Abstract
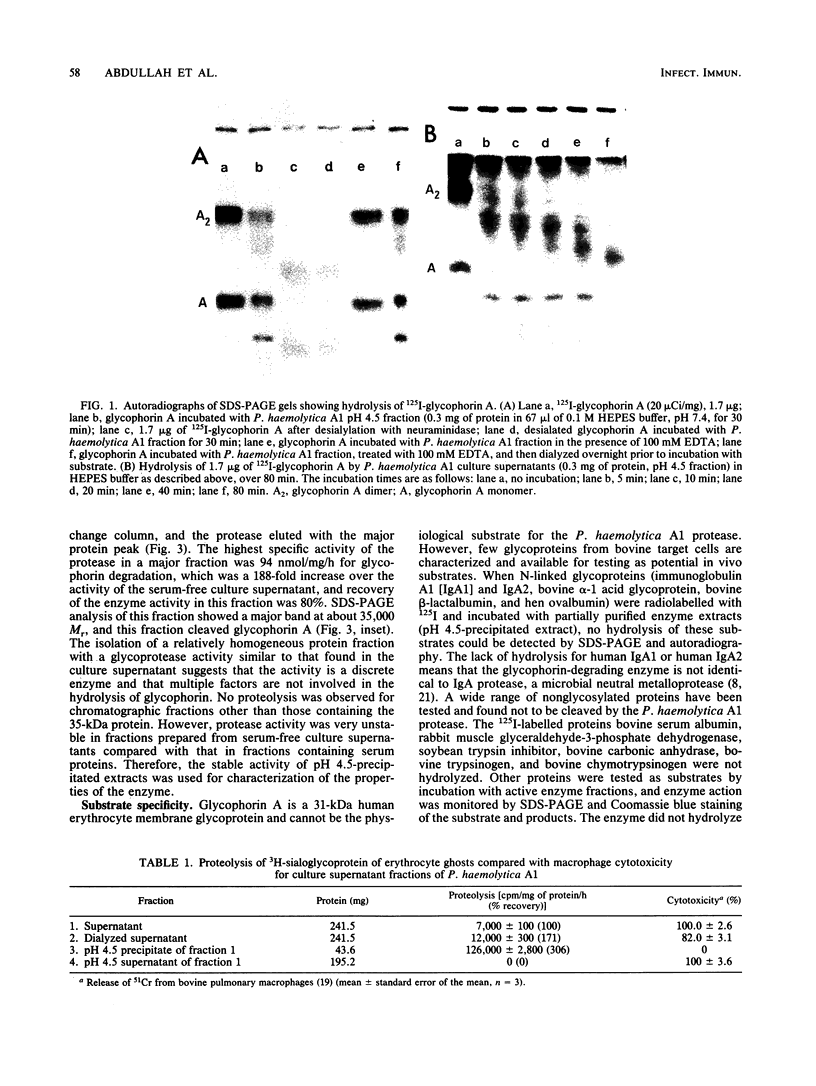
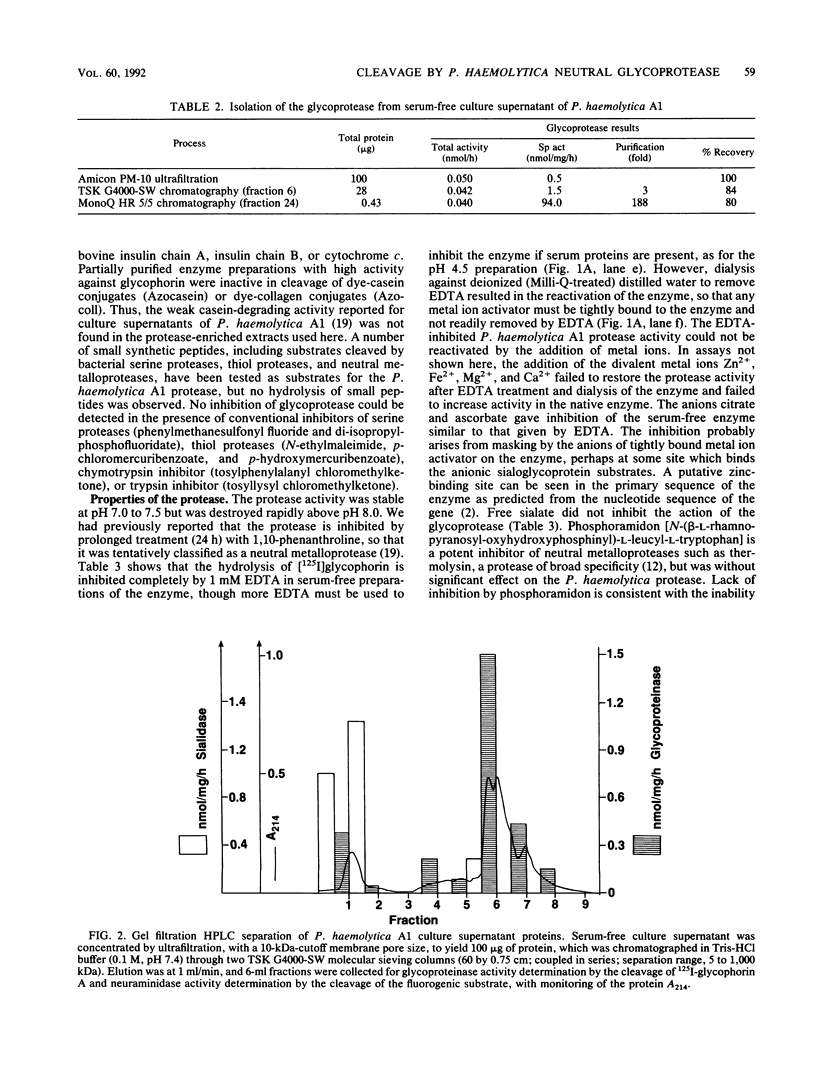
A neutral metalloprotease with marked specificity for an O-sialoglycoprotein has been isolated from culture supernatants of Pasteurella haemolytica A1. The 35-kDa enzyme cleaves human erythrocyte glycophorin A, which is O glycosylated, but does not cleave N-glycosylated proteins or nonglycosylated proteins. Glycophorin A was cleaved when it was present in situ in erythrocyte ghost plasma membranes or when it was free in solution. The glycoprotease did not hydrolyze glycophorin A from which sialate residues had been removed by neuraminidase treatment. An immobilized preparation of the enzyme cleaved glycophorin A at several positions, with a major site of cleavage at Arg-31-Asp-32. The glycoprotease is inhibited by EDTA, citrate, and ascorbate, but inhibition appears to be due to the masking of metal ion activators rather than to their removal. The enzyme is not inhibited by phosphoramidon, an inhibitor of other bacterial neutral metalloproteases.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdullah K. M., Lo R. Y., Mellors A. Cloning, nucleotide sequence, and expression of the Pasteurella haemolytica A1 glycoprotease gene. J Bacteriol. 1991 Sep;173(18):5597–5603. doi: 10.1128/jb.173.18.5597-5603.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdullah K. M., Lo R. Y., Mellors A. Distribution of glycoprotease activity and the glycoprotease gene among serotypes of Pasteurella haemolytica. Biochem Soc Trans. 1990 Oct;18(5):901–903. doi: 10.1042/bst0180901. [DOI] [PubMed] [Google Scholar]
- Blumenfeld O. O., Gallop P. M., Liao T. H. Modification and introduction of a specific radioactive label into the erythrocyte membrane sialoglycoproteins. Biochem Biophys Res Commun. 1972 Jul 11;48(1):242–251. doi: 10.1016/0006-291x(72)90369-5. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Carlsson S. R. Leukosialin, a major sialoglycoprotein on human leukocytes as differentiation antigens. Med Biol. 1986;64(6):335–343. [PubMed] [Google Scholar]
- Furthmayr H. Structural comparison of glycophorins and immunochemical analysis of genetic variants. Nature. 1978 Feb 9;271(5645):519–524. doi: 10.1038/271519a0. [DOI] [PubMed] [Google Scholar]
- Jentoft N. Why are proteins O-glycosylated? Trends Biochem Sci. 1990 Aug;15(8):291–294. doi: 10.1016/0968-0004(90)90014-3. [DOI] [PubMed] [Google Scholar]
- Labib R. S., Calvanico N. J., Tomasi T. B., Jr Studies on extracellular proteases of Streptococcus sanguis. Purification and characterization of a human IgA1 specific protease. Biochim Biophys Acta. 1978 Oct 12;526(2):547–559. doi: 10.1016/0005-2744(78)90145-6. [DOI] [PubMed] [Google Scholar]
- Lo R. Y., Shewen P. E., Strathdee C. A., Greer C. N. Cloning and expression of the leukotoxin gene of Pasteurella haemolytica A1 in Escherichia coli K-12. Infect Immun. 1985 Dec;50(3):667–671. doi: 10.1128/iai.50.3.667-671.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo R. Y., Strathdee C. A., Shewen P. E. Nucleotide sequence of the leukotoxin genes of Pasteurella haemolytica A1. Infect Immun. 1987 Sep;55(9):1987–1996. doi: 10.1128/iai.55.9.1987-1996.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfroy B., Schwartz J. C. Comparison of dipeptidyl carboxypeptidase and endopeptidase activities in the three enkephalin-hydrolysing metallopeptidases: "angiotensin-converting enzyme", thermolysin and "enkephalinase". Biochem Biophys Res Commun. 1985 Jul 16;130(1):372–378. doi: 10.1016/0006-291x(85)90427-9. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Andrews E. P. Glycoproteins: isolation from cellmembranes with lithium diiodosalicylate. Science. 1971 Dec 17;174(4015):1247–1248. doi: 10.1126/science.174.4015.1247. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Furthmayr H., Tomita M. The red cell membrane. Annu Rev Biochem. 1976;45:667–698. doi: 10.1146/annurev.bi.45.070176.003315. [DOI] [PubMed] [Google Scholar]
- Markham R. J., Wilkie B. N. Interaction between Pasteurella haemolytica and bovine alveolar macrophages: cytotoxic effect on macrophages and impaired phagocytosis. Am J Vet Res. 1980 Jan;41(1):18–22. [PubMed] [Google Scholar]
- Martin S. W., Meek A. H., Davis D. G., Thomson R. G., Johnson J. A., Lopez A., Stephens L., Curtis R. A., Prescott J. F., Rosendal S. Factors associated with mortality in feedlot cattle: the Bruce County Beef Cattle Project. Can J Comp Med. 1980 Jan;44(1):1–10. [PMC free article] [PubMed] [Google Scholar]
- Otulakowski G. L., Shewen P. E., Udoh A. E., Mellors A., Wilkie B. N. Proteolysis of sialoglycoprotein by Pasteurella haemolytica cytotoxic culture supernatant. Infect Immun. 1983 Oct;42(1):64–70. doi: 10.1128/iai.42.1.64-70.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Plaut A. G. The IgA1 proteases of pathogenic bacteria. Annu Rev Microbiol. 1983;37:603–622. doi: 10.1146/annurev.mi.37.100183.003131. [DOI] [PubMed] [Google Scholar]
- Remold-O'Donnell E., Davis A. E., 3rd, Kenney D., Bhaskar K. R., Rosen F. S. Purification and chemical composition of gpL115, the human lymphocyte surface sialoglycoprotein that is defective in Wiskott-Aldrich syndrome. J Biol Chem. 1986 Jun 5;261(16):7526–7530. [PubMed] [Google Scholar]
- Segrest J. P., Kahane I., Jackson R. L., Marchesi V. T. Major glycoprotein of the human erythrocyte membrane: evidence for an amphipathic molecular structure. Arch Biochem Biophys. 1973 Mar;155(1):167–183. doi: 10.1016/s0003-9861(73)80019-0. [DOI] [PubMed] [Google Scholar]
- Tomita M., Furthmayr H., Marchesi V. T. Primary structure of human erythrocyte glycophorin A. Isolation and characterization of peptides and complete amino acid sequence. Biochemistry. 1978 Oct 31;17(22):4756–4770. doi: 10.1021/bi00615a025. [DOI] [PubMed] [Google Scholar]
- Tomita M., Marchesi V. T. Amino-acid sequence and oligosaccharide attachment sites of human erythrocyte glycophorin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2964–2968. doi: 10.1073/pnas.72.8.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]