Abstract
Expression of a NUP98-HOXD13 (NHD13) fusion gene, initially identified in a patient with myelodysplastic syndrome (MDS), leads to a highly penetrant MDS in mice that recapitulates all of the key features of the human disease. Expansion of undifferentiated lineage negative (linneg) hematopoietic precursors that express NHD13 was markedly inhibited (30-fold) in vitro. Decreased expansion was accompanied by decreased production of terminally differentiated cells, indicating impaired differentiation of NHD13 precursors. Rather than differentiate, the majority (80%) of NHD13 linneg precursors underwent apoptotic cell death when induced to differentiate. These findings demonstrate that NHD13 linneg cells provide a tractable in vitro system for studies of MDS.
Keywords: myelodysplastic syndrome, mouse model, NUP98, HOXD13, apoptosis
Introduction
Myelodysplastic syndrome (MDS) is a clonal stem-cell disorder characterized by ineffective hematopoiesis in one or more hematopoietic cell lineages. Despite peripheral blood cytopenias, the bone marrow (BM) of patients with MDS usually shows normal or increased cellularity (1). This paradox can be explained by an increase in apoptosis, and several studies have demonstrated an increased rate of apoptosis in the BM of patients with early-stage MDS (2-5). In addition to increased cell death or apoptosis, failure of differentiation contributes to the lack of terminally differentiated blood cells in patients with MDS (6). In vitro colony assays demonstrate abnormal colony formation by granulocyte-macrophage (CFU-GM), erythroid (BFU-E, CFU-E) and megakaryocyte (CFU-Meg) progenitors in many patients with MDS (7-9).
Important clues to the etiology of many hematologic malignancies, including MDS have come from cytogenetic studies. Although rare, translocations of the NUP98 gene, especially those leading to fusions of NUP98 with clustered homeobox (HOX) genes have been associated with both MDS and AML (10). Recently, we developed a mouse model of MDS that recapitulates all of the critical features of the human disease by expressing a NUP98-HOXD13 (hereafter NHD13) fusion gene in hematopoietic tissues under control of the Vav promoter (11). In our initial studies, we showed that unmanipulated BM from NHD13 and wild type (WT) mice gave rise to similar numbers of colony forming units (CFU) in vitro, and that BM from the NHD13 mice showed increased replating potential (11). However, those studies did not assess the growth, differentiation, and death of the hematopoietic stem cell (HSC) population. In this study, we demonstrate that NHD13 lineage negative (linneg) murine bone marrow cells, which contain the hematopoietic stem and progenitor population, show increased apoptosis and differentiation failure, similar to the human disease.
Materials and Methods
Mice
All of the NHD13 transgenic mice were 7 to 9 months old on a C57Bl6 background. Bone marrow nucleated cells (BMNC) were obtained by flushing the femur and tibia. Diagnosis of MDS was confirmed by complete blood counts (CBCs) and cytospin examination of BM cells. CBCs were determined using a HEMAVET Multispecies Hematology Analyzer (CDC Technologies, Oxford, CT). These studies were approved by the National Cancer Institute Animal Care and Use Committee.
Selection of lineage-negative (linneg) cells
We used the StemCep™ Mouse Progenitor Enrichment Kit (Stemcell Technologies, Canada) following the manufacturer's recommendations. The lineage cocktail contained the following antibodies: CD5 (Ly-1), Mac-1, B220, Gr-1, and Ter119. Purity of the purified linneg population was assessed by fluorescence activated cell sorting (FACS).
Liquid cultures and colony forming cell (CFC) assays
Linneg cells were cultured at 37°C/5% CO2 in 6-well culture clusters (Corning Incorporated, NY) in 3 ml of Iscove's Modified Dulbecco's Medium (Invitrogen, CA) supplemented with 15% fetal bovine serum and the following cytokines: recombinant mouse stem cell factor (SCF, 100 ng/ml), recombinant mouse interleukin 3 (rmIL-3, 6 ng/ml) and recombinant mouse interleukin 6 (rhIL-6, 10 ng/ml) (all from R&D Systems, MN). On the 2nd, 4th, and 7th day, cells were harvested, counted, and assayed for apoptosis. For CFC assays, 4X104 whole BM or 2X103 linneg cells were plated onto 35-mm Petri dishes in Methocult M3434 methylcellulose medium (Stemcell Technologies, Canada), respectively. Methocult M3434 is 2% methylcellulose supplemented with cytokines (SCF [50 ng/mL], rmIL-3 [10 ng/mL], rhIL-6 [10 ng/mL], and rhEpo [3 U/mL]). The CFC plates were incubated at 37°C in a 5% CO2 incubator, and the number of colonies was counted 12 days after plating.
Apoptosis assay
Harvested cells from suspension culture were stained with FITC Annexin V (BD Biosciences, CA) and propidium iodide (PI) (Sigma-Aldrich, MO) using the manufacturer's recommended protocol. FACS analyses was performed using a dual laser FACScan (BD Biosciences, CA).
Statistical analysis
Data are expressed as the mean ± standard errors of the mean (SEM). Differences between groups were analyzed by student's t-test. P-values less than or equal to 0.05 were considered to be significant.
Results and Discussion
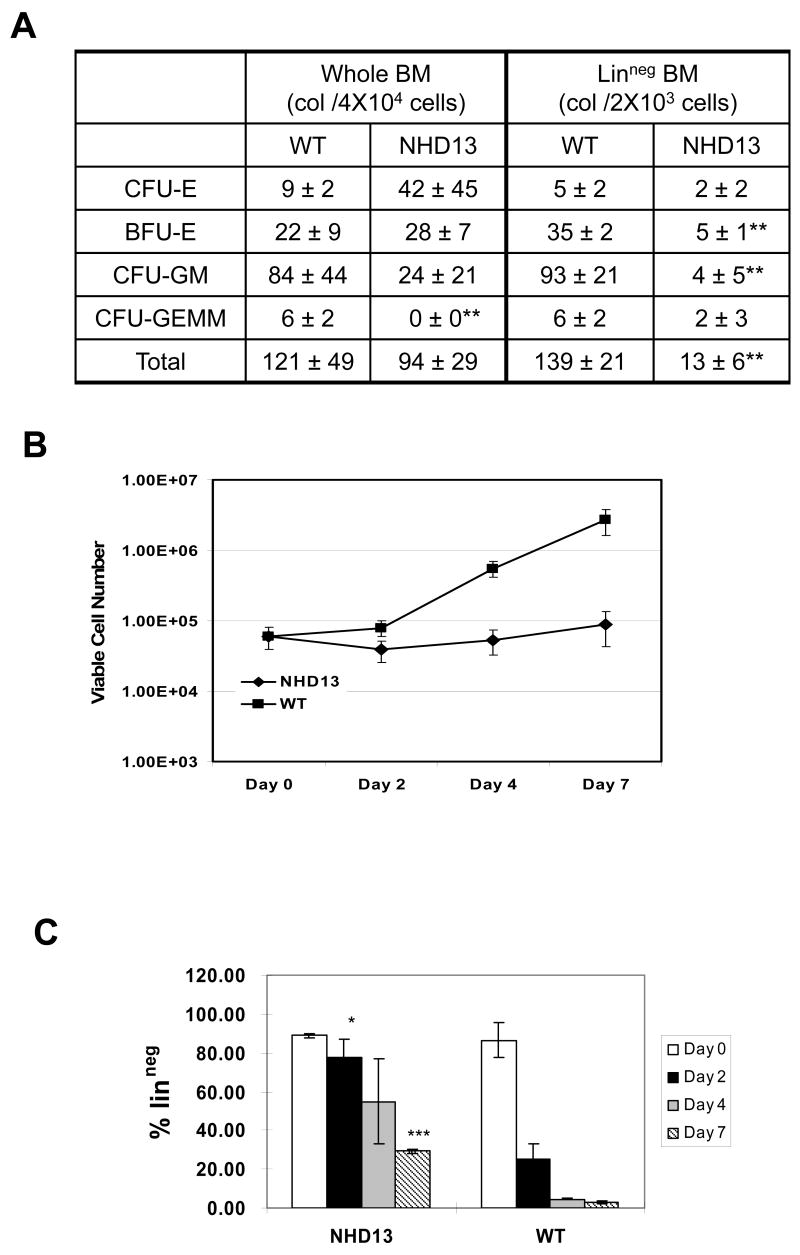
To determine whether the NHD13 bone marrow nucleated cells (BMNC) were impaired in their ability to differentiate, we performed a standard colony forming cell (CFC) assay in methylcellulose. Although there was not a dramatic difference in the numbers of colonies produced from whole BM, there was a clear difference in the type of colonies generated (Figure 1A). Whereas most of the colonies generated from WT BMNC were CFU-GM and CFU-GEMM, most of the NHD13 colonies were abnormally small BFU-E and CFU-E colonies. To assess the differentiation potential of more primitive hematopoietic progenitors, we isolated linneg BMNC using magnetic beads as described above, and repeated the CFC assay with purified linneg BMNC. In these experiments there was an almost 8-fold decrease in the number of colonies produced by the NHD13 linneg BMNC compared to WT linneg BMNC, suggesting a marked inhibition of growth and/or differentiation of the linneg progenitors. These results are similar to studies of MDS patients comparing in vitro CFC results using CD34+ purified cells instead of whole BM (12, 13). We therefore focused our subsequent studies on linneg BMNC.
Figure 1. In vitro expansion and differentiation of linneg cells.
(A) CFC assay of whole BM and linneg BM cells from NHD13 or WT mice. Colony counts represent total number from two plates for each sample, n=3 NHD13 and WT mice each. CFU-E, colony forming unit-erythroid; BFU-E, burst forming unit-erythroid; CFU-GM, colony forming unit-granulocyte, macrophage; CFU-GEMM, colony forming unit-granulocyte, erythroid, macrophage, megakaryocyte; **p<0.01.
(B) Expansion of linneg cells from WT and NHD13 mice grown in liquid culture. Cell counts represent pooled data from 3 independent experiments. (C) Percent linneg cells according to the culture day, pooled data from 3 independent experiments. (*p=0.051, ***p<0.01).
On day zero, 6X104 linneg cells were placed in liquid culture with SCF, IL-3, and IL-6 as described above. The total number of viable cells was decreased in the NHD13 cultures on day 2; thereafter, the total cell number slowly increased. In contrast, the WT cells accumulated briskly after a brief lag phase, such that by day 7 there was a 30-fold difference in cell number between the NHD13 and WT cultures (Fig 1B).
As murine hematopoietic cells differentiate, they acquire cell surface markers such as Mac1 (monocyte/granulocyte), Gr1 (granulocyte), Ter119 (erythroid), B220 (B-lymphoid), and CD5 (T-lymphoid). We evaluated acquisition of these markers to determine whether the cells had differentiated after in vitro culture in the presence of SCF, IL-3, and IL-6. As early as day 2, 77.6±13.1 % of the NHD13 cells remained linneg, whereas only 25.2±10.7 % of the WT cells remained linneg (Fig 1C). This trend continued, and on day 7 the fraction of NHD13 linneg cells was almost 10-fold greater than the fraction of WT linneg cells (29.2±1.58 % vs. 3.1±0.88%, p<0.01) (Fig 1C). These results demonstrate that NHD13 BM cells are impaired in the ability to differentiate in vitro, consistent with the in vivo results demonstrating peripheral blood cytopenias and increased immature granulocytes in the BM of NHD13 mice (11).
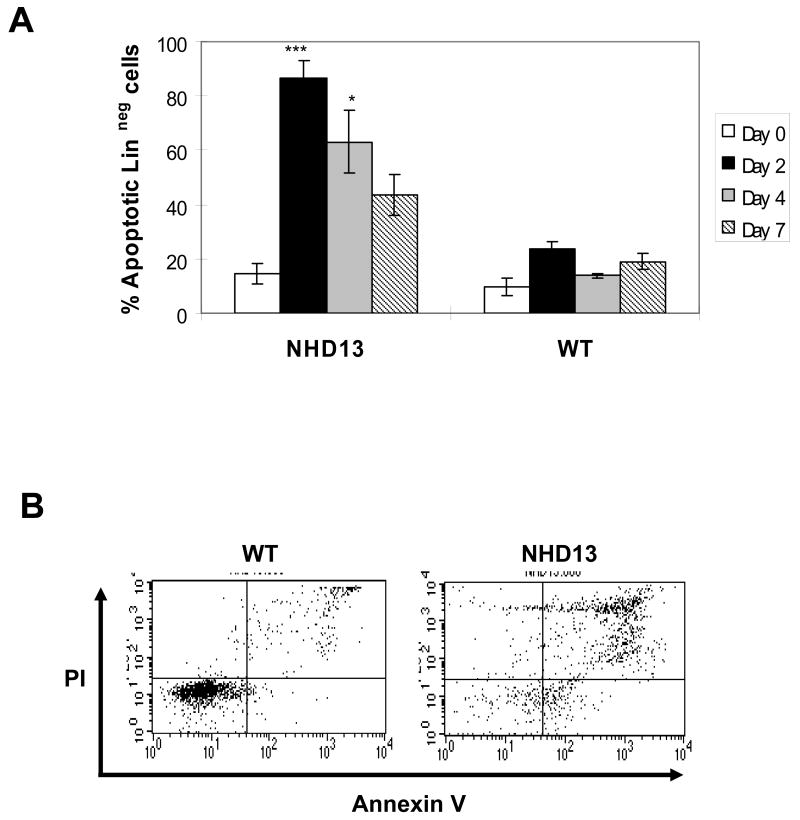
To determine whether increased apoptosis of NHD13 linneg BMNC might contribute to the decreased number of NHD13 compared to WT cells, we used annexin V and PI staining to evaluate apoptotic cells. Linneg cells were again placed in liquid culture with SCF, IL-3, and IL-6. An increased proportion of apoptotic cells was evident in the NHD13 culture as early as day 2 (Fig. 2), and persisted for up to 7 days. However, there was no clear increase in apoptosis of freshly isolated NHD13 linneg cells (Fig. 2). These results suggest apoptosis of the NHD13 cells is induced in vitro after the cells receive a differentiation stimulus. Alternatively, increased apoptosis of NHD13 BM may be more difficult to detect in vivo as the apoptotic cells are quickly and efficiently cleared by phagocytes. In this context, it is important to note that increased apoptosis in BM from MDS patients is often difficult to appreciate, and has been attributed to rapid clearance of apoptotic hematopoietic cells in vivo, this hypothesis is supported by studies which show marked apoptosis of BMNC cells from MDS patients following culture in vitro (14-16). The hypothesis that myeloid cells which are blocked in their ability to terminally differentiate undergo apoptosis in response to differentiation signals is supported by previous studies which showed that murine BMNC with enforced Myc expression had decreased terminal differentiation and increased apoptosis when treated with GM-CSF (17).
Figure 2. Apoptosis of linneg NHD13 cells grown in liquid culture.
(A) Apoptosis (%) of linneg cells in liquid culture (n=3, *p=0.051, ***p<0.01) (B) Representative FACS profiles of Annexin V and PI on culture day 2.
In this report, we show that linneg hematopoietic precursors from NHD13 mice are impaired in their ability to differentiate, and undergo apoptosis when induced to differentiate, similar to findings with human MDS. These findings are consistent with findings that embryonic stem (ES) cells with a NHD13 “knock-in” allele were impaired in their ability to generate hematopoietic colonies in vitro (18). Given that authenticated stable cell lines which recapitulate MDS characteristics in vitro and in vivo are not available (2, 6, 19), we believe that BM obtained from an accurate mouse model of MDS can provide a useful in vitro platform for MDS studies. This in vitro approach is more amenable to high throughput studies than are in vivo models, and provide an accessible, in vitro culture system with which to study apoptosis and impaired differentiation associated with MDS.
Acknowledgments
We would like to thank Dave Caudell, Helge Hartung, Sarah Beachy, Dwayne Barber, and Eli Estey for insightful discussions. This research was supported by the Intramural Research Program of the NIH, NCI.
References
- 1.Heaney ML, Golde DW. Myelodysplasia. N Engl J Med. 1999;340:1649–60. doi: 10.1056/NEJM199905273402107. [DOI] [PubMed] [Google Scholar]
- 2.Kerbauy DB, Deeg HJ. Apoptosis and antiapoptotic mechanisms in the progression of myelodysplastic syndrome. Exp Hematol. 2007;35:1739–46. doi: 10.1016/j.exphem.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gersuk GM, Beckham C, Loken MR, Kiener P, Anderson JE, Farrand A, et al. A role for tumour necrosis factor-alpha, Fas and Fas-Ligand in marrow failure associated with myelodysplastic syndrome. Br J Haematol. 1998;103:176–88. doi: 10.1046/j.1365-2141.1998.00933.x. [DOI] [PubMed] [Google Scholar]
- 4.Parker JE, Mufti GJ. The role of apoptosis in the pathogenesis of the myelodysplastic syndromes. Int J Hematol. 2001;73:416–28. doi: 10.1007/BF02994003. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida Y, Mufti GJ. Apoptosis and its significance in MDS: controversies revisited. Leuk Res. 1999;23:777–85. doi: 10.1016/s0145-2126(99)00094-6. [DOI] [PubMed] [Google Scholar]
- 6.Corey SJ, Minden MD, Barber DL, Kantarjian H, Wang JC, Schimmer AD. Myelodysplastic syndromes: the complexity of stem-cell diseases. Nat Rev Cancer. 2007;7:118–29. doi: 10.1038/nrc2047. [DOI] [PubMed] [Google Scholar]
- 7.Haak HL, Kerkhofs H, van der Linden JS, Schonewille H, van der Sanden-van der Meer HS, Hermans J. Significance of in vitro cultures in myelodysplastic syndromes. Scand J Haematol. 1986;37:380–9. doi: 10.1111/j.1600-0609.1986.tb02625.x. [DOI] [PubMed] [Google Scholar]
- 8.Juvonen E, Aimolahti A, Volin L, Ruutu T. The prognostic value of in vitro cultures of erythroid and megakaryocyte progenitors in myelodysplastic syndromes. Leuk Res. 1999;23:889–94. doi: 10.1016/s0145-2126(99)00104-6. [DOI] [PubMed] [Google Scholar]
- 9.Ruutu T, Partanen S, Lintula R, Teerenhovi L, Knuutila S. Erythroid and granulocyte-macrophage colony formation in myelodysplastic syndromes. Scand J Haematol. 1984;32:395–402. doi: 10.1111/j.1600-0609.1984.tb00695.x. [DOI] [PubMed] [Google Scholar]
- 10.Slape C, Aplan PD. The role of NUP98 gene fusions in hematologic malignancy. Leuk Lymphoma. 2004;45:1341–50. doi: 10.1080/10428190310001659325. [DOI] [PubMed] [Google Scholar]
- 11.Lin YW, Slape C, Zhang Z, Aplan PD. NUP98-HOXD13 transgenic mice develop a highly penetrant, severe myelodysplastic syndrome that progresses to acute leukemia. Blood. 2005;106:287–95. doi: 10.1182/blood-2004-12-4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez-Jaramillo G, Flores-Figueroa E, Sanchez-Valle E, Gutierrez-Espindola G, Gomez-Morales E, Montesinos JJ, et al. Comparative analysis of the in vitro proliferation and expansion of hematopoietic progenitors from patients with aplastic anemia and myelodysplasia. Leuk Res. 2002;26:955–63. doi: 10.1016/s0145-2126(02)00042-5. [DOI] [PubMed] [Google Scholar]
- 13.Sawada K. Impaired proliferation and differentiation of myelodysplastic CD34+ cells. Leuk Lymphoma. 1994;14:37–47. doi: 10.3109/10428199409049649. [DOI] [PubMed] [Google Scholar]
- 14.Claessens YE, Bouscary D, Dupont JM, Picard F, Melle J, Gisselbrecht S, et al. In vitro proliferation and differentiation of erythroid progenitors from patients with myelodysplastic syndromes: evidence for Fas-dependent apoptosis. Blood. 2002;99:1594–601. doi: 10.1182/blood.v99.5.1594. [DOI] [PubMed] [Google Scholar]
- 15.Span LF, Rutten E, Gemmink A, Boezeman JB, Raymakers RA, de Witte T. Bone marrow mononuclear cells of MDS patients are characterized by in vitro proliferation and increased apoptosis independently of stromal interactions. Leuk Res. 2007;31:1659–67. doi: 10.1016/j.leukres.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Span LF, Vierwinden G, Pennings AH, Boezeman JB, Raymakers RA, de Witte T. Programmed cell death is an intrinsic feature of MDS progenitors, predominantly found in the cluster-forming cells. Exp Hematol. 2005;33:435–42. doi: 10.1016/j.exphem.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Amanullah A, Liebermann DA, Hoffman B. p53-independent apoptosis associated with c-Myc-mediated block in myeloid cell differentiation. Oncogene. 2000;19:2967–77. doi: 10.1038/sj.onc.1203638. [DOI] [PubMed] [Google Scholar]
- 18.Slape C, Chung YJ, Soloway PD, Tessarollo L, Aplan PD. Mouse embryonic stem cells that express a NUP98-HOXD13 fusion protein are impaired in their ability to differentiate and can be complemented by BCR-ABL. Leukemia. 2007;21:1239–48. doi: 10.1038/sj.leu.2404648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drexler HG. Malignant hematopoietic cell lines: in vitro models for the study of myelodysplastic syndromes. Leuk Res. 2000;24:109–15. doi: 10.1016/s0145-2126(99)90169-8. [DOI] [PubMed] [Google Scholar]