Concepts of genetic and hormonal induction of vertebrate sexual differentiation in the twentieth century, with special reference to the brain. In: Pfaff DW, Arnold AP, Etgen A, Fahrbach S, Rubin R, editors. Hormones, Brain, and Behavior. Academic Press; 2002. pp. 105–135. [Google Scholar]
- 2.Arnold AP. Sex chromosomes and brain gender. Nature Reviews Neuroscience. 2004;5:701–708. doi: 10.1038/nrn1494. [DOI] [PubMed] [Google Scholar]
- 3.Arnold AP, Burgoyne PS. Are XX and XY brain cells intrinsically different? Trends in Endocrinology and Metabolism. 2004;15:6–11. doi: 10.1016/j.tem.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Barr GA, Limon E, Luthmann RA, Barr GA, Cheng J, Wang S. Analgesia induced by local plantar injections of opiates in the formalin test in infant rats. Dev Psychobiol. 2003;42:111–122. doi: 10.1002/dev.10089. [DOI] [PubMed] [Google Scholar]
- 5.Berkley KJ. Sex differences in pain. Behavioral and Brain Sciences. 1997;20:371–380. doi: 10.1017/s0140525x97221485. [DOI] [PubMed] [Google Scholar]
- 6.Bryant CD, Eitan S, Sinchak K, Fanselow MS, Evans CJ. NMDA receptor antagonism disrupts the development of morphine analgesic tolerance in male, but not female C57BL/6J mice. Am J Physiol-Reg Int Comp Physiol. 2006;291:R315–R326. doi: 10.1152/ajpregu.00831.2005. [DOI] [PubMed] [Google Scholar]
- 7.Carruth LL, Reisert I, Arnold AP. Sex chromosome genes directly affect brain sexual differentiation. Nat Neurosci. 2002;5:933–934. doi: 10.1038/nn922. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Watkins R, Delot E, Releine R, Schistl RH, Burgoyne PS, Arnold AP. Sex difference in neural tube defects in p53-null mice is caused by differences in the complement of X not Y genes. Develop Neurobiol. 2008;68:265–273. doi: 10.1002/dneu.20581. [DOI] [PubMed] [Google Scholar]
- 9.Cicero TJ, Nock B, O'Connor L, Meyer ER. Role of steroids in sex differences in morphine-induced analgesia: activational and organizational effects. J Pharmacol Exp Ther. 2002;300:695–701. doi: 10.1124/jpet.300.2.695. [DOI] [PubMed] [Google Scholar]
- 10.Craft RM. Sex differences in drug- and non-drug-induced analgesia. Life Sci. 2003;72:2675–2688. doi: 10.1016/s0024-3205(03)00178-4. [DOI] [PubMed] [Google Scholar]
- 11.Craft RM. Sex differences in opioid analgesia: "from mouse to man". Clin J Pain. 2003;19:175–186. doi: 10.1097/00002508-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Craft RM, Mogil JS, Aloisi AM. Sex differences in pain and analgesia: the role of gonadal hormones. Eur J Pain. 2004;8:397–411. doi: 10.1016/j.ejpain.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Davies W, Isles AR, Burgoyne PS, Wilkinson LS. X-linked imprinting: effects on brain and behaviour. BioEssays. 2006;28:35–44. doi: 10.1002/bies.20341. [DOI] [PubMed] [Google Scholar]
- 14.De Vries GJ. Minireview: Sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinol. 2004;145:1063–1068. doi: 10.1210/en.2003-1504. [DOI] [PubMed] [Google Scholar]
- 15.De Vries GJ. Sex steroids and sex chromosomes at odds? Endocrinol. 2005;146:3277–3279. doi: 10.1210/en.2005-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci. 2002;22:9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dewing P, Chiang CWK, Sinchak K, Sim H, Fernagut PO, Kelly S, Chesselet MF, Micevych PE, Albrecht KH, Harley VR, Vilain E. Direct regulation of adult brain function by the male-specific factor SRY. Current Biology. 2006;16:415–420. doi: 10.1016/j.cub.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Fillingim RB. Sex differences in analgesic responses: evidence from experimental pain models. Eur J Anaesthesiol Suppl. 2002;26:16–24. doi: 10.1097/00003643-200219261-00004. [DOI] [PubMed] [Google Scholar]
- 19.Fitzgerald M. The development of nociceptive circuits. Nat Rev Neurosci. 2005;6:507–520. doi: 10.1038/nrn1701. [DOI] [PubMed] [Google Scholar]
- 20.Gatewood JD, Wills A, Shetty S, Xu J, Arnold AP, Burgoyne PS, Rissman EF. Sex chromosome complement and gonadal sex influence aggressive and parental behaviors in mice. J Neurosci. 2006;26:2335–2342. doi: 10.1523/JNEUROSCI.3743-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gioiosa L, Chen X, Watkins R, Klanfer N, Bryant CD, Evans CJ, Arnold AP. Sex chromosome complement affects nociception in tests of acute and chronic exposure to morphine in mice. Horm Behav. 2008;53:124–130. doi: 10.1016/j.yhbeh.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodwin GA, Barr GA. Developmental changes in the behavioral and autonomic effects of kappa opioid receptor stimulation of the midbrain periaqueductal gray. Dev Psychobiol. 2005;46:47–56. doi: 10.1002/dev.20039. [DOI] [PubMed] [Google Scholar]
- 23.Haberny KA, Paule MG, Scallet AC, Sistare FD, Lester DS, Hanig JP, Slikker W., Jr Ontogeny of the N-methyl-D-aspartate (NMDA) receptor system and susceptibility to neurotoxicity. Toxicol Sci. 2002;68:9–17. doi: 10.1093/toxsci/68.1.9. [DOI] [PubMed] [Google Scholar]
- 24.Kehoe P, Boylan CB. Behavioral effects of kappa-opioid-receptor stimulation on neonatal rats. Behav Neurosci. 1994;108:418–423. doi: 10.1037//0735-7044.108.2.418. [DOI] [PubMed] [Google Scholar]
- 25.Krzanowska EK, Ogawa S, Pfaff DW, Bodnar RJ. Reversal of sex differences in morphine analgesia elicited from the ventrolateral periaqueductal gray in rats by neonatal hormone manipulations. Brain Res. 2002;929:1–9. doi: 10.1016/s0006-8993(01)03350-9. [DOI] [PubMed] [Google Scholar]
- 26.Lovell-Badge R, Robertson E. XY female mice resulting from a heritable mutation in the primary testis-determining gene. Tdy. Dev. 1990;109:635–646. doi: 10.1242/dev.109.3.635. [DOI] [PubMed] [Google Scholar]
- 27.Loyd DR, Murphy AZ. Sex differences in the anatomical and functional organization of the periaqueductal gray-rostral ventromedial medullary pathway in the rat: A potential circuit mediating the sexually dimorphic actions of morphine. J Comp Neurol. 2006;496:723–738. doi: 10.1002/cne.20962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahadevaiah SK, Odorisio T, Elliott DJ, Rattigan A, Szot M, Laval SH, Washburn IL, McCarrey JR, Cattanach BM, Lovell-Badge R, Burgoyne PS. Mouse homologues of the human AZF candidate gene RBM are expressed in spermatogonia and spermatids, and map to a Y chromosome deletion interval associated with a high incidence of sperm abnormalities. Hum Mol Genet. 1998;7:715–727. doi: 10.1093/hmg/7.4.715. [DOI] [PubMed] [Google Scholar]
- 29.Markham JA, Jurgens HA, Auger CJ, De Vries GJ, Arnold AP, Juraska JM. Sex differences in mouse cortical thickness are independent of the complement of sex chromosomes. Neurosci. 2003;116:71–75. doi: 10.1016/s0306-4522(02)00554-7. [DOI] [PubMed] [Google Scholar]
- 30.Mayer EA, Berman S, Lin C, Naliboff BD. Sex-based differences in gastrointestinal pain. Eur J Pain. 2004;8:451–463. doi: 10.1016/j.ejpain.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 31.McLaughlin CR, Tao Q, Abood ME. Analysis of the antinociceptive actions of the kappa-opioid agonist enadoline (CI-977) in neonatal and adult rats: comparison to kappa-opioid receptor mRNA ontogeny. Drug Alcohol Depend. 1995;38:261–269. doi: 10.1016/0376-8716(95)01124-h. [DOI] [PubMed] [Google Scholar]
- 32.Mogil JS, Chesler EJ, Wilson SG, Juraska JM, Sternberg WF. Sex differences in thermal nociception and morphine antinociception in rodents depend on genotype. Neurosci Biobehav Rev. 2000;24:375–389. doi: 10.1016/s0149-7634(00)00015-4. [DOI] [PubMed] [Google Scholar]
- 33.Mogil JS, Sternberg WF, Kest B, Marek P, Liebeskind JC. Sex-differences in the antagonism of swim stress-induced analgesia - effects of gonadectomy and estrogen replacement. Pain. 1993;53:17–25. doi: 10.1016/0304-3959(93)90050-Y. [DOI] [PubMed] [Google Scholar]
- 34.Mogil JS, Wilson SG, Chesler EJ, Rankin AL, Nemmani KVS, Lariviere WR, Groce MK, Wallace MR, Kaplan L, Staud R, Ness TJ, Glover TL, Stankova M, Mayorov A, Hruby VJ, Grisel JE, Fillingim RB. The melanocortin-1 receptor gene mediates female-specific mechanisms of analgesia in mice and humans. Proc Natl Acad Sci U S A. 2003;100:4867–4872. doi: 10.1073/pnas.0730053100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy AZ. Sex differences in the antihyperalgesic actions of morphine. Neuropsychopharm. 2005;30:S74. [Google Scholar]
- 36.Nandi R, Fitzgerald M. Opioid analgesia in the newborn. Eur J Pain. 2005;9:105–108. doi: 10.1016/j.ejpain.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 37.Nemmani KVS, Grisel JE, Stowe JR, Smith-Carliss R, Mogil JS. Modulation of morphine analgesia by site-specific N-methyl-D-aspartate receptor antagonists: dependence on sex, site of antagonism, morphine dose, and time. Pain. 2004;109:274–283. doi: 10.1016/j.pain.2004.01.035. [DOI] [PubMed] [Google Scholar]
- 38.Palaszynski KM, Smith DL, Kamrava S, Burgoyne PS, Arnold AP, Voskuhl RR. A Yin-Yang effect between sex chromosome complement and sex hormones on the immune response. Endocrinol. 2005;146:3280–3285. doi: 10.1210/en.2005-0284. [DOI] [PubMed] [Google Scholar]
- 39.Spain JW, Roth BL, Coscia CJ. Differential ontogeny of multiple opioid receptors (mu, delta, and kappa) J Neurosci. 1985;5:584–588. doi: 10.1523/JNEUROSCI.05-03-00584.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sternberg WF, Ritchie J, Mogil JS. Qualitative sex differences in kappa-opioid analgesia in mice are dependent on age. Neurosci Lett. 2004;363:178–181. doi: 10.1016/j.neulet.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 41.Sternberg WF, Smith L, Scorr L. Nociception and antinociception during the first week of life in mice: sex differences and test dependence. J Pain. 2004;5:420–426. doi: 10.1016/j.jpain.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 42.Stoffel EC, Ulibarri CM, Folk JE, Rice KC, Craft RM. Gonadal hormone modulation of mu, kappa, and delta opioid antinociception in male and female rats. Journal of Pain. 2005;6:261–274. doi: 10.1016/j.jpain.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wagner CK, Xu J, Pfau JL, Quadros PS, De Vries GJ, Arnold AP. Neonatal mice possessing an Sry transgene show a masculinized pattern of progesterone receptor expression in the brain independent of sex chromosome status. Endocrinol. 2004;145:1046–1049. doi: 10.1210/en.2003-1219. [DOI] [PubMed] [Google Scholar]
- 44.Xu J, Burgoyne PS, Arnold AP. Sex differences in sex chromosome gene expression in mouse brain. Hum Mol Genet. 2002;11:1409–1419. doi: 10.1093/hmg/11.12.1409. [DOI] [PubMed] [Google Scholar]
- 45.Zhu H, Barr GA. Opiate withdrawal during development: are NMDA receptors indispensable? Trends Pharmacol Sci. 2001;22:404–408. doi: 10.1016/s0165-6147(00)01792-2. [DOI] [PubMed] [Google Scholar]
- 46.Zhu H, Barr GA. Ontogeny of NMDA receptor-mediated morphine tolerance in the postnatal rat. Pain. 2003;104:437–447. doi: 10.1016/S0304-3959(03)00051-4. [DOI] [PubMed] [Google Scholar]