Abstract
The effects of genistein on 30-day survival and delayed lung injury were examined in C57BL/6J female mice. A single subcutaneous injection of vehicle (PEG-400) or genistein (200 mg/kg) was administered 24 h before total body irradiation (7.75 Gy 60Co, 0.6 Gy/min). Experimental groups were: No treatment + Sham (NC), Vehicle + Sham (VC), Genistein + Sham (GC), Radiation only (NR), Vehicle + Radiation (VR), Genistein + Radiation (GR). Thirty-day survivals after 7.75 Gy were: NR 23%, VR 53%, and GR 92%, indicating significant protection from acute radiation injury by genistein. Genistein also mitigated radiation-induced weight loss on days 13–28 postirradiation. First generation lung fibroblasts were analyzed for micronuclei 24 h postirradiation. Fibroblasts from the lungs of GR-treated mice had significantly reduced micronuclei compared with NR mice. Collagen deposition was examined by histochemical staining. At 90 days postirradiation one half of the untreated and vehicle irradiated mice had focal distributions of small collagen-rich plaques in the lungs, whereas all of the genistein-treated animals had morphologically normal lungs. Radiation reduced the expression of COX-2, transforming growth factor-β receptor (TGFβR) I and II at 90 days after irradiation. Genistein prevented the reduction in TGFβRI. However, by 180 days postirradiation, these proteins normalized in all groups. These results demonstrate that genistein protects against acute radiation-induced mortality in female mice and that GR-treated mice have reduced lung damage compared to NR or VR. These data suggest that genistein is protective against a range of radiation injuries.
Keywords: Total body irradiation, Lung fibrosis, Genistein, Radioprotection, Micronuclei, Pneumonitis
INTRODUCTION
Exposure to high-dose, total body irradiation (TBI) in humans results in a dose-dependent, severe, and potentially fatal illness known as the acute radiation syndrome (ARS). ARS is characterized by the hematopoietic (> 1 Gy), gastrointestinal (GI; > 10 Gy), and cerebrovascular syndromes (CVS; > 20 Gy).1 Following ARS, radiation can also induce delayed effects, including damage to tissues such as the lungs, kidneys, and eyes.2 The extent of radiation injuries depends upon a variety of factors including total radiation dose, dose rate, delivery as a single or fractionated doses, internal or external exposure, and amount of the body exposure (total or partial body irradiation).3 Recently there has been a great deal of interest in the discovery of radiation protective agents, i.e. compounds administered before radiation exposure.4,5 These radiation countermeasure agents are being developed to protect individuals from a terrorist threat involving radiation sources, or for emergencies such as a nuclear power plant accident. Many research investigations are primarily focused on protection from the acute effects of TBI, with experiments frequently concentrating on survival studies in the 30-day postirradiation period.6,7 Little attention has been given to protection against late effects that may develop after survival from the acute radiation syndrome, as is the case when a radiation countermeasure agent has been administered.
Survival from the acute effects of radiation may not provide protection against delayed radiation-induced injuries. Among the most devastating late effects from radiation damage is radiation injury to the lung. Radiation exposure to the lung results in two phases of injury, first pulmonary inflammation (pneumonitis), and second, pulmonary scarring and remodeling (fibrosis). Both of these phases of injury are associated with high mortality.8–11 Individuals accidentally exposed to 8–24 Gy radiation in the Tokaimura, Japan radiation accident were successfully treated for acute radiation injuries but succumbed to delayed respiratory failure between 82–210 days postirradiation.12,13 A radiation accident victim in Belarus also recovered from acute injuries but died from radiation-induced pneumonitis 130 days following a 10 Gy exposure.9 In patients treated with TBI for bone marrow transplantation, a high dose rate exposure was found to be an adverse prognostic factor for developing interstitial pneumonitis,14 and it is believed that any dose of thoracic irradiation, such as used in clinical applications, induces some degree of lung injury.8,11
Genistein, a soy isoflavone, is currently being developed as a radiation protective agent in our laboratory. This compound has antioxidant, free radical scavenging,15,16 anti-inflammatory,17,18 and antimicrobial properties,19 making it an ideal candidate for ameliorating both the acute and late effects of ionizing radiation. It was previously demonstrated that male mice receiving repeated daily oral administration of genistein,20,21 or a single subcutaneous (SC) injection of genistein 24 h before exposure, survive an acute dose of gamma radiation that would otherwise be lethal.22 The improvement in survival was related to accelerated neutrophil and platelet recovery, resulting from earlier and more pronounced multilineage, hematopoietic progenitor cell reconstitution in the marrow compartment.23 At radioprotective doses, genistein is nontoxic as evidenced by the absence of behavioral toxicity and gross morphological and histopathological analysis.22
In mice exposed to irradiation, two phases of radiation-induced delayed lung injury have been observed, pneumonitis and pulmonary fibrosis. The rate of onset of each of these phases is dependent upon the area of the body irradiated, the dose of radiation, and the strain of mouse. The C57BL/6 mouse strain is susceptible to both radiation-induced pneumonitis and lung fibrosis.24,25 Although few studies have addressed the effects of lung damage following whole body exposure, we selected this method of irradiation since it is of growing concern due to potential nuclear terrorism.26 Mattos et al.27 reported that C57BL mice exposed to a sublethal dose (7 Gy 60Co) TBI resulted in vascular congestion, thickening of the alveolar septa, and collagen deposition detectable within 30 days following irradiation These effects were more pronounced at 90 days postirradiation. Using a lethal dose of TBI (10 Gy, 137Cs), Van der Meeren et al. (2003) found alveolar edema and alveolar wall thickening 14 days postirradiation in C57BL/6 mice.28
In this paper, we examined effects of genistein administered to C57BL/6J mice 24 h before TBI irradiation (7.75 Gy, 60Co). Acute hematopoietic radiation injury was assessed using an endpoint of 30-day survival. Because radiation-induced pulmonary inflammation and subsequent fibrotic remodeling are believed to be related to genotoxicity in the lung, the cytochalasin-blocked micronucleus (CBMN) assay was used to quantify radiation-induced DNA damage. Radiation-induced damage in the lung was also assessed by histology to examine increases in inflammatory cell infiltration consistent with pneumonitis and collagen deposition consistent with fibrotic remodeling. In addition, Western blotting of lung lysates was performed to identify changes in the levels of four proteins shown to have altered expression in either radiation- or bleomycin-induced fibrotic remodeling. These proteins were angiotensin converting enzyme (ACE),29 cyclooxygenase-2 (COX-2),30 and transforming growth factor-beta receptor (TGFβR) I and II.31,32 Our results demonstrate that genistein reduces acute and delayed radiation-induced injuries as shown by increased 30-day survival, decreased DNA damage in the lung, and decreased collagen deposition in the lung. Genistein also prevented alterations in several other biomarkers of lung injury.
MATERIALS AND METHODS
Animals
Female C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME, USA) were used at 12–14 weeks of age (17.5–21.5 g) at the time of irradiation. Female mice were used because of lower aggression tendencies and the extensive evaluation period (180 day) for lung injury. Mice were housed in groups of four in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. Animal rooms were maintained at 21 ± 2°C, 50% ± 10% humidity, and 12-h light/dark cycle. Commercial rodent ration (Harlan Teklad Rodent Diet 8604) was freely available. Acidified (pH = 2.5–3.0) water was also freely available to control opportunistic infections.33 All animal handling procedures were performed in compliance with guidelines from the National Research Council, and were approved by the Institutional Animal Care and Use Committee of the Armed Forces Radiobiology Research Institute (AFRRI, Bethesda, MD, USA).
Isoflavone preparation
Genistein was prepared as previously described.22 Briefly, genistein (Sigma Chemical Company, St. Louis, MO) was freshly solubilized on the day of the experiment in modified polyethylene glycol, MW 400 (PEG; Sigma Chemical Company (St Louis, MO, USA), and sonicated for 20 sec (Heat Systems-Ultrasonics Inc., Plainview, NY, USA). All drugs were administered SC in the nape of the neck in a volume of 0.1 ml. The day of radiation was considered day 0.
Irradiation
Mice received TBI in a bilateral gamma radiation field at AFRRI’s 60Co facility. The midline tissue dose to the animals was 7–9 Gy at a dose rate of 0.6 Gy/min. Control animals were sham irradiated. The alanine/electron spin resonance (ESR) dosimetry system (American Society for Testing and Materials, Standard E 1607) was used to measure dose rates (to water) in the cores of acrylic mouse phantoms. To simulate a mouse, the phantoms were three inches in length and one inch in diameter. For field mapping, all exposure rack compartments contained phantoms, and alternate phantoms contained alanine dosimeters. The ESR signals were measured using a calibration curve based on the standard calibration dosimeters (National Institute of Standards and Technology (NIST), Gaithersburg, MD, USA). The overall uncertainty in the doses given to the calibration dosimeters at NIST was approximately 1.8% at 2 standard deviations. The accuracy of the calibration curve was verified by parallel measurements of doses to selected dosimeters at AFRRI and the National Physical Laboratory (Middlesex, UK). Corrections were applied to the dose rates in phantoms for the decay of 60Co and differences in the mass energy-absorption coefficients for water and soft tissue.
Animal Survival Studies
We previously determined that genistein-induced radio-protection was optimal at 200 mg/kg genistein administered SC 24 h prior to TBI.22 This regimen was used for all experiments. We conducted two acute injury radiation experiments. The first experiment was to determine the LD50/30 for C57BL/6J mice in our radiation facility. Naïve (untreated) mice were randomized and exposed to one of seven radiation doses between 7 Gy and 9 Gy (n = 20/group). Based on the results of the LD50/30 study, two additional experiments were performed to determine the radioprotective effects of genistein on C57BL/6J female mice and the data were pooled. Animals were randomly assigned to one of three groups of (i) no treatment (n = 56), (ii) PEG-400 vehicle (n = 32), and (iii) 200 mg/kg genistein (n = 26). PEG vehicle or genistein were administered SC 24 h prior to TBI. Survival was monitored for 30 days after all mice received a single dose of 7.75 Gy TBI.
In another set of experiments, we determined genistein protection of mice surviving an acutely lethal radiation dose from the development of radiation-induced late effects in the lung. These experiments utilized the ~LD75/30 dose (7.75 Gy) of radiation and evaluated endpoints at 90, 120 or 180 days postirradiation. Experimental groups were: (i) no treatment + sham irradiation (n = 9) (Control), (ii) PEG-400 vehicle + sham irradiation (n = 10) (Vehicle), (iii) genistein + sham irradiation (n = 10) (Genistein), (iv) no treatment + radiation (n = 40) (Radiation), (v) PEG-400 vehicle + radiation (n = 20) (Vehicle + Radiation), and (vi) genistein + radiation (n = 10) (Genistein + Radiation). PEG-400 vehicle (0.1 ml/animal) and the 200 mg/kg genistein groups received a single SC injection 24 h prior to TBI. The body weights of individual mice were recorded routinely through day 36 postirradiation.
Micronucleus assay
The CBMN assay is a standard method for assessing genotoxicity induced by physical and chemical agents, including radiation, in both in vivo and in vitro systems.34 Radiation-induced DNA damage in lungs was assessed by the cytochalasin-blocked micronucleus, CBMN assay in fibroblasts harvested from lungs 24 h postirradiation as previously described with minor modifications.34 Lungs (n = 6/group) were aseptically removed en bloc into sterile PBS on ice, mechanically minced in buffered HBSS (Mg+2-Ca+2-free Hank’s Balanced Salt Solution (HBSS), (Invitrogen, Carlsbad, CA, USA), and digested with dispase (5 mg/ml, Stem Cell Technologies, Vancouver, BC, Canada) and 0.3 mg/ml collagenase type IV (Sigma) in HBSS for 1 h at 37°C on a shaker. Digested tissue was passed through a 10 ml pipette to obtain a cell suspension, which was then centrifuged and resuspended in DMEM/F12 medium (Invitrogen, Carlsbad, CA, USA), 10% FBS (Gemini BioProducts, West Sacramento, CA, USA); cells were incubated for 1 h at 37°C before washing and application of fresh growth medium. After 18 h incubation, cytochalasin B was added (2 μg/ml, Sigma) for 72 h. Cell cultures were fixed for 2 h with acetic acid: methanol (1:3 respectively) fixative. Cells were stained for 5 min at ambient temperature with 250 μg/ml propidum iodide (Sigma) in PBS. Micronuclei were scored positive if they were distinguishable from the main nuclei (about one third of the nuclear size), using an LSM-510 Pascal microscope (Carl Zeiss MicroImaging, Inc., Thornwood, NY, USA) with an oil 100× objective. For each treatment we scanned 6 slides (n = 6) from 4 independent experiments and on each slide 120 fields were counted for total amount of binuclear cells with or without micronuclei.
Western blots and lung lysates
Reagents for Western blotting were from BioRad (Hercules, CA, USA). Primary antibodies for TGFβRI (Alk5, sc-9048), TGFβRII (sc-7791), COX-2 (sc-7951), ACE (sc-2079), and tubulin (sc-8035) were from Santa Cruz Biotechnology, Inc (Santa Cruz, CA, USA); mouse anti-actin (A5316) was from Sigma (St. Louis, MO, USA). Secondary HRP-linked antibodies were acquired from Amersham Biosciences (Piscataway, NJ, USA). At endpoints, animals were euthanized by injection with a lethal dose of pentobarbitol. Lungs were removed en bloc, flash frozen in liquid nitrogen, and stored at −70°C. Lungs were homogenized in RIPA buffer with protease and phosphatase inhibitors (Tris base 50 mM, NP-40 1%, sodium deoxycholate 1%, NaCl 150 mM, EDTA 2.5 mM, and NaF 50 mM, sodium vanadate 100 mM, aprotinin 10 μg/ml, leupeptin 10 μg/ml, and phenylmethylsulfonyl fluoride 1 mM); lysates were sonicated on ice twice for 30 seconds. Lung lysates were used in SDS-PAGE and electroblotted onto either nitrocellulose membrane (BioRad), or PVDF membrane (Invitrogen, Carlsbad, CA, USA). Blots were blocked in 5% non-fat milk in TTBS (154 mM NaCl, 20 mM Tris HCl, 0.05% Tween 20) for 1 h and incubated overnight with 1:500 dilution of primary antibody in TTBS/0.5% non-fat milk, 4°C. Blots were washed three times in TTBS for 10 min. Secondary antibodies were diluted 1:1000 in TTBS for 1 h and were washed two hours. ECL (Amersham, Piscataway, NJ, USA) was applied according to the manufacturer’s instructions before film exposure. For normalization, blots were stripped and reprobed with either tubulin or actin. Blots were stripped (Tris-HCl 62.5 mM, 2% SDS, 0.8% β-mercaptoethanol) for 10 min at 40°C and then washed with TTBS and reblocked for 1 h at room temperature before probing. Densitometry was done using the IR LAS-1000 Pro v2.7 and Image Gauge v4.01 software for the Macintosh, accompanying the FujiFilm LAS-1000/Intelligent Dark Box II hardware used for imaging the Western blots. Experiments were repeated three times using at least 3 animals per group.
Lung fixation and staining
To obtain lungs for histochemical staining, animals were euthanized by intraperitoneal injection of sodium pentobarbital. After dissection, the heart was perfused through the right ventricle with 10 ml of phosphate buffered saline (PBS) to remove blood cells. Following perfusion, the trachea was canulated and the heart-lung block was then removed and fixed for 15 min at RT by infusing 4% paraformadehyde (PFA)/PBS using 20 cm gravity pressure. Lungs were immersed in PFA/PBS for an additional 2 h at 4°C. Lungs were washed twice with sterile PBS for 30 min each, before embedding in paraffin. Lung tissue was embedded in paraffin and then cut into 6 microns sections and stained routinely with hematoxylin and eosin stain or Masson’s trichrome stain.
Statistical analysis
Statistical analysis for determination of the LD50/30 was determined by probit analysis. The Fisher’s exact test was used for analysing the 30-day survival data. For Western blot and micronuclei data analysis, statistics were performed using one-way ANOVA using the Student-Newman-Keuls Method of pairwise multiple comparisons. Statistical significance was determined at p ≤ 0.05.
RESULTS
Survival Studies: Determination of LD50/30 and the effects of genistein on protection from acute radiation injury
In the first experiment, untreated female mice were exposed to one of seven doses of 60Co radiation. The effects of radiation doses on 30-day survival for female C57BL/6J mice are illustrated in Fig. 1A. The LD50/30 with 95% confidence intervals was 7.52 Gy (7.44, 7.59). Previous results from our laboratory showed that a single SC injection of genistein, 24 h prior to radiation exposure, protected male CD2F1 mice from acute radiation injury.22,23 The optimal dosage for protection was 200 mg/kg body weight.22 Female C57BL/6J mice, receiving a single SC dose of genistein (200 mg/kg) administered 24 h before 7.75 Gy had a survival rate of 92% (p < 0.001), compared to 53% for vehicle (p < 0.006), and 23% for non-treated irradiated mice (Fig. 1B). Survival of genistein treated mice was significantly (p < 0.002) greater than vehicle treated animals. All mice that survived through day 30 also survived through day 120 with the exception of one mouse in the genistein + radiation group that died on day 64 postirradiation.
Fig. 1.
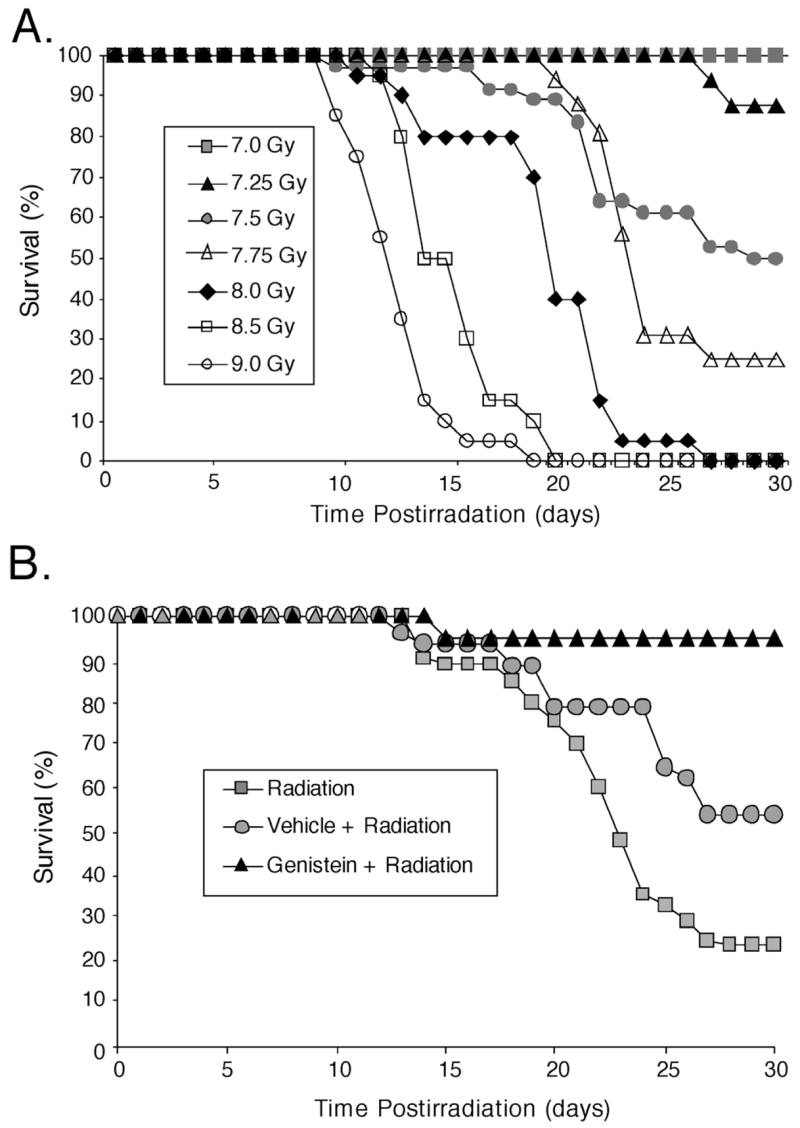
Thirty-day survival plots for female C57BL/6J mice after exposure to 60Co total body irradiation at a dose rate of 0.6 Gy/min. A) Radiation (7–9 Gy, n = 20) dose response. The LD50/30 was determined to be 7.52 Gy by probit analysis. B) Survival for animals with no pretreatment (Radiation, n = 56), a single subcutaneous injection of PEG-400 vehicle (Vehicle + Radiation, n = 32), or genistein (200 mg/kg, Genistein + Radiation, n = 26), 24 h before exposure to 7.75 Gy radiation.
Effects of genistein on radiation-induced weight loss
The body weights of individual mice were recorded through day 36 and are expressed as a change in weight from baseline (2 days before irradiation) for all surviving mice. There was a significant reduction in body weight for all irradiated groups. Genistein mitigated radiation-induced weight loss between days 13–27 postirradiation (Fig. 2). The maximal effect of genistein on weight loss was observed on days 18–23 postirradiation. On the day of maximal weight loss, day 20 postirradiation, average weight loss for irradiated mice was 4.1 g, while mice pretreated with genistein lost on average only 1.6 g (p < 0.05). Mice treated with the vehicle lost an average of 3.0 g. These results demonstrate that genistein protected female C57BL/6J mice against acute radiation-induced mortality and associated weight loss.
Fig. 2.
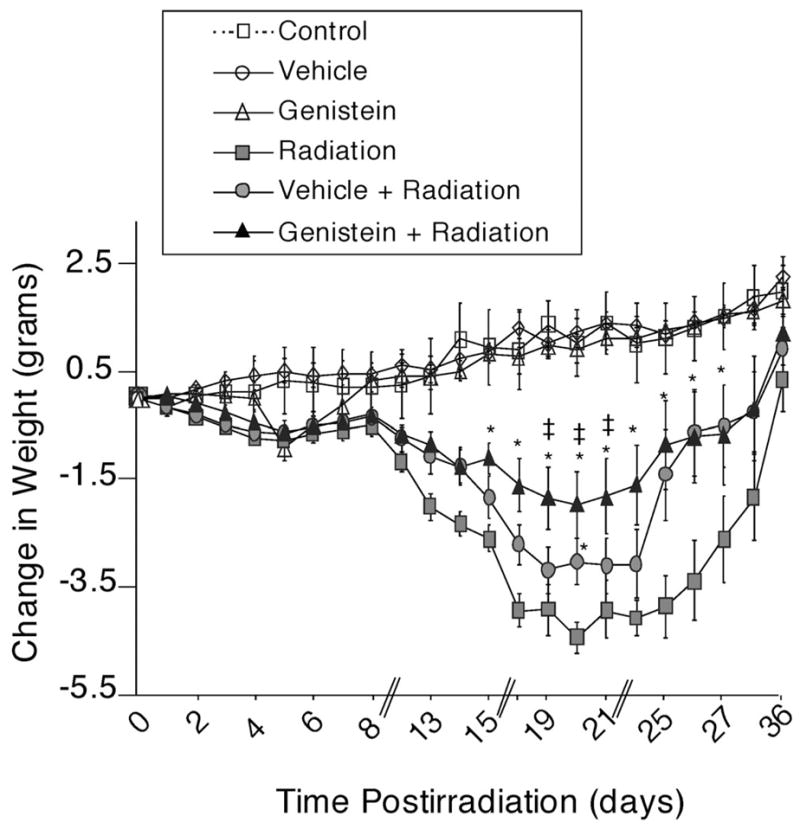
Effect of genistein on radiation-induced weight loss after exposure to 7.75 Gy 60Co. Mice were either sham-irradiated or irradiated with 7.75 Gy 60Co and received no-treatment (control), PEG-400 vehicle, or genistein (200 mg/kg, sc, 24 h before irradiation). Animals were individually marked and weighed daily for the first week, and 5 times weekly through 36 days. The changes in sampling rates are indicated by double breaks on the X-axis. Data show the mean +/− SEM change in body weight from baseline levels in surviving animals. Control (no treatment) (n = 9), Vehicle (n = 10), Genistein (n = 10), Radiation alone, (n = 8), Vehicle + Radiation (n = 10), Genistein + Radiation (n = 9). * p < 0.05 compared to radiation group; ‡ p < 0.05 compared to vehicle + radiation group.
Genistein pretreatment reduces radiation-induced micronuclei in pulmonary fibroblasts
The CBMN assay was used to determine induction of DNA damage in pulmonary fibroblasts isolated after acute radiation exposure, and to assess whether genistein could mitigate DNA damage. Figure 3A shows a binucleated cell with a radiation-induced micronucleus in a pulmonary fibroblast following ex vivo culture. The micronucleus is less than one-third the size of a complete nucleus and has similar staining properties. We compared the number of binucleated cells cultured ex vivo from sham irradiated or 7.75 Gy irradiated mice, with and without pretreatments. The number of binucleated cells was between 10–16% of the total cells in all six treatment groups, with no significant difference between the groups (Fig. 3B).
Fig. 3.
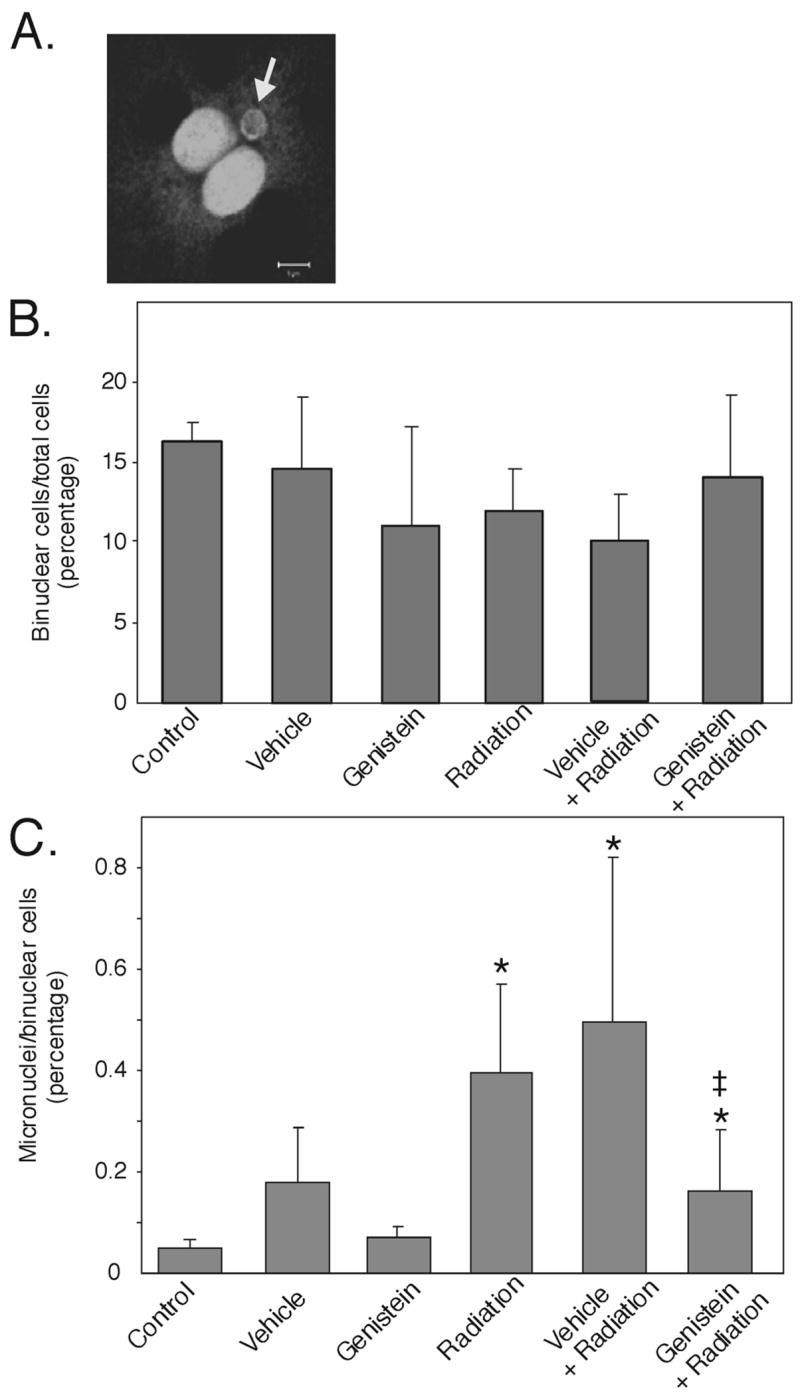
Genistein treatment reduces radiation-induced micronuclei in lung fibroblasts. C57BL/6J mice were either sham-irradiated or irradiated with 7.75 Gy 60Co and received no-treatment (control), PEG-400 vehicle, or genistein (200 mg/kg, sc, 24 h before irradiation). 24 h following irradiation, lung fibroblasts were cultured and used for CBMN assays to evaluate radiation-induced genotoxicity. A). A typical micronucleus-containing binuclear cell. Arrow indicates the micronucleus (100× magnification). B). The number of binuclear cells were counted and the percentage of binuclear cells per total cells was determined for each experiment. C). Percentage of micronuclei-containing binuclear cells/per total binuclear cells was calculated for each experiment. Sham irradiation groups: Control (no treatment), Vehicle, and Genistein. Irradiated groups: Radiation (7.75 Gy 60Co alone), Vehicle + Radiation, and Genistein + Radiation. Data show means +/− standard deviation. (n = 6 from 3 independent experiments). * p < 0.05 compared to untreated control; ‡ p < 0.05 compared to radiation alone.
Isolated pulmonary fibroblasts from non-treated irradiated mice had an approximately eight-fold increase in the frequency of micronuclei, relative to controls (0.4 ± 0.17 radiation; 0.05 ± 0.02 control, p < 0.05) (Fig. 3C). Fibroblasts from mice pretreated with genistein exhibited a significant decrease in the number of micronuclei (0.17 ± 0.12, p < 0.05) compared to control irradiated mice, although this number was still significantly higher than non-irradiated control mice. No significant reduction of radiation-induced micronuclei was observed in mice pretreated with vehicle. There was no significant change in micronuclei in nonirradiated mice injected with genistein or vehicle. Therefore, genistein administered 24 h before irradiation protects the lungs of mice from radiation-induced genotoxicity.
Assessment of fibrosis-associated protein alterations at 90 days and effects of genistein
To determine whether pretreatment with genistein reduced pneumonitis or other structural alterations, we examined histochemical staining of the lungs starting at 90 days postirradiation. Hematoxylin and eosin staining of the lung tissue from day 90 postirradiation in any experimental group failed to show significant increases in lymphocyte infiltration, which would be consistent with development of pneumonitis. However, Masson’s trichrome stained lung tissue from the lungs of untreated irradiated mice at 90 days postirradiation revealed small collagen-rich foci, populated with fibroblasts (Fig. 4A). Examination of lung tissue from irradiated animals pretreated with vehicle at 90 days showed that 50% of the animals also had collagen-rich foci. In contrast, no collagen-rich foci were identified in control non-irradiated animals or in irradiated mice that were pretreated with genistein at 90 days postirradiation (data not shown). The foci were localized just beneath the surface or pleura of the lung. However, neither diffuse alveolar septal thickening due to fibrosis nor other major histological abnormalities were noted, as would be expected in cases of advanced radiation-induced fibrosis.
Fig. 4.
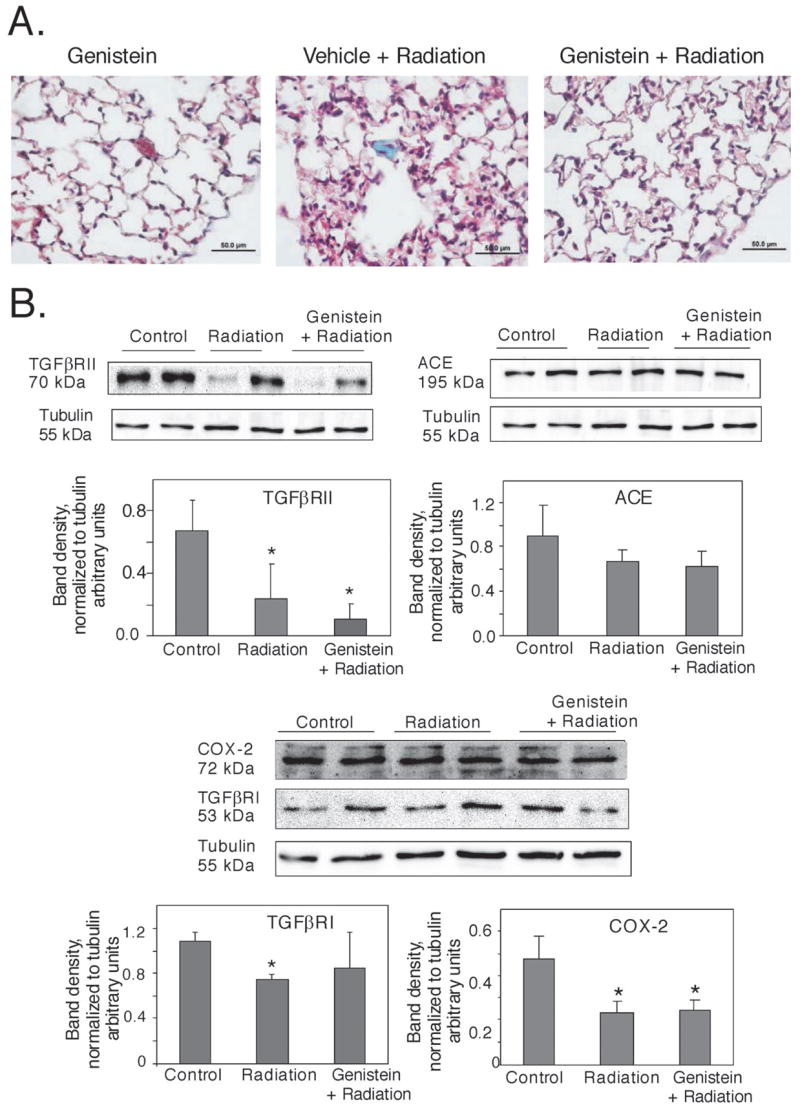
Protein expression in C57BL/6J mouse lungs at 90 days postirradiation (7.75 Gy 60Co) A). Masson’s trichrome stain of left lungs (600 × magnification). Groups: Genistein (sham irradiation), Vehicle + Radiation, Genistein + Radiation. The irradiated lung from mice pretreated with vehicle contains a small collagen-rich lesion (blue). No collagen lesions were observed in genistein (sham irradiation) or genistein + radiation-treated mice. B). Lung lysates were blotted for TGFβRII, ACE, COX-2, or TGFβRI. Densitometry of protein levels was normalized to densitometry of tubulin or actin on the same blots. Groups: Control (no treatment), Radiation (radiation alone), Genistein + Radiation. Representative data are shown. (n = 3–4/group) * p < 0.05 compared to no treatment control group.
We next examined the expression of several proteins which have been shown by others to be associated with pulmonary damage and/or fibrotic remodeling in either radiation- or bleomycin-induced lung injury: angiotensin converting enzyme (ACE),29 cyclooxygenase-2 (COX-2),30 and transforming growth factor β receptor I (TGFβR-I, Alk5),31,32 and TGFβR-II.31,32 Total cell lysates, harvested from lungs 90 days postirradiation, were analyzed by Western blotting (Fig. 4B). Protein levels were normalized by blotting for β-actin or tubulin. At day 90 postirradiation, no significant changes were observed in ACE expression, however, TGFβR-I, TGFβR-II and COX-2 expression levels were significantly reduced by radiation treatment alone (p < 0.05). In mice receiving genistein and irradiation, the expression of TGFβR-I in the lung was not significantly different from that of untreated control lungs. However, genistein did not prevent the radiation-induced alterations in TGFβR-II or COX-2 levels, indicating that genistein was not protective against these changes. Together these findings suggest that genistein can protect from some radiation-induced alterations in the lung, as detected at 90 days.
TBI 7.75 Gy does not induce observable injury to the lung at 120–180 days
Because we observed the presence of small foci and expression alterations in injury- or remodeling-associated proteins in the lungs of mice at 90 days postirradiation, we anticipated an increase in severity of the alterations at later time points, in both lung histochemistry and protein expression. However, histological examination of lungs at 120 and 180 days postirradiation did not reveal any collagen-rich foci or other gross abnormalities in all treatment goups (data not shown). H&E staining of the lungs also showed normal levels of lymphocytes in the lungs of all experimental groups at the 120–180 time points postirradiation, indicating the absence of pneumonitis at these time points. When lung protein expression levels were assayed at 120 days postirradiation, no significant changes in ACE, COX-2 or TGFβR-I protein levels were observed between the different treatment groups (Fig. 5A). However, TGFβR-II expression level remained significantly reduced in irradiated mice, with the exception of genistein pretreated, irradiated mice where TGFβR-II levels recovered to above control levels (Fig. 5A). At day 180 postirradiation, the levels of expression of all the measured proteins were similar to control levels (Fig. 5B).
Fig. 5.
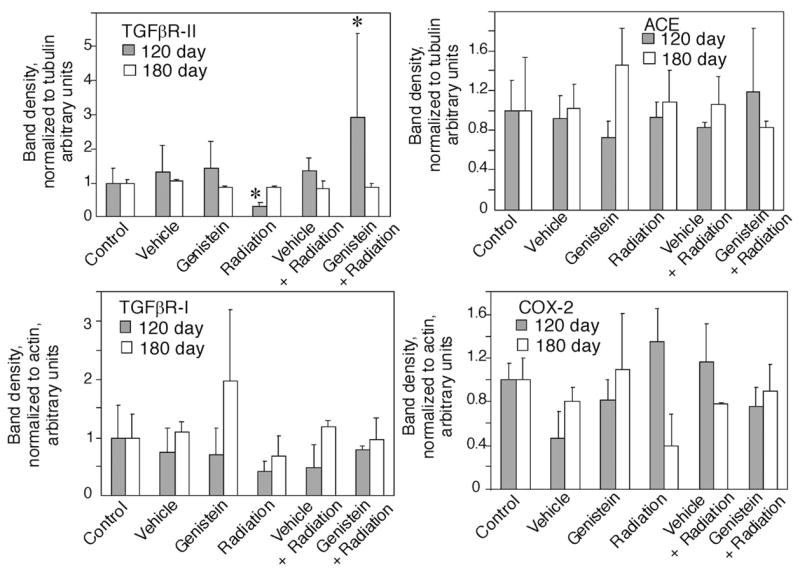
Protein expression in C57BL/6J mouse lungs 120 and 180 days postirradiation (7.75 Gy 60Co). Protein lysates were blotted for TGFβRII, ACE, COX-2, or TGFβRI. Densitometry of protein levels was normalized to densitometry of tubulin or actin on the same blots. Gray bars: 120 days postirradiation; White bars: 180 days postirradiation. Sham irradiation groups: Control (untreated), Vehicle, Genistein. Irradiation groups: Radiation (7.75 Gy 60Co alone), Vehicle + Radiation, and Genistein + Radiation. (n = 3–4). * p < 0.05 compared to untreated control group.
DISCUSSION
Radiation exposure can generate a cascade of molecular and cellular events in a variety of cell types in the lungs that lead to the activation of pro-inflammatory and pro-fibrotic processes. The level of damage to the lung has been shown to be dependent on total radiation dose, whether the dose was delivered in single or multiple fractions, the locality and amount of irradiated tissue, and whether the dose was received externally or internally (radionuclides).3 Here, we investigated the ability of genistein to protect against acute radiation injury and delayed radiation-induced damage to the lung in a total body irradiation model. The results of this study show that genistein, given as a single SC injection of 200 mg/kg body weight 24 h before irradiation, significantly reduced acute radiation-induced mortality and radiation-induced weight loss in C57BL/6J female mice. This demonstrates that the radioprotection by genistein against ARS previously observed with CD2F1 male mice is neither strain- nor gender-dependent.22,23 Although we did not observe classic radiation-induced pneumonitis or fibrosis in our TBI model, we did observe some injuries in the lung including DNA damage and transient alterations in protein expression. Our investigation of genistein protection against lung injury shows that genistein reduced radiation-induced DNA damage. The genotoxicity protection correlated with genistein protection against radiation-induced collagen deposition and some other alterations in protein expression in the lung.
The CBMN assay revealed that mice receiving TBI developed micronuclei in pulmonary fibroblasts, and that genistein reduced this radiation-induced DNA damage. The micronucleus index is a standard test used in genetic toxicology to assess chromosome damage.35 This assay is the preferred method for measuring micronuclei in cultured mammalian cells because scoring is restricted to once-divided cells, eliminating cell division kinetics which can influence micronuclei frequency. We observed an eight-fold increase in the incidence of micronuclei in ex vivo pulmonary fibroblast cultures from mice exposed to 7.75 Gy TBI, indicating the presence of radiation-induced DNA damage. Khan et al. (2003)36 reported a higher incidence of micronuclei in primary ex vivo fibroblast cultures obtained from rats 16–18 h after partial volume lung irradiation with 10 Gy 60Co compared to fibroblasts from unirradiated lungs. The relationship between radiation-induced micronuclei and lung fibrosis, however, is not clear. Our studies showed that fibroblasts from mice treated with genistein prior to irradiation had decreased micronuclei frequency, demonstrating genistein protection against radiation-induced genotoxicity in pulmonary fibroblasts.
Histological analyses of the lung tissue from irradiated and control animals at 90, 120, and 180 days did not identify pneumonitis or classic fibrotic remodeling following 7.75 Gy (60Co, 0.6 Gy/min) whole body irradiation. It is possible that pneumonitis occurred at earlier time points that were not examined in our experiments. Van der Meeren et al. (2003) examined the lung from days 10–18 following 10 Gy total body irradiation and found increased expression of genes involved in pro-inflammatory and thrombotic processes.28 Mattos et al. (2002) reported that C57BL mice exposure to 7 Gy TBI (60Co, 0.97 Gy/min) resulted in significant collagen deposition with vascular congestion and alveolar septal thickening deposition within 30 days, and significant collagen deposition after 90 days.27,37 Although we were unable to observe classic histochemical evidence of pneumonitis or fibrosis, we did identify small, focal collagen-rich deposits or plaques in the lungs at 90 days postirradiation in animals receiving vehicle or no treatment. These lesions were not present in irradiated mice pretreated with genistein. The differences observed in our study may be the result of our lower radiation dose rate, differences in the genetic background of the mice obtained from the different suppliers, or differences in collagen detection methodologies.38
In addition to examination of lung histology, we selected four proteins as biomarkers for pulmonary injury (TGFβRI, TGFβRII, COX-2 and ACE). Three of these (TGFβRI, RII and COX-2) have previously been identified as markers for lung injury in the bleomycin model system. Bleomycin, a chemotherapeutic agent, induces DNA damage as well as oxidative stress, similar mechanisms as radiation. The time course of injury, however, is compressed to 30–60 days, compared with 120–180 days for radiation injuries. Our study showed radiation-induced reductions in TGFβRI, RII and COX-2 but not in ACE. Studies by others suggest that ACE is biphasically regulated in radiation-damaged lung tissue, where initial loss is attributed to the loss of pulmonary endothelial cells and delayed increase is due to fibrotic remodeling.39,40 It is possible that ACE expression was modified prior to our first time point at 90 days postirradiation. COX-2 levels have been demonstrated to be decreased in lung cells from patients with idiopathic pulmonary fibrosis.41 Prostaglandin E2, a downstream product of COX-2, inhibits fibroblast proliferation and transdifferentiation to the myofibroblast phenotype, suggesting that reduction of COX-2 may be associated with progression of fibrosis.42 TGFβRs have also been reported to have altered expression following lung injury induced by bleomycin.31,32 Khalil et al. used in situ immunohistochemistry to identify a transient decrease of TGFβRI expression on alveolar epithelial cells in the early stages following bleomycin-induced injury in rats (day 4–7), believed to correlate with proliferation of these cells in the early repair process.31 This group found no alterations in TGFβRII. However, Zhao et al., examining overall expression patterns of the receptors, reported increased TGFβRI expression rat lungs following bleomycin treatment for the full time course of their experiments (day 3–12).32 Zhao and coworkers also reported a transient decrease TGFβRII expression during the early reparative stage. We observed a significant transient reduction in TGFβRII expression at 90 days, similar to that previously reported by Zhao.32 We also found a transient reduction in TGFβRI (Alk5 isoform), but this is the opposite of findings in the bleomycin model, where this receptor was reported to have transient increased expression.32 At 90 days postirradiation we found that genistein prevented radiation-induced reduction in the level of TGFβRI expression, but not that of TGFβRII. However, by 180 days postirradiation, all protein expression levels had recovered to control values. Future studies will examine the regulation of TGFβRs in relation to TGF ligands β1, β2 and β3, which are also expressed in lung injury and fibrosis.
In our studies, mice administered the PEG-400 excipient also exhibited an enhancement in 30-day survival (53%) in comparison to the non-treated irradiated mice (23%) animals. The radioprotective properties observed for PEG-400 have previously been demonstrated22,43 and are similar to the protective effects of other excipients such as Emulphor,44 dimethyl sulfoxide,45 and the polyethoxylated castor oil vehicle Cremophor.46 The modest radioprotective activity of these excipients is believed to be mediated through modulation of the immune system.
Our laboratory has shown that in vivo genistein prevents acute injuries from radiation.22,47 However, a number of studies have demonstrated in vivo and in vitro that genistein may act as a radiosensitizing agent. Genistein increases radiation-induced apoptosis in several cancer cell lines, including human prostate carcinoma cells, leukemic cells, and cervical cancer cells.48–50 In orthotopic prostate tumor transplants in mice, genistein combined with radiation treatment had greater inhibitory effects on prostate tumor growth than radiation alone.51 The differential effect of genistein on cancer cells compared with normal cells may be related to the arrest of cancer cells in the G2/M phases of the cell cycle.52–54 The G2/M arrest of cancer cells by genistein has been the topic of a number of studies that have identified both the inactivation of nuclear factor-kappa B and the stable activation of ERK1/2 mitogen activated protein kinases as potential mechanisms.52–54 Unlike the G1/G0 and S phases of the cell cycle which have active DNA repair mechanisms, the G2/M phase of the cell is not associated with DNA repair activity.55 The activity of genistein in sensitizing cells to DNA damaging agents has been shown in cell culture studies to be selective toward cancer cells and not toward normal cells.56 The mechanism(s) by which genistein selectively causes radiosensitization versus radioprotection has not been elicidated.
The relative sensitivity of the lung to radiation-induced damage is hypothesized to be due to the lung’s high oxygen content compared with other organs. While primary radiation injury results in direct damage to cells, secondary effects can arise from the ionization of oxygen, which can form radicals that initiate and propagate chain reactions.57,58 Pneumonitis and pulmonary fibrosis in both humans and animal models are dependent upon the total radiation dose, fraction size, and volume of lung exposure.8,59,60 Although anti-inflammatory treatments (steroidal and non-steroidal) are effective in treating inflammation associated with early radiation-induced pneumonitis, they do not prevent the subsequent fibrosis.61,62 Using TBI in C57BL/6J mice, our results indicate that genistein protects against acute radiation-induced mortality, radiation-induced DNA damage in lung fibroblasts, and some transient radiation-induced alterations in protein expression in the lung. Genistein has a variety of biological properties that may reduce lung injury. These include its anti-inflammatory properties,17,18 antioxidant capacity,15,16 and effects on the cell cycle.52,63 Further experiments are required to establish the mechanism(s) responsible for the radioprotective effects of genistein. Additional studies using high dose thoracic radiation will determine whether administration of genistein can prevent radiation-induced pneumonitis and/or fibrotic remodeling in the lung.
Acknowledgments
We would like to thank Mr. Travis R. Crooks, Ms. Autumn J. Griffin, Ms. GuiFang Chen, and Ms. Shwetha Manoharan for technical support on this project. We thank Dr. Joseph F. Weiss for critically reading an earlier version of this manuscript. Portions of this work were presented at the 2006 American Lung Association/American Thoracic Association International Conference (Proc Amer Thoracic Soc A187). This work was supported in part by National Institutes of Health grant HL73929 (to RMD), USUHS Research Grant (to RMD), and DTRA grant H.10025_07_US_R (to RMD and MRL). Some of the authors are employees of the U.S. Government. This work was prepared as part of their official duties. Title 17 U.S.C. §105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.’ Title 17 U.S.C §101 defined a U.S. Government work as a work prepared by a military service member or employees of the U.S. Government as part of that person’s official duties. The views in this article are those of the authors and do not necessarily reflect the views, official policy or position of the Uniformed Services University of the Health Sciences, the Armed Forces Radiobiology Research Institute, Department of the Navy, Department of Defense or the U.S. Federal Government.
References
- 1.Waselenko JK, MacVittie TJ, Blakely WF, Pesik N, Wiley AL, Dickerson WE, Tsu H, Confer DL, Coleman CN, Seed T, Lowry P, Armitage JO, Dainiak N. Medical management of the acute radiation syndrome: Recommendations of the strategic national stockpile radiation working group. Ann Intern Med. 2004;140:1037–1051. doi: 10.7326/0003-4819-140-12-200406150-00015. [DOI] [PubMed] [Google Scholar]
- 2.Moulder JE. Post-irradiation approaches to treatment of radiation injuries in the context of radiological terrorism and radiation accidents: A review. Int J Radiat Biol. 2004;80:3–10. doi: 10.1080/09553000310001642920. [DOI] [PubMed] [Google Scholar]
- 3.Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. Lipincott Williams and Williams; New York, NY: 2005. [Google Scholar]
- 4.Stone HB, Moulder JE, Coleman CN, Ang KK, Anscher MS, Barcellos-Hoff MH, Dynan WS, Fike JR, Grdina DJ, Greenberger JS, Hauer-Jensen M, Hill RP, Kolesnick RN, Macvittie TJ, Marks C, McBride WH, Metting N, Pellmar T, Purucker M, Robbins ME, Schiestl RH, Seed TM, Tomaszewski JE, Travis EL, Wallner PE, Wolpert M, Zaharevitz D. Models for evaluating agents intended for the prophylaxis, mitigation and treatment of radiation injuries report of an NCI workshop, December 3–4, 2003. Radiat Res. 2004;162:711–728. doi: 10.1667/rr3276. [DOI] [PubMed] [Google Scholar]
- 5.Pellmar T, Rockwell S Group tRNTCW. Meeting report: Priority list of research areas for radiological nuclear threat countermeasures. Radiat Res. 2005;163:115–123. doi: 10.1667/rr3283. [DOI] [PubMed] [Google Scholar]
- 6.Seed TM. Radiation protectants: Current status and future prospects. Health Phys. 2005;89:531–545. doi: 10.1097/01.hp.0000175153.19745.25. [DOI] [PubMed] [Google Scholar]
- 7.Weiss JF, Landauer MR. Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology. 2003;189:1–20. doi: 10.1016/s0300-483x(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 8.Hill RP. Radiation effects on the respiratory system. B J R Suppl. 2005;27:75–81. [Google Scholar]
- 9.Baranov AE, Selidovkin GD, Butturini A, Gale RP. Hematopoietic recovery after 10-Gy acute total body radiation. Blood. 1994;83:596–599. [PubMed] [Google Scholar]
- 10.Crawford SW. Diagnosis and management of pulmonary problems associated with radiation injury. The Medical Basis for Radiation-Accident Preparedness. In: Ricks RC, Berger ME, O’Hara FM, editors. The Clinical Care of Victims. The Parthenon Publishing Group; Boca Ratan: 2002. pp. 131–138. [Google Scholar]
- 11.Morgan GW, Breit SN. Radiation and the lung: A reevaluation of the mechanisms mediating pulmonary injury. Int J Radiat Oncol Biol Phys. 1995;31:361–369. doi: 10.1016/0360-3016(94)00477-3. [DOI] [PubMed] [Google Scholar]
- 12.Ishii T, Futami S, Nishida M, Suzuki T, Sakamoto T, Suzuki N, Maekawa K. Brief note and evaluation of acute-radiation syndrome and treatment of a Tokai-mura criticality accident patient. J Radiat Res (Tokyo) 2001;42(Suppl):S167–S182. doi: 10.1269/jrr.42.s167. [DOI] [PubMed] [Google Scholar]
- 13.Uozaki H, Fukayama M, Nakagawa K, Ishikawa T, Misawa S, Doi M, Maekawa K. The pathology of multi-organ involvement: Two autopsy cases from the Tokai-mura criticality accident. B J R Suppl. 2005;27:13–16. [Google Scholar]
- 14.Carruthers SA, Wallington MM. Total body irradiation and pneumonitis risk: A review of outcomes. Br J Cancer. 2004;90:2080–2084. doi: 10.1038/sj.bjc.6601751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kruk I, Aboul-Enein HY, Michalska T, Lichszteld K, Kladna A. Scavenging of reactive oxygen species by the plant phenols genistein and oleuropein. Luminescence. 2005;20:81–89. doi: 10.1002/bio.808. [DOI] [PubMed] [Google Scholar]
- 16.Zhang P, Li HF, Tian ZF, Qiu XQ, Wu JX, Jia ZJ. Effects of phytoestrogens and 17beta-estradiol on vasoconstriction elicited by reactive oxygen species. Pharmazie. 2007;62:378–381. [PubMed] [Google Scholar]
- 17.Verdrengh M, Jonsson IM, Holmdahl R, Tarkowski A. Genistein as an anti-inflammatory agent. Inflamm Res. 2003;52:341–346. doi: 10.1007/s00011-003-1182-8. [DOI] [PubMed] [Google Scholar]
- 18.Marotta F, Mao GS, Liu T, Chui DH, Lorenzetti A, Xiao Y, Marandola P. Anti-inflammatory and neuroprotective effect of a phytoestrogen compound on rat microglia. Ann NY Acad Sci. 2006;1089:276–281. doi: 10.1196/annals.1386.033. [DOI] [PubMed] [Google Scholar]
- 19.Hong H, Landauer MR, Foriska MA, Ledney GD. Antibacterial activity of the soy isoflavone genistein. J Basic Microbiol. 2006;46:329–335. doi: 10.1002/jobm.200510073. [DOI] [PubMed] [Google Scholar]
- 20.Landauer MR, Srinivasan SV, Shapiro A, Takimoto C, Seed TM. Protection against lethal irradiation by genistein. Int J Toxicol. 2000;19:37. [Google Scholar]
- 21.Zhou Y, Mi MT. Genistein stimulates hematopoiesis and increases survival in irradiated mice. J Radiat Res (Tokyo) 2005;46:425–433. doi: 10.1269/jrr.46.425. [DOI] [PubMed] [Google Scholar]
- 22.Landauer M, Srinivasan V, Seed T. Genistein treatment protects mice from ionizing radiation injury. J Appl Toxicol. 2003;23:379–385. doi: 10.1002/jat.904. [DOI] [PubMed] [Google Scholar]
- 23.Davis TA, Clarke TK, Mog SR, Landauer MR. Subcutaneous administration of genistein prior to lethal irradiation supports multilineage, hematopoietic progenitor cell recovery and survival. Int J Radiat Biol. 2007;83:141–151. doi: 10.1080/09553000601132642. [DOI] [PubMed] [Google Scholar]
- 24.O’Brien TJ, Letuve S, Haston CK. Radiation-induced strain differences in mouse alveolar inflammatory cell apoptosis. Can J Physiol Pharmacol. 2005;83:117–122. doi: 10.1139/y05-005. [DOI] [PubMed] [Google Scholar]
- 25.Epperly MW, Travis EL, Sikora C, Greenberger JS. Manganese [correction of magnesium] superoxide dismutase (MNSOD) plasmid/liposome pulmonary radioprotective gene therapy: Modulation of irradiation-induced mRNA for IL-1, TNF-alpha, and TGF-beta correlates with delay of organizing alveolitis/fibrosis. Biol Blood Marrow Transplant. 1999;5:204–214. doi: 10.1053/bbmt.1999.v5.pm10465100. [DOI] [PubMed] [Google Scholar]
- 26.Fliedner TM, Graessle D, Meineke V, Dorr H. Pathophysiological principles underlying the blood cell concentration responses used to assess the severity of effect after accidental whole-body radiation exposure: An essential basis for an evidence-based clinical triage. Exp Hematol. 2007;35:8–16. doi: 10.1016/j.exphem.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Mattos M, Kimura E, Silva M, Egami M, Segreto R, Segreto H. Latent TGFbeta1 activation in the lung irradiated in vivo. Rev Assoc Med Bras. 2002;48:329–334. doi: 10.1590/s0104-42302002000400039. [DOI] [PubMed] [Google Scholar]
- 28.Van der Meeren A, Vandamme M, Squiban C, Gaugler MH, Mouthon MA. Inflammatory reaction and changes in expression of coagulation proteins on lung endothelial cells after total-body irradiation in mice. Radiat Res. 2003;160:637–646. doi: 10.1667/rr3087. [DOI] [PubMed] [Google Scholar]
- 29.Ward WF, Solliday NH, Molteni A, Port CD. Radiation injury in rat lung. II Angiotensin-converting enzyme activity. Radiat Res. 1983;96:294–300. [PubMed] [Google Scholar]
- 30.Ogushi F, Endo T, Tani K, Asada K, Kawano T, Tada H, Maniwa K, Sone S. Decreased prostaglandin E2 synthesis by lung fibroblasts isolated from rats with bleomycin-induced lung fibrosis. Int J Exp Pathol. 1999;80:41–49. doi: 10.1046/j.1365-2613.1999.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khalil N, Parekh TV, O’Connor RN, Gold LI. Differential expression of transforming growth factor-beta type I and II receptors by pulmonary cells in bleomycin-induced lung injury: Correlation with repair and fibrosis. Exp Lung Res. 2002;28:233–250. doi: 10.1080/019021402753570527. [DOI] [PubMed] [Google Scholar]
- 32.Zhao Y, Shah DU. Expression of transforming growth factor-beta type I and type II receptors is altered in rat lungs undergoing bleomycin-induced pulmonary fibrosis. Exp Mol Pathol. 2000;69:67–78. doi: 10.1006/exmp.2000.2319. [DOI] [PubMed] [Google Scholar]
- 33.McPherson C. Reduction of Psuedomonas aeruginosa and coliform bacteria in mouse drinking water following treatment with hyrdochloric acid and chlorine. Lab Animal Care. 1963;13:737–744. [PubMed] [Google Scholar]
- 34.Kirsch-Volders M, Fenech M. Inclusion of micronuclei in non-divided mononuclear lymphocytes and necrosis/apoptosis may provide a more comprehensive cytokinesis block micronucleus assay for biomonitoring purposes. Mutagenesis. 2001;16:51–58. doi: 10.1093/mutage/16.1.51. [DOI] [PubMed] [Google Scholar]
- 35.Fenech M. Cytokinesis-block micronucleus cytome assay. Nat Protoc. 2007;2:1084–1104. doi: 10.1038/nprot.2007.77. [DOI] [PubMed] [Google Scholar]
- 36.Khan MA, Van Dyk J, Yeung IW, Hill RP. Partial volume rat lung irradiation; assessment of early DNA damage in different lung regions and effect of radical scavengers. Radiother Oncol. 2003;66:95–102. doi: 10.1016/s0167-8140(02)00325-0. [DOI] [PubMed] [Google Scholar]
- 37.Olejar T, Zadinova M, Hlouskova D, Pouckova P. Production of TGF-beta1 in lungs after low-dose whole-body radiation exposure in fibrosing (C57BL/6) and non-fibrosing (C3H/J) mouse strain. Folia Biol (Praha) 2001;47:55–61. [PubMed] [Google Scholar]
- 38.Taft RA, Davisson M, Wiles MV. Know thy mouse. TRENDS in Genetics. 2006;22:649–653. doi: 10.1016/j.tig.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 39.Movas B, Raffin TA, Epstein AH, Link CJ. Pulmonary radiation injury. Chest. 1997;111:1061–1076. doi: 10.1378/chest.111.4.1061. [DOI] [PubMed] [Google Scholar]
- 40.Molteni A, Moulder J, Cohen E, Ward W, Fish B, Taylor J, Wolfe L, Brizio-Molteni L, Veno P. Control of radiation-induced pneumopathy and lung fibrosis by angiotensin-converting enzyme inhibitors and an angiotensin II type 1 receptor blocker. Int J Radiat Biol. 2000;76:523–532. doi: 10.1080/095530000138538. [DOI] [PubMed] [Google Scholar]
- 41.Wilborn J, Crofford LJ, Burdick MD, Kunkel SL, Strieter RM, Peters-Golden M. Cultured lung fibroblasts isolated from patients with idiopathic pulmonary fibrosis have a diminished capacity to synthesize prostaglandin E2 and to express cyclooxygenase-2. J Clin Invest. 1995;95:1861–1868. doi: 10.1172/JCI117866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lovgren AK, Jania LA, Hartney JM, Parsons KK, Audoly LP, Fitzgerald GA, Tilley SL, Koller BH. COX-2-derived prostacyclin protects against bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2006;291:L144–156. doi: 10.1152/ajplung.00492.2005. [DOI] [PubMed] [Google Scholar]
- 43.Shaeffer J, Schellenberg KA, Seymore CH, Schultheiss TE, el-Mahdi AM. Radioprotective effect of polyethylene glycol. Radiat Res. 1986;107:125–135. [PubMed] [Google Scholar]
- 44.Landauer MR, Castro CA, Benson KA, Hogan JB, Weiss JF. Radioprotective and locomotor responses of mice treated with nimodipine alone and in combination with WR-151327. J Appl Toxicol. 2001;21:25–31. doi: 10.1002/jat.728. [DOI] [PubMed] [Google Scholar]
- 45.Moos WS, LeVan H, Mason HC. Radioprotective effects of dimethyl sulfoxide vapor on mice. Experientia. 1967;23:923. doi: 10.1007/BF02136222. [DOI] [PubMed] [Google Scholar]
- 46.Bertoncello I, Kriegler AB, Woodcock DM, Williams B, Barber L, Nilsson SK. Haematopoietic radioprotection by cremophorel: A polyethoxylated castor oil. Int J Radiat Biol. 1995;67:57–64. doi: 10.1080/09553009514550071. [DOI] [PubMed] [Google Scholar]
- 47.Landauer M, Srinivasan S, Wang P, Wang M, Golubcow-Teglasi J, Smith C, Seed T. Behavioral and radioprotective effects of acute subcutaneous administration of genistein. The Toxicologist. 2001;60:116. [Google Scholar]
- 48.Hillman GG, Forman JD, Kucuk O, Yudelev M, Maughan RL, Rubio J, Layer A, Tekyi-Mensah S, Abrams J, Sarkar FH. Genistein potentiates the radiation effect on prostate carcinoma cells. Clin Cancer Res. 2001;7:382–390. [PubMed] [Google Scholar]
- 49.Yashar CM, Spanos WJ, Taylor DD, Gercel-Taylor C. Potentiation of the radiation effect with genistein in cervical cancer cells. Gyn Oncol. 2005;99:199–205. doi: 10.1016/j.ygyno.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 50.Papazisis KT, Zambouli D, Kimoundri OT, Papadakis ES, Vala V, Geromichalos GD, Voyatzi S, Markala D, Destouni E, Boutis L, Kortsaris AH. Protein tyrosine kinase inhibitor, genistein, enhances apoptosis and cell cycle arrest in K562 cells treated with gamma-irradiation. Cancer Lett. 2000;160:107–113. doi: 10.1016/s0304-3835(00)00569-3. [DOI] [PubMed] [Google Scholar]
- 51.Hillman GG, Wang Y, Che M, Raffoul JJ, Yudelev M, Kucuk O, Sarkar FH. Progression of renal cell carcinoma is inhibited by genistein and radiation in an orthotopic model. B M C Cancer. 2007;7:4. doi: 10.1186/1471-2407-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raffoul JJ, Wang Y, Kucuk O, Forman JD, Sarkar FH, Hillman GG. Genistein inhibits radiation-induced activation of NF-kappaB in prostate cancer cells promoting apoptosis and G2/M cell cycle arrest. B M C Cancer. 2006;6:107. doi: 10.1186/1471-2407-6-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mohammad RM, Al-Katib A, Aboukameel A, Doerge DR, Sarkar F, Kucuk O. Genistein sensitizes diffuse large cell lymphoma to chop (cyclophosphamide, doxorubicin, vincristine, prednisone) chemotherapy. Mol Cancer Ther. 2003;2:1361–1368. [PubMed] [Google Scholar]
- 54.Li Z, Li J, Mo B, Hu C, Liu H, Qi H, Wang X, Xu J. Genistein induces G(2)/M cell cycle arrest via stable activation of ERK1/2 pathway in MDA-MB-231 breast cancer cells. Cell Biol Toxicol. 2008 Jan. 26;2008 doi: 10.1007/s10565-008-9054-1. Epub; in press. [DOI] [PubMed] [Google Scholar]
- 55.Tamulevicius P, Wang M, Iliakis G. Homology-directed repair is required for the development of radioresistance during s phase: Interplay between double-strand break repair and checkpoint response. Radiat Res. 2007;167:1–11. doi: 10.1667/RR0751.1. [DOI] [PubMed] [Google Scholar]
- 56.Lee R, Kim YJ, Lee YJ, Chung HW. The selective effect of genistein on the toxicity of bleomycin in normal lymphocytes and HL-60 cells. Toxicology. 2004;195:87–95. doi: 10.1016/j.tox.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 57.Riley PA. Free radicals in biology: Oxidative stress and the effects of ionizing radiation. Int J Radiat Biol. 1994;65:27–33. doi: 10.1080/09553009414550041. [DOI] [PubMed] [Google Scholar]
- 58.Augustine AD, Gondre-Lewis T, McBride W, Miller L, Pellmar TC, Rockwell S. Animal models for radiation injury, protection and therapy. Radiat Res. 2005;164:100–109. doi: 10.1667/rr3388. [DOI] [PubMed] [Google Scholar]
- 59.Rosen II, Fischer TA, Antolak JA, Starkschall G, Travis EL, Tucker SL, Hogstrom KR, Cox JD, Komaki R. Correlation between lung fibrosis and radiation therapy dose after concurrent radiation therapy and chemotherapy for limited small cell lung cancer. Radiology. 2001;221:614–622. doi: 10.1148/radiol.2213992043. [DOI] [PubMed] [Google Scholar]
- 60.Abid SH, Malhotra V, Perry MC. Radiation-induced and chemotherapy-induced pulmonary injury. Curr Opin Oncol. 2001;13:242–248. doi: 10.1097/00001622-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 61.Hong JH, Jung SM, Tsao TC, Wu CJ, Lee CY, Chen FH, Hsu CH, McBride WH, Chiang CS. Bronchoalveolar lavage and interstitial cells have different roles in radiation-induced lung injury. Int J Radiat Biol. 2003;79:159–167. doi: 10.1080/0955300031000076894. [DOI] [PubMed] [Google Scholar]
- 62.Johnston CJ, Williams JP, Elder A, Hernady E, Finkelstein JN. Inflammatory cell recruitment following thoracic irradiation. Exp Lung Res. 2004;30:369–382. doi: 10.1080/01902140490438915. [DOI] [PubMed] [Google Scholar]
- 63.Yu Z, Li W, Liu F. Inhibition of proliferation and induction of apoptosis by genistein in colon cancer HT-29 cells. Cancer Lett. 2004;215:159–166. doi: 10.1016/j.canlet.2004.06.010. [DOI] [PubMed] [Google Scholar]


