Abstract
Hepatitis E virus (HEV) is a single-stranded RNA virus that causes large-scale epidemics of acute viral hepatitis, particularly in developing countries. In men and non pregnant women, the disease is usually self-limited and has a case-fatality rate of less than <0.1%. However in pregnant women particularly from certain geographic areas in India, HEV infection is more severe, often leading to fulminant hepatic failure and death in a significant proportion of patients. In contrast, reports from Egypt, Europe and the USA have shown that the course and severity of viral hepatitis during pregnancy is not different from that in non-pregnant women. The reasons for this geographical difference are not clear. The high mortality rate in pregnancy has been thought to be secondary to the associated hormonal (estrogen and progesterone) changes during pregnancy and consequent immunological changes. These immunological changes include down regulation of p65 component of NF κB with a predominant Th2 bias in the T-cell response along with host susceptibility factors, mediated by HLA expression. Thus far, researchers were unable to explain the high HEV morbidity in pregnancy, why it is different from other hepatitis viruses such as hepatitis A with similar epidemiological features, and the reason behind the difference in HEV morbidity in pregnant women in different geographical regions. The recent developments in understanding the immune response to HEV have encouraged us to review possible mechanisms for these differences. Further research in the immunology of HEV and pregnancy is required to conquer this disease in the near future.
Keywords: hepatitis E, pregnancy, fulminant liver failure, immunology, hormonal
Introduction
Enterically transmitted hepatitis E virus (HEV) infection is the most frequent cause of acute viral hepatitis (AVH) in developing countries 1. The disease was first recognized in the Indian subcontinent in the 1950s. Initially thought of as Hepatitis A infection, 2it took almost 30 years to recognize it as a different virus when the sera from persons during two water borne epidemics in India were negative for Hepatitis A and B 3, 4. As research progressed in HEV infection, HEV genome was isolated and the enzyme-linked immunosorbent (ELISA) and polymerase chain reaction (PCR) assays to the HEV became available over the last decade. 5
In the industrialized countries, hepatitis E is considered as an emerging disease of global importance and has been reported in a number of developed countries. A recent survey of acute hepatitis E cases in France highlighted that hepatitis E clearly is an emerging disease in France as in other developed countries.6 Also, a recent study from France,7 has shown that post transplant patients who are immunocompromised progressed to chronic HEV infection.
In addition to this peculiar trait of progressing to chronic hepatitis E in immunocompromised patients, HEV has an interesting course in pregnant women in certain geographical regions of the world. Studies from various developing countries have shown that the incidence of HEV infection in pregnancy is high and a significant proportion of pregnant women can progress to fulminant hepatitis with a mortality rate varying from 30−100%. 8-16The mechanism of severe liver injury in pregnant women with Hepatitis E remains a mystery. In this review article, we would initially review the normal hormonal and immunological changes occurring during gestation and later discuss in depth the interaction of hepatitis E and pregnancy and the potential mechanisms for its high mortality.
We searched MEDLINE from 1980 to the present using the Medical Subject Headings terms Hepatitis E, Immunological changes and pregnancy, Hormones and pregnancy, Hepatitis E and pregnancy, Hepatitis E in pregnancy and mortality. Important clinical guidelines, large case series from centers of excellence, consensus conference and our own research developments form the basis of this review article.
Before going into the interaction of HEV and pregnancy, we would discuss some unique features of hepatitis E infection that may play a role in pathogenesis.
Molecular virology of HEV
HEV, a single stranded RNA virus was first described in 1983 as spherical, 27−30-nm virus-like particles. Analysis of its RNA helicase and RNA-dependent RNA polymerase regions show HEV forms a phylogenetically distinct group that was recently placed into a separate genus, Hepevirus.1
Characterization of HEV genomes from geographically distinct locations has identified at least four major genotypes that may differ up to 20% at the nucleotide level. 17While these are diverse in all three ORF regions, they are serologically indistinguishable and cross-reactive.
Genotype 1 includes isolates from Asia, the Middle East, and North Africa. Genotype 2 has been found in Mexico and Nigeria. Genotype 3 was recovered from swine in North America, Europe, Egypt, Asia and New Zealand and from humans in North and South America, Europe, Japan and China. Genotype 4 was found in humans and swine in Asia.18
HEV genotypes are also important as they correlate with the severity of infection. 19, 20 Accumulating evidence suggests that genotypes 3 and 4 are less pathogenic in humans, while genotype 1 isolated has been shown to be more pathogenic. This explains the high severity of infection in India where genotype 1 is the commonest subtype in comparison to US, where genotype 3 is the commonest type seen.
Epidemiology
HEV is classically transmitted feco-orally, although person-to-person transmission has also been reported 21. HEV has been occasionally linked to nosocomial spread22. Vertical transmission from mother to infant is also known to occur23. It is infrequently transmitted by transfusion of blood or blood products24-25.
Hepatitis E can occur either in large epidemics2, 4, 21, or in the form of sporadic cases. Although hepatitis E infection is endemic in Southeast and Central Asian countries, outbreaks have also been reported from several parts of the Middle East, Africa and Mexico.
The outbreaks of Hepatitis E are large and the overall attack rates ranges from 1 to 15%, varying from 3−30% in adults to 0.2−10% in children 4, 21, 26-27. Children have a high rate of sub clinical infection. In the US and Western Europe, less than 1% of patients of acute viral hepatitis have hepatitis E as the etiology of their infection and was thought to be associated with their travel to HEV-endemic regions28-29. However the recent paper from France highlighting that 90% of acute hepatitis E patients acquired by the indigenous route by contaminated water supplies and uncooked shellfish may change this current perception of hepatitis E epidemiology. 6
Clinical presentation of Hepatitis E in pregnancy
The relationship between Hepatitis E and pregnancy is quite interesting. Hepatitis E has both a high incidence and severe course in pregnant women in some geographic regions of HEV endemic countries, such as Northern India, 12-13while in other HEV endemic countries, such as Egypt, it has been shown to have a benign course with little or no morbidity. 31In a recent large prospective study from Northern India on the maternal and fetal outcomes of Hepatitis E infection, close to 60% of viral hepatitis in pregnant women was attributed to hepatitis E infection. Fulminant hepatic failure was more common among HEV-infected women (55%) who were 2.7 times at higher risk than non-HEV infected women (20%); maternal mortality was also higher secondary to fulminant hepatic failure in the HEV infected group (41%) vs. 7% in the non-HEV group 13.
Sporadic hepatitis E infection is also associated with increased incidence and severity in pregnant women as reported by a study from India. Hepatitis E alone contributed to approximately 50% of patients with acute viral hepatitis. Fulminant liver failure was significantly higher in pregnant women with HEV infection as supposed to other causes of acute viral hepatitis (69.2% vs. 10%, p<0.001). Also the prevalence and the severity of HEV infection in pregnant women did not differ significantly in various stages of gestation. 16
In contrast, in Egypt, where prevalence of anti-HEV in rural communities is very high, severe HEV-caused AVH in pregnant women has not been reported. In one study of 2428 pregnant women, the anti-HEV prevalence was 84.3%. No patients with AVH were reported. 31
Reasons for the differences in the outcome of HEV in different geographical areas remain unclear but could be the result of early childhood HEV exposures, producing long-lasting immunity and/or modify subsequent responses to exposure to the virus. Alternatively, the predominant HEV genotype(s) in Egypt could be less virulent than those in Asia. 30, 31 The high risk of vertical Transmission of HEV infection from mother to infant was investigated in a study of 469 pregnant women and reported a mother-to-infant transmission of 100%, although there may be a selection bias. Nonetheless the high transmission rate signifies the importance of vertical transmission of HEV infection. A small percentage of the babies born to mothers with active disease were either pre-tem, or had anicteric hepatitis. Two of the babies died within 48 hours, while the remaining alive 24 infants had full recovery. 32
Studies in animals to better understand the pathogenesis too have been non-contributory. In an animal study to investigate the changes induced by HEV in pregnant and non-pregnant primates 33, the course of liver injury was similar in both groups. However, this is not surprising considering that HEV infection in primates leads only to a milder form of liver injury. The severe liver injury due to HEV infection during pregnancy may be related to one of several possible host factors, such as differences in immune and hormonal factors occurring during pregnancy, the genetic and environmental factors with its occurrence in certain developing countries and we would explore all these issues over in this article.
Immunological changes during pregnancy
During pregnancy, the maternal immune system is clearly altered to tolerate genetically different fetus. 34-35The outer layer of the placenta is made of trophoblasts, which forms the interface between the maternal and fetal circulations. Trophoblasts do not express Major Histocompatibility Complex (MHC) class proteins and hence resistant to T-cell mediated injury, which is a protective phenomenon to sustain the fetus. However the Natural Killer (NK) cells do not require MHC proteins and the trophoblasts are protected against the NK cells as they express a unique Human Leukocyte Antigen (HLA) molecule called HLA-G, which binds to NK receptors CD 16, and CD 56 and inactivates it. 36 The placenta also expresses an enzyme called indoleamine 2, 3-dioxygenase which inactivates and depletes tryptophan, an amino acid essential to T-cell function and hence suppresses cell mediated immunity at the fetus-placental interface. 37
Cytokines also contribute to the immunological tolerance as both the placenta and the trophoblasts secrete cytokines, including TGF-β, IL-4, and IL-10, which inhibit cell, mediated immunity. In an attempt to understand the immunological changes in pregnancy, Orsi et al measured serum cytokines in mouse models during different stages of pregnancy; levels of TNF-α, IL-1β, IL-2, IL-6, IL-10, IL-12 (p40), IL-12(p70), and IL-17 (p<0.05)were low during the initial phase of pregnancy and increased markedly in late pregnancy and post partum 38. Dudley et al. in his murine model also had observations, which reinforced the hypothesis that cytokine production during pregnancy favors antibody production over cytotoxic T cell responses. 39
T-cells are markedly reduced during early pregnancy up to the 20th week of gestation leading to reduced level of immunity. 40-42This modulation of cell-mediated immunity occurs to allow fetal allograft retention, but it also alters the immune response mounted against infections 43. The decrease in T cell activity has been suggested to increase susceptibility to viral infections such as hepatitis, rubella, herpes and human papilloma virus and also infections like malaria during pregnancy 44, but also explains why cell mediated immune diseases like rheumatoid arthritis improve during gestation.
While some studies have argued that there is no alteration in the number of total T-lymphocytes or in CD4+ lymphocytes in pregnancy 45-46; others have suggested an initial decrease until 20 weeks to sustain the fetus during the implantation phase and then increase or normalization later during pregnancy or the postpartum period. 47-53The apparent response to pregnancy of CD8 lymphocytes, although less well studied, is either a slight decrease or stability throughout gestation 46, 50-52, 54-55.
To summarize, the immunological changes during pregnancy promote the maintenance of the antigenic fetus in the maternal environment by suppression of T cell mediated immunity. There is a clear shift in the Th1:Th2 cell paradigm during pregnancy with definite skew towards Th2 cells. The levels of most cytokines are depressed particularly during the initial 20 weeks of pregnancy, which is an important phase to sustain the fetus. Whether this suppressed immune system translates into increased risk of infections during pregnancy is still not clear with the available data.
Hormonal Factors in pregnancy
Hormonal factors during pregnancy may also play a significant role in altering immune regulation or viral replication56, 57. Progesterone, estrogen and human chorionic gonadotropin (HCG) increase with pregnancy. In animal studies, these hormones have been shown to have a clear suppressive effect on the cell-mediated immunity. HCG has been shown to inhibit cell mediated immunity in guinea pigs 58, while estrogen produce shrinkage of thymus and deplete the CD4 and CD8 populations in mice 59, 60 and progesterone produces involution of the thymus and blocks T cell development and inhibits Th1 cell and promotes Th2 cell development 61. Progesterone has been specifically shown to impair the transition of pro T-cells to early pre T-cells in mice models. 61 The expression of thymic stromal progesterone receptors is required to produce involution of the thymus and they play a greater role than estrogen for produce thymic involution. 60, 61 Despite these changes, the numbers of peripheral T and B cells is unchanged as the half life of peripheral lymphocytes is higher. Studies have also shown that there is also a decrease in bone marrow B cell production, mainly pre-B and immature (fractions B–D) bone marrow B cells of pregnant mice due to increase in estrogen and progesterone during pregnancy. 62
In addition, there are evidences indicating that steroid hormones may influence viral replication. 63-64 For example, hormonal enhancement of cytomegalovirus (CMV) replication may be a possible mechanism for the increased incidence of CMV infection observed during human pregnancy 63. There are also reports of increased predisposition to viral infection in certain high-estrogen states 64.
Mechanisms for high morbidity of hepatitis E in pregnancy
As discussed previously, pregnancy is associated with high levels of steroid hormones. These steroid hormones may promote viral replication. It also has a direct inhibition on hepatic cells, which may predispose to hepatic dysfunction/failure when exposed to infectious pathogens.65 Steroid hormones are immunosuppressive66 and mediate lymphocyte apoptosis through NF-κB. NF-κB is a eukaryotic dimeric transcription factor which has a multiple cellular effects, including liver development and regeneration and its implications on the immune response67. Animal experiments in mice studying the p65 component of NF-κB have shown their primary role in liver development and regeneration. Mice lacking the p65 component of NF-κB had evidence of widespread apoptosis, which lead to recent attempts to study this phenomenon in humans. Prusty et al studied the changes in NF-κB activity using electrophoretic assays of the p50 and p65 component in pregnant and non pregnant patients with fulminant hepatic failure (FHF) due to hepatitis B,C and E.68 Their results replicated the results in animal experiments and they found that the activity of the p65 component of NF-κB was diminished in both the peripheral blood mononuclear cells (PBMC) and post mortem liver biopsy specimens in pregnant patients with fulminant liver failure. There was a higher than normal level of p50 expression, but there was a near complete absence or a minimal expression of p65. The absence of p65 from the NF-κB complex produced fulminant liver damage68. Their results established that the absence of p65 was probably responsible for severe liver damage in pregnant FHF patients. This hypothesis has been further supported in other viruses too where recent studies have shown decreased expression of p65 causing liver fibrosis and liver damage in patients with HCV-induced chronic liver disease 69. The expression of NF-κB, physiologically down regulated during pregnancy also plays an important role in sustaining the fetus during pregnancy70.
Jilani et al found that HEV infected pregnant women with fulminant hepatic failure had lower CD4 count and higher CD8 counts. They also observed that the levels of estrogens, progesterone and beta-HCG were significantly higher in the above-mentioned group when compared to HEV negative patients or control healthy pregnant females. 71Although the levels of hormones were physiologically high in the normal control population; patients with HEV infection seem to have significantly higher levels than controls, which probably explain the direct interaction of HEV with the immune system.
Pal et al studied the cellular immune response in both pregnant and non pregnant women with acute hepatitis E and the control population 72 found that pregnant women with HEV had generalized immune suppression characterized by decrease in lymphocyte response to phytohemagglutinin (PHA) with a predominant Th2 bias as compared to non pregnant women with hepatitis E and normal healthy controls. This challenged the previously existing hypothesis that normal pregnancy is associated with systemic immune suppression with an increased risk of infections. 73-76This study was important from a number of perspectives. The thought that normal pregnancy is an immunosuppressed state is challenged because normal healthy pregnant women did not demonstrate decreased response to PHA. Also non-pregnant patients with HEV did not show any defective PHA response either highlighting that HEV by itself does not produce the immunological changes and needs a pregnancy as a physiological state to produce the above-mentioned changes. The Th2 bias observed in the present study was specific to HEV infection during pregnancy. It may be just that the Th2 bias is very much prominent in HEV infection as compared to normal pregnancy. The mechanism by which Th2 bias may lead to more severe disease course in pregnant women with hepatitis E needs further investigation. With this Th2 bias, it was suggested that decreased cellular-mediated immunity is considered a major cause of death in Asian pregnant women with fulminant hepatitis caused by HEV infection.
If all the hypothesis of immunological and hormonal factors interacting with the genetic susceptibility in Asian women holds true, we should expect a high mortality in pregnancy from all HEV endemic regions. But this is not always true. Two studies, from Chennai, southern India and Egypt, although highlighting the high prevalence of Hepatitis E infection in pregnancy also had very interesting observations. The mortality rate of hepatitis E infection was very low (3.4%) 77 and absent 31respectively as against 30−100% reported in various studies in HEV endemic regions 8-16. Also most of the HEV infected pregnant women had normal term deliveries. These studies may underline the importance that viral genotypes in the pathogenesis and severity of HEV infection. The results of various studies from endemic regions have been summarized in table 1. We hypothesized that the difference in the genotype or its subtypes of the Hepatitis E virus infection could be the answer 77. Genotype 1 is the commonest subtype causing HEV infection in India, while genotype 3 predominates in the US. Genotype 1 has been further classified into 4 subtypes and most of them have been grouped to genotype 1A. Sub-genotype shift, 78 may have been responsible for the different geographic morbidity in pregnant women in Southern India and Egypt. If this hypothesis holds true, it opens up the intriguing possibility of the exploration of the genotype in pregnancy.
Table 1.
Studies on Hepatitis E infection and Pregnancy
| Study | Patients (n) | Prevalence of HEV infection (%) | Prevalence of fulminant liver failure (%) | Mortality rate (%) |
|---|---|---|---|---|
| Jaiswal et al, 2001 (North India) 15 | 127 | 58 | 58 | 45 |
| Singh et al, 2003 (North India) 14 | 60 | 37 | 64 | 64 |
| Khuroo et al, 2003 (North India) 16 | 76 | 86 | 69 | 55 |
| Beniwal et al, 2003 (North India) 8 | 97 | 47.4 | 75 | 39.1 |
| Tsega et al, 1993 (Ethiopia) 10 | 32 | 59 | - | 42 |
| Kumar et al, 2004 (North India) 12 | 65 | 45 | 32 | 73 |
| Patra et al, 2007 (North India) 13 | 220 | 60 | 55 | 41 |
| *Stoszek SK et al, 2006 (Egypt) 31 | 2428 | 84.3 | 0 | 0 |
| *Rasheeda et al, 2008 (South India) 77 | 115 | 75 | 3.4 | 3.4 |
Studies with low morbidity and mortality in pregnancy
In addition to the above mentioned factors, Khuroo et al79 suggested that infection of the fetus with HEV may be responsible for the increased severity of the disease in the mother; Variations in the major histocompatibility complex (MHC) which mediate antigen presentation may also explain some of the difference in the mortality in different geographical areas in women infected with HEV 80-81. Recently an editorial commented that HEV infected pregnant women as a group may be more commonly taking herbal medications, which could explain the high mortality in certain geographic regions. 82However, in our center back home where herbal medication use is very common, we have observed that the mortality rate is low. 77, 83At the same time, we observed that the use of herbal medications was an independent predictor for poor prognosis in patients with acute liver failure due to other etiologies. 83
Also the recent study of post transplant patients from France, highlighting the increased risk of acute hepatitis E progressing to chronicity has reiterated the importance of immune response to protect against infection. 7 However, as observed all these patients were post transplant and patients who progressed to chronic hepatitis had significantly lower levels of CD3 and CD4 cells highlighting the importance particularly of T-cell mediated immunity for pathogen clearance. This difference in presentation is really interesting. The progression to fulminant liver failure in pregnant women could be due to immunological injury, while chronic hepatitis could be mediated by failure to inhibit viral replication given that these patients were immunosuppressed and immunological injury is absent. Future research is required to understand the implications of immunology and hepatitis E infection.
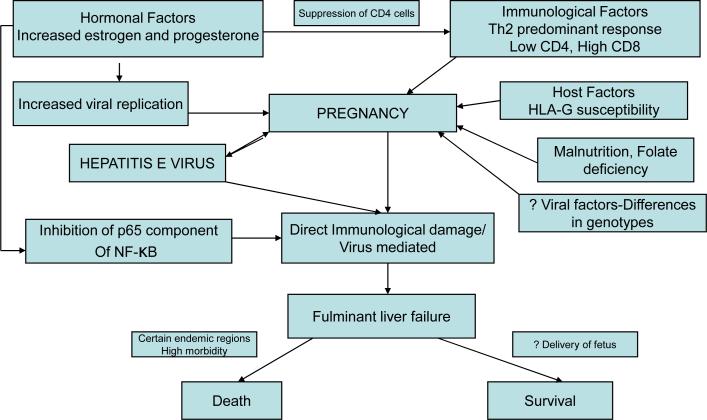
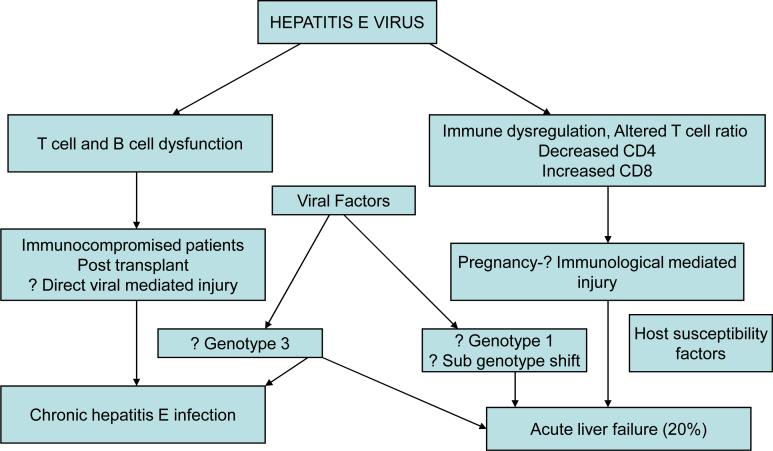
Thus a complex interaction of viral, host, immunological and hormonal factors interact with each other producing a paradigm of severe liver damage in pregnancy, probably immunologically, which is summarized in figure 1. The probable hypothesis of pathogenesis of hepatitis E depending on the host immune function is summarized in figure 2.
Figure 1.
Pathogenesis of HEV in pregnancy
Figure 2.
Probable hypotheses for the variable pathogenesis of HEV
Management
The mechanism of liver injury in hepatitis E is not clear and all the hypotheses put forth has not been yet conclusively proved. In this situation of uncertainty, the management of HEV infection induced liver failure assumes more importance than ever before. All the studies have shown that pregnant women have the differential immune response which triggers fulminant liver failure. So the logical treatment should be to deliver the fetus as soon as possible. Unfortunately, very few such studies have been undertaken in this field. Therapeutic termination of pregnancy, which has been proved to be beneficial in pregnancy specific disorders like HELLP syndrome and acute fatty liver of pregnancy 84, have not been fully, explored in hepatitis E infection. However in a retrospective study from India, Banait et al, 85 studied 42 patients with HEV induced liver failure, there was no difference in maternal mortality in pregnant women who delivered and those who did not questioning the role of therapeutic termination. The literature at present is not supportive of the fact that delivery of the baby may decrease the maternal mortality. However this was a small retrospective study and must not discourage physicians from pursuing that option considering that HEV infection produces immunological changes in the fetus too. Studies have also explored the role of preventing HEV in high-risk endemic countries given the high maternal and fetal morbidity and mortality. Arankalle et al, 86 used an Indian made preparation of immune serum globulin based on locally prevalent genotypes in preventing hepatitis E among pregnant women during an epidemic. Patients who received immune globulin had lower frequency of HEV infection than in control population (18.1% versus 33.9%). However the study was limited by the small number of patients and did not have sufficient power to be conclusive. At present, although there is no consensus to treat patients with HEV infection in pregnancy, early delivery of the fetus if possible to prevent maternal mortality should be tried. Randomized studies are required in the future to decide upon the best way of treating patients with HEV infection in pregnancy.
Conclusions
The interaction of Hepatitis E and pregnancy is fascinating and has provided new insights into the pathophysiology and understanding of the immunology and host susceptibility factors and their interaction to produce the disease processes. The severe liver injury due to HEV infection during pregnancy may be related to several possible factors, such as differences in immune and hormonal factors occurring during pregnancy, the genetic and environmental factors with its occurrence in certain developing countries. From a molecular biology aspect, a more interesting target in the future for scientists will be for discovering the probable modifier genes that might explain the differences in the presentation of hepatitis E in different populations and targeting them to alter the immune response. Immunological research in the future might provide us more information if the management strategies are to be improved and conquer this disease in the near future.
Acknowledgements
This article is supported by NIH grant R21 AI067868 to Dr. Shata
Contributor Information
Dr. Udayakumar Navaneethan, Department of Internal Medicine, University of Cincinnati Medical Center, Ohio, USA.
Dr. Mayar Al Mohajer, Department of Internal Medicine, University of Cincinnati Medical Center, Ohio, USA.
Dr. Mohamed T Shata, Associate Research Prof. of Medicine, Division of Digestive diseases University of Cincinnati College of Medicine, Ohio, USA.
References
- 1.Purcell R, Emerson S. Viral Hepatitis. In: Mandell GL, Douglas RG, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennet's principles and practice of infectious diseases. 6th ed. Elsevier/Churchill Livingstone; New York: 2005. [Google Scholar]
- 2.Vishwanathan R. Infectious hepatitis in Delhi (1955−56): a critical study. Epidemiology. Indian J Med Res. 1957;45(Suppl 1):1–29. [Google Scholar]
- 3.Wong DC, Purcell RH, Sreenivasan MA, Prasad SR, Pavri KM. Epidemic and endemic hepatitis in India: evidence for non-A, non-B hepatitis virus etiology. Lancet. 1980;2:876–79. doi: 10.1016/s0140-6736(80)92045-0. [DOI] [PubMed] [Google Scholar]
- 4.Khuroo MS. Study of an epidemic of non-A, non-B hepatitis. Possibility of another human hepatitis virus distinct from post transfusion non-A, non-B type. Am. J. Med. 1980;68:818–24. doi: 10.1016/0002-9343(80)90200-4. [DOI] [PubMed] [Google Scholar]
- 5.Dawson GJ, Chau KH, Cabol CM, Yarbough PO, Reyes GR, Mushahwar IK. Solid-phase enzyme-linked immunosorbent assay for hepatitis E virus IgG and IgM antibodies utilizing recombinant antigens and synthetic peptides. J. Virol. Methods. 1992;38:175–86. doi: 10.1016/0166-0934(92)90180-l. [DOI] [PubMed] [Google Scholar]
- 6.Renou C, Moreau X, Pariente A, et al. A national survey of acute Hepatitis E in France. Aliment Pharmacol Ther. 2008 Mar 14; doi: 10.1111/j.1365-2036.2008.03679.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Kamar N, Selves J, Mansuy JM, et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med. 2008;21(358):811–7. doi: 10.1056/NEJMoa0706992. [DOI] [PubMed] [Google Scholar]
- 8.Beniwal M, Kumar A, Kar P, Jilani N, Sharma JB. Prevalence and Severity Of Acute Viral Hepatitis And Fulminant Hepatitis During Pregnancy: A Prospective Study From North India. Indian J Medical Microbiol. 2003;21:184–5. [PubMed] [Google Scholar]
- 9.Medhat A, el-Sharkawy MM, Shaaban MM, Makhlouf MM, Ghaneima SE. Acute viral hepatitis in pregnancy. Int J Gynaecol Obstet. 1993;40:25–31. doi: 10.1016/0020-7292(93)90768-r. [DOI] [PubMed] [Google Scholar]
- 10.Tsega E, Krawczynski K, Hansson BG, Nordenfelt E. Hepatitis E virus infection in pregnancy in Ethiopia. Ethiop Med J. 1993;31:173–81. [PubMed] [Google Scholar]
- 11.Strand RT, Franque-Ranque M, Bergstrom S, Weiland O. Infectious etiology of jaundice among pregnant women in Angola. Scand J Infect Dis. 2003;35:401–3. doi: 10.1080/00365540310010930. [DOI] [PubMed] [Google Scholar]
- 12.Kumar A, Beniwal M, Kar P, Sharma JB, Murthy NS. Hepatitis E in pregnancy. Int J Gynaecol Obstet. 2004;85:240–4. doi: 10.1016/j.ijgo.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 13.Patra S, Kumar A, Trivedi SS, Puri M, Sarin SK. Maternal and fetal outcomes in pregnant women with acute hepatitis E virus infection. Ann Intern Med. 2007;147:28–33. doi: 10.7326/0003-4819-147-1-200707030-00005. [DOI] [PubMed] [Google Scholar]
- 14.Singh S, Mohanty A, Joshi YK, Deka D, Mohanty S, Panda SK. Mother to child transmission of hepatitis E virus infection. Indian J Pediatr. 2003;70:37–9. doi: 10.1007/BF02722743. [DOI] [PubMed] [Google Scholar]
- 15.Jaiswal SPB, Jain AK, Naik G, Soni N, Chitnis DS. Viral hepatitis during pregnancy. Int J Gynaec Obstet. 2001;72:103–108. doi: 10.1016/s0020-7292(00)00264-2. [DOI] [PubMed] [Google Scholar]
- 16.Khuroo MS, Kamili S. Etiology, clinical course and outcome of sporadic acute viral hepatitis in pregnancy. J Viral Hepat. 2003;10:61–69. doi: 10.1046/j.1365-2893.2003.00398.x. [DOI] [PubMed] [Google Scholar]
- 17.Schlauder GG, Mushahwar IK. Genetic heterogeneity of hepatitis E virus. J Med Virol. 2001;65:282–292. doi: 10.1002/jmv.2031. [DOI] [PubMed] [Google Scholar]
- 18.Fields BN, Knipe DM. Fields virology. xix. 2nd ed. Vol. 2. Raven Press; New York: 1990. p. 2336. [Google Scholar]
- 19.Mizuo H, Yazaki Y, Sugawara K, Tsuda F, Takahashi M, Nishizawa T, Okamoto H. Possible risk factors for the transmission of hepatitis E virus and for the severe form of hepatitis E acquired locally in Hokkaido. Japan. J Med Virol. 2005;76:341–349. doi: 10.1002/jmv.20364. [DOI] [PubMed] [Google Scholar]
- 20.Emerson SU, Purcell RH. Hepatitis E virus. Rev Med Virol. 2003;13:145–154. doi: 10.1002/rmv.384. [DOI] [PubMed] [Google Scholar]
- 21.Naik SR, Aggrawal R, Salunke PN, Mehrotra NN. A large waterborne hepatitis E epidemic in Kanpur. India. Bull WHO. 1992;70:597–604. [PMC free article] [PubMed] [Google Scholar]
- 22.Robson SC, Adams S, Brink N, Woodruff B, Bradley D. Hospital outbreak of hepatitis E. Lancet. 1992;339:1425–1425. doi: 10.1016/0140-6736(92)91250-c. [DOI] [PubMed] [Google Scholar]
- 23.Khuroo MS, Kamili S, Jameel S. Vertical transmission of hepatitis E virus. Lancet. 1995;345:1025–1026. doi: 10.1016/s0140-6736(95)90761-0. [DOI] [PubMed] [Google Scholar]
- 24.Psichogiou M, Tzala E, Boletis J, et al. Hepatitis E virus infection in individuals at high risk of transmission of non-A, non-B hepatitis and sexually transmitted diseases. Scand. J, Infect. Dis. 1996;28:443–445. doi: 10.3109/00365549609037936. [DOI] [PubMed] [Google Scholar]
- 25.Brazilai A, Schulman S, Karetnyi YV, et al. Hepatitis E virus infection in hemophiliacs. J. Med. Virol. 1995;46:153–156. doi: 10.1002/jmv.1890460213. [DOI] [PubMed] [Google Scholar]
- 26.Tsega E, Hansson BG, Krawczynski K, Nordefelt E. Acute Sporadic viral hepatitis in Ethiopia: causes, risk factors, and effects on pregnancy. Clin, Infect Dis. 1992;14:961–965. doi: 10.1093/clinids/14.4.961. [DOI] [PubMed] [Google Scholar]
- 27.Kane MA, Bradely DW, Shrestha SM, et al. Epidemic non-A, non-B hepatitis in Nepal: Recovery of a possible etiologic agent and transmission studies in marmosets. JAMA. 1984;252:3140–3145. doi: 10.1001/jama.252.22.3140. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention Hepatitis E among US travelers, 1989−1992. MMWR Morb. Mortal. Wkly Rep. 1993;42:1–4. [PubMed] [Google Scholar]
- 29.De Cock KM, Bradley DW, Sandford NL, Govindarajan S, Maynard JE, Redekar AG. Epidemic non-A, non-B hepatitis in patients from Pakistan. Ann. Intern. Med. 1987;106:227–230. doi: 10.7326/0003-4819-106-2-227. [DOI] [PubMed] [Google Scholar]
- 30.Stoszek SK, Engle RE, Abdel-Hamid M, et al. Hepatitis E antibody seroconversion without disease in highly endemic rural Egyptian communities. Trans R Soc Trop Med Hyg. 2006;100:89–94. doi: 10.1016/j.trstmh.2005.05.019. Epub 2005 Oct 28. [DOI] [PubMed] [Google Scholar]
- 31.Stoszek SK, Abdel-Hamid M, Saleh DA, et al. High prevalence of hepatitis E antibodies in pregnant Egyptian women. Trans R Soc Trop Med Hyg. 2006;100:95–101. doi: 10.1016/j.trstmh.2004.12.005. Epub 2005 Oct 28. [DOI] [PubMed] [Google Scholar]
- 32.Kumar RM, Uduman S, Rana S, Kochiyil JK, Usmani A, Thomas L. Sero-prevalence and mother-to-infant transmission of hepatitis E virus among pregnant women in the United Arab Emirates. Eur J Obstet Gynecol Reprod Biol. 2001;10(100):9–15. doi: 10.1016/s0301-2115(01)00448-1. [DOI] [PubMed] [Google Scholar]
- 33.Aggarwal R. Hepatitis E and pregnancy. Indian J Gastroenterol. 2007;26:3–5. [PubMed] [Google Scholar]
- 34.Hunt JS. Cytokine networks in the uteroplacental unit: macrophages as pivotal regulatory cells. J. Reprod. Immunol. 1989;16:1–17. doi: 10.1016/0165-0378(89)90002-8. [DOI] [PubMed] [Google Scholar]
- 35.Marzi M, Vigano A, Trabattoni D, et al. Characterization of type 1 and type 2 cytokine production profile in physiologic and pathologic human pregnancy. Clin. Exp. Immunol. 1996;106:127–133. doi: 10.1046/j.1365-2249.1996.d01-809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 37.R Mellor AL, Sivakumar J, Chandler P, et al. Prevention of T cell–driven complement activation and inflammation by tryptophan catabolism during pregnancy. Nat Immunol. 2001;2:64. doi: 10.1038/83183. [DOI] [PubMed] [Google Scholar]
- 38.Orsi NM, Gopichandran N, Ekbote UV, Walker JJ. Murine serum cytokines throughout the estrous cycle, pregnancy and post partum period. Anim Reprod Sci. 2006;96:54–65. doi: 10.1016/j.anireprosci.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 39.Dudley DJ, Chen C, Mitchell MD, Daynes RA, Araneo BA. Adaptive immune responses during murine pregnancy: pregnancy-induced regulation of lymphokine production by activated T lymphocytes. Am. J. Obstet. Gynecol. 1993;168:1155–63. doi: 10.1016/0002-9378(93)90361-l. [DOI] [PubMed] [Google Scholar]
- 40.Cahill KM. Hepatitis in pregnancy. Surg. Gynaecol. Obstet. 1962;114:545–52. [PubMed] [Google Scholar]
- 41.Purtilo DT, Hallgren HM, Yunis EJ. Depressed maternal response to phytohaemagglutinin in human pregnancy. Lancet. 1972;1:769–71. doi: 10.1016/s0140-6736(72)90522-3. [DOI] [PubMed] [Google Scholar]
- 42.Lewis J, Jr, Whang J, Nagel B, Oppenheim JJ, Perry S. Lymphocyte transformation in mixed leukocyte cultures in women with normal pregnancy or tumor of placental origin. A preliminary report. Am. J. Obstet. Gynecol. 1966;96:287–90. doi: 10.1016/0002-9378(66)90327-9. [DOI] [PubMed] [Google Scholar]
- 43.Meeusen EN, Bischof RJ, Lee CS. Comparative T-cell responses during pregnancy in large animals and humans. Am. J. Reprod. Immunol. 2001;46:169–79. doi: 10.1111/j.8755-8920.2001.460208.x. [DOI] [PubMed] [Google Scholar]
- 44.Riley EM, Schneider G, Sambou I, Greenwood BM. Suppression of cell-mediated immune responses to malaria antigens in pregnant Gambian women. Am. J. Trop. Med. Hyg. 1989;40:141–4. doi: 10.4269/ajtmh.1989.40.141. [DOI] [PubMed] [Google Scholar]
- 45.Priddy KD. Immunologic adaptations during pregnancy. J. Obstet. Gynecol. Neonatal Nurs. 1997;26:388. doi: 10.1111/j.1552-6909.1997.tb02720.x. [DOI] [PubMed] [Google Scholar]
- 46.Biggar RJ, Pahwa S, Minokoff H, et al. Immunosuppression in pregnant women infected with human immunodeficiency virus. Am J Obstet Gynecol. 1989;161:1239–44. doi: 10.1016/0002-9378(89)90674-1. [DOI] [PubMed] [Google Scholar]
- 47.Coulam CB, Silverfield JC, Kazmar RE, Fathman CG. T-Lymphocyte subsets during pregnancy and the menstrual cycle. Am J Reprod Immunol. 1983;4:88–90. doi: 10.1111/j.1600-0897.1983.tb00259.x. [DOI] [PubMed] [Google Scholar]
- 48.Glassman AB, Bennett CE, Christopher JB, Self S. Immunity during pregnancy: Lymphocyte subpopulations and mitogen responsiveness. Ann Clin Lab Sci. 1985;15:357–62. [PubMed] [Google Scholar]
- 49.Sridama V, Pacini F, Yang SL, Moawad A, Reilly M, Degroot LJ. Decreased levels of helper T cells: A possible cause of immunodeficiency in pregnancy. N Engl J Med. 1982;307:352–6. doi: 10.1056/NEJM198208053070606. [DOI] [PubMed] [Google Scholar]
- 50.Tallon DF, Corcoran DJ, O'Dwyer GM, Greally JF. Circulating lymphocyte subpopulation in pregnancy: A longitudinal study. J immunol. 1984;132:1784–7. [PubMed] [Google Scholar]
- 51.Barnett MA, Learmonth RP, Pihl E, Wood EC. T-helper lymphocyte depression in early human pregnancy. J Repred Immunol. 1983;5:55–7. doi: 10.1016/0165-0378(83)90021-9. [DOI] [PubMed] [Google Scholar]
- 52.Malinowshki A, Szpakowski M, Tchorzewski H, Zeman K, Pawlowicz P, Wozniac P. T-lymphocyte subpopulations and lymphocyte proliferative activity in normal and pre-eclamptic pregnancies. Eur J Obstet Gynecol Reprod Biol. 1994;53:27–31. doi: 10.1016/0028-2243(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 53.Degenne D, Canepa S, Lecomte C, Renoux M, Bardos P. Serial study of T-lymphocyte subsets in women during very early pregnancy. Clin Immun Immunopathol. 1988;48:187–91. doi: 10.1016/0090-1229(88)90082-7. [DOI] [PubMed] [Google Scholar]
- 54.Canapa S, Horowitz R, Degenne D, Magnin G, Valat C, Bardos P. Correlation of plasma hormone levels and peripheral circulating lymphocyte subpopulation during human pregnancy. Immunol Lett. 1984;8:159–63. doi: 10.1016/0165-2478(84)90070-1. [DOI] [PubMed] [Google Scholar]
- 55.Castilla JA, Reuda R, Vargas ML, Gonzalez-Gomez F, Garcia-Olivares E. Decreased levels of circulating CD4+ T lymphocytes during normal human pregnancy. J repred Immunol. 1989;15:103–11. doi: 10.1016/0165-0378(89)90030-2. [DOI] [PubMed] [Google Scholar]
- 56.Arankalle VA, Chadha MS, Dama BM, Tsarvey SA, Purcell RM, Banerjee K. Role of immune serum globulins in pregnant women during an epidemic of hepatitis E. J viral hepat. 1998;5:199–204. doi: 10.1046/j.1365-2893.1998.00096.x. [DOI] [PubMed] [Google Scholar]
- 57.Ponta H, Kennedy N, Scroch P, Hynes NE, Groner B. Hormonal response region in the mouse mammary tumor virus long terminal repeat can be dissociated from the proviral promoter and has enhancer properties. Proc. Natl Acad. Sci. USA. 1985;82:1020–24. doi: 10.1073/pnas.82.4.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Han T. Human chorionic gonadotropin. Its inhibitory effect on cell-mediated immunity in vivo and in vitro. Immunology. 1975;29:509–15. [PMC free article] [PubMed] [Google Scholar]
- 59.Boll G, Reimann J. Estrogen treatment depletes extrathymic T cells from intestinal lymphoid tissues. Scand. J. Immunol. 1996;43:345–50. doi: 10.1046/j.1365-3083.1996.d01-41.x. [DOI] [PubMed] [Google Scholar]
- 60.Rijhisinghami AG, Thompson K, Bhatia SK, Waldscmidt TJ. Estrogen blocks early T cell development in the thymus. Am. J. Reprod. Immunol. 1996;36:267–77. doi: 10.1111/j.1600-0897.1996.tb00176.x. [DOI] [PubMed] [Google Scholar]
- 61.Tibbets TA, de Mayo F, Rich S, Conneely OM, Omalley BW. Progesterone receptors in the thymus are required for thymic involution during pregnancy and for normal fertility. Proc. Natl Acad. Sci. USA. 1999;96:12021–6. doi: 10.1073/pnas.96.21.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Medina KL, Smithson G, Kincade PW. Suppression of B lymphopoiesis during normal pregnancy. J. Exp. Med. 1993;178:1507. doi: 10.1084/jem.178.5.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Styrt B, Sugarman B. Estrogens and infection. Rev. Infect. Dis. 1991;13:1139–50. doi: 10.1093/clinids/13.6.1139. [DOI] [PubMed] [Google Scholar]
- 64.Hussaini SH, Skidmore SJ, Richardson P, Sherratt LM, Cooper BT, O'Grady JG. Severe hepatitis E infection during pregnancy. J. Viral hepat. 1997;4:51–4. doi: 10.1046/j.1365-2893.1997.00123.x. [DOI] [PubMed] [Google Scholar]
- 65.Barbara H, McGovern, Jeremy S, et al. Hepatic Steatosis is associated with fibrosis, nucleoside analogue use, and hepatitis C virus genotype 3 infection in HIV-seropositive patients. Clin. Infect. Dis. 2006;43:365–72. doi: 10.1086/505495. [DOI] [PubMed] [Google Scholar]
- 66.Mellor AM, Munn DH. Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation? Immunol. Today. 1999;20:469–73. doi: 10.1016/s0167-5699(99)01520-0. [DOI] [PubMed] [Google Scholar]
- 67.Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-kappa B. Ann. Rev. Cell Biol. 1994;10:405–55. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 68.Prusty BK, Hedau S, Singh A, Kar P, Das BC. Selective Suppression of NF-kBp65 in Hepatitis Virus-Infected Pregnant Women Manifesting Severe Liver Damage and High Mortality. Mol Med. 2007;13:518–26. doi: 10.2119/2007-00055.Prusty. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boya P, Larrea E, Sola I, Majano PL, Jiménez C, Civeira MP, Prieto J. Nuclear factor-kappa B in the liver of patients with chronic hepatitis C: decreased RelA expression is associated with enhanced fibrosis progression. Hepatology. 2001;34:1041–8. doi: 10.1053/jhep.2001.29002. [DOI] [PubMed] [Google Scholar]
- 70.McCracken SA, Drury CL, Lee HS, Morris JM. Pregnancy is associated with suppression of the nuclear factor kappaB/IkappaB activation pathway in peripheral blood mononuclear cells. J. Reprod. Immunol. 2003;58:27–47. doi: 10.1016/s0165-0378(02)00081-5. [DOI] [PubMed] [Google Scholar]
- 71.Jilani N, Das BC, Husain SA, Baweja UK, Chattopadhya D, Gupta RK, Sardana S, Kar P. Hepatitis E virus infection and fulminant hepatic failure during pregnancy. J Gastroenterol Hepatol. 2007;22:676–82. doi: 10.1111/j.1440-1746.2007.04913.x. [DOI] [PubMed] [Google Scholar]
- 72.Pal R, Aggarwal R, Naik SR, Das V, Das S, Naik S. Immunological alterations in pregnant women with acute hepatitis E. J Gastroenterol Hepatol. 2005;20:1094–101. doi: 10.1111/j.1440-1746.2005.03875.x. [DOI] [PubMed] [Google Scholar]
- 73.Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272:1502–4. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 74.Morell V. Zeroing in on how hormones affect the immune system. Science. 1995;269:773–5. doi: 10.1126/science.7638587. [DOI] [PubMed] [Google Scholar]
- 75.Singh N, Shukla MM, Sharma VP. Epidemiology of malaria in pregnancy. Bull. WHO. 1999;77:567. [PMC free article] [PubMed] [Google Scholar]
- 76.Matthiesen L, Berg G, Ernerudh J, Hakansson L. Lymphocyte subsets and mitogen stimulation of blood lymphocytes in normal pregnancy. Am. J. Reprod. Immunol. 1996;35:70–9. doi: 10.1111/j.1600-0897.1996.tb00010.x. [DOI] [PubMed] [Google Scholar]
- 77.Rasheeda CA, Navaneethan U, Jayanthi V. Liver Disease in pregnancy and its influence on maternal and fetal mortality- A prospective study from Chennai, Southern India. Eur J Gastroenterol Hepatol. 2008;20:362–4. doi: 10.1097/MEG.0b013e3282f246d6. [DOI] [PubMed] [Google Scholar]
- 78.Arankalle VA, Paranjape S, Emerson SU, Purcell RH, Walimbe AM. Phylogenetic analysis of hepatitis E virus isolates from India (1976−1993). J Gen Virol. 1999;80:1691–700. doi: 10.1099/0022-1317-80-7-1691. [DOI] [PubMed] [Google Scholar]
- 79.Khuroo MS, Kamili S, Yattoo GN. Severe fetal hepatitis E virus infection is the possible of increased severity of hepatitis E virus infection in the mother: Another example of mirror syndrome. Indian J Gastroenteral. 2004;23(Suppl 1):A1. [Google Scholar]
- 80.Hohler T, Reuss E, Evers N, et al. Differential genetic determination of immune responsiveness to hepatitis B surface antigen and to hepatitis. a virus: a vaccination study in twins. Lancet. 2002;360:991–95. doi: 10.1016/S0140-6736(02)11083-X. [DOI] [PubMed] [Google Scholar]
- 81.Thio CL, Carrington M, Marti D, et al. Class II HLA alleles and hepatitis B virus persistence in African Americans. J. Infect. Dis. 1999;179:1004–6. doi: 10.1086/314684. [DOI] [PubMed] [Google Scholar]
- 82.Bernuau JR, Durand F. Herbal medicines in acute viral hepatitis: a ticket for more trouble. Eur J Gastroenterol Hepatol. 2008;20:161–3. doi: 10.1097/MEG.0b013e3282f2bbf7. [DOI] [PubMed] [Google Scholar]
- 83.Udayakumar N, Subramaniam K, Umashankar L, Verghese J, Jayanthi V. Predictors of mortality in hepatic encephalopathy in acute and chronic liver disease: a preliminary observation. J Clin Gastroenterol. 2007;41:922–6. doi: 10.1097/01.mcg.0000225639.45157.ee. [DOI] [PubMed] [Google Scholar]
- 84.Mjahed K, Charra B, Hamoudi D, Noun M, Barrou L. Acute fatty liver of pregnancy. Arch Gynecol Obstet. 2006;274:349–53. doi: 10.1007/s00404-006-0203-6. [DOI] [PubMed] [Google Scholar]
- 85.Banait VS, Sandur V, Parikh F, Murugesh M, Ranka P, Ramesh VS, et al. Outcome of acute liver failure due to acute hepatitis E in pregnant women. Indian J Gastroenterol. 2007;26:6–10. [PubMed] [Google Scholar]
- 86.Arankalle VA, Chadha MS, Dama BM, Tsarev SA, Purcell RH, Banerjee K. Role of immune serum globulins in pregnant women during an epidemic of hepatitis E. J Viral Hepat. 1998;5:199–204. doi: 10.1046/j.1365-2893.1998.00096.x. [DOI] [PubMed] [Google Scholar]