Abstract
Background
In a blinded, placebo controlled study, we investigated whether intracoronary infusion of autologous mononuclear cells from G-CSF mobilised apheresis product or bone marrow (BM) improved sensitive outcome measures in a swine model of large MI.
Methods and Results
Four days after LAD occlusion and reperfusion, cells from BM or apheresis product of saline (Placebo) or G-CSF injected animals were infused into the LAD. Large infarcts were created: baseline ejection fraction (EF) by MRI of 35.3 ± 8.5%, no difference between the Placebo, G-CSF and BM groups (p=0.16 by ANOVA). At 6 weeks EF fell to a similar degree in the Placebo, G-CSF and BM groups (−7.9±6.0%, −8.5±8.8% and −10.9±7.6%, p=0.78 by ANOVA). Left ventricular volumes and infarct size by MRI deteriorated similarly in all 3 groups. Quantitative PET demonstrated significant decline in FDG uptake rate in the LAD territory at follow-up, with no histological, angiographic or PET perfusion evidence of functional neovascularisation. Immunofluorescence failed to demonstrate transdifferentiation of infused cells.
Conclusion
Intracoronary infusion of mononuclear cells from either bone marrow or G-CSF mobilised apheresis product may not improve or limit deterioration in systolic function, adverse ventricular remodelling, infarct size or perfusion in a swine model of large MI.
Keywords: Angiogenesis, imaging, myocardial infarction, myogenesis, stem cell
Introduction
Preclinical investigations of bone marrow derived progenitor cell therapy in large animal models of acute myocardial infarction, with prognostically significant reductions of left ventricular function, have not been reported. This would appear valuable given the increasing clinical interest in cardiac regenerative strategies for such patients1. We therefore performed a blinded placebo controlled study of intracoronary infusion of autologous mononuclear cells derived from either cytokine mobilised apheresis product or bone marrow in a swine model of large myocardial infarction (MI).
Methods
Animal Procedures
Animal procedures were approved by the NHLBI Animal Care and Use Committee. The experimental protocol is summarised in Figure 1. Twenty-four Yucatan miniswine (wt 37–52 kg) underwent 90 minute balloon occlusion of the left anterior descending (LAD) artery as described previously2.
Figure 1.
Schematic describing the study design. Abbreviations: AP – apheresis product mononuclear cells; BM – bone marrow mononuclear cells; CFU – colony forming unit; IC –intracoronary; LAD – left anterior descending coronary artery; MRI – magnetic resonance imaging; PB – peripheral blood mononuclear cells; PET – positron emission tomography; R + L Ht Cath – right and left heart cardiac catheterisation.
In a blinded fashion, animals were alternately assigned to receive no injections or daily subcutaneous injections of either human recombinant granulocyte colony stimulating factor (G-CSF, n=8, 10 μg/kg, Neupogen, Amgen) or placebo (n=7, equal volume of normal saline) for five days. The first injection was administered after LAD occlusion and daily thereafter. All injected animals underwent apheresis. Animals receiving no injections underwent bone marrow aspiration (BM, n=7) without apheresis. Animals in the placebo group formed the cellular control against which the G-CSF and BM groups were compared.
Apheresis and Bone Marrow Aspiration
Apheresis was performed using citrate anticoagulant in a 1:13 ratio to the whole blood flow rate. Aseptic bone marrow aspiration from both iliac crests was performed under general anaesthesia.
Cell Preparation
Immediately after apheresis or bone marrow aspiration, the cellular product was prepared for infusion. The product was incubated with ammonium chloride (ACK, BioWhittaker), washed, labelled with CM-DiI (Molecular Probes) according to manufacturer’s instructions, and resuspended in plasmalyte A/2% human serum albumin which was heparinised (30u/mL).
Colony Forming Assays
Haematopoietic colony forming assays were performed as an index of progenitor cell activity (see Supplementary Methods). The combined number of myeloid (CFU G/M) and erythroid (BFU E) colonies counted 2 weeks after plating determined the total number of colony forming units (CFU). The number of colony forming cells (CFC) was calculated assuming one CFU per CFC. All procedures were performed by investigators blinded to treatment allocation and imaging results.
Intracoronary Cell Infusion
Intracoronary cell infusion was performed into the LAD as reported previously3, by investigators blinded to the identity of the cell product, followed by coronary angiography to confirm vessel patency.
PET
Dynamic PET was performed using a GE Advance scanner (GE Medical Systems). Myocardial blood flow was measured at rest and during adenosine infusion (140 μg/kg/min) using a bolus administration of 50 mCi of oxygen-15 labeled water (H215O) 4. Myocardial FDG uptake was measured after intravenous fluorine-18 fluorodeoxyglucose (FDG, 15 mCi) under an hyperinsulinaemic euglycaemic glucose clamp 5.
MRI
MRI was performed at 1.5 T (Sonata, Siemens Medical Systems) using an 8 channel phased array surface coil (Nova Medical Inc). Breath-held, ECG-gated cine steady state free precession (SSFP) MRI was acquired (TR/TE, 3.6/1.8 ms; flip angle, 65°; field of view (FOV), 300 × 244 mm; matrix, 256 × 127 pixels, slice thickness, 8 mm, bandwidth 1085 Hz/pixel). Delayed enhancement (DE-MRI) images were acquired after intravenous Gd-DTPA (0.2 mmol/kg) using a phase sensitive inversion recovery sequence (TR/TE, 11/4.45 ms; flip angle, 30°; inversion-time, 300 ms; FOV, 350 × 241 mm; matrix, 256 × 141 pixels; slice-thickness, 8 mm, bandwidth 140 Hz/pixel).
Follow-up
At six weeks, animals returned for cardiac catheterisation, MRI and PET after which they were euthanised. Tissues were prepared for histology (Data Supplement).
Data Analysis
All analyses were performed by investigators blinded to treatment allocation after 6 animals in each group had completed the protocol.
MRI
All images were analysed on a Leonardo workstation (Siemens). Ejection fraction (EF), end-systolic volume (ESV), end-diastolic volume (EDV), and stroke volume (SV) were calculated. Regional wall thickening was determined using a 16-sector model. Sectors were categorised as either hypokinetic (wall thickening > 0 mm and <1.5 mm) or akinetic (wall thickening ≤ 0 mm). Infarct volumes were quantified from DE-MRI images using a previously validated tool which automatically windows, levels, and contours images of infarcted regions6.
PET
PET and MRI images were registered (Data Supplement). Manually segmented infarct, peri-infarct and remote myocardial regions on MR images were transformed on to PET images for kinetic analysis. Myocardial blood flow values in the infarct, peri-infarct and remote regions were calculated from the dynamic H215O data4. The ratio of myocardial blood flow during adenosine infusion to resting myocardial blood flow was defined as the adenosine vasodilator response. Rate constants for net myocardial FDG uptake (Ki) were calculated using Patlak analysis. Parametric Ki images were generated on a pixel by pixel basis in each animal7. Partial volume correction of the FDG data was based on the H215O data for corresponding regions.
Statistics
The primary endpoint was change in EF from baseline to 6 weeks. Secondary endpoints included EF, EDV, ESV, SV, and infarct volume at 6 weeks. Data were assessed for normality using the Kolmogorov-Smirnov method. Continuous parameters are shown as mean ± SD. Data were analysed using one way ANOVA with specific intergroup comparisons made using unpaired Student t-tests with Bonferroni correction for multiple comparisons. p<0.05 was considered significant.
Results
Six of 24 animals failed to complete or were excluded from the protocol due to death before intracoronary cell infusion (n=4), severe biventricular failure due to extensive left and right ventricular infarction 7 days after cell delivery (n=1, G-CSF), or baseline MRI scanner failure (n=1, BM). Data are presented from 18 animals. G-CSF increased total leukocyte count and CFC concentration in peripheral blood (See Data Supplement). The composition of infused cells is summarised in Table 1. Trypan blue viability for all cellular products was > 90%. The weight-adjusted dose of colony forming cells administered by intracoronary infusion was similar in the G-CSF and BM groups were similar (8.04 ± 4.13 × 103 v 7.27 ± 3.38 × 103 colony forming cells/kg) (Table 1).
Table 1.
Composition of infused cells
| Placebo | G-CSF | BM | |
|---|---|---|---|
| Total Nucleated Cells (×108) | 47.4±17.6 | 186±231 | 10.1±6.29 |
| Mononuclear Cells (×106/kg) | 110±42.9 | 98.1±50.3 | 5.33±2.48 |
| Colony Forming Cells (×102/kg) | 3.62±1.42 | 80.4±41.3 | 72.7±33.8 |
| Granulocytes (×108) | 1.63±3.04 | 147±225 | 7.58±5.33 |
| Platelets (×109) | 16.2±11.6 | 27.1±35.1 | 13.3±8.8 |
Apheresis was tolerated in all animals despite recent large myocardial infarctions. Intracoronary cell infusion was performed successfully and without complication in 18/19 animals. In one G-CSF treated animal, receiving the highest infused dose of total nucleated cells (1.57 ×109 cells/kg), no-reflow occurred.
At 6 weeks, there was no haemodynamic improvement in any group. Cardiac output was maintained by increased heart rate, not improved stroke volume (Data Supplement). Coronary angiography revealed no collateralisation of the LAD territory.
Large infarcts were created as evidenced by baseline EF of 34.3±9.7%, 31.2±7.8%, and 40.4±6.0% in the Placebo (n=6), G-CSF (n=6) and BM (n=6) groups respectively (p=0.16 by ANOVA) (Table 1). EF, ESV and EDV deteriorated in all groups at 6 weeks. There was no significant difference in any parameter between treatment groups (Figure 2). At 6 weeks, the number of sectors with a wall motion abnormality increased to a similar degree in all 3 groups (Figure 3), with an increasing proportion of akinetic segments (Table 3). After completing blinded analyses, assuming the null hypothesis could be rejected, we estimated that n=134 pigs per group would be required to achieve 80% power to detect a significant difference (p<0.05) in change in EF from baseline to 6 weeks follow-up. The study was therefore terminated on grounds of futility.
Figure 2.

Global systolic function and remodelling assessed by SSFP-MRI (Placebo n=6; G-CSF n=5; BM n=5). Increased end-systolic volume and end-diastolic volume without significant change in stroke volume occurred in all groups (Panel A). There was no significant difference between the 3 groups for the change stroke volume (p=0.86), end-systolic volume (p=0.34) or end-diastolic volume (p=0.38) by ANOVA. EF deteriorated in all 3 groups (Panel B) without significant differences between groups by ANOVA (p=0.78).
Figure 3.
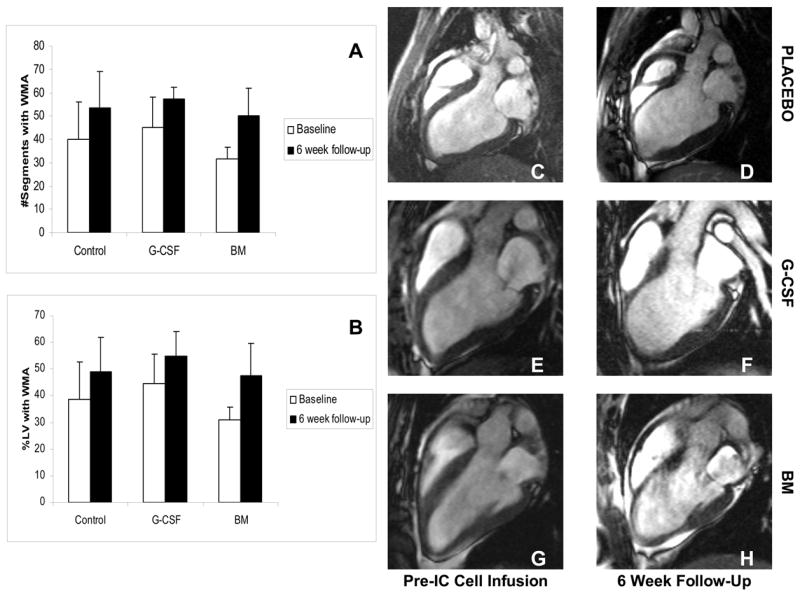
Regional wall thickening analysis (Placebo n=6; G-CSF n=5; BM n=5). The number of segments with a regional wall motion abnormality expressed as an absolute number (Panel A) or as a proportion of the left ventricle (Panel B) increased in all treatment groups at 6 weeks compared with baseline. This is illustrated on the end-diastolic SSFP MRI images for the placebo (Panels C and D), G-CSF (Panels E and F) and BM (Panels G and H) groups.
Table 3.
Regional Wall Motion Analysis of MRI Data
| Pre-IC Cell Infusion | 6 Wk Follow-Up | |||||
|---|---|---|---|---|---|---|
| #WMA | %Hypo | %Akin | #WMA | %Hypo | %Akin | |
| Placebo | 40±16 | 74±6 | 26±6 | 54±15* | 63±13 | 37±13 |
| G-CSF | 45±13 | 60±12 | 40±12 | 57±4.8* | 50±12* | 50±12* |
| BM | 32±5 | 64±7 | 36±7 | 50±12† | 56±13* | 44±13* |
WMA – number of sectors with a wall motion abnormality; %Hypo - % of hypokinetic sectors; %Akin - % of akinetic sectors.
p<0.05 v Pre-IC Cell Infusion;
p<0.02 v Pre-IC Cell Infusion
Transmural delayed enhancement was evident in the antero-septal wall in all animals at baseline (Figure 4). At 6 weeks, transmural delayed enhancement persisted consistent with nonviable myocardium. These regions underwent significant thinning and expansion, and accordingly, the mass of tissue showing delayed enhancement at follow-up was reduced (Figure 4).
Figure 4.

DE-MRI demonstrated transmural infarction prior to intracoronary cell delivery in all 3 groups. At 6 weeks, there was significant thinning of the infarcted segments with persistent transmural delayed enhancement. The graph shows that the mass of tissue exhibiting delayed enhancement significantly reduced by 6 weeks in all treatment groups (Placebo n=6; G-CSF n=5; BM n=5).
Quantitative myocardial FDG uptake (Ki) images before intracoronary cell infusion demonstrated preserved or enhanced FDG uptake in sectors showing transmural delayed enhancement (Figure 5). At follow-up, these regions thinned, and exhibited markedly reduced FDG uptake despite partial volume correction, suggesting non-viable infarcted myocardium (Figure 5 and 6). Ki calculated on remote, infarct and peri-infarct regions reflected a similar trend.
Figure 5.
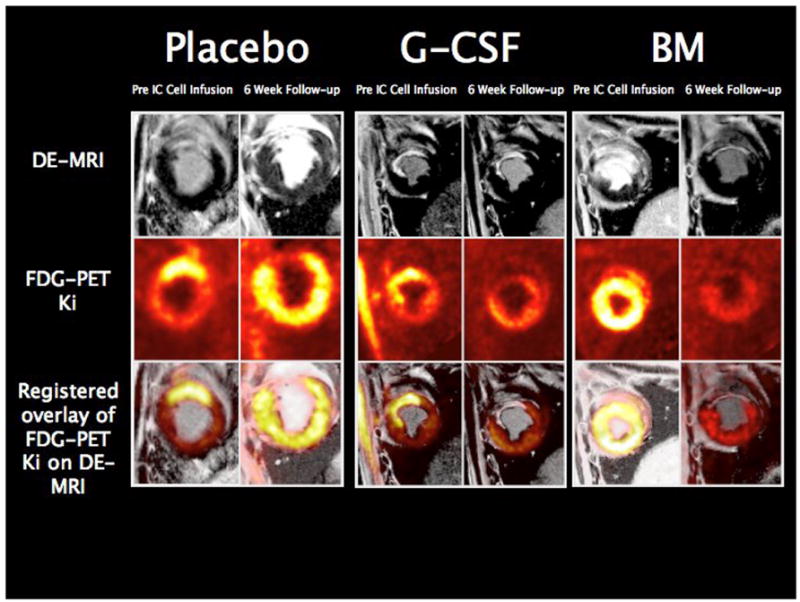
Overlay of registered short axis DE-MRI and FDG uptake (Ki) images. FDG uptake in myocardium with transmural delayed enhancement on MRI before intracoronary cell infusion is preserved or increased. By 6 weeks, there was concordance between the FDG uptake images and the DE-MRI images with all treatment groups showing reduced FDG uptake in regions showing thinning and persistent transmural delayed enhancement.
Figure 6.

Vertical long axis projections of quantitative FDG uptake (Ki) images before and 6 weeks after intracoronary cell during hyperinsulinaemic euglycaemic clamp. FDG uptake rate (Ki) in the peri-infarct and infarcted myocardium are shown for all 3 groups. There is a consistent fall in the rate of FDG uptake in all 3 treatment groups at 6 week follow-up (Placebo n=6; G-CSF n=5; BM n=5).
Adenosine vasodilator response, an index of coronary microcirculatory function, was measured in the infarcted and peri-infarct myocardium at baseline and at six weeks follow-up and was not different in any of the 3 groups in the peri-infarct regions (Figure 7).
Figure 7.
The upper panels show measurements of adenosine vasodilator response in the peri-infarct and infarcted myocardium pre-intracoronary cell infusion and at 6 weeks measured by H215O-PET. The lower panels show histologically measured vessel scores. No increase in vessel density or improvement in vasodilator response was observed in any treatment group.
Haematoxylin and Eosin and Masson-Trichrome stained sections from the border and infarct zones of the hearts in all 3 groups demonstrated equivalent degrees of myocyte hypertrophy, myocyte degeneration, oedema, fibrosis, inflammatory cell infiltrate, hemorrhage, and vascular density. These findings are inconsistent with myocyte regeneration or neovascularisation in any group.
Confocal microscopy demonstrated persistent CM-DiI labelled cells. There was no difference in the density of arterioles staining positive for alpha smooth muscle actin between the 3 groups (Data Supplement). There was no specific colocalisation of CM-DiI labelled cells with stains for CD31, desmin, or vimentin (Figure 8). There was evidence of colocalisation of CM-DiI cells with CD45 staining suggesting that some of the labelled cells retained a leukocyte lineage (Data Supplement). These observations do not support transdifferentiation of infused cells.
Figure 8.
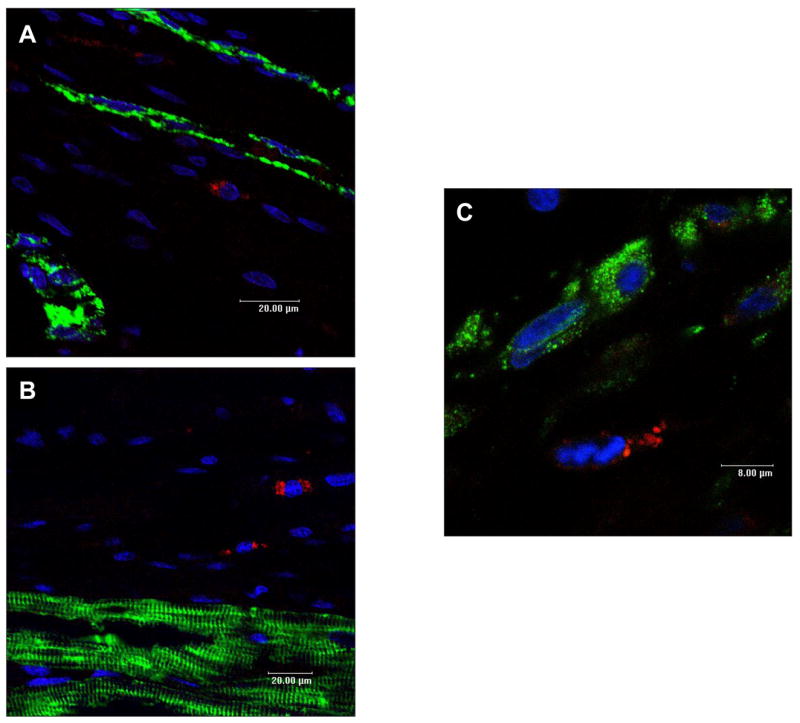
Representative high power laser confocal micrographs showing no colocalisation of CM-DiI positive cells (red) with cells staining positive for CD31 (panel A), desmin (panel B) and vimentin (panel C). Similar findings were observed in all treatment groups.
Discussion
In this blinded study, intracoronary infusion of mononuclear cells from bone marrow or G-CSF mobilised apheresis product failed to improve adverse remodelling, global or regional contractile function, rest and stress perfusion or viability in an animal model of large prognostically significant reperfused antero-septal myocardial infarction. If cell treatment were efficacious, a noticeable treatment effect could be anticipated in this experimental model, given the combination of large infarct size and assessments using highly sensitive measurement tools capable of detecting subtle changes in global and regional parameters of myocardial function. Additionally, there was no evidence of myocyte regeneration, or neovascularisation in the G-CSF or BM groups. While a treatment effect cannot be excluded, we thought this unlikely and hence were doubtful that submission of additional animals to this protocol would alter the result. Therefore, consistent with previously reported pilot studies8, our investigation was terminated and unblinded.
Our observations were made in a model where mean baseline EF was 35.3±8.5%. This is analogous to post-infarct patients at high risk of developing adverse ventricular remodelling and cardiac failure. Such patients would benefit considerably from safe and effective regenerative strategies and are the focus of a number of clinical trials of cellular therapy. Cardiac MRI is the modality of choice for quantifying ventricular remodelling as well as global and regional left ventricular function. This technique is infrequently used in preclinical investigations of cellular therapies9. Consistent with the findings of our study, recent clinical reports employing quantitative assessments of ventricular function by MRI failed to demonstrate early or long term benefit of G-CSF therapy 10–12 or intracoronary administration of bone marrow derived mononuclear cells 13–15 in subjects with acute myocardial infarction. Additionally, we did not observe improvements in regional contractile function, with an increasing proportion of akinetic segments at follow-up.
We quantified infarct size from DE-MRI using a semi-automated tool that reduces potential bias introduced by manual windowing, leveling and segmentation of images6. Imaging performed before intracoronary cell infusion demonstrated extensive transmural delayed enhancement in the antero-septal myocardium. The mass of tissue showing delayed enhancement was similar in all 3 groups (Figure 6). At follow-up, this measurement declined significantly, consistent with recent reports3, 9, 13. This appears due to tissue loss, wall thinning and scar contracture rather than replacement of infarcted myocardium with viable tissue (Figure 6). These observations are characteristic of the natural history of DE-MRI in reperfused myocardial infarction16, 17. The decrease in the mass of tissue showing delayed enhancement may therefore not represent evidence of myocardial regeneration as proposed by other investigators3, 13. Interestingly, FDG-PET imaging performed before intracoronary cell infusion demonstrated preserved and in many cases enhanced uptake in regions of myocardium showing transmural delayed enhancement. We believe this is explained by FDG uptake by the intense inflammatory cell infiltrate that occurs in reperfused myocardium18 rather than FDG being taken up by metabolically active viable cardiac myocytes. At follow-up, FDG uptake in the infarcted myocardium reduced (Figures 7 and 8), indicative of metabolically inactive non-viable myocardium. Histopathological examination demonstrated extensive transmural infarction with no evidence of transdifferentiation (Figure 8), cardiac regeneration or neovascularisation (Data Supplement).
Certain haematopoietic progenitor cell populations may be angiogenic19. Histological assessment of the density of vascular structures in peri-infarct and infarcted regions was similar in all three groups (Data Supplement). Furthermore, the number of arterioles staining positive for anti-smooth muscle actin was similar in all 3 groups (Data Supplement). Adenosine vasodilator response measured quantitatively in the peri-infarct regions with H215O-PET showed no improvement and was similar in all 3 groups (Figure 7). Selective coronary angiography failed to demonstrate collateral growth. These data do not support functional neovascularisation in this model.
Clinical studies have reported few immediate procedural complications from infusion of mononuclear cells into infarct related coronary arteries. The total nucleated cell dose in these studies has progressively increased from 9×106 to 24.6± 8.4×108 3, 13, 15, 20, 21. In one animal, no-reflow was observed at the highest infused total nucleated cell dose. Pending results from further dose escalation studies, we would caution against exceeding this intracoronary cell dose in clinical studies.
Study Limitations
We recognise the limitations of simulating human clinical disease using animal models. Unlike many clinical observations where EF improves after reperfusion in myocardial infarction22, we observed a consistent decrease in EF in all groups. This has been observed in clinical studies 23 and may be due to the extensive myocardial infarctions created in this study24. The small number of animals reflects the pilot nature of this protocol and is consistent with sample sizes in previously published preclinical investigations using large animals25, 26. We estimated that ~400 animals should be studied to detect a significant difference (beneficial or otherwise) between the treatment groups. While the sample size in this study is small, the measurement techniques were highly sensitive and reproducible, and performed in a model of large infarction in which any benefits should have been easily discerned. Our results in part may be explained by the use of an unselected cell product. Currently, clinically relevant progenitor cell populations cannot be selected in swine due to lack of appropriate antibodies. Furthermore, intramyocardial rather than intracoronary cell delivery may be more efficacious in this model system. The follow-up timepoint in the current study was chosen as previous preclinical investigations have demonstrated functional improvements by this time which plateau thereafter 9, 25–28. A cellular control (mononuclear cells from non-mobilised apheresis product) was chosen to maintain study blind and help determine if the test cell populations would exert any effect over and above simple interstitial reinforcement of the infarct region29. We cannot exclude the possibility that all 3 cell populations administered in the current study conferred improvement relative to an acellular control, though this seems unlikely as previous studies have not shown benefit over intracoronary administration of medium on ejection fraction in reperfused myocardial infarction9.
Conclusions
Intracoronary infusion of mononuclear cells from either bone marrow or G-CSF mobilised apheresis product may not improve or limit deterioration of left ventricular systolic function, rest or stress perfusion and infarct size in a swine model of large prognostically significant reperfused antero-septal myocardial infarction. These cell populations may not necessarily confer benefit to analogous patient populations. Studies in animal models of large reperfused myocardial infarction which employ local delivery systems and endpoint assessments equivalent to those used in contemporary clinical studies may represent an essential step in selecting cellular products that ultimately demonstrate sustained therapeutic benefit in clinical trials30.
Supplementary Material
Table 2.
Global Measures of Systolic Function and Remodeling from MRI Data
| Placebo | G-CSF | BM | ||||
|---|---|---|---|---|---|---|
| Pre-IC Rx | 6 Wk FU | Pre-IC Rx | 6 Wk FU | Pre-IC Rx | 6 Wk FU | |
| n=6 | n=6 | n=6 | n=5 | n=6 | n=5 | |
| EF (%) | 34.3±9.7 | 26.5±6.8* | 31.2±7.8 | 24.3±5.1* | 40.4±6.0 | 29.6±8.1* |
| ESV (mL) | 56.5±18.4 | 69.2±14.1* | 49.5±12.2 | 72.3±12.9* | 35.7±11.3 | 52.9±22.8* |
| EDV (mL) | 84.2±16.0 | 93.9±14.8† | 72.1±15.7 | 95.8±19.2† | 60.1±16.7 | 73.4±23.7† |
| SV (mL) | 27.7±4.1 | 24.7±6.3 | 22.6±7.2 | 23.7±8.5 | 24.2±6.9 | 20.5±3.9 |
| LVM (g) | 80.1±11.2 | 80.7±6.2 | 68.6±13.8 | 79.5±12.1 | 83.0±8.7 | 81.7±12.0 |
EF – ejection fraction; EDV – end-diastolic volume; ESV – end-systolic volume; SV – stroke volume; LVM – left ventricular mass; Pre-IC Rx – Pre-Intracoronary Cell Infusion; 6Wk FU – 6 Week Follow-up.
p<0.01 v pre-IC Rx;
p<0.05 v pre-IC Rx.
Acknowledgments
We thank Kathryn Hope, Katherine Lucas and Joni Taylor for their expert animal care; William Schenke and Victor Wright for technical assistance; Cengizhan Ozturk and Ludovic Le Meunier for assistance with MRI and PET imaging; Peter Kellman for the phase sensitive inversion recovery sequence; and Li-Yueh Hsu for infarct volume analysis software. We are grateful to Dawson Smith of Gambro BCT for providing the Cobe Spectra system. Amgen provided Neupogen (recombinant human G-CSF) under a materials collaborative research and development agreement with NIH. Finally we pay tribute to and posthumously thank Charles Carter for his invaluable contribution without which this study would not have been possible.
Funding Sources
This work was supported by NIH Z01-HL005062-04 (RJL).
Footnotes
Conflicts of Interest Disclosures
None.
References
- 1.Penn MS. Stem-cell therapy after acute myocardial infarction: the focus should be on those at risk. Lancet. 2006;367(9505):87–88. doi: 10.1016/S0140-6736(05)67895-6. [DOI] [PubMed] [Google Scholar]
- 2.de Silva R, Gutierrez LF, Raval AN, McVeigh ER, Ozturk C, Lederman RJ. X-ray fused with magnetic resonance imaging (XFM) to target endomyocardial injections: validation in a swine model of myocardial infarction. Circulation. 2006;114(22):2342–2350. doi: 10.1161/CIRCULATIONAHA.105.598524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364(9429):141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 4.Bonow RO, Dilsizian V, Cuocolo A, Bacharach SL. Identification of viable myocardium in patients with chronic coronary artery disease and left ventricular dysfunction. Comparison of thallium scintigraphy with reinjection and PET imaging with 18F-fluorodeoxyglucose. Circulation. 1991;83(1):26–37. doi: 10.1161/01.cir.83.1.26. [DOI] [PubMed] [Google Scholar]
- 5.Chareonthaitawee P, Schaefers K, Baker CS, Turkheimer F, Stegger L, Banner NR, Yacoub M, Bonser RS, Iozzo P, Camici PG, Rimoldi O. Assessment of infarct size by positron emission tomography and [18F]2-fluoro-2-deoxy-D-glucose: a new absolute threshold technique. Eur J Nucl Med Mol Imaging. 2002;29(2):203–215. doi: 10.1007/s00259-001-0685-1. [DOI] [PubMed] [Google Scholar]
- 6.Hsu LY, Natanzon A, Kellman P, Hirsch GA, Aletras AH, Arai AE. Quantitative myocardial infarction on delayed enhancement MRI. Part I: Animal validation of an automated feature analysis and combined thresholding infarct sizing algorithm. J Magn Reson Imaging. 2006;23(3):298–308. doi: 10.1002/jmri.20496. [DOI] [PubMed] [Google Scholar]
- 7.Choi Y, Hawkins RA, Huang SC, Gambhir SS, Brunken RC, Phelps ME, Schelbert HR. Parametric images of myocardial metabolic rate of glucose generated from dynamic cardiac PET and 2-[18F]fluoro-2-deoxy-d-glucose studies. J Nucl Med. 1991;32(4):733–738. [PubMed] [Google Scholar]
- 8.Penicka M, Horak J, Kobylka P, Pytlik R, Kozak T, Belohlavek O, Lang O, Skalicka H, Simek S, Palecek T, Linhart A, Aschermann M, Widimsky P. Intracoronary injection of autologous bone marrow-derived mononuclear cells in patients with large anterior acute myocardial infarction: a prematurely terminated randomized study. J Am Coll Cardiol. 2007;49(24):2373–2374. doi: 10.1016/j.jacc.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Moelker AD, Baks T, van den Bos EJ, van Geuns RJ, de Feyter PJ, Duncker DJ, van der Giessen WJ. Reduction in infarct size, but no functional improvement after bone marrow cell administration in a porcine model of reperfused myocardial infarction. Eur Heart J. 2006;27(24):3057–3064. doi: 10.1093/eurheartj/ehl401. [DOI] [PubMed] [Google Scholar]
- 10.Ripa RS, Haack-Sorensen M, Wang Y, Jorgensen E, Mortensen S, Bindslev L, Friis T, Kastrup J. Bone marrow derived mesenchymal cell mobilization by granulocyte-colony stimulating factor after acute myocardial infarction: results from the Stem Cells in Myocardial Infarction (STEMMI) trial. Circulation. 2007;116(11 Suppl):I24–30. doi: 10.1161/CIRCULATIONAHA.106.678649. [DOI] [PubMed] [Google Scholar]
- 11.Ripa RS, Jorgensen E, Wang Y, Thune JJ, Nilsson JC, Sondergaard L, Johnsen HE, Kober L, Grande P, Kastrup J. Stem cell mobilization induced by subcutaneous granulocyte-colony stimulating factor to improve cardiac regeneration after acute ST-elevation myocardial infarction: result of the double-blind, randomized, placebo-controlled stem cells in myocardial infarction (STEMMI) trial. Circulation. 2006;113(16):1983–1992. doi: 10.1161/CIRCULATIONAHA.105.610469. [DOI] [PubMed] [Google Scholar]
- 12.Zohlnhofer D, Ott I, Mehilli J, Schomig K, Michalk F, Ibrahim T, Meisetschlager G, von Wedel J, Bollwein H, Seyfarth M, Dirschinger J, Schmitt C, Schwaiger M, Kastrati A, Schomig A. Stem cell mobilization by granulocyte colony-stimulating factor in patients with acute myocardial infarction: a randomized controlled trial. Jama. 2006;295(9):1003–1010. doi: 10.1001/jama.295.9.1003. [DOI] [PubMed] [Google Scholar]
- 13.Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, Kalantzi M, Herbots L, Sinnaeve P, Dens J, Maertens J, Rademakers F, Dymarkowski S, Gheysens O, Van Cleemput J, Bormans G, Nuyts J, Belmans A, Mortelmans L, Boogaerts M, Van de Werf F. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367(9505):113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 14.Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, Hecker H, Schaefer A, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months’ follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006;113(10):1287–1294. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 15.Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, Endresen K, Ilebekk A, Mangschau A, Fjeld JG, Smith HJ, Taraldsrud E, Grogaard HK, Bjornerheim R, Brekke M, Muller C, Hopp E, Ragnarsson A, Brinchmann JE, Forfang K. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355(12):1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 16.Fieno DS, Hillenbrand HB, Rehwald WG, Harris KR, Decker RS, Parker MA, Klocke FJ, Kim RJ, Judd RM. Infarct resorption, compensatory hypertrophy, and differing patterns of ventricular remodeling following myocardial infarctions of varying size. J Am Coll Cardiol. 2004;43(11):2124–2131. doi: 10.1016/j.jacc.2004.01.043. [DOI] [PubMed] [Google Scholar]
- 17.Baks T, van Geuns RJ, Biagini E, Wielopolski P, Mollet NR, Cademartiri F, van der Giessen WJ, Krestin GP, Serruys PW, Duncker DJ, de Feyter PJ. Effects of primary angioplasty for acute myocardial infarction on early and late infarct size and left ventricular wall characteristics. J Am Coll Cardiol. 2006;47(1):40–44. doi: 10.1016/j.jacc.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Go LO, Murry CE, Richard VJ, Weischedel GR, Jennings RB, Reimer KA. Myocardial neutrophil accumulation during reperfusion after reversible or irreversible ischemic injury. Am J Physiol. 1988;255(5 Pt 2):H1188–1198. doi: 10.1152/ajpheart.1988.255.5.H1188. [DOI] [PubMed] [Google Scholar]
- 19.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9(6):702–712. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 20.Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, Tonn T, Dimmeler S, Zeiher AM. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355(12):1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 21.Assmus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, Grunwald F, Aicher A, Urbich C, Martin H, Hoelzer D, Dimmeler S, Zeiher AM. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI) Circulation. 2002;106(24):3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 22.Baks T, van Geuns RJ, Biagini E, Wielopolski P, Mollet NR, Cademartiri F, Boersma E, van der Giessen WJ, Krestin GP, Duncker DJ, Serruys PW, de Feyter PJ. Recovery of left ventricular function after primary angioplasty for acute myocardial infarction. Eur Heart J. 2005;26(11):1070–1077. doi: 10.1093/eurheartj/ehi131. [DOI] [PubMed] [Google Scholar]
- 23.Tarantini G, Razzolini R, Cacciavillani L, Bilato C, Sarais C, Corbetti F, Marra MP, Napodano M, Ramondo A, Iliceto S. Influence of transmurality, infarct size, and severe microvascular obstruction on left ventricular remodeling and function after primary coronary angioplasty. Am J Cardiol. 2006;98(8):1033–1040. doi: 10.1016/j.amjcard.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 24.Chareonthaitawee P, Christian TF, Hirose K, Gibbons RJ, Rumberger JA. Relation of initial infarct size to extent of left ventricular remodeling in the year after acute myocardial infarction. J Am Coll Cardiol. 1995;25(3):567–573. doi: 10.1016/0735-1097(94)00431-O. [DOI] [PubMed] [Google Scholar]
- 25.Fuchs S, Baffour R, Zhou YF, Shou M, Pierre A, Tio FO, Weissman NJ, Leon MB, Epstein SE, Kornowski R. Transendocardial delivery of autologous bone marrow enhances collateral perfusion and regional function in pigs with chronic experimental myocardial ischemia. J Am Coll Cardiol. 2001;37(6):1726–1732. doi: 10.1016/s0735-1097(01)01200-1. [DOI] [PubMed] [Google Scholar]
- 26.Silva GV, Litovsky S, Assad JA, Sousa AL, Martin BJ, Vela D, Coulter SC, Lin J, Ober J, Vaughn WK, Branco RV, Oliveira EM, He R, Geng YJ, Willerson JT, Perin EC. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111(2):150–156. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- 27.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7(4):430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 28.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410(6829):701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 29.Wall ST, Walker JC, Healy KE, Ratcliffe MB, Guccione JM. Theoretical Impact of the Injection of Material Into the Myocardium: A Finite Element Model Simulation. Circulation. 2006;114(24):2627–2635. doi: 10.1161/CIRCULATIONAHA.106.657270. [DOI] [PubMed] [Google Scholar]
- 30.Unger EF. All is not well in the world of translational research. J Am Coll Cardiol. 2007;50(8):738–740. doi: 10.1016/j.jacc.2007.04.067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




