Abstract
Background
Sexually transmitted infection (STI) clinics provide an opportune setting for HIV prevention efforts. This randomized controlled trial evaluated a unique, two-step approach to sexual risk reduction at a publicly-funded STI clinic.
Methods
During an initial visit, patients completed an audio-computer assisted self-interview (ACASI), were randomized to and received one of two brief interventions, obtained medical care, and completed a post-assessment. Next, two-thirds of the patients were assigned to attend an intensive sexual risk reduction workshop. At 3, 6, and 12 months, patients completed additional ACASIs and provided urine specimens to assess behavior change and incident STIs.
Results
During a 28-month interval, 5613 patients were screened, 2691 were eligible, and 1483 consented to participate and were randomized; the modal reason for declining was lack of time (82%). Consenting patients included 688 women and 795 men; 64% of participants were African-American. The sample was low-income with 57% reporting an annual income of less than $15,000; most participants (62%) had a high school education or less, and 51% were unemployed. Sexual risk behavior was common, as indicated by multiple sexual partners (mean = 32.8, lifetime; mean = 2.8, past 3 months), unprotected sex (mean = 17.3 episodes, past 3 months), and prior STIs (mean = 3.3, lifetime; 23% at baseline). Bivariate analyses confirmed our prediction that HIV-related motivation and behavioral skills would be related to current sexual risk behavior. All patients received a brief intervention; patient satisfaction ratings were uniformly high for both interventions (all means ≥ 3.7 on 4-point scales). Fifty-six percent of invited patients attended the intensive workshop, and attendance did not differ as a function of brief intervention. Patient satisfaction ratings were also uniformly positive for the workshop interventions (all means ≥ 3.6). Return to follow-up assessments exceeded 70%.
Conclusions
Results demonstrate that implementing an HIV preventive program in a busy, public clinic is feasible and well-accepted by patients. Ongoing evaluation will determine if the interventions reduce sexual risk behavior and lower incident STIs.
Keywords: Randomized controlled trial, sexual behavior, HIV infections/prevention and control, sexually transmitted diseases/prevention and control, intervention studies, African-Americans, health promotion, unsafe sex
1. Introduction
Sexually transmitted infections (STIs) remain a major public health problem [1]. For example, there are 40,000 new cases of HIV reported each year in the United States. Since the epidemic began, more than 500,000 people have died of AIDS in the U.S.; despite recent treatment advances, 17,000 people died of AIDS in 2005 [2]. Nearly one million cases of chlamydia and 340,000 cases of gonorrhea were reported in 2005 [3]. STIs can have serious health consequences, including pelvic pain, pelvic inflammatory disease, ectopic pregnancy, cervical cancer, and infertility [4]. In addition, infection with any sexually transmitted disease increases the risk of contracting HIV [5].
1.1 Sexual risk reduction for HIV/STI prevention
Sexual health promotion and disease prevention programs often seek to help people to reduce their risk for STIs by providing information about STI epidemiology, transmission and sequelae, and by encouraging risk reduction strategies such as condom use and avoiding multiple sexual partners. Publicly-funded STI clinics provide an opportune venue in which to provide sexual risk reduction interventions because rates of STIs and HIV are higher among patients compared to the general population [6, 7].
Several studies have investigated the use of brief, individual counseling in STI clinics. For example, Project RESPECT investigated two active behavioral interventions. One intervention involved two brief (20-minute) behavioral counseling sessions before and after HIV-antibody testing, whereas the second intervention comprised four sessions of individual counseling. Compared to standard care (a 5-minute primarily educational session), both individual counseling interventions were found to be efficacious with a one year follow-up [8]. Other brief, individual-level interventions have also been found to be efficacious [e.g., 9]. These brief, individual-level interventions, which can be tailored to a patient’s unique circumstances and needs, are promising, but they cannot address the full range of clients’ needs due to time constraints; moreover, brief, individual counseling interventions have not been widely adopted in standard STI care, perhaps because of the perception that providing such counseling is not feasible because of time and staffing constraints [10]. To address this barrier to adoption, Rietmeijer [10] suggests that counseling should be viewed as a new way of interacting with a client during standard care (rather than as a new service); he also suggested the use of computer-delivered counseling interventions, for which there is encouraging initial evidence of efficacy [11]. Both of these suggestions warrant further study.
Intensive, group-based behavioral interventions for STI clinic patients have also been investigated. For example, Kalichman et al. [12] recruited 105 women to a single 150-minute, group intervention based on the IMB model [13]. Compared to a time-matched control, women who received the IMB-based intervention reported greater use of the female condom at a three-month follow-up. Other investigators have also reported that intensive group-based interventions help to reduce sexual risk behavior among clients at STI clinics [14, 15]. Although these interventions allow for in-depth coverage and discussion of relevant material, a major limitation of intensive, group-based interventions is the requirement that clients return to the clinic at a time separate from their initial clinic visit; due to this requirement, participation rates for group-based interventions tend to be lower than participation rates for individual interventions. To address this challenge, research on substance use suggests that brief motivational interventions can increase participation in more intensive interventions [16, 17]. To our knowledge, this strategy has not been used in the context of sexual risk reduction.
In summary, prior evidence suggests that both brief (individual) HIV prevention counseling at the time of STI treatment, as well more intensive (group-based) sexual risk reduction interventions can be efficacious when implemented in STI clinic settings. Individual, clinic-based interventions need to be relatively brief because of space and staffing constraints, and because the process of diagnosing and treating STIs is time consuming. More intensive clinic-based interventions that utilize a group format need to address the challenge of motivating patients to attend group sessions outside of a standard clinic visit, a challenge that is made more difficult because STIs are highly stigmatized and most patients want to minimize their time in STI clinics. Given both the successes and the limitations of individual- and group-based behavioral interventions, we were interested in determining the extent to which a combined, multi-level intervention would optimize the strengths and minimize the limitations of both approaches. Therefore, we designed a two-step approach to sexual risk reduction that involved a brief, clinic-based intervention followed by a more intensive, group-based intervention. All interventions were informed by behavioral science theory to optimize their efficacy.
1.2 Theoretical guidance for sexual risk reduction interventions
Behavioral science theory can provide helpful guidance to optimize the acceptability, feasibility, and efficacy of sexual risk reduction interventions [18]. Prominent among behavioral theories in the sexual behavior domain is the Information—Motivation—Behavioral Skills (IMB) model [13]. The IMB model posits that HIV-related information, motivation (to engage in safer sexual behaviors), and behavioral skills (e.g., condom use and assertiveness skills) facilitate sexual risk behavior change. Constructs derived from the IMB model have been associated with sexual risk behavior in a variety of samples [19-21], and interventions targeting information, motivation, and skills are more efficacious than control interventions in reducing sexual risk behavior [22-24]. Indeed, recent meta-analyses have concluded that motivational and skills components were important components of effective sexual risk reduction interventions [25, 26].
A second theoretical framework that has been widely used in the context of health behavior change programs is the Transtheoretical Model (TTM) [27, 28]. The TTM proposes that individuals hold differing levels of motivation, or readiness-to-change, and that behavior change interventions should be tailored to each individual’s stage of change. According to this approach, individuals are unlikely to benefit from action-oriented (e.g., skills training) interventions if they are not sufficiently motivated (i.e., “ready for action”). Thus, from this theoretical perspective, it is important to tailor and sequence intervention components according to each individual’s readiness-to-change.
1.3 Purpose
The main purpose of this paper is to provide an overview of a randomized controlled trial (RCT) designed to evaluate the efficacy of our two-step intervention program, implemented in a public STI clinic. We also describe participant recruitment and flow and present baseline characteristics of participants, including rates of sexual risk behavior. In addition, we characterize relationships among theoretically-important baseline variables to understand better the nature of the sample and risk characteristics. Finally, we describe participant satisfaction with the interventions, present results of the short-term effects of the brief individual-level interventions, and provide attendance rates for follow-up assessments.
2. Methods
2.1 Study aims
The primary aims of this RCT were to investigate the independent and interactive effects of our two-step intervention system (i.e., brief and intensive interventions). The first step involved testing two clinic-based, brief interventions; during this step, we assessed patients’ readiness-to-change and provided primarily informational and motivational components. At the second step of the program, we provided two intensive workshops. One of the two intensive workshops targeted all three constructs suggested by the IMB model, whereas the other group intervention provided only HIV-related information. We measured the efficacy of the interventions using three classes of outcomes: (a) theoretical antecedents of sexual behavior change (i.e., information, motivation, and behavioral skills), (b) sexual risk behavior, and (c) STI incidence.
2.2 Research design
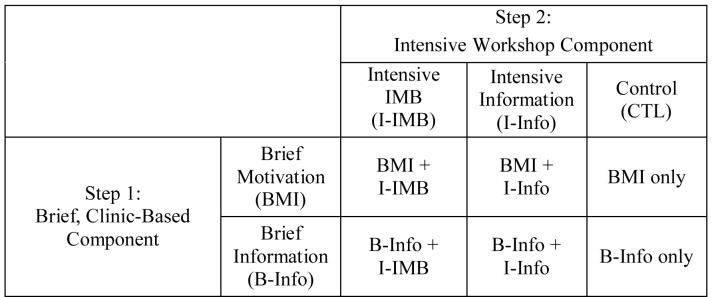
Patients were randomly assigned to one of the six conditions that are formed by crossing the Step 1 and Step 2 intervention components (see Figure 1). They completed the brief clinic-based intervention the same day they were enrolled in the study, and completed the intensive workshop intervention within two weeks of study enrollment.
Figure 1.
The six intervention conditions formed by crossing a brief clinic-based intervention with an intensive workshop condition
Participants were assessed on five occasions [a] immediately before and [b] immediately after receiving the brief clinic-based intervention, and [c] 3-months, [d] 6-months, and [e] 12-months following receipt of the intensive workshop intervention. The immediate post-intervention [b] provides patient satisfaction information and immediate cognitive-motivational changes resulting from the brief clinic intervention. The follow-up assessments [c, d, e] afford the opportunity to evaluate shorter-term effects (at 3 and 6 months), and longer-term effects (at 12 months). Having three follow-up occasions provides the opportunity to explore behavior change over time (e.g., sleeper or decay effects).
2.3 Participants
Patients attending a public STI clinic in upstate New York were recruited for the trial. Inclusion criteria were: (a) age 18 or older; (b) engaged in sexual risk behavior in the past 3 months (i.e., in the past 3 months, did not use a condom every time for vaginal or anal intercourse, and had more than one sexual partner, had an STI, had a sexual partner who had other partners, or had a sexual partner who injected drugs, was diagnosed with an STI, or had HIV); and (c) willing to take an HIV test. Exclusion criteria were: (a) mental impairment that would interfere with the ability to participate meaningfully; (b) planning to move from the area in the next year; (c) receiving inpatient substance use treatment; and (d) HIV positive (because the intervention was designed to prevent HIV infection and was not tailored to the unique sexual risk reduction needs of individuals who were HIV positive). Mental impairment was determined through behavioral observation by a trained Research Assistant.
To ensure adequate power to detect the smallest anticipated effect (i.e., differences in STI rates among intervention groups), we determined that we would need to recruit 1554 participants. We expected an attrition rate of 21% from baseline to the final follow-up assessment; thus, we expected to have 1228 participants remaining at the final follow-up, or approximately 204 participants in each of the six intervention conditions.
2.4 Measures
The baseline survey assessed (a) demographic and other descriptive characteristics, (b) substance use, (c) hypothesized moderators of intervention efficacy, (d) hypothesized antecedents of sexual risk behavior, and (e) current and lifetime sexual behavior.
2.4.1 Sociodemographic characteristics
Items requested information about patient sex, age, race/ethnicity, educational attainment, employment status, income, marital status, living status, sexual orientation, and number of children.
2.4.2 Antecedents of sexual risk behavior
Sixty-nine items addressed the IMB constructs that are hypothesized to influence of sexual risk behavior, including knowledge, safer sex motivation, and safer sex skills. Knowledge was measured with 28 items from the Brief HIV Knowledge Questionnaire (HIV-KQ) [29, 30] and a subset of items from the STD Knowledge Questionnaire (STD-KQ) [31]; these items were supplemented with 4 items designed to assess HIV-testing knowledge. Internal reliability in the current sample was strong (α = .85). In previous studies, test-retest reliabilities for the HIV-KQ over three months ranged from .76 to .88 [30]; test-retest reliability for the STD-KQ was .88 over 2 weeks [31]. Evidence for the validity of these measures was obtained from individuals who received a sexual risk reduction intervention, and demonstrated greater increases in HIV-KQ and STD-KQ scores than did control participants [30, 31].
Motivation was assessed with 29 items designed to measure: (a) condom use attitudes [adapted from 32, 33], (b) risk reduction attitudes, (c) HIV testing attitudes [adapted from 34], and (d) behavioral intentions for safer sex [adapted from 23, 35, 36]. Internal consistency reliabilities for the current sample were satisfactory, with α = .70 for condom attitudes, .71 for risk reduction attitudes, .61 for testing attitudes, and .64 for behavioral intentions.
Behavioral skills were measured with three indicators: (a) a subset of 7 items from the Condom Influence Strategy Questionnaire (CISQ) [37, 38]; (b) 15 items adapted from the safer sex strategies survey [39]; and (c) 12 items adapted from a Self-Efficacy measure [40]. Internal consistency reliability for the CISQ in the present sample was .89. Previous studies have found an association between the CISQ and measures of sexual assertiveness, partner communication, condom use self-efficacy, negotiation self-efficacy, intentions to use condoms consistently, and condom use at last intercourse [37, 38]. Internal consistency reliability for the self-efficacy measure in the present study was .83.
All items were carefully selected based on prior item and factor analyses (when available) with the goals of (a) minimizing the participant burden while (b) maintaining the psychometric strength of the measures.
2.4.3 Sexual risk behavior
Items to assess sexual risk behavior were adapted from previous research [23, 35, 41], and assessed the number of male and female sexual partners (lifetime and past 3 months), and the number of occasions of protected and unprotected vaginal and anal sex (past 3 months) with steady and non-steady partners. Responses were summed to determine participants’ number of episodes of unprotected sex (vaginal plus anal) in the past 3 months with their steady partner, their non-steady partners, and all partners, consistent with prior recommendations [42, 43]. Responses also determined the percentage of total sexual episodes that involved unprotected sex with a steady partner, non-steady partners, and all partners.
A series of items assessed the most recent sexual experience, including whether the experience was with a steady or non-steady partner, the type of sex (i.e., oral, vaginal, or anal sex), and whether a condom was used. Additional items asked about sex trading (i.e., the number of times sex was exchanged for money or drugs); history of STI treatment; age at first oral, vaginal, and anal sex; and the co-occurrence of alcohol use and sex as well as the co-occurrence of drug use and sex, by partner type. All items had been used in previous research [23, 35, 41].
2.4.4 Incident sexually transmitted infections (STIs)
To assess incident STIs, participants provided urine specimens at the 3, 6, and 12-month follow-up occasions.
In addition, we determined, from electronic clinic records, whether participants tested positive for an incident STI (i.e., gonorrhea, Chlamydia, trichomonasis, syphilis, or HIV) at the clinic at any time during their year of follow-up assessments. Whether or not a positive syphilis test indicated a new infection was determined by consult with a nurse practitioner. In addition, we obtained county records of positive test results for reportable STIs (i.e., gonorrhea, Chlamydia, syphilis, and HIV). Thus, we were able to determine whether participants were diagnosed at any time during their year of follow-up with an incident STI at the clinic, or with a reportable STI anywhere in the county.
2.4.5 Potential moderators of intervention efficacy
Depressive symptoms were assessed with the 9-item version of the Center for Epidemiologic Studies Depression Scale (CES-D) [44, 45]. Internal consistency reliability for the current sample was .87. In previous studies, individuals who were diagnosed with depression had higher CES-D scores than non-depressed individuals [45], and CES-D scores correlate with other self-report measures of depression [44].
Future time perspective was assessed with a subset of 4 items from the Future Orientation scale [46]. Internal consistency reliability for the present sample was .79.
2.4.6 Substance use
Patients were asked to indicate how often they used marijuana, crack, cocaine, nitrite inhalants, speed or methamphetamine, cigarettes or tobacco, heroin, or ecstasy in the past three months (5-point scale: every day to never). These items were used to calculate the number of different drugs used in the past 3 months. Two items asked about the number of drinking days in a typical week, and the average number of drinks on a typical drinking day; responses to these items were multiplied to derive participants’ average number of alcoholic drinks per week. To determine the number of binge drinking episodes in which participants engaged in the past 3 months, men reported the number of times they had ≥ 5 drinks on one occasion (women reported how often they had ≥ 4 drinks on one occasion) [47].
To detect the likelihood of hazardous alcohol consumption, we used the Alcohol Use Disorders Identification Test (AUDIT) [48]. Internal consistency reliability for the AUDIT in the current sample was .90. High AUDIT scores have been associated with harmful alcohol use [48] and current alcohol problems [49].
To detect the likelihood of a drug use disorder, we used the Drug Abuse Screening Test (DAST) [50]. In the current sample, internal consistency reliability was .89. DAST scores are correlated with the frequency of drug use in the past 12 months [50], and with other measures of drug use [51].
2.5 Interventions
All participants received a brief, clinic-based intervention. Two-thirds of the participants also received an intensive intervention with the remaining third assigned to a no-intensive intervention (standard care) control condition.
2.5.1 Brief interventions
The two brief interventions were administered to patients individually during their clinic visit. Participants were randomly assigned to either a brief information intervention, or to a brief motivational intervention. These interventions were delivered during the participant’s clinic visit by a clinic nurse or nurse practitioner (nurses on the clinic staff delivered both interventions), and took the place of the traditional HIV pre- and post-test counseling delivered in most STI clinic settings.
2.5.1.1 Brief information (B-Info)
The B-Info intervention utilized digital video-disc (DVD) technology to present state-of-the-science information about HIV, testing, and sexual risk reduction options. The content presented on the DVD was based on a DVD previously shown to lead to greater rates of HIV testing [52]. Modifications were made to emphasize safer sex rather than HIV testing, and to present the local epidemiology of STIs and HIV. The DVD lasted 13 minutes, 22 seconds. Before beginning the DVD, the nurse met with each patient and asked a series of closed-ended questions to assess recent sexual risk behavior and stage of change for condom use (5 mins). The participant was then left alone in the room to watch the DVD. All nurses and nurse practitioners who delivered the DVD attended a brief training session, and received a detailed protocol for administering the DVD and responding to patient inquires.
2.5.1.2 Brief motivation intervention (BMI)
The form of BMI implemented in this study was stage-based behavioral counseling (SBC). SBC is a brief (15-20 minute) intervention, influenced by the TTM, and designed to target an individual’s sexual risk reduction needs based on his or her stage of change [27, 28]. Consistent with a motivational interviewing approach [53], SBC is client-centered, nonjudgmental, and tailored to the patient’s unique circumstances. Open-ended questions are used to elicit information about patients’ circumstances. The first part of the session was focused on determining a patient’s sexual risk behaviors and stage of change for using a condom; the second part of the session was focused on delivering a counseling strategy that was appropriate to the patient’s stage of change. For example, a patient who was precontemplative for condom use (i.e., did not see a need to use condoms) might be given information about the connection between STIs and HIV; in contrast, a patient who was ready for action (i.e., ready to start using condoms) might work with the counselor to develop a specific plan for condom use.
SBC was delivered by trained clinic nurses and nurse practitioners. Although many of the nurse counselors had been delivering SBC for several years, all study counselors attended a 1-day training refresher course to ensure a standard competence level prior to working with patients in the trial. Moreover, all counselors were provided with a study-specific manual, and they participated in weekly peer supervision meetings for the duration of the study, during which they listened to tapes of SBC sessions and discussed their counseling. To further ensure ongoing intervention fidelity, all counseling sessions were audiotaped and rated by SBC trainers. These ratings were used primarily for quality assurance purposes.
2.5.2 Intensive interventions
The two intensive interventions were administered to patients in small groups within two weeks of their clinic visit. Participants were randomly assigned to one of three intensive intervention conditions: (a) Intensive Information workshop (I-Info); (b) Intensive Information-Motivation-Behavioral Skills workshop (I-IMB); or (c) no workshop control. Both workshops were held in a single, 4-hour session, and were led by two trained co-facilitators. Separate workshops were held for men and women.
The twelve facilitators came from diverse backgrounds, including nursing, psychology, social work, and public health. Having a diverse facilitator team strengthened facilitation of the workshops by assuring that multiple perspectives, both theoretical and practical, were represented on the team. Most had post-baccalaureate education, although several of the facilitators were clinically skilled paraprofessionals. An effort was made to pair paraprofessional facilitators with professionally-trained facilitators. Most workshops were facilitated by a male and female co-facilitator; at least one member of each facilitator team was African-American.
All facilitators participated in eight hours of training, including a 2-hour orientation session that addressed the theoretical framework of the interventions, counseling and group management techniques, ethical issues, and intervention content. Facilitators also completed readings about HIV and STIs, the IMB model, group facilitation, and ethics. They read and followed detailed manuals. Most prepared by facilitating a mock workshop. After the initial group of facilitators was trained, subsequent facilitators were required to observe at least two I-Info and two I-IMB sessions before facilitating (the initial facilitators were given additional training covering manual content, because there were no ongoing workshops to observe). Facilitators also attended bi-weekly peer supervision meetings; during these meetings, facilitators discussed intervention content and fidelity, and problem-solved issues that arose during the workshops. To further ensure intervention quality and fidelity, the Project Coordinator periodically observed facilitators delivering the interventions. In addition, all sessions were audiotaped for quality assurance purposes.
2.5.2.1 Intensive information (I-Info)
The I-Info workshop included information about HIV, STIs, and safer sex, without motivation or skills elements. To begin, index cards with statements about HIV and STIs were passed out to participants. The participants took turns reading the cards aloud, and facilitators engaged all the workshop participants in a discussion of whether or not the statement on the card was true or false. The topics discussed included: (a) background information about HIV; (b) HIV transmission; (c) HIV prevention; (d) HIV testing; (e) HIV treatment; (f) and STIs. Participants also completed a risk continuum activity, in which they placed different sexual activities on a continuum from no risk to low risk to high risk. The session ended with a question-and-answer game show-style activity, reviewing the information covered earlier in the workshop.
2.5.2.2 Intensive information-motivation-behavioral skills (I-IMB)
The I-IMB workshop included information, motivation, and skills components. Intervention activities and manual content were based on previous interventions, including Partners in Prevention [54], the Women’s Health Project [35], the Health Improvement Project [41], and the NIMH Multisite Project [14]. The workshop began with a brief overview of important HIV information, including information about how HIV is transmitted and information about how to prevent HIV. Participants were given a brief amount of time to ask questions.
The motivation section included information about local STI and HIV rates, to sensitize participants to their risk of contracting an STI. Participants then watched a video of people living with HIV; after the video, participants discussed whether they knew anyone who was HIV positive, and what it would be like for themselves and for their families if they contracted HIV. Finally, participants completed a risk continuum activity, similar to the activity in the I-Info workshop, except that the participants were asked to personalize the risk continuum by thinking about their own sexual behaviors, and where they fell on the risk continuum.
The majority of the I-IMB workshop was focused on skills. First, participants learned trigger management, including how to identify personal triggers for sexual risk behavior, and how to problem-solve managing these triggers. Participants then learned the important parts of an assertive statement, and practiced using them during a role playing exercise. Finally, participants were shown how to use the male and female condom, and practiced putting these condoms on penis and pelvic models (women only). The I-IMB workshop concluded with a brief review. Participants were given a card and asked to write down a personal safer sex goal.
2.6 Procedures
Clinic charts were screened to determine which patients were age 18 or older, not HIV positive, had not been to the clinic in the past 3 months, and were at the clinic for a full STI screening (e.g., not just for a vaccination or test results). The RA called a patient by clinic identification number from the waiting room, brought him/her to a private exam room, explained that a study was being conducted in the clinic to improve sexual health in the community, and asked if the patient was willing to answer a few brief questions to determine whether he/she was eligible to participate. Patients who agreed were asked a series of questions about recent sexual behavior, participation in substance use treatment, willingness to take an HIV test, and previous HIV test results, to determine eligibility to participate. In addition, trained RAs made an informal assessment of whether the patient was impaired mentally. The study was explained in detail to patients who were eligible to participate. Those who were interested in participating were asked to read and sign an informed consent form.
Participants who agreed to participate and signed the consent form were asked for detailed contact information so they could be contacted for interventions and follow-up surveys. Participants then completed a calendar of important events over the past 3 months (e.g., birthdays, holidays, trips out of town, starting or stopping a job), to orient them to the time frame of 3 months used in many of the questions. Next, participants were seated at a laptop computer and guided by the RA through a series of sample questions using software programmed to administer an audio, computer-assisted, self-interview (ACASI). After they were comfortable answering the sample questions, patients completed the survey in private.
After completing the baseline ACASI, patients participated in a routine clinic visit, including an intake, a physical examination, and a rapid HIV test (OraQuick®). They then received either a brief clinic-based intervention (B-Info or BMI, which was randomly assigned using a computer-generated random number table), preliminary STI and HIV test results, and STI treatment (if indicated). (The latter were part of standard clinical care, not part of the research protocol.) All components of the clinic visit were delivered by a nurse or nurse practitioner, and all occurred on the day participants were enrolled in the study.
Before leaving the clinic, participants completed a brief post-assessment, which included information, motivation, and skills questions, and ratings of the two brief intervention conditions. They were randomly assigned (using a random number table) to one of the three intensive intervention conditions: (a) a 4-hour informational workshop; (b) a 4-hour informational, motivational, and skills workshop; or (c) a no-intervention control group. Participants who were randomized to a workshop condition received a written and a verbal invitation (from the RA) to attend the workshop. The RA asked patients if they anticipated any difficulties attending the workshop, and generated possible solutions to anticipated barriers. If needed, bus tokens were provided to participants, and occasionally child care was provided at the workshop, if the participant had no other option for child care. All participants were reimbursed $20 for their time, and were given an appointment card for their next survey.
All participants in a 2-week period (wave) were assigned to the same workshop. If a participant could not attend a workshop, he or she was invited to the next scheduled workshop. If a participant could not attend either of these two workshops, he/she was not invited to another workshop. Participants who attended one of the workshops were asked to complete a brief survey about their experiences during the workshop. Participants were reimbursed $40 for attending the workshop, and were given a safer sex kit and a certificate for attending.
All participants were asked to return 3, 6, and 12 months after they were scheduled to attend a workshop (participants in the no-workshop control condition were asked to return 3, 6, and 12 months after the workshop would have been scheduled had they been recruited during an intervention wave). Participants were given reminder cards with dates during which they could return for these follow-up visits (encompassing a 4 week period, from 2 weeks before the target date to two weeks after the target date), were sent a reminder letter, and were called on the phone. In addition, clinic charts were reviewed each day to determine whether any of the clinic patients were study participants and were due for a follow-up visit.
At these follow-up visits, participants provided a urine sample, which was tested for Chlamydia and gonorrhea. New contact information was obtained, and RAs again helped the participant complete a calendar of important events in the past 3 months. Participants completed an ACASI survey, answering questions similar to those asked at the baseline assessment. They were reimbursed $30 for their time, and given an appointment card for their next follow-up visit, or a certificate of completion at their final survey.
Patients also gave permission for their STI clinic records to be reviewed during their year of study participation. From these records, we determined whether participants had been diagnosed with an incident STI (chlamydia, gonorrhea, trichomonas, syphilis, or HIV) at any time during the year after they enrolled in the study. Finally, because chlamydia, gonorrhea, syphilis, and HIV must be reported to the county, we determined from county health department records whether participants were diagnosed with one of these STIs at any medical facility in the county (other than the STI clinic) in the year following study enrollment.
Participants were enrolled in the study for approximately one year. If they attended an intensive workshop and completed the baseline and three follow-up assessments, they were reimbursed a total of $150. The amount of time invested by participants in the assessments and the intensive workshop was approximately 6.5 hours.
All procedures were approved by the Institutional Review Boards of the participating institutions (i.e., Syracuse University, the University of Rochester, and the Monroe County Health Department). In addition, to further protect participant privacy, we obtained a Federal Certificate of Confidentiality.
2.7 Data analyses
The analyses were designed to: (a) characterize the sample regarding demographic, sexual risk behavior, and substance use; (b) explore the inter-relationships among the baseline variables; (c) confirm that randomization produced equivalent groups at baseline; (d) determine whether participants found the two brief interventions and the two intensive interventions equally acceptable; (e) evaluate the short-term effects of the brief interventions on knowledge, attitudes, and workshop attendance; and (f) describe follow-up rates. Descriptive statistics (means, standard deviations, and percentages) were used to characterize the sample and follow-up rates. Correlations were conducted to explore inter-relationships among baseline variables. Analyses of variance (ANOVAs; for continuous variables) and chi-square analyses (for categorical variables) were conducted to determine whether randomization resulted in equivalent groups at baseline, and to determine whether satisfaction ratings differed by workshop condition. Repeated measures ANOVAs were conducted to evaluate the short-term effects of the brief interventions.
3. Results
3.1 Patient recruitment and flow
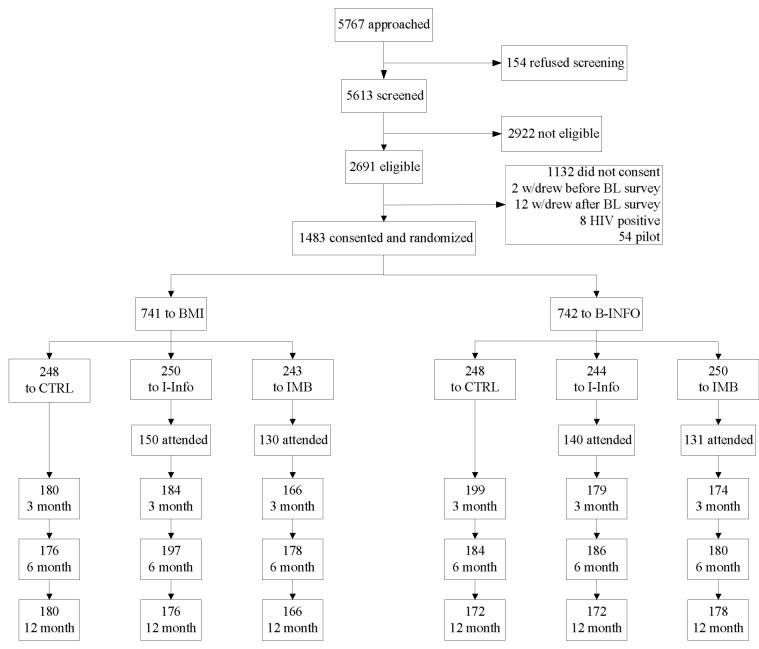
As depicted in Figure 2, a total of 5767 patients were approached and 5613 (97%) agreed to the screening. Among those screened, 2691 (48%) were eligible to participate. The most common reason for ineligibility was no sexual risk behavior (e.g., sexually abstinent, or all sexual experiences were condom protected) in the past 3 months (reported by 77% of those who were not eligible), followed by HIV test refusal (14%) and mental impairment (5%).
Figure 2.
Study flow indicating patient screening, recruitment, randomization, intervention, and follow-up assessment
Of those who were eligible, 1559 (58%) consented to participate. The most common reason for declining participation was lack of time (82%). Two participants withdrew before completing the baseline survey, 12 withdrew after the survey but before receiving any counseling, eight participants tested positive for HIV and were withdrawn from the study and provided with more specific and intensive counseling and referrals, and 54 patients were pilot participants, leaving 1483 participants (95%) who were randomized to the interventions.
Approximately one-half of participants (n = 741) were randomized to the BMI with the other participants randomized to the B-Info (n = 742). One-third of participants were randomized to the I-Info workshop (n = 494; 33%), one-third were randomized to the I-IMB workshop (n = 493; 33%), and one-third were randomized to the no-workshop control group (n = 496; 33%). Among those randomized to one of the two workshop conditions, n = 551 (56%) attended a workshop. These details are depicted in Figure 2.
3.2 Participant characteristics
3.2.1 Demographic variables
As detailed in Table 1, 54% of the participants were male and 46% were female. Nearly two-thirds (64%) self-identified as African American, 24% as Caucasian, and 12% as other or mixed race; 9% of the sample were Hispanic. On average, participants were 29.2 years of age. One-quarter of the sample had less than a high school education; 38% had attended at least some college. Approximately one-half of the sample (51%) was unemployed, and 57% had an income less than $15,000 per year. The majority of the sample (93%) was unmarried, had children (58%), and identified themselves as heterosexual (88%).
Table 1. Demographic characteristics of study participants at baseline (N = 1483).
| n | % | |
|---|---|---|
| Gender | ||
| Female | 688 | 46% |
| Male | 795 | 54% |
| Race | ||
| Caucasian | 360 | 24% |
| African American | 947 | 64% |
| Other | 176 | 12% |
| Hispanic | ||
| Yes | 129 | 9% |
| No | 1354 | 91% |
| Education | ||
| Less than high school | 374 | 25% |
| High school / GED | 545 | 37% |
| At least some college | 563 | 38% |
| Employment | ||
| Unemployed | 760 | 51% |
| Employed | 723 | 49% |
| Income | ||
| < $15,000/year | 837 | 57% |
| $15,000/year to $30, 0000/year | 410 | 28% |
| ≥ $30,000/year | 233 | 16% |
| Married | ||
| Yes | 98 | 7% |
| No | 1385 | 93% |
| Live with partner | ||
| Yes | 345 | 23% |
| No | 1138 | 77% |
| Self-identified sexual orientation | ||
| Homosexual | 23 | 2% |
| Bisexual | 113 | 8% |
| Heterosexual | 1306 | 88% |
| Don’t know | 40 | 3% |
| Children | ||
| No | 618 | 42% |
| Yes (at least 1) | 865 | 58% |
| M | SD | |
| Age (years) | 29.2 | 9.7 |
| Number of children | 1.5 | 1.8 |
3.2.2 Sexual behavior
Lifetime and recent sexual behavior are summarized in Table 2. The average participant became sexually active at a relatively early age; the mean age at first vaginal intercourse was 14.7 years old (median = 15 years) and participants reported an average of 32.8 (median = 20) lifetime sexual partners. Most patients (76%) reported being treated for at least one STI in their lifetime, and the mean number of times treated for an STI was 3.3.
Table 2. Sexual behavior patterns of study participants at baseline (N = 1483).
| n | Median | M | SD | Observed Range | Skewness | Kurtosis | |
|---|---|---|---|---|---|---|---|
| Sexual partners, lifetime | 1472 | 20 | 32.8 | 35.6 | 1-139 | 1.8 | 5.4 |
| Sexual partners, past 3 months | 1483 | 2 | 2.8 | 2.2 | 0-10 | 1.8 | 6.1 |
| Unprotected sex (count), past 3 months | 1476 | 9 | 17.3 | 21.3 | 0-84 | 1.9 | 5.8 |
| Unprotected sex with steady partner (count), past 3 months | 1478 | 6 | 14.0 | 20.2 | 0-81 | 2.0 | 6.4 |
| Unprotected sex with outside partners (count), past 3 months | 1479 | 1 | 2.8 | 4.3 | 0-17 | 2.1 | 6.7 |
| Treated for an STI (count) | 1482 | 2 | 3.3 | 3.9 | 0-18 | 2.0 | 7.5 |
| Unprotected sex (proportion), past 3 months (0-1) | 1476 | .74 | .66 | .33 | 0-1 | -0.6 | 2.0 |
| Unprotected sex with steady partner (proportion), past 3 months (0-1) | 1478 | .69 | .56 | .43 | 0-1 | -0.2 | 1.3 |
| Unprotected sex with outside partners (proportion), past 3 months (0-1) | 1478 | .25 | .37 | .39 | 0-1 | 0.5 | 1.7 |
| Co-occurrence of alcohol use and sex (1-5) | 1482 | 2.3 | 1.2 | 1-5 | 0.7 | 2.4 | |
| Co-occurrence of drug use and sex (1-5) | 1481 | 2.0 | 1.3 | 1-5 | 1.1 | 2.8 | |
| n | % | Median | |||||
| Age at 1st oral sex < 13 years | 148 | 11% | 17 | ||||
| Age at 1st vaginal sex < 13 years | 252 | 18% | 15 | ||||
| Age at 1st anal sex < 13 years | 41 | 5% | 20 | ||||
| Used condom at last intercourse | 380 | 27% | |||||
| Diagnosed with an STI at baseline | 341 | 23% | |||||
| Ever traded sex | 320 | 22% |
Data for sexual behavior in the past 3 months indicated continuing high levels of sexual risk behavior. On average, participants reported 2.8 sexual partners (median = 2) in the past 3 months and an average of 17.3 episodes of unprotected sex in the past 3 months. Sixty-six percent of sex occasions were not condom protected, and only 27% of participants reported using a condom at last sexual intercourse. The number of partners was correlated with the number of unprotected sexual events, r (n = 1476) = .09, p < .001. Twenty-three percent of the sample was diagnosed with an STI at baseline (these infections were confirmed biologically).
3.2.3 Substance use
Table 3 provides a summary of participants’ substance use. Overall, participants reported an average of 7.3 drinks per week, and an average of 3.6 binge drinking episodes in the past 3 months. However, 72% of participants reported any alcohol use, and these participants reported an average of 10.1 drinks per week, and an average of 4.9 binge drinking episodes in the past 3 months. Thirty-three percent of participants reported an AUDIT score ≥ 8, indicative of hazardous drinking patterns.
Table 3. Substance use characteristics of study participants at baseline (N = 1483).
| n | Median | Mean | SD | Observed Range | Skewness | Kurtosis | Number of Items | α | |
|---|---|---|---|---|---|---|---|---|---|
| Alcohol Use Disorders Identification Test (AUDIT), summary score | 1449 | 4 | 6.9 | 7.9 | 0-40 | 1.6 | 5.4 | 10 | .90 |
| Drug Abuse Screening Test (DAST), summary score | 1286 | 1 | 1.9 | 2.6 | 0-10 | 1.5 | 4.2 | 10 | .88 |
| Drinks, average weekly | 1478 | 3 | 7.3 | 10.3 | 0-41 | 1.8 | 5.7 | ||
| Binge drinking episodes, past 3 months | 1478 | 1 | 3.6 | 5.6 | 0-21 | 2.0 | 6.3 | ||
| Drugs used, past 3 months | 1481 | 1 | 1.0 | 1.0 | 0-8 | 1.4 | 6.2 |
The most commonly used drug was marijuana, with 46% of the sample reporting marijuana use in the past 3 months (not tabled). Use of other drugs, including crack cocaine (9%), cocaine powder (6%), ecstasy (2%), heroin (1%), speed (1%), and nitrite inhalants (<1%) was reported much less frequently. Tobacco use was reported by about one-third (32%) of the sample. Overall, participants scored an average of 1.9 on the DAST, but 28% of participants scored ≥ 3 on the DAST, indicative of problematic drug use.
3.2.4 IMB constructs
Data from the IMB constructs are summarized in Table 4. Participants were moderately knowledgeable about HIV and STIs, correctly answering 67% of HIV, STI, and testing knowledge questions correctly. Participants also had relatively positive attitudes towards risk reduction, with an average of 2.9 on Behavioral Intentions (1 to 4 scale where 4 indicates strongest safer sex intentions), 4.5 on Condom Attitudes (1 to 6 scale where 6 indicates strongest agreement with positive condom attitudes), 4.8 on Risk Reduction Attitudes (1 to 6 scale where 6 indicates strongest agreement with positive attitudinal statements), and 5.4 on Testing Attitudes (1 to 6 scale where 6 indicates strongest agreement with positive testing attitude statements). Participants reported moderate safer sex skills. They reported using an average of 4.3 risk reduction strategies in the past 3 months (out of 15 possible strategies). On the self-efficacy measure, participants scored an average of 7.8 (0 to 10 scale; 10 indicates greatest self-efficacy). Participants scored an average of 2.4 on the CISQ (1 to 5 scale, where 5 indicates most frequent use of condom influence strategies).
Table 4. HIV-related information, motivation, and skills scores of study participants at baseline (N = 1483).
| n | M | SD | Observed Range | Skewness | Kurtosis | # Items | α | |
|---|---|---|---|---|---|---|---|---|
| Information | ||||||||
| Knowledge (% correct, 0-100%) | 1483 | 67.2 | 19.2 | 0-100 | -0.9 | 3.7 | 28 | 0.85 |
| Motivation | ||||||||
| Behavioral Intentions (1-4) | 1482 | 2.9 | 0.7 | 1-4 | -0.2 | 2.2 | 4 | 0.64 |
| Condom Attitudes (1-6) | 1482 | 4.5 | 1.0 | 1-6 | -0.5 | 3.2 | 5 | 0.70 |
| Risk Reduction Attitudes (1-6) | 1483 | 4.8 | 0.6 | 2.1-6 | -0.4 | 3.5 | 14 | 0.70 |
| Testing Attitudes (1-6) | 1481 | 5.4 | 0.6 | 2.8-6 | -1.1 | 4.3 | 6 | 0.61 |
| Skills | ||||||||
| CISQ (1-5) | 1479 | 2.4 | 1.1 | 1-5 | 0.4 | 2.2 | 7 | 0.89 |
| Risk Reduction Strategies (0-15) | 1480 | 4.3 | 2.7 | 0-15 | 0.5 | 2.8 | 15 | 0.67 |
| Self-Efficacy (0-10) | 1482 | 7.8 | 1.6 | 0-10 | -1.0 | 4.0 | 6 | 0.82 |
3.2.5 Moderators
Five hundred (34%) participants met criteria for being at risk for a major depressive episode, based on endorsing 4 or more of the 9 items on the CES-D; on average, participants scored 9.6 (maximum = 27) on the CES-D. Despite endorsing items suggestive of depression, participants reported feeling moderately optimistic about their future. On the Future Time Perspective measure, they scored an average of 4.8 (1 to 6 scale, where 6 indicates the most optimistic outlook toward the future).
3.3 Hypothesized correlates of sexual risk behavior
Based on the IMB model, we expected that information, motivation, and behavioral skills at baseline would be inversely related to recent sexual behavior, including number of sexual partners in the past 3 months and number of episodes of unprotected sex in the past 3 months. Correlations between IMB constructs and sexual behavior variables are presented in Table 5.
Table 5. Correlations between sexual behavior and IMB constructs at baseline (N = 1483).
| Number of sexual partners | Unprotected Sex (no. of episodes) | Knowledge | Behavioral Intentions | Condom Attitudes | Risk Reduction Attitudes | Testing Attitudes | Condom Influence Strategies | Risk Reduction Strategies | Self-Efficacy | |
|---|---|---|---|---|---|---|---|---|---|---|
| Number of Sexual Partners | 1 | |||||||||
| Unprotected Sex (number of episodes) | .09** | 1 | ||||||||
| Knowledge | .02 | .03 | 1 | |||||||
| Behavioral Intentions | -.27** | -.10** | -.02 | 1 | ||||||
| Condom Attitudes | -.04 | -.12** | .18** | .24** | 1 | |||||
| Risk Reduction Attitudes | -.27** | -.08* | .10** | .50** | .32** | 1 | ||||
| Testing Attitudes | -.08* | -.002 | .17** | .18** | .26** | .42** | 1 | |||
| Condom Influence Strategies | .15** | -.34** | -.07* | .21** | .22** | .09** | -.01 | 1 | ||
| Risk Reduction Strategies | .005 | -.13** | .18** | .18** | .15** | .20** | .13** | .33** | 1 | |
| Self-Efficacy | -.08* | -.08* | .10** | .36** | .29** | .35** | .22** | .21** | .22** | 1 |
Note. IMB = Information—Motivation—Behavioral Skills model of HIV risk reduction. The natural log of the number of partners and number of episodes of unprotected sex was used
p < .01
p < .001.
Contrary to the IMB model, information scores were unrelated to sexual risk behavior, r (number of partners) = .02, r (number episodes unprotected sex) = .03, ps > .05. As predicted by the IMB model, however, both motivation and behavioral skills were related to recent sexual behavior. For the motivational variables, the number of partners in the past 3 months was negatively correlated with behavioral intentions (r = -.27, p < .001), risk reduction attitudes (r = -.27, p < .001), and testing attitudes (r = -.08, p < .01). The number of episodes of unprotected sex in the past 3 months was negatively correlated with behavioral intentions for risk reduction (r = -.10, p < .001), condom attitudes (r = -.12, p < .001), and risk reduction attitudes (r = -.08, p < .01).
The pattern of correlations between sexual behavior and behavioral skills was more complicated. As predicted, the number of episodes of unprotected sex in the past 3 months was negatively correlated with use of condom influence strategies (r = -.34, p < .001), risk reduction strategies (r = -.13, p < .001), and self-efficacy (r = -.08, p < .01). The number of partners in the past 3 months was also negatively correlated with self-efficacy (r = -.08, p < .01). The number of partners in the past 3 months was positively correlated with use of condom influence strategies (r = .15, p < .001), perhaps because having more partners creates a greater number of opportunities to use differing risk reduction strategies.
3.4 Effects of randomization
Overall, randomization succeeded in producing equivalent groups at baseline. There were no differences among the six intervention groups on any of these variables: (a) demographics (gender, race, ethnicity, employment, income, marital status, co-habitation, sexual orientation); (b) lifetime sexual behavior (number of sexual partners, number of times treated for an STI); (c) recent sexual behavior (number of sexual partners, number of episodes of unprotected sex total, with a steady partner, and with outside partners, proportion of episodes of unprotected sex with a steady partner or with outside partners, condom use at last intercourse, co-occurrence of alcohol use and sex, co-occurrence of drug use and sex during the past three months); (d) STI diagnosis at baseline; (e) substance use (AUDIT, DAST, number of drinks per week, number of binge drinking episodes, number of different drugs used); or (f) risk behavior antecedents, including information (knowledge scores), motivation (behavioral intentions, condom attitudes, risk reduction attitudes, testing attitudes), or behavioral skills (CISQ, self-efficacy; all ps > .05).
Overall, there were few group differences; however, the groups did differ on education, χ2(1, N = 1482) 6.88, p < .01, age, F(2, 1477) = 3.48, p < .05, and proportion of episodes of unprotected sex with all partners in the past 3 months, F(1, 1470) = 8.70, p < .01. On average, participants who received the BMI intervention had less education compared to participants who received the B-Info intervention (35% vs. 41% had some college, respectively). Follow-up analyses also indicated that patients in the BMI + IMB condition tended to be younger (M = 27.6 years) than patients in the BMI + I-Info condition (M = 30.2 years) and those in the B-Info + CTRL (M = 30.7 years) groups. Those in the BMI conditions reported a lower percentage of episodes of unprotected sex in the past 3 months (M = 63%) compared to those in the B-Info conditions (M = 69%). Applying a Bonferroni correction based on the number of tests conducted rendered even these findings non-significant; nonetheless, when we conduct outcome analyses, we will use these baseline variables as covariates.
3.5 Patient satisfaction with the brief interventions
To assess patient satisfaction, all patients were asked to respond to a set of items regarding their experience with the brief intervention [55]. The average score in response to an item asking how well the counseling met patients’ needs was 3.7 (on a 4-point scale, with 4 indicating “almost all needs were met”). Participants were also very satisfied with the counseling (M = 3.8, on a 4-point scale, with 4 indicating they were “very satisfied”), and they reported they would come back to the program (M = 3.8 on a 4-point scale, with 4 indicating “definitely yes”). Patient satisfaction ratings did not differ between the BMI and B-Info interventions, all ps > .05.
3.6 Short-term effects of the brief interventions
To determine if the brief interventions achieved the short-term goals of increasing knowledge and enhancing motivation for risk reduction, we conducted separate two (intervention: BMI vs. B-Info) × two (time: pre- vs. post-intervention) repeated measures ANOVAS on the HIV-related information and motivational variables. These analyses revealed a main effect of time such that patients in both the BMI and B-Info conditions improved their HIV-related knowledge, F (1, 1433) = 634.91, p < .0001 (Mpre = 60% and Mpost = 73%), behavioral intentions, F (1, 1448) = 50.46, p < .0001 (Mpre = 2.99 and Mpost = 3.13), condom attitudes, F (1, 1462) = 94.05, p < .0001 (Mpre = 4.50 and Mpost = 4.74), and risk reduction attitudes, F (1, 1447) = 54.89, p < .0001 (Mpre = 4.97 and Mpost =5.07) from the pre-intervention assessment to the post- intervention assessment.
In addition, there was a significant intervention-by-time interaction for the knowledge measure, F (1, 1433) = 59.90, p < .0001. Follow-up analyses showed that the BMI and B-Info groups did not differ in baseline knowledge, F (1, 1433) = 1.16, p > .05 (MBMI = 60%; MB-Info 61% correct), but the groups did differ in knowledge post-intervention, F (1, 1433) = 78.46, p < .0001 (MBMI = 68%; MB-Info = 77% correct). Although both groups improved in knowledge from pre- to post-intervention, the B-Info group had greater knowledge gains than did the BMI group. The intervention-by-time interaction was nonsignificant for the motivational variables (ps > .05). A chi-square analysis indicated that attendance at the intensive workshop did not differ as a function of brief intervention condition, χ2 (1, N = 987) = 0.38, p > .05.
3.7 Patient satisfaction with the intensive interventions
Participants also evaluated the intensive interventions using a 4-point scale with items similar to those used to evaluate the brief interventions. They reported almost all of their needs were met (M = 3.6), and they were very satisfied (M = 3.8). In addition, participants thought the workshops were very interesting (M = 3.9, on a 4 point scale with 4 indicating very interesting), they were comfortable in the workshops (M = 3.8, on a 4 point scale with 4 indicating very comfortable), and they thought the topics discussed were very important (M = 3.9 on a 4 point scale, with 4 indicating very important). Participants also reported they would recommend the workshop to a friend (M = 3.9, on a 4 point scale with 4 indicating definitely yes). There were no differences in patient satisfaction ratings for the two intensive interventions.
3.8 Initial follow-up rates
Patients were asked to return at 3, 6, and 12 months to provide data that will be used for the intervention efficacy analyses. Return rates for 3, 6, and 12 month follow-ups were 73%, 74%, and 70%, respectively. At 3 months, more participants in the control condition (76%) returned than in the IMB condition (69%), χ2 (1, N = 989) = 6.91, p < .01. However, the percentage of participants who returned at 6 and 12 months did not differ by condition. In addition, the percentage of participants who returned for at least one follow-up assessment did not differ by condition. Finally, condition was not associated with the number of follow-ups (0, 1, 2, or 3) for which participants returned.
4. Discussion
STI clinics serve low-income patients who often have limited access to health care, and who are at elevated risk for HIV and other STIs. Clearly, such clinics provide an opportune setting in which to implement needed public health and clinical interventions, and to test those interventions in RCTs.
4.1 Innovative study features
The trial described in this paper is noteworthy because of several methodological, clinical, and practical features that are rarely evident in a single trial. First, we implemented and are evaluating a unique two-step approach to intervention delivery. Patients initially received a brief, clinic-based, individual intervention while they were waiting to receive STI test results; then, within the next several weeks, the patients were invited to attend an intensive, group-based, sexual risk reduction workshop. The brief, clinic-based intervention allowed patients to receive individual counseling tailored to their unique circumstances, utilized the time that patients were waiting to receive test results, and capitalized on the possibly motivating anxiety that patients may feel as they are awaiting HIV test results [56]. The intensive intervention allowed for more in-depth discussion and practice, and capitalized on the support from other participants and other group processes that emerge in group-based interventions [57, 58].
Second, the clinic based interventions were implemented in a busy, publicly-funded clinic, by existing clinic staff. Nurses and nurse practitioners either delivered the stage-based behavioral counseling, or set up patients with the educational DVD, while the patients waited for their HIV test results. Thus, with both of these clinic-based interventions, no additional or specialized personnel was needed beyond the usual clinic staffing. Although some clinics may have the resources to hire counseling personnel such as health educators or behavioral counselors, most do not have this luxury. Although there is some cost to using existing clinic staff, if the outcome evaluation indicates that these interventions reduce risk behavior and incident infections, the additional effort required will likely be cost-saving, because there is no need to hire additional counseling staff.
Third, the use of a video-based intervention delivered via laptop computer (i.e., the B-Info intervention) is noteworthy. As the importance of behavior for health is more widely accepted [59], and as the cost of health care continues to escalate, the need for widespread health promotion and disease prevention increases. Traditional clinical and public health interventions cannot fully address this need because of resource constraints; in response to this resource constraint, self-administered interventions, such as computer-delivered interventions (CDIs), have been touted as an efficient way to meet the need for cost-effective interventions. Evaluation of the efficacy of CDIs in a variety of health domains suggests that they can lead to improved short-term health outcomes [11]. However, few trials have examined self-administered interventions in the context of HIV and STI prevention, a gap that this trial will help to fill.
Fourth, we employed audio-computer assisted self-interviewing (ACASI) to collect self-report data. Survey administration using ACASI has several advantages over traditional interviews or self-administered questionnaires, including increased patient privacy, lower literacy skills needed by patients, flexible branching and skip patterning to allow more detailed assessments based on sexual behavior or partner type, and more accurate and efficient data management. Evidence suggests that data collected by ACASI is likely to be more valid than data collected by other assessment modalities, particularly for socially sensitive data [60-62].
Fifth, evaluation of the intervention will involve four sources of data: patient self-report (of sexual risk behavior), patient behavior (workshop attendance as an outcome of the brief intervention), biological specimen collection (point prevalence of STIs), and chart abstraction (of STIs that might have been diagnosed and treated between follow-up assessments). This multi-method and bio-behavioral evaluation strategy allows for a more comprehensive and fine-grained evaluation of intervention efficacy [63].
Finally, the multidisciplinary research team represents a unique partnership among university-based behavioral scientists, nurses, physicians, and public health practitioners. Although the translation of empirically-tested HIV prevention interventions is widely recognized as being essential to the improvement of public health [64], typically this entreaty implies moving from scientific discovery to public health practice. Our approach has been more collaborative and bidirectional, benefiting from the expertise of both scientists and practitioners. In addition, the collaboration between researchers and clinic staff helped to ensure that the clinic staff were supportive of the study, and that the study could be integrated into the clinic flow. Overall, this and the other features just described have strengthened the scientific integrity of the trial while optimizing the ecological validity of its results.
4.2 Short-term results
In addition to its innovative features, this trial has yielded some interesting short-term results. The success of any public health intervention depends upon its reach [65, 66]. In this regard, we were able to recruit 58% of the eligible patients. Although this rate of participation can be improved, it compares favorably to rates reported in other major RCTs, which have ranged from 33% to 44% in similar settings [8, 9]. That some patients decline to participate in research is not surprising, especially given that the research required an extra (unplanned) hour on that day plus agreeing to three follow-up assessments over the next year. Indeed, the modal reason for declining to participate was time. It is likely that a greater percentage of patients would have taken part in the interventions if not for the burden of completing four, 45-minute long ACASIs, urine sampling, and other commitments required by the research protocol. Nonetheless, increased attention should be devoted to making interventions (and research protocols) patient-friendly and optimally convenient.
Participants in this study were predominately African-American and low income, reflecting the demography of patients served by the clinic. The participants in this study reported high levels of sexual risk behaviors, including multiple partners and many episodes of unprotected sex. Most patients had been diagnosed with an STI during their lifetimes, and 23% were diagnosed during the baseline evaluation. Although not surprising, these findings demonstrate that STI clinics provide an opportune setting for sexual risk reduction.
In addition to reporting high levels of sexual risk behaviors, participants also reported high levels of substance use (mostly alcohol and marijuana use) and high levels of depression. Others have similarly found high levels of substance use [67] and depression [68] among STI clinic patients. Some authors have used the term “syndemic” to refer to the additive effect of multiple health problems in a community [69, 70], and noted that the co-occurrence of multiple health threats makes intervention and behavior change both more important, and more challenging [71]. Thus, patients attending STI clinics appear to be at risk for multiple problems, in addition to being at elevated risk for STIs and HIV. Both substance use and depression could interfere with sexual behavior change, and should be considered when designing sexual risk reduction interventions for patients attending an STI clinic.
Participants were very satisfied with the two brief and the two intensive interventions, rating them very positively on all satisfaction items. The short-term effects of the brief interventions on knowledge and motivational antecedents of behavior change are promising, especially given that the motivational antecedents were associated with risk behavior at baseline. Overall, these results are very encouraging, indicating that once patients attend sexual risk reduction programs, they typically benefit and find the experience worthwhile. However, a continuing challenge for such prevention programs is getting patients to attend sessions that occur at another time and place.
In this trial, 56% of those who were invited to a workshop attended. These attendance rates are similar to those obtained in other behavioral intervention trials with socioeconomically disadvantaged populations [e.g., 9, 72, 73]. We plan to conduct outcome analyses using an intent-to-treat approach, because this approach provides the best estimation of the true public health impact of interventions; however, because only 56% of participants attended the intensive interventions, this intent-to-treat approach will reduce the power to detect group differences. Therefore, we will likely conduct supplemental “as-treated” outcome analyses.
As noted in previous reports, we have found that, in general, men and younger persons are less likely to attend workshops [74], and that financial incentives increase attendance rates [75]. These results corroborate results obtained by other investigators, and underscore the importance of developing improved social marketing strategies, optimizing the convenience of the sessions, and providing incentives to overcome initial reluctance to attend. Clearly, once patients attend, they find the workshops interesting, important, and comfortable.
Overall, the relationships among variables was as expected based on the IMB model [13]. Thus, as predicted, motivation and most skills variables were associated with current sexual behavior. Contrary to the IMB model, information was unrelated to current sexual behavior; however, the model (and previous empirical work) does suggest that motivation and skills may be more influential determinants of sexual risk behavior (and sexual risk reduction interventions). It should be noted that these results should be considered to be preliminary, because they reflect cross-sectional analyses. Future analyses will examine the predictive nature of the IMB constructs using longitudinal data available from the 3, 6, and 12 month follow-ups. A strength of the study design is the ability to test the IMB model using longitudinal data.
4.3 Study limitations
Several limitations of the study design and implementation should be acknowledged. First, despite randomization, a few group differences did emerge at baseline; even though these differences are likely due to chance, we will control (statistically) for these baseline differences when analyzing outcome data. Second, the 58% participation rate compares favorably to other RCTs conducted in STI clinics [8, 9]; however, 42% of eligible patients declined to participate and, therefore, the results may not generalize to all STI clinic patients. Because time was the primary reason for declination, further attempts to streamline research protocols may help to increase participation rates in future trials. It is likely that participation in the interventions would be improved if offered as stand-alone services (i.e., separated from the intensive assessments needed for research purposes), but we did not evaluate this hypothesis. Third, because non-attendance at the intensive intervention can make the detection of intervention effects even more challenging, both “intent-to-treat” [76] and “as treated” [77] analyses will be conducted.
4.4 Conclusions
This study demonstrates the feasibility and acceptability of implementing a brief clinic-based sexual risk reduction intervention in a busy STI clinic as well as the feasibility and acceptability of intensive risk reduction workshops. Participation and retention rates demonstrate that, despite the burdens of a comprehensive evaluation protocol, patients will join RCTs in impressive numbers. Moreover, the sample that was recruited evinced considerable risk for HIV and other STIs, substance use and misuse, and mental health concerns. These “syndemic” characteristics confirm that STI clinics provide a setting where investigators can implement and evaluate a variety of behavioral health promotion strategies with an at-risk but underserved population.
5.0 Acknowledgments
This research was supported by a grant from the National Institute on Mental Health (#R01-MH068171) awarded to Michael P. Carey. The authors would like to acknowledge the contributions of the participants, the clinic nurses and staff, and our research team, including Mary-Leah Albano, LuAnne Cori, Nicoy Douglas, Joyce Jones, Tracy Montesano, and Tricia Santa-Ferrara.
Funding source: NIH, National Institute of Mental Health # R01-MH068171.
6.0 References
- [1].Eng TR, Butler WT, editors. The Hidden Epidemic: Confronting Sexually Transmitted Diseases. National Academy Press; Washington, DC: 1997. [PubMed] [Google Scholar]
- [2].Centers for Disease Control and Prevention . HIV/AIDS surveillance report, 2005. US Department of Health and Human Services; Atlanta, GA: 2006. [Google Scholar]
- [3].Centers for Disease Control and Prevention . Sexually transmitted diseases surveillance, 2005. US Department of Health and Human Services; Atlanta, GA: 2006. [Google Scholar]
- [4].Centers for Disease Control and Prevention . Trends in reportable sexually transmitted diseases in the United States, 2005. US Department of Health and Human Services; Atlanta, GA: 2006. [Google Scholar]
- [5].Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: The contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75:3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Weinstock HS, Sidhu J, Gwinn M, Karon J, Petersen LR. Trends in HIV seroprevalence among persons attending sexually transmitted disease clinics in the United States, 1988-1992. J Acquir Immune Defic Syndr Hum Retrovirology. 1995;9:514–522. [PubMed] [Google Scholar]
- [7].Weinstock HS, Sweeney S, Satten GA, Gwinn M, STD Clinic HIV Seroincidence Study Group HIV seroincidence and risk factors among patients repeatedly tested for HIV attending sexually transmitted disease clinics in the United States, 1991-1996. J Acquir Immune Defic Syndr Hum Retrovirology. 1998;19:506–512. doi: 10.1097/00042560-199812150-00010. [DOI] [PubMed] [Google Scholar]
- [8].Kamb ML, Fishbein M, Douglas JM, et al. Efficacy of risk-reduction counseling to prevent Human Immunodeficiency Virus and sexually transmitted diseases. JAMA. 1998:280. doi: 10.1001/jama.280.13.1161. [DOI] [PubMed] [Google Scholar]
- [9].Boyer CB, Barrett DC, Peterman TA, Bolan G. Sexually transmitted disease (STD) and HIV risk in heterosexual adults attending a public STD clinic: Evaluation of a randomized controlled behavioral risk-reduction intervention trial. AIDS. 1997;11:359–367. doi: 10.1097/00002030-199703110-00014. [DOI] [PubMed] [Google Scholar]
- [10].Rietmeijer CA. Risk reduction counseling for prevention of sexually transmitted infections: How it works and how to make it work. Sex Transm Infect. 2007;83:2–9. doi: 10.1136/sti.2006.017319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Portnoy DB, Scott-Sheldon LAJ, Johnson BT, Carey MP. Computer-delivered interventions for health promotion and behavioral risk reduction: A meta-analysis of 75 randomized controlled trials, 1988-2007. Prev Med. doi: 10.1016/j.ypmed.2008.02.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kalichman SC, Williams E, Nachimson D. Brief behavioural skills building intervention for female controlled methods of STD-HIV prevention: Outcomes of a randomized clinical field trial. Int J STD AIDS. 1999;10:174–181. doi: 10.1258/0956462991913844. [DOI] [PubMed] [Google Scholar]
- [13].Fisher JD, Fisher WA. Changing AIDS-risk behavior. Psychol Bull. 1992;111:455–474. doi: 10.1037/0033-2909.111.3.455. [DOI] [PubMed] [Google Scholar]
- [14].NIMH Multisite HIV Prevention Trial Group The NIMH multisite HIV prevention trial: Reducing HIV sexual risk behavior. Science. 1998;280:1889–1894. doi: 10.1126/science.280.5371.1889. [DOI] [PubMed] [Google Scholar]
- [15].Shain RN, Piper JM, Newton ER, Perdue ST, Ramos R, Champion JD, Guerra FA. A randomized, controlled trial of a behavioral intervention to prevent sexually transmitted disease among minority women. N Engl J Med. 1999;340:93–100. doi: 10.1056/NEJM199901143400203. [DOI] [PubMed] [Google Scholar]
- [16].Moyer A, Finney JW, Swearingen CE, Vergun P. Brief interventions for alcohol problems: A meta-analytic review of controlled investigations in treatment-seeking and non-treatment-seeking populations. Addiction. 2002;97:279–292. doi: 10.1046/j.1360-0443.2002.00018.x. [DOI] [PubMed] [Google Scholar]
- [17].Dunn C, Deroo L, Rivara FP. The use of brief interventions adapted from motivational interviewing across behavioral domains: A systematic review. Addiction. 2001;96:1725–1742. doi: 10.1046/j.1360-0443.2001.961217253.x. [DOI] [PubMed] [Google Scholar]
- [18].Fishbein M, Triandis HC, Kanfer FH, et al. Factors influencing behavior and behavior change. In: Baum A, Revenson TA, Singer JE, editors. Handbook of Health Psychology. Erlbaum; Mahwah, NJ: 2001. [Google Scholar]
- [19].Boyer CB, Shafer M, Wibbelsman CJ, et al. Associations of sociodemographic, psychosocial, and behavioral factors with sexual risk and sexually transmitted diseases in teen clinic patients. J Adolesc Health. 2000;27:102–111. doi: 10.1016/s1054-139x(99)00113-5. [DOI] [PubMed] [Google Scholar]
- [20].Kalichman SC, Simbayi LC, Cain D, et al. Generalizing a model of health behavior change and AIDS stigma for use with sexually transmitted infection clinic patients in Cape Town, South Africa. AIDS Care. 2006;18:178–182. doi: 10.1080/09540120500456292. [DOI] [PubMed] [Google Scholar]
- [21].Mustanski B, Donenberg G, Emerson E. I can use a condom, I just don’t: The importance of motivation to prevent HIV in adolescent seeking psychiatric care. AIDS Behav. 2006;10:753–762. doi: 10.1007/s10461-006-9098-2. [DOI] [PubMed] [Google Scholar]
- [22].Jaworski BC, Carey MP. Effects of a brief, theory-based STD-prevention program for female college students. J Adolesc Health. 2001;29:417–425. doi: 10.1016/s1054-139x(01)00271-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Carey MP, Braaten LS, Maisto SA, et al. Using information, motivational enhancement, and skills training to reduce the risk of HIV infection for low-income urban women: A second randomized clinical trial. Health Psychol. 2000;19:3–11. doi: 10.1037//0278-6133.19.1.3. [DOI] [PubMed] [Google Scholar]
- [24].Cornman DH, Schmiege SJ, Bryan A, Benziger TJ, Fisher JD. An information-motivation-behavioral skills (IMB) model-based HIV prevention intervention for truck drivers in India. Soc Sci Med. 2007;64:1572–1584. doi: 10.1016/j.socscimed.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Johnson BT, Carey MP, Chaudoir SR, Reid AE. Sexual risk reduction for persons living with HIV: Research synthesis of randomized controlled trials, 1993 to 2004. J Acquir Immune Defic Syndr. 2006;41:642–650. doi: 10.1097/01.qai.0000194495.15309.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Smoak ND, Scott-Sheldon LAJ, Johnson BT, Carey MP. SHARP Research Team. Sexual risk reduction interventions do not inadvertently increase the overall frequency of sexual behavior: A meta-analysis of 174 studies with 116,735 participants. J Acquir Immune Defic Syndr. 2006;41:374–384. doi: 10.1097/01.qai.0000185575.36591.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Prochaska JO, DiClemente CC, Norcross JC. In search of how people change: Applications to addictive behaviors. Am Psychol. 1992;47:1102–1114. doi: 10.1037//0003-066x.47.9.1102. [DOI] [PubMed] [Google Scholar]
- [28].Prochaska JO, Redding CA, Harlow LL, Rossi JS, Velicer WF. The transtheoretical model of change and HIV prevention: A review. Health Educ Q. 1994;21:471–486. doi: 10.1177/109019819402100410. [DOI] [PubMed] [Google Scholar]
- [29].Carey MP, Morrison-Beedy D, Johnson BT. The HIV-Knowledge Questionnaire: Development and evaluation of a reliable, valid, and practical self-administered questionnaire. AIDS Behav. 1997;1:61–74. [Google Scholar]
- [30].Carey MP, Schroder KE. Development and psychometric evaluation of the brief HIV Knowledge Questionnaire. AIDS Educ Prev. 2002;14:172–182. doi: 10.1521/aeap.14.2.172.23902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jaworski BC, Carey MP. Development and psychometric evaluation of a self-administered questionnaire to measure knowledge of sexually transmitted diseases. AIDS Behav. 2007;11:557–574. doi: 10.1007/s10461-006-9168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Brown IS. Development of a scale to measure attitude toward the condom as a method on birth control. J Sex Res. 1984;20:255–263. [Google Scholar]
- [33].Sacco WP, Levine B, Reed DL, Thompson K. Attitudes about condom use as an AIDS-relevant behavior: Their factor structure and relation to condom use. Psychol Assess. 1991;3:265–272. [Google Scholar]
- [34].Kalichman SC, Simbayi LC. HIV testing attitudes, AIDS stigma, and voluntary HIV counseling and testing in a black township in Cape Town, South Africa. Sex Transm Infect. 2003;79:442–447. doi: 10.1136/sti.79.6.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Carey MP, Maisto SA, Kalichman SC, et al. Enhancing motivation to reduce the risk of HIV infection for economically disadvantaged urban women. J Consult Clin Psychol. 1997;65:531–541. doi: 10.1037//0022-006x.65.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kalichman SC, Cherry C, Brown F. Effectiveness of a video-based motivational skills-building HIV risk-reduction intervention for inner-city African American men. J Consult Clin Psychol. 1999;67:959–966. doi: 10.1037//0022-006x.67.6.959. [DOI] [PubMed] [Google Scholar]
- [37].Noar SM, Morokoff PJ, Harlow LL. Condom influence strategies in a community sample of ethnically diverse men and women. J Appl Soc Psychol. 2004;38:1730–1751. [Google Scholar]
- [38].Noar SM, Morokoff PJ, Harlow LL. Condom negotiation in heterosexually active men and women: Development and validation of a Condom Influence Strategy Questionnaire. Psychol Health. 2002;17:711–731. [Google Scholar]
- [39].Kalichman SC, Weinhardt LS, Benotsch E, et al. Experimental components analysis of brief theory-based HIV-AIDS risk reduction counseling for sexually transmitted infection patients. Health Psychol. 2005;24:198–208. doi: 10.1037/0278-6133.24.2.198. [DOI] [PubMed] [Google Scholar]
- [40].Murphy DA, Stein JA, Schlenger W, Mailbach E, National Institute of Mental Health Multisite HIV Prevention Trial Group Conceptualizing the multidimensional nature of self-efficacy: Assessment of situational context and level of behavioral challenges to maintain safer sex. Health Psychol. 2001;20:281–290. [PubMed] [Google Scholar]
- [41].Carey MP, Carey KB, Maisto SA, et al. Reducing HIV-risk behavior among adults receiving outpatient psychiatric treatment: Results from a randomized controlled trial. J Consult Clin Psychol. 2004;72:252–268. doi: 10.1037/0022-006X.72.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Schroder KEE, Carey MP, Vanable PA. Methodological challenges in research on sexual risk behavior: I. Item content, scaling, and data analytical options. Ann Behav Med. 2003;26:76–103. doi: 10.1207/s15324796abm2602_02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Weinhardt LS, Forsyth AD, Carey MP, Jaworski BC, Durant LE. Reliability and validity of self-report measures of HIV-related sexual behavior: Progress since 1990 and recommendations for research and practice. Arch Sex Behav. 1998;27:155–180. doi: 10.1023/a:1018682530519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- [45].Santor DA, Coyne JC. Shortening the CES-D to improve its ability to detect cases of depression. Psychol Assess. 1997;9:233–243. [Google Scholar]
- [46].Heimberg LK. The measurement of future time perspective. Vanderbilt University: 1963. Unpublished dissertation. [Google Scholar]
- [47].Weschler H, Dowdall GW, Davenport A, Rimm EB. A gender-specific measure of binge drinking among college students. Am J Public Health. 1995;85:982–985. doi: 10.2105/ajph.85.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- [49].Barry KL, Fleming MF. The Alcohol Use Disorders Identification Test (AUDIT) and the SMAST-13: Predictive validity in a rural primary care sample. Alcohol Alcohol. 1993;28:33–42. [PubMed] [Google Scholar]
- [50].Skinner HA. The Drug Abuse Screening Test. Addict Behav. 1982;7:363–371. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- [51].Cocco KM, Carey KB. Psychometric properties of the Drug Abuse Screening Test in psychiatric outpatients. Psychol Assess. 1998;4:408–414. [Google Scholar]
- [52].Apanovitch AM, McCarthy D, Salovey P. Using message framing to motivate HIV testing among low-income, ethnic minority women. Health Psychol. 2003;22:60–67. doi: 10.1037//0278-6133.22.1.60. [DOI] [PubMed] [Google Scholar]
- [53].Miller WR, Rollnick S. Motivational interviewing: Preparing people for change. 2nd ed. Guilford Press; New York: 2002. [Google Scholar]
- [54].Center for AIDS Intervention Research . Partners in Prevention (intervention manual) 2007. [Google Scholar]
- [55].Larsen DL, Attkisson CC, Hargreaves WA, Nguyen TD. Assessment of client/patient satisfaction: Development of a general scale. Eval Program Plan. 1979;2:197–207. doi: 10.1016/0149-7189(79)90094-6. [DOI] [PubMed] [Google Scholar]
- [56].Metcalf CA, Douglas JM, Malotte CK, et al. Relative efficacy of prevention counseling with rapid and standard HIV testing: A randomized, controlled trial (RESPECT-2) Sex Transm Dis. 2005;32:130–138. doi: 10.1097/01.olq.0000151421.97004.c0. [DOI] [PubMed] [Google Scholar]
- [57].Kelly JA. Changing HIV Risk Behavior: Practical Strategies. Guilford Press; New York: 1995. [Google Scholar]
- [58].Kalichman SC. Preventing AIDS: A Sourcebook for Behavioral Interventions. Erlbaum; Hillsdale, NJ: 1998. [Google Scholar]
- [59].Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- [60].Des Jarlais DC, Paone D, Milliken J, et al. Audio-computer interviewing to measure risk behavior for HIV among injecting drug users: A quasi-randomized trial. Lancet. 1999;353:1657–1671. doi: 10.1016/s0140-6736(98)07026-3. [DOI] [PubMed] [Google Scholar]
- [61].Kurth AE, Martin DP, Golden MR, et al. Comparison between audio computer-assisted self-interviews and clinician interviews for obtaining the sexual history. Sex Transm Dis. 2004;31:719–726. doi: 10.1097/01.olq.0000145855.36181.13. [DOI] [PubMed] [Google Scholar]
- [62].Metzger DS, Koblin B, Turner CF, et al. Randomized controlled trial of audio computer-assisted self-interviewing: Utility and acceptability in longitudinal studies. Am J Epidemiol. 2000;152:99–106. doi: 10.1093/aje/152.2.99. [DOI] [PubMed] [Google Scholar]
- [63].Fishbein M, Pequegnat W. Evaluating AIDS prevention interventions using behavioral and biological outcome measures. Sex Transm Dis. 2000;27:101–110. doi: 10.1097/00007435-200002000-00008. [DOI] [PubMed] [Google Scholar]
- [64].Glasgow RE, Lichtenstein E, Marcus AC. Why don’t we see more translation of health promotion research to practice? Rethinking the efficacy-to-effectiveness transition. Am J Public Health. 2003;93:1261–1267. doi: 10.2105/ajph.93.8.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Glasgow RE, McKay HG, Piette JD, Reynolds KD. The RE-AIM framework for evaluating interventions: What can it tell us about approaches to chronic illness management? Patient Educ Couns. 2001;44:119–127. doi: 10.1016/s0738-3991(00)00186-5. [DOI] [PubMed] [Google Scholar]
- [66].Rose G. The strategy of preventive medicine. New York: Oxford: 1992. [Google Scholar]
- [67].Cook RL, Comer DM, Wiesenfeld HC, et al. Alcohol and drug use and related disorders: An underrecognized health issue among adolescents and young adults attending sexually transmitted disease clinics. Sex Transm Dis. 2006;33:565–570. doi: 10.1097/01.olq.0000206422.40319.54. [DOI] [PubMed] [Google Scholar]
- [68].Erbelding EJ, Hummel B, Hogan T, Zenilman J. High rates of depressive symptoms in STD clinic patients. Sex Transm Dis. 2001;28:281–284. doi: 10.1097/00007435-200105000-00008. [DOI] [PubMed] [Google Scholar]
- [69].Singer MC, Erickson PI, Badiane L, et al. Syndemics, sex and the city: Understanding sexually transmitted diseases in the social and cultural context. Soc Sci Med. 2006;63:2010–2021. doi: 10.1016/j.socscimed.2006.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Stall R, Mills TC, Williamson J, et al. Association of co-occurring psychosocial health problems and increased vulnerability to HIV/AIDS among urban men who have sex with men. Am J Public Health. 2003;93:939–942. doi: 10.2105/ajph.93.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Mustanski B, Garofalo R, Herrick A, Donenberg G. Psychosocial health problems increase risk for HIV among urban young men who have sex with men: Preliminary evidence of a syndemic in need of attention. Ann Behav Med. 2007;34:37–45. doi: 10.1080/08836610701495268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Branson BM, Peterman TA, Cannon RO, Ransom R, Zaidi AA. Group counseling to prevent sexually transmitted disease and HIV: A randomized controlled trial. Sex Transm Dis. 1998;25:553–560. doi: 10.1097/00007435-199811000-00011. [DOI] [PubMed] [Google Scholar]
- [73].Maher JE, Peterman TA, Osewe PL, Odusanya S, Scerba JR. Evaluation of a community-based organization’s intervention to reduce the incidence of sexually transmitted diseases: A randomized, controlled trial. South Med J. 2003;96:248–253. doi: 10.1097/01.SMJ.0000054605.31081.07. [DOI] [PubMed] [Google Scholar]
- [74].Senn TE, Carey MP, Vanable PA, Coury-Doniger P, Urban MA. Even if you build it, we may not come: Correlates of non-attendance at a sexual risk reduction workshop for STD clinic patients. AIDS Behav. doi: 10.1007/s10461-007-9218-7. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Carey MP, Vanable PA, Senn TE, Coury-Doniger P, Urban MA. Recruiting patients from a sexually transmitted disease clinic to sexual risk reduction workshops: Are monetary incentives necessary? J Public Health Manag Pract. 2005;11:516–521. doi: 10.1097/00124784-200511000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Fergusson D, Aaron SD, Guyatt G, Hebert P. Post-randomisation exclusions: The intention to treat principle and excluding patients from analysis. BMJ. 2002;325:652–654. doi: 10.1136/bmj.325.7365.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Gross D, Fogg L. A critical analysis of the intent-to-treat principle in prevention research. J Prim Prev. 2004;25:475–489. [Google Scholar]