Abstract
Background
Women and men are at risk for different types of stress-related disorders, with women at greater risk for depression and anxiety and men at greater risk for alcohol-use disorders. The present study examines gender differences in emotional and alcohol craving responses to stress that may relate to this gender divergence in disorders.
Method
Healthy adult social drinkers (27 men, 27 women) were exposed to individually developed and calibrated stressful, alcohol-related, and neutral-relaxing imagery, 1 imagery per session, on separate days and in random order. Subjective emotions, behavioral/bodily responses, cardiovascular arousal [heart rate (HR), blood pressure (BP)], and self-reported alcohol craving were assessed.
Results
Women reported and displayed greater sadness and anxiety following stress than men and men had greater diastolic BP response than women. No gender differences in alcohol craving, systolic BP or HR were observed. Subjective, behavioral, and cardiovascular measures were correlated in both genders. However, for men, but not women, alcohol craving was associated with greater subjective emotion and behavioral arousal following stress and alcohol cues.
Conclusions
These data suggest that men and women respond to stress differently, with women experiencing greater sadness and anxiety, while men show a greater integration of reward motivation (craving) and emotional stress systems. These findings have implications for the gender- related divergence in vulnerability for stress-related disorders, with women at greater risk for anxiety and depression than men, and men at greater risk for alcohol-use disorders than women.
Keywords: Gender/Sex Differences, Emotion, Stress, Craving, Alcohol Use
Women and men are at risk for different types of stress-related disorders, with women at greater risk for depression and anxiety and men at greater risk for alcohol- use disorders (Kajantie and Phillips, 2005; Kessler et al., 1993). Although the specific basis for gender differences in prevalence of these disorders have not been well studied, it has been suggested that gender differences in emotional and craving responses to stress may underlie the differential risk for these psychological disorders (Kajantie and Phillips, 2005; Levenson et al., 1987; Sinha and Rounsaville, 2002; Taylor et al., 2006; Zahn-Waxler, 2000).
Studies of subjective emotion experience find that women report greater sadness (Brebner, 2003; Fischer et al., 2004) and anxiety/fear (Feingold, 1994; Fischer et al., 2004; Ollendick et al., 1995) than men. Studies of behavioral/bodily indicators of emotion are limited. The few existing studies show that women express greater sadness and anxiety behaviorally and in their bodies than men while interacting with a spouse (Barnes and Buss, 1985) or viewing a sad film (Rottenberg et al., 2002).
In contrast to subjective and behavioral studies, studies of physiological markers of emotional response to stress find that men have greater blood pressure (BP) response than women (Allen et al., 1993; Stoney et al., 1988), although women show greater heart rate (HR) response than men (Allen et al., 1993; Stoney et al., 1988). Also, while previous research has shown that stress increases drinking behavior (Cooper et al., 1992; Lang et al., 1989; Marlatt et al., 1975; Sinha, 2001a; Tucker et al., 1981), few laboratory studies have assessed gender differences in the urge to drink following stress.
Previous research on gender differences in responses to stress has primarily focused on 1 domain of response (either subjective, behavioral, or physiological) and the association between emotional response and the urge to drink has rarely been explored. Furthermore, previous studies of physiological stress responses (discussed above) commonly utilize stressors that are more relevant for men than women, specifically achievement-related stressors (see Stroud et al., 2002; for discussion). Therefore as pointed out by Taylor et al. (2000), less is known about stress responses in women than men. Studies are needed that examine stress response to stressors that are equivalent in distress experience for men and women.
The present study will address the question of whether there are gender differences in emotional stress responses measured across subjective, behavioral/bodily, and physiological (cardiovascular) domains in social drinkers when they are exposed to stressful, alcohol-cue, and neutral-relaxing conditions. We induced these conditions using an individually calibrated guided imagery procedure adapted from the work of Lang and his colleagues (Lang, 1979; McNeil et al., 1993; Miller et al., 1987) and used extensively in our studies examining the effects of stress on addictive processes (Fox et al., 2005, 2006; Sinha et al., 1999, 2000, 2003, 2006). Alcohol-cue conditions were examined to determine whether these social drinkers, particularly men, would show a craving response to the drinking-related stories. Alcohol cues can also lead to arousal, for example, alcoholics show increases in subjective anxiety and fear in the cue condition (Fox et al., 2007). As such, we wanted to examine gender differences in the cue condition in addition to the stress condition in social drinkers in the present study.
On the basis of research cited in earlier sections, we hypothesized that women will report greater subjective sad and anxious experience and greater behavioral/bodily arousal in response to stress than men. We predict, however, that women will show lower physiological arousal in response to stress than men, as indicated by lower cardiovascular response, especially for BP. Further, we predict that men will show greater craving in response to stress than women. We will also explore gender differences in the alcohol-cue and neutral-relaxing conditions, in which alcohol craving and positive emotion were likely. Lastly, we will examine correlations among subjective, behavioral, cardiovascular, and alcohol-craving systems during stress and alcohol-cue conditions for men and women.
MATERIALS AND METHODS
Participants
Fifty-four healthy adults (27 women, 27 men) were recruited via advertisements placed in local newspapers for healthy volunteers. These healthy adults were part of a larger study comparing them to adults with alcohol dependence. Adults were included in the study if they were physically in good health, were not taking medication, did not have a current psychiatric or substance use diagnosis, and did not have a history of substance use, psychotic disorder, or bipolar disorder diagnosis. Participants underwent stringent medical assessments that included electrocardiography and laboratory tests of renal, hepatic, pancreatic, hematopoietic, and thyroid function. Structured Clinical Interviews (First et al., 1995) and self-reports were conducted to assess psychiatric and substance use disorders and breathalyzer and urine samples were obtained as an objective assessment of illicit drug use and current drinking levels. In addition, women were scheduled to participate in the laboratory sessions during the follicular or luteal phases of their menstrual cycle and were excluded if they reported irregular menstrual cycle or were taking hormonal birth control, to control for the possible influence of sex steroid hormone fluctuation on stress response (e.g., Kirschbaum et al., 1999). All participants were social drinkers; that is, they reported consuming <6 drinks per week. No participants reported current or past alcohol abuse or dependence. All participants provided written informed consent and were compensated $450 for study participation. The research was approved by the University’s Human Investigation Committee (IRB).
Design
A mixed design was used with gender (male, female) as the between subjects factor and condition (stress, neutral/relaxing, and alcohol cue) as a repeated measures factor. The 3 conditions were presented on separate testing days with only 1 stimulus presentation per day. Condition order was assigned randomly and counterbalanced across participants by gender. Research staff was unaware of imagery condition and the content of the personalized imagery scripts assigned to each laboratory session. Participants also were unaware of condition until imagery presentation.
Procedures
Participants completed an imagery script development session and were then admitted to a hospital research unit for a 3-night inpatient stay for participation in the 3 laboratory sessions. Given the importance of imagery ability for imagining the stress, alcohol cue, and neutral scenes, all subjects (both men and women) were given the Questionnaire of Mental Imagery (QMI; Sheehan, 1967) before enrolling in the study. Participants were included only if they scored an average of 3 or lower on the QMI across scenes, indicating that they saw the images at least “moderately clearly and vividly”.
Imagery Script Development
In a session prior to the laboratory sessions, scripts for the guided imagery induction were developed. The stress imagery script was based on participants’ description of a recent personal stressful event that was experienced as “most stressful”. “Most stressful” was determined by having the participants rate their individual level of distress experienced by them on a 10-point Likert scale where “1 = not at all stressful” and “10 = the most stress they felt recently in their life”. Only situations rated as 8 or above on this scale were accepted as appropriate for script development. In this manner, each stress script was individually calibrated on the basis of distress level experienced by each participant. Examples of acceptable stressful situations include breakup with a significant other, a verbal argument with a significant other or family member or unemployment-related stress, such as being fired or laid off from work (see Appendix for sample stress, alcohol cue, and neutral/relaxing scripts for men and women). Notably, trauma-related situations were not used for script development, as reliving traumatic events may be related to unique stress responses.
Alcohol-Cue Script
The alcohol-cue script was based on individual situations that included alcohol-related stimuli and resulted in subsequent alcohol use (e.g., buying alcohol, being at a bar, and watching others drink alcohol). Alcohol-related situations that occurred in the context of negative affect or psychological distress were not allowed, e.g., when participants went to a bar after a fight, or were feeling depressed and called a drinking buddy. Finally, a neutral/relaxing script was developed as a control for the stress and appetitive/alcohol-cue scripts. The neutral/relaxing script was developed from the participants’ individual experiences of commonly experienced neutral relaxing situations, such as a summer day relaxing at the beach, taking hot shower or bubble bath, and a Fall day reading at the park.
Details of each elicited situation was described using the Scene Construction Questionnaires (Miller et al., 1987; Sinha et al., 1992), which obtain specific stimulus and response details, including specific physical and interpersonal context details, verbal/cognitive attributions regarding the people involved, and physiological and bodily sensations experienced for the situation being described. A 5-minute “script” was then written in a standardized style and format for the stress, alcohol cue, and neutral situations for each participant and these were recorded on an audiotape in randomized order for presentation in the laboratory sessions. Scripts were developed, written, and recorded by upper-level research associates who underwent at least 10 hours of training. Script development procedures were based on methods developed by Lang and his colleagues (Lang et al., 1979; Lang et al., 1983; Miller et al., 1987), and further adapted in our previous studies (Sinha, 2001a; Sinha and Parsons, 1996; Sinha et al., 1992, 2000, 2003).
Gender Equivalency of the Stress Scripts and Imagery Vividness
In addition to the stress scripts being individually calibrated by each participant for the experience of distress, the stress scripts were rated for stressfulness on a scale from 1 to 5 by 2 independent raters (1 male, 1 female) who were unaware of study hypotheses, and raters’ scores were averaged. Mean stressfulness ratings were high for both men’s (M = 4.48, SD = 0.49) and women’s (M = 4.41, SD = 0.66) stress stories and were not statistically different (t = −0.42, p = 0.68). The 2 raters also rated scripts for the type of stress as either: Interpersonal (argument, parenting problem, betrayal, and personal violation), Environmental (economic problems), Achievement (employment/career concerns, role in household), Medical (accident, injury, illness of self or others). The most common type of stressor was Interpersonal (83%), with no differences in type of stressor by gender, χ2= 0.96, p > 0.10. Also, immediately following the presentation of each scene (stress, neutral, and alcohol cue), participants reported the “vividness” of the scene on a scale from 1 to 10. Both men and women reported high levels of vividness (from 8.42 to 9.54), with no significant differences between men’s and women’s vividness scores.
Habituation Session
On a day prior to the laboratory sessions, participants were brought into the testing room in order to acclimate them to study procedures (e.g., rating forms) and to train them in imagery and relaxation, as described in Sinha (2001b) and Sinha et al. (2003). Both men and women received 1 hour of training in imagery and relaxation techniques. During this time, they were asked to image 4 situations. If they had difficulty imagining these situations clearly (i.e., they rated the scene’s clarity below 7 on a scale from 1 to 10), further training was given.
Laboratory Sessions
On each testing day, participants abstained from breakfast (in order to minimize impact of recent food consumption on measurements of cortisol that were taken as part of the larger study). They were brought into the testing room by 8:00 am. After settling into a sitting position in a hospital bed, a BP cuff was placed on the participant’s preferred arm to monitor BP and a pulse sensor was placed on the participant’s forefinger to obtain a measure of pulse. This was followed by a 1-hour adaptation period during which the participants were instructed to practice relaxation.
At 9:10 am, participants were provided headphones and given the following instructions for the imagery procedure: “Close your eyes and imagine the situation being described, ‘as if’ it were happening right now. Let your body and mind get completely involved in the situation, doing what you would do in the real situation. Stop imagining when you hear the voice on the tape tell you to stop imaging.” The length of each script was approximately 5 minutes. After imagery, participants remained in the testing room for an additional 75 minutes to examine recovery from exposure to stress, alcohol cues, and neutral imagery. No instructions for relaxation were given during this period. After the last assessment at 10:30 am, the participant was disconnected from the apparatus and was served breakfast.
Measures of subjective emotion experience, behavioral/bodily response, alcohol craving, and cardiovascular response were taken before the imagery (baseline), immediately after the imagery (peak), and at repeated timepoints up to 75 minutes postimagery.
Measures
Subjective Emotion Experience
The Differential Emotions Scale (DES) -Revised short form (Izard, 1972) was used as a measure of subjective emotional experience. The present study used 5 subscales from the DES—sadness, anger, joy, fear, and anxiety. Each subscale is made up of 5 adjectives describing the particular emotion state. Participants rate on a 5-point scale the extent to which each word describes the way they feel at the present moment. The DES shows good psychometric properties (Izard, 1972). The DES has been used to examine subjective emotion following laboratory mood inductions (Schwartz and Weinberger, 1980; Sinha et al., 1999). Subjective emotion was assessed at baseline (−5), immediately following imagery exposure (0 timepoint), and at +5, +15, and +30 minutes postimagery.
Behavioral/Bodily Response
An independent rater, unaware of imagery condition, rated participants’ behavioral expressions of emotional arousal in person during the laboratory session, using the Behavioral Observation Scale (BOS; Sinha, 2004). They rated the following 12 behaviors as present or absent based on their observations and, when necessary, additional questioning of participants: muscle twitching, muscle tremor, restlessness, muscle tension, muscle ache, headache, quickened breathing, yawning, talking/facial movements, crying, sweating, and stomach/abdominal changes. The BOS was developed based on Lang’s (1979) bio-informational theory of emotions and BOS items were adapted from Lang and colleague’s list of physical and behavioral arousal responses provided in the “Scene Construction Questionnaire” (Miller et al., 1987). In addition, because we compare healthy adults with alcohol and drug-dependent adults in other studies, items associated with both emotional arousal and alcohol and drug withdrawal, including muscle twitching, muscle ache, and yawning were added (from the Clinical Institute Narcotics Assessment Scale for Withdrawal, Peachey and Lei, 1988).
In the present study, the internal consistency of the BOS was good, α= 0.74. Behaviors were double coded for 10 participants by 2 independent raters. Inter-rater reliability was assessed using Cohen’s kappa following Rovine’s (1994) procedure for redistributing low-frequency cells along the diagonal. Agreement on the presence of the behaviors was high (κ = 0.88 for stress condition, 0.92 for neutral/relaxing, and 0.93 for alcohol cue). The present study used the total number of behaviors as the measure of behavioral and bodily responses to emotional stress. BOS ratings were taken at baseline (−5 minutes), immediately following imagery exposure (0 timepoint) and at+15 minutes after imagery.
Cardiovascular Response
A Critikon Dinamap 120 Patient Monitor (Cheshire, CT) was used to assess BP. A pulse sensor was attached to the participant’s finger and connected to the Dinamap Monitor to provide a continuous measure of pulse. Multiple measures of pulse and BP were averaged to obtain mean responses for the baseline (4 readings during the 5-minute preimagery baseline period), during the imagery period (4 readings, 1 per minute during the imagery story, averaged for the 0 timepoint) and single readings at +5, +10, and+15 minutes postimagery.
Alcohol Craving
The desire for using alcohol was assessed using a 10-point visual analog scale in which 1 = not at all and 10 = extremely high. Similar items phrased for alcohol and cocaine craving have been used in previous research and have been found to be associated with measures of alcohol and cocaine relapse (Breese et al., 2005; Sinha et al., 2006). Craving was measured at baseline (−5), immediately following imagery exposure (0 timepoint), and at +5, +15, and+30 minutes after imagery.
Statistical Analysis
Linear Mixed Effect Models (LME; Laird and Ware, 1982) were used to test the hypothesis of gender differences in emotional response, using SAS software (SAS Institute, Cary, NC). First, any condition or gender differences on baseline measures were assessed using condition (stress, neutral, and alcohol cue) and gender (females = 1, males = 2) as the fixed effects factors and subjects as the random effects factor. Second, response to the imagery condition was assessed using change from baseline scores (i.e., score at each timepoint–baseline score) for all measures. For response analyses, the within-subjects factors of condition (stress, neutral, and alcohol cue), timepoint (varying levels), and the between-subjects factors of gender (females, males) were the fixed effects and subjects was the random effects factor. LMEs are particularly well suited when the design calls for repeated measurements within the same individual that can lead to correlations between measurements.
Correlations were performed to ascertain the relationship among subjective emotion, behavioral and bodily responses, HR, BP, and craving in the stress and alcohol-cue conditions, separately for males and females. Difference from baseline scores was examined. Peak timepoint was used for all measures.
RESULTS
Demographics
The sample ranged from age 20 to 50 with a mean age of 33.67 years (SD = 9.34 years). Participants’ mean education level was 14.67 years of schooling (SD = 2.54 years).
As shown in Table 1, women and men were similar in age and education level. Women were more likely to identify as African-American (48%) than were men (7%). To account for this, we examined race as a covariate in each of the LME’s. Race was not a significant predictor for any variable except subjective joy, so models without race are presented below, except for subjective joy. When race was included in the mixed models analyses, the results for all variables was the same, except the gender difference in diastolic BP (DBP) dropped out of significance (to p = 0.15).
Table 1.
Sample Characteristics for Women and Men
| Women (n = 27) | Men (n = 27) | |
|---|---|---|
| Age in years: mean (SD) | 33.19 (9.18) | 34.15 (9.65) |
| Race: number (%)* | ||
| Caucasian | 11 (40.7) | 18 (66.6) |
| African-American | 13 (48.1) | 2 (7.4) |
| Hispanic | 2 (7.4) | 6 (22.2) |
| Others | 1 (3.7) | 1 (3.7) |
| Years of education: mean (SD) | 14.52 (3.04) | 14.81 (1.96) |
| Lifetime psychiatric disorders: number (%) | ||
| Posttraumatic stress disorder (PTSD) | 3 (11.1) | 1 (3.7) |
| Anxiety disorder without PTSD | 0 (0) | 0 (0) |
| Unipolar mood disorder* | 6 (22.2) | 1 (3.7) |
| Positive family history of alcohol-use disorders: number (%) | 11 (40.74) | 11 (40.74) |
| Smoking behavior | ||
| Smoker: number (%) | 2 (7.41) | 5 (18.52) |
| If smoker, number of cigarettes per week? | 2.67 (3.33) | 5.60 (3.33) |
| Alcohol use: mean (SD) | ||
| Age of onset | 16.07 (5.24) | 15.52 (2.46) |
| Frequency (no. of days drank in past month)* | 2.30 (2.67) | 6.59 (7.42) |
| Amount (if drank, no. of drinks in past month) | n = 12, 6.19 (5.57) | n = 16, 11.43 (9.12) |
| Recent use (no. of days since last drink) | 54 (16.75) | 69 (24.27) |
Indicates significant gender difference at p < 0.05. Gender differences were examined with chi-squared analyses for categorical variables and t-tests for continuous variables.
Consistent with the literature, women reported a greater history of unipolar mood disorders than men, although as mentioned above, women and men did not report any current mood or other psychiatric disorder. Alcohol use was measured with the Addiction Severity Index structured interview (McLellan et al., 1992). Men reported a greater number of days per month of alcohol use than women (p = 0.02).1
Baseline Analyses
As expected, no main effects of condition or condition × gender interactions were observed at baseline for any dependent variable. There was 1 gender main effect—women had significantly higher HR than men at baseline (p = 0.02). As stated above, all analyses used change from baseline scores, which controlled for this difference.
Response to Imagery Exposure
Subjective Emotion Experience
Significant main effects of Condition were found for sadness [F (2, 103) = 102.02, p < 0.0001], anxiety [F (2, 103) = 96.34, p < 0.0001], anger [F (2, 103) = 84.93, p < 0.0001], fear [F (2, 103) = 37.98, p < 0.0001], and joy [F (2, 103) = 169.72, p < 0.0001] experience. All participants (regardless of gender) reported higher negative emotion (sadness, anxiety, anger, and fear) and lower joy experience in the stress condition when compared with the neutral and alcohol-cue conditions. The neutral and alcohol-cue conditions did not differ for any emotion. There were no significant main effects of gender on any subjective emotion measure. Below we report findings for the hypothesized gender × condition interactions for each emotion separately.
Sadness
A significant gender × condition interaction [F (2, 103) = 3.16, p = 0.046] indicated that women reported significantly higher sadness than men in the stress condition(estimate = 0.87, p = 0.01), but not in the neutral or alcohol- cue conditions (see Fig. 1).
Fig. 1.
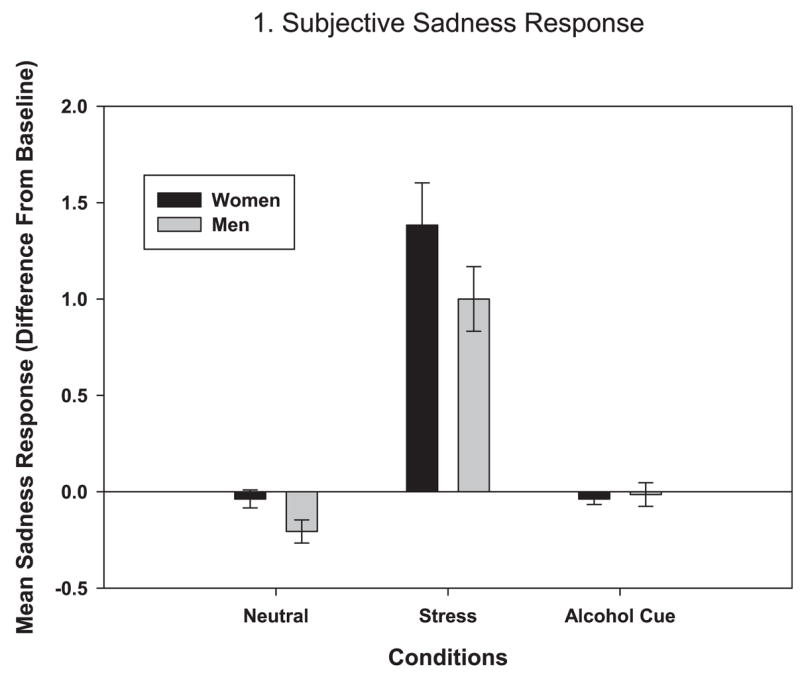
Average subjective sadness response over time to neutral, stress, and alcohol-cue conditions by gender (in stress: women > men, p = 0.01).
Anxiety
A significant gender × condition interaction for anxiety [F (2, 103) = 14.80, p < 0.0001] indicated that women reported greater anxiety than men in the stress condition (estimate = 1.08, p < 0.0001), but not in the neutral or alcohol-cue conditions (see Fig. 2).
Fig. 2.
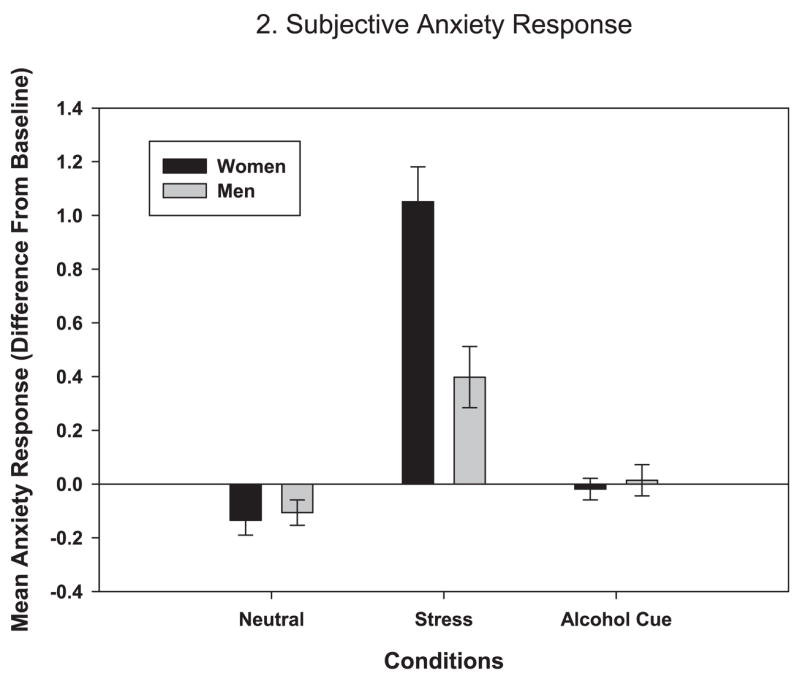
Average subjective anxiety response over time to neutral, stress, and alcohol-cue conditions by gender (in stress: women > men, p < 0.0001).
Joy
A significant gender × condition interaction emerged for joy [F (2, 103) = 8.00, p = 0.001], with women (but not men) reporting greater joy in the neutral than the cue condition (p = 0.04).
Anger and Fear
The gender × condition interactions were not significant for anger or fear.
Behavioral/Bodily Responses
The main effect of condition was significant [F (2, 104) = 42.01, p < 0.0001], indicating higher behavioral/bodily arousal in the stress when compared with the neutral and alcohol-cue conditions, with no difference between the neutral and alcohol-cue conditions. A significant gender × condition interaction for behavioral emotion expression [F (2, 103) = 3.75, p = 0.03] indicated that women showed greater behavioral and bodily responses than men in the stress condition (estimate = 0.89, p = 0.01), but not in the neutral or alcohol-cue conditions (see Fig. 3).
Fig. 3.
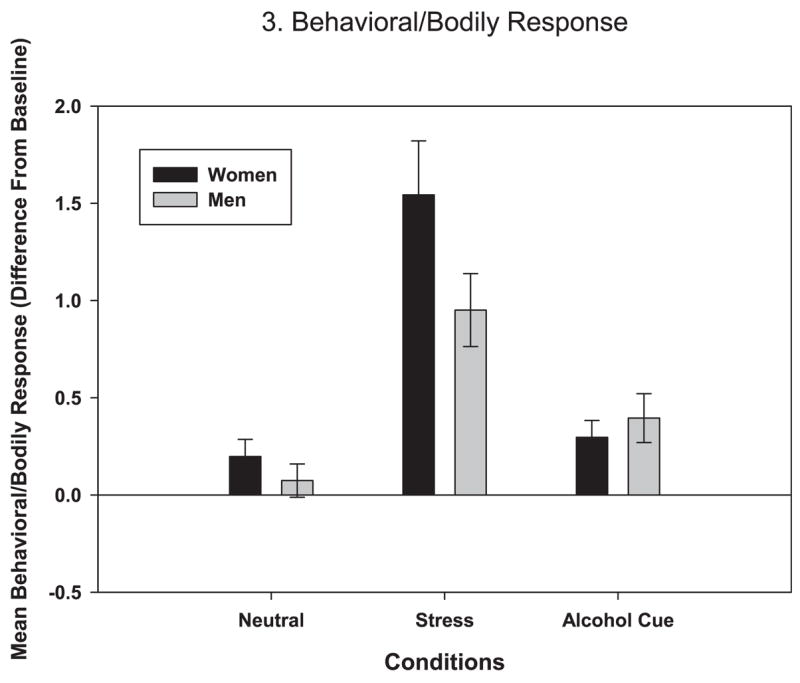
Average subjective joy response over time to neutral, stress, and alcohol-cue conditions by gender (in stress: women > men, p = 0.04).
Cardiovascular Measures
Significant main effects of condition were seen for HR [F (2, 103) = 3.93, p = 0.02), systolic BP (SBP) [F (2, 103) = 4.48, p = 0.01], and DBP [F (2, 103) = 4.11, p = 0.02], all indicating higher cardiovascular arousal in the stress when compared with the neutral and alcohol-cue conditions, with no difference between the neutral and alcohol-cue conditions. A significant main effect of Gender was found for DBP [F (1, 52) = 3.92, p = 0.05], indicating that men had significantly higher DBP response than women. There were no significant main effects of gender for HR or SBP and no significant condition × gender interactions for HR, SBP, or DBP.
Alcohol Craving
A significant main effect of condition [F (2, 103) = 9.93, p < 0.0001] indicated greater craving in the alcohol cue when compared with the neutral (p < 0.0001) or stress (p = 0.02) conditions and greater craving in the stress than the neutral (p = 0.03) condition. The gender main effect and gender × condition interaction were not significant for alcohol craving. While there were no gender effects on craving, because there were gender differences in baseline alcohol-use frequency (see Table 1), we assessed correlations between craving and alcohol use frequency for each gender, but these failed to achieve significance (r’s < 0.19, NS).
Correlations Among Measures of Emotional Arousal by Gender
As stated above, all correlation analyses used difference from baseline scores.
Stress Condition
For women in the stress condition, subjective sadness, anxiety, and fear were all significantly correlated with HR (r’s = 0.47, 0.46, and 0.49, respectively) and with behavioral arousal (r’s = 0.45, 0.54, and 0.65, respectively). In addition, behavioral arousal was significantly correlated with lower reported joy (r = −0.51) and higher HR (r = 0.38). Subjective anger, craving, SBP, and DBP were not significantly correlated with other types of measures in the stress condition.
For men, subjective anxiety was significantly correlated with DBP (r = 0.46). Subjective sadness, anxiety and anger were all significantly correlated with behavioral arousal (r’s = 0.55, 0.46, and 0.52, respectively). In addition to correlations with subjective emotion, behavioral arousal was also correlated with SBP (r = 0.38). Also, only in men, alcohol craving was significantly correlated with subjective sadness, anxiety, fear, and behavioral/bodily response (r’s = 0.62, 0.69, 0.50, and 0.44, respectively). HR was not significantly correlated with other measures.
Alcohol-Cue Condition
For women, subjective fear was significantly correlated with lower SBP (r = −0.48), and higher behavioral/bodily arousal (r = 0.52) in the alcohol-cue condition. Subjective joy, anxiety, anger, sadness, DBP, HR, and craving were not significantly correlated with other types of measures in the alcohol-cue condition.
For men, subjective anger was significantly correlated with higher SBP (r = 0.58) and subjective anxiety was correlated with greater behavioral arousal (r = 0.82) in the alcohol-cue condition. Also, in men, alcohol craving was significantly correlated with higher subjective anxiety (r = 0.45) and HR (r = 0.39) in the alcohol-cue condition. Subjective joy, fear, sadness, and DBP were not significantly correlated with other measures in the alcohol-cue condition.
DISCUSSION
The present study found gender differences in response to individually calibrated imagery of personal stressful situations when compared with neutral relaxing situations and alcohol-cue situations across subjective, behavioral, and physiological domains. These differences may have implications for gender differences in risk for stress-related disorders. Consistent with hypotheses, women reported and expressed greater sad and anxious emotion than men following stress, even though they experienced equal (for HR, SBP) or lower (for DBP) physiological arousal than men. Furthermore, stress- and alcohol-cue-related alcohol craving was correlated with greater subjective negative emotion for men but not for women.
As predicted, women reported greater subjective sadness and anxiety following the stress induction than men. Also, women showed greater emotion behaviorally and in their bodily response than men. Our measure of behavioral/bodily arousal largely tapped anxiety (e.g., restlessness) or sadness (e.g., crying) behaviors and bodily sensations and thus may best be considered a behavioral or bodily indicator of anxiety/sadness. Taken together, these findings for subjective and behavioral measures are consistent with past findings that women report experiencing and expressing more sadness and anxiety than men (e.g., Brody, 1999; Levenson et al., 1994) and extend these findings to personally relevant emotional stress situations.
It is interesting, however, that although women had greater subjective and behavioral emotional arousal following stress, their HR and SBP responses were not different from men’s and their DBP was actually lower than men’s (although baseline HR was higher for women than men, consistent with the literature—Allen et al., 1993). Women may be more likely to label the same or lower physiological arousal as sadness/anxiety related than men or may experience it subjectively and express it behaviorally with greater intensity. Women may also focus cognitively on sadness/anxiety more than men—-for example, women are more likely to ruminate on sad and anxious emotions than men whereas men are more likely to distract attention away from these emotional states (Nolen-Hoeksema et al., 1999). Rumination has been linked to depressive and anxious symptoms, symptoms which are more common for women than men (Nolen-Hoeksema, 2000). Alternatively, as hypothesized by Taylor et al. (2000), it may be that cardiovascular arousal and the traditional “fight” or “flight” response is not a prominent domain of stress experience for women.
For both men and women, subjective emotion was related to behavioral arousal and with some marker of cardiovascular arousal, although this measure was HR for women and BP for men. This may be because women tend to have higher HR and men higher BP at rest and following stressors (Kudielka et al., 2004; Matthews and Stoney, 1988; Stoney et al., 1988), leading to greater range and more ability to find significance. Interestingly, the type of subjective emotion that showed correlations was different for men versus women. Behavioral and bodily response was associated with subjective anger for men (but not women) and subjective fear for women (but not men). This is consistent with gender roles in U.S. culture that fear is more acceptable for women and anger for men (Brody, 1999), leading those systems to be most integrated with behaviors. One implication of this finding is that men may be more at risk for disorders involving low fear and high anger, such as externalizing disorders (e.g., Antisocial Personality Disorder) (Chaplin and Cole, 2005).
For both men and women, the different response domains were moderately associated with one another. This is an important finding, given that some research has failed to find correlations across different markers of emotional arousal (Baum et al., 1992). We may have found greater convergence than past studies due to the use of personally relevant emotional stress stories, which leads to greater range of response, permitting the ability to detect associations.
Contrary to prediction, the present study did not find gender differences in alcohol craving in response to stress or alcohol-cue imagery. However, craving was associated with subjective emotion and behavioral/bodily responses to stress, and with subjective emotion and HR response to alcohol cue, for men but not for women. These data suggest a closer association between emotional stress reward motivation in these social drinking men than women. It is important to note that while men on average drank more frequently than women (see Table 1), and this is consistent with national norms (Russell et al., 2004), drinking levels were not associated with alcohol craving measures in either men or women. Thus, these findings are not simply due to men drinking more frequently than women. The findings may suggest that, even among light to moderate social drinkers, men are more likely to cope with elevated negative emotion with desire for alcohol. This is consistent with past findings that men are more likely than women to report using alcohol as a way to cope with stress (Nolen-Hoeksema and Harrell, 2002; Park and Levenson, 2002). If this is true, it may help to explain the greater vulnerability among men for alcohol-use disorders (Kessler et al., 1994).
SUMMARY AND LIMITATIONS.FUTURE DIRECTIONS
While the present study found intriguing gender differences in responses to stress, it is unclear how these differences develop and how they relate to risk for psychopathology. For example, in the present study, women reported greater histories of depressive disorders than men. It is unclear without longitudinal study whether these women’s depression histories lead them to be more sensitized towards sadness/anxiety following stress or whether their sadness/anxiety responses to stress preceded their development of depressive disorders. Future longitudinal studies should examine relations between gender differences in stress response and in psychopathology over time. In addition, the present study had a relatively small sample without full distribution across racial/ethnic groups, which limited the power to examine whether gender differences in stress response differed by race/ethnicity. Theorists have argued that we must consider the intersection between gender and other social categories (e.g., ethnicity, socioeconomic status) in order to fully understand when and in what setting gender differences occur (e.g., Crenshaw, 1997). Thus, future research on the intersection of gender and ethnicity in terms of emotional response to stress is warranted.
Despite limitations of the study, using individually calibrated stress induction procedures, the present study found significant gender differences in subjective, behavioral, physiological, and craving responses to stress. Women were more likely than men to report and show sadness and anxiety following stress. Further, negative emotion was related to alcohol craving for men but not women. Men and women’s different responses to stress may have implications for the known divergences between men and women in vulnerability for stress-related disorders. Women’s focus on sadness and anxiety may lead to risk for depression and anxiety, disorders which are more common in women than men. Men’s stronger integration between negative emotion and craving may contribute to risk for the development of alcohol-use disorders, disorders which are more common for men than women. If this is true, it would have implications for the prevention and treatment of depression/anxiety and alcohol-use disorders. Treatment of anxiety and depression in women may benefit from addressing the tendency to ruminate on sad/anxious feelings. And, treatment of men might focus on encouraging men to cope with stress and negative emotions in ways other than substance use.
Supplementary Material
The following supplementary material is available for this article:
Appendix S1. Sample Stress, Alcohol-Cue, and Neutral Imagery Scripts.
This material is available as part of the online article from: http://www.blackwell-synergy.com/doi/abs/10.1111/j.1530-0277.2008.00679.x (This link will take you to the article abstract).
Please note: Blackwell Publishing are not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Acknowledgments
Funding for this study was provided by National Institute of Health Grants R01-AA113892 and K02-DA17232 (PI: Sinha).
Footnotes
We re-ran analyses with days of alcohol use per month as a covariate. Significance levels of findings were unchanged, except that the significant gender difference in DBP fell to a trend level (p = 0.08).
References
- Allen MT, Stoney CM, Owens JF, Matthews KA. Hemodynamic adjustments to laboratory stress: the influence of gender and personality. Psychosom Med. 1993;55:505–517. doi: 10.1097/00006842-199311000-00006. [DOI] [PubMed] [Google Scholar]
- Barnes ML, Buss D. Sex differences in the interpersonal behavior of married couples. J Pers Soc Psychol. 1985;48:654–661. [Google Scholar]
- Baum A, Grunberg NE, Singer JE. Biochemical measurement in the study of emotion. Psychol Sci. 1992;3:56–60. [Google Scholar]
- Brebner J. Gender and emotions. Pers Individ Dif. 2003;34:387–394. [Google Scholar]
- Breese GR, Chu K, Dayas CV, Funk DK, Darin J, Koob GF, Le DA, O’Dell LE, Overstreet DH, Roberts AJ, Sinha R, Valdez GR, Weiss F. Stress enhancement of craving during sobriety: a risk for relapse. Alcohol Clin Exp Res. 2005;29:185–195. doi: 10.1097/01.alc.0000153544.83656.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody L. Gender, Emotion, and the Family. Harvard University Press; Cambridge, MA: 1999. [Google Scholar]
- Chaplin TM, Cole PM. The role of emotion regulation in the development of Psychopathology. In: Hankin BL, Abela JRZ, editors. Development of Psychopathology: A Vulnerability- Stress Perspective. Sage; Thousand Oaks, CA: 2005. pp. 49–74. [Google Scholar]
- Cooper ML, Russell M, Skinner JB, Frone MR, Mudar P. Stress and alcohol use: moderating effects of gender, coping, and alcohol expectancies. J Abnorm Psychol. 1992;101:139–152. doi: 10.1037//0021-843x.101.1.139. [DOI] [PubMed] [Google Scholar]
- Crenshaw K. Intersectionality and identity politics: learning from violence against women of color. In: Shanley M, Narayan U, editors. Reconstructing Political Theory: Feminist Perspectives. The Pennsylvania State University Press; University Park, PA: 1997. pp. 178–193. [Google Scholar]
- Feingold A. Gender differences in personality: a meta-analysis. Psychol Bull. 1994;116:429–456. doi: 10.1037/0033-2909.116.3.429. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV. Patient Edition. American Psychiatric Press Inc.; Washington, DC: 1995. [Google Scholar]
- Fischer AH, Mosquera PMR, van Vianen AEM, Manstead ASR. Gender and culture differences in emotion. Emotion. 2004;4:87–94. doi: 10.1037/1528-3542.4.1.87. [DOI] [PubMed] [Google Scholar]
- Fox HC, Berquist KL, Hong KI, Sinha R. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol dependent individuals. Alcohol Clin Exp Res. 2007;31:395–403. doi: 10.1111/j.1530-0277.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- Fox HC, Garcia M, Kemp K, Milivojevic V, Kreek MJ, Sinha R. Gender differences in cardiovascular and corticoadrenal response to stress and drug-cue in cocaine dependent individuals. Psychopharmacology. 2006;185:348– 357. doi: 10.1007/s00213-005-0303-1. [DOI] [PubMed] [Google Scholar]
- Fox HC, Talih M, Malison R, Anderson GM, Kreek MJ, Sinha R. Frequency of recent cocaine and alcohol use affects drug craving and associated responses to stress and to drug-related cues. Psychoneuroendocrinology. 2005;30:880–891. doi: 10.1016/j.psyneuen.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Izard CE. Patterns of Emotions: a new Analysis of Anxiety and Depression. Academic Press; New York: 1972. [Google Scholar]
- Kajantie E, Phillips DIW. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2005;31:151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus–pituitary–adrenal axis. Psychosom Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. Differential heart rate reactivity and recovery after psychosocial stress (TSST) in healthy children, younger adults, and elderly adults: the impact of age and gender. Int J Behav Med. 2004;2:116–121. doi: 10.1207/s15327558ijbm1102_8. [DOI] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Lang P. A bio-informational theory of emotional imagery. Psychophysiology. 1979;16:496–512. doi: 10.1111/j.1469-8986.1979.tb01511.x. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Levin DN, Miller GA, Kozak MJ. Fear behavior, fear imagery, and the psychophysiology of emotion: the problem of affective response integration. J Abnorm Psychol. 1983;92:276–306. doi: 10.1037//0021-843x.92.3.276. [DOI] [PubMed] [Google Scholar]
- Lang AR, Pelham WE, Johnston C, Gelernter S. Levels of adult alcohol consumption induced by interactions with child confederates exhibiting normal versus externalizing behaviors. J Abnorm Psychol. 1989;98:294–299. doi: 10.1037//0021-843x.98.3.294. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Carstensen LL, Gottman JM. Influence of age and gender on affect, physiology, and their interrelations: a study of long-term marriages. J Pers Soc Psychol. 1994;67:56–68. doi: 10.1037//0022-3514.67.1.56. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Oyama ON, Meeks PS. Greater reinforcement from alcohol for those at risk: parental risk, personality risk, and sex. J Abnorm Psychol. 1987;96:242–253. doi: 10.1037//0021-843x.96.3.242. [DOI] [PubMed] [Google Scholar]
- Marlatt GA, Kosturn CF, Lang AR. Provocation to anger and opportunity for retaliation as determinants of alcohol consumption in social drinkers. J Abnorm Psychol. 1975;84:652–659. [PubMed] [Google Scholar]
- Matthews K, Stoney CM. Influences of sex and age on cardiovascular responses during stress. Psychosom Med. 1988;50:46–56. doi: 10.1097/00006842-198801000-00006. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The fifth edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- McNeil DW, Vrana SR, Melamed BG, Cuthbert BN, Lang PJ. Emotional imagery in simple and social phobia: fear versus anxiety. J Abnorm Psychol. 1993;102:212–225. doi: 10.1037//0021-843x.102.2.212. [DOI] [PubMed] [Google Scholar]
- Miller G, Levin D, Kozak M, Cook E, McLean A, Lang P. Individual differences in imagery and the psychophysiology of emotion. Cogn Emot. 1987;1:367–390. [Google Scholar]
- Nolen-Hoeksema S. The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. J Abnorm Psychol. 2000;109:504–511. [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Harrell ZA. Rumination, depression, and alcohol use: tests of gender differences. J Cogn Psychother. 2002;16:391–403. [Google Scholar]
- Nolen-Hoeksema S, Larson J, Grayson C. Explaining the gender difference in depressive symptoms. J Pers Soc Psychol. 1999;77:1061–1072. doi: 10.1037//0022-3514.77.5.1061. [DOI] [PubMed] [Google Scholar]
- Ollendick T, Yang B, Dong Q, Xia Y, Lin L. Perceptions of fear in children and adolescents: the role of gender and friendship status. J Abnorm Child Psychol. 1995;23:439–452. doi: 10.1007/BF01447207. [DOI] [PubMed] [Google Scholar]
- Park CL, Levenson MR. Drinking to cope among college students: prevalence, problems and coping processes. J Stud Alcohol. 2002;63:486–497. doi: 10.15288/jsa.2002.63.486. [DOI] [PubMed] [Google Scholar]
- Peachey JE, Lei H. Assessment of opioid dependence with naloxone. Br J Addict. 1988;83:193–201. doi: 10.1111/j.1360-0443.1988.tb03981.x. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Gross JJ, Wilhelm FH, Najmi S, Gotlib IH. Crying threshold and intensity in major depressive disorder. J Abnorm Psychol. 2002;111:302–312. [PubMed] [Google Scholar]
- Rovine M. Latent variable models and missing data analyses. In: von Eye A, Clogg C, editors. Latent Variable Analysis: Applications for Developmental Research. Sage; Thousand Oaks, CA: 1994. pp. 181–225. [Google Scholar]
- Russell M, Light JM, Gruenewald JP. Alcohol consumption and drinking problems: the relevance of drinking patterns. Alcohol Clin Exp Res. 2004;28:921–930. doi: 10.1097/01.alc.0000128238.62063.5a. [DOI] [PubMed] [Google Scholar]
- Schwartz GE, Weinberger DA. Patterns of emotional responses to affective situations: relations among happiness, sadness, anger, fear, depression and anxiety. Motiv Emot. 1980;4:175–191. [Google Scholar]
- Sheehan PW. A shortened form of Bett’s questionnaire on mental imagery. J Clin Psychol. 1967;23:386–389. doi: 10.1002/1097-4679(196707)23:3<386::aid-jclp2270230328>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001a;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R. Imagery Script Development Procedures. Yale University; New Haven, CT: 2001b. Unpublished Manual. [Google Scholar]
- Sinha R. Behavioral Observation System. Yale University; New Haven, CT: 2004. Unpublished Manual. [Google Scholar]
- Sinha R, Catapano D, O’Malley S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology (Berl) 1999;142:343–351. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Renee-Aubin l, O’Malley SS. Psychological stress, drug cues and cocaine craving. Psychopharmacology (Berl) 2000;152:140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress induced cocaine craving and hypothalamic–pituitary–adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63:324– 331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Sinha R, Lovallo WR, Parsons OA. Cardiovascular differentiation of emotions. Psychosom Med. 1992;54:422–435. doi: 10.1097/00006842-199207000-00005. [DOI] [PubMed] [Google Scholar]
- Sinha R, Parsons O. Multivariate response patterning of fear and anger. Cogn Emot. 1996;10:173–198. [Google Scholar]
- Sinha R, Rounsaville BJ. Sex differences in depressed substance abusers. J Clin Psychiatry. 2002;63:616–627. doi: 10.4088/jcp.v63n0715. [DOI] [PubMed] [Google Scholar]
- Sinha R, Talih M, Malison R, Cooney N, Anderson GM, Kreek MJ. Hypothalamic–pituitary–adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology (Berl) 2003;170:62–72. doi: 10.1007/s00213-003-1525-8. [DOI] [PubMed] [Google Scholar]
- Stoney CM, Matthews KA, McDonald RH, Johnson CA. Sex differences in lipid, lipoprotein, cardiovascular, and neuroendocrine responses to acute stress. Psychophysiology. 1988;25:645–656. doi: 10.1111/j.1469-8986.1988.tb01902.x. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Salovey P, Epel ES. Sex differences in stress responses: social rejection versus achievement stress. Biol Psychiatry. 2002;52:318–327. doi: 10.1016/s0006-3223(02)01333-1. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RA, Updegraff JA. Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychol Rev. 2000;107:411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Way BM, Welch WT, Hilmert CJ, Lehman BJ, Eisenberger NI. Early family environment, current adversity, the serotonin transporter promoter polymorphism, and depressive symptomatology. Biol Psychiatry. 2006;60:671–676. doi: 10.1016/j.biopsych.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Tucker JA, Vuchinich RE, Sobell M. Alcohol consumption as a self-handicapping strategy. J Abnorm Psychol. 1981;90:220–230. doi: 10.1037//0021-843x.90.3.220. [DOI] [PubMed] [Google Scholar]
- Zahn-Waxler C. The development of empathy, guilt, and internalization of distress: implications for gender difference in internalizing and externalizing problems. In: Davidson RJ, editor. Anxiety, Depression, and Emotion. Oxford University Press; New York: 2000. pp. 222–265. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The following supplementary material is available for this article:
Appendix S1. Sample Stress, Alcohol-Cue, and Neutral Imagery Scripts.
This material is available as part of the online article from: http://www.blackwell-synergy.com/doi/abs/10.1111/j.1530-0277.2008.00679.x (This link will take you to the article abstract).
Please note: Blackwell Publishing are not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.


