Abstract
Objectives
The objective of this paper is to describe the complex mixed-methods design of a study conducted to assess health-related quality of life (HRQOL) outcomes and ostomy-related obstacles and adjustments among long-term (>five years) colorectal cancer (CRC) survivors with ostomies (cases) and without ostomies (controls). In addition, details are provided regarding the study sample and the psychometric properties of the quantitative data collection measures used. Subsequent manuscripts will present the study findings.
Research Design and Methods
The study design involved a cross-sectional mail survey for collecting quantitative data and focus groups for collecting qualitative data. The study subjects were individuals identified as long-term CRC survivors within a community-based health maintenance organization's enrolled population. Focus groups comprised of cases and divided by gender and HRQOL high and low quartile contrasts (based on the mail survey data) were conducted.
Main Outcome Measures
The modified City of Hope Quality of Life (mCOH-QOL)-Ostomy and SF-36v2 questionnaires were used in the mail survey. An abridged version of the mCOH-QOL-Ostomy was used for the control subjects. Focus groups explored ostomy-related barriers to self-care, adaptation methods/skills, and advice for others with an ostomy.
Results
The survey response rate was 52% (679/1308) and 34 subjects participated in focus groups. The internal consistency reliability estimates for the mCOH-QOL-Ostomy and SF-36v2 questionnaires were very acceptable for group comparisons. In addition, evidence supports the construct validity of the abridged version of the mCOH-QOL-Ostomy. Study limitations include potential non-response bias and limited minority participation.
Conclusions
We were able to successfully recruit long-term CRC survivors into this study and the psychometric properties of the quantitative measures used were quite acceptable. Mixed-methods designs, such as the one used in this study, may be useful in identification and further elucidation of common problems, coping strategies, and HRQOL outcomes among long-term cancer survivors.
Keywords: Colorectal cancer; Health-related quality of life; Ostomy; Patient-reported outcomes; Stomas, Qualitative research
INTRODUCTION
Colorectal cancer (CRC) is the third most frequent cancer in the US, accounting for 10% of all new cancer cases in men and 11% in women1. Five-year estimates of overall CRC survival from 1996−2002 were 64.1%, and recurrence-free survival rates were 37%−50%1,2. There are currently over 1.1 million persons alive in the US with a history of CRC3. Many of these individuals, especially those with low-rectal cancers, have received temporary or permanent intestinal stomas (ostomies), leaving a critical need to better understand how ostomies affect these individuals’ lives.
Ostomies are the surgical exteriorization of the small (ileostomy) or large (colostomy) bowel to the anterior abdominal wall, allowing intestinal waste to flow directly into an external pouch (appliance). Ostomies can be permanent, as in the case of low-rectal cancer, or temporary, to divert fecal flow related to emergent procedures or as protection of bowel anastomoses. While ostomies are much more common in rectal cancer patients, they may be necessary in the surgical treatment of colon cancer for several reasons, including an obstructing cancer, metastatic disease, or other medical concerns (e.g. currently taking steroids). The quality of life implications of the various CRC treatment options are a major consideration for patients4. A 2005 Cochrane review found that health-related quality of life (HRQOL) may not be better in rectal cancer survivors who were reconstructed without an ostomy when compared with those in whom an ostomy was required5. This review challenged the assumption that patients who received anterior resection without colostomies universally fared better, and concluded that larger, better-designed and executed prospective studies were needed.
There is a long history of research that has addressed the physical and psychosocial consequences of having an ostomy6-16. Regardless of the type of or reason for the ostomy, it can result in profound changes in a person's functioning and well-being17-19. Hence, it is well documented that ostomies adversely affect the lives of ostomates, but why some people adjust more easily than others or what best facilitates their adjustment, is not well understood. In the past, ostomy interventions have been based largely on clinician assumptions and researcher interests, rather than on an informed understanding of patient-centered concerns and needs. This study was designed to ascertain the subjective experience of living with an ostomy and to explore how it affects functioning and well-being by focusing on the self-assessed HRQOL of long-term CRC survivors living with, and without, an ostomy.
This study builds upon a program of research at the City of Hope National Medical Center17-19 and the Veterans Health Administration20-25. The current study focuses on patients in a community-based health maintenance organization (HMO) and broadens our program of HRQOL research to better reflect the general population of CRC survivors. The objective of this paper is to describe our complex mixed-methods design and the study sample. In addition, the psychometric properties of our quantitative measurement tools in the study sample are discussed. Subsequent manuscripts will present the study findings.
RESEARCH DESIGN AND METHODS
This study was focused on long-term (greater than five years) colorectal cancer survivors recruited from three regions of a national prepaid group practice HMO. Our data collection strategy was designed explicitly to combine quantitative elements of a structured survey instrument with qualitative elements of open-ended questions and focus group data collected in a sequential manner, integrating emerging data into each sequential step. By combining mixed methods in this study, our goal was to elicit important new insight into the causes and consequences of the challenges ostomates face, their adaptation processes, and the strategies they adopt for self-care.
Study Setting and Coordination
This multi-site study included three Kaiser Permanente (KP) Regions: Northern California (KPNC), Northwest (KPNW), and Hawaii (KPHI). The study was coordinated at the Southern Arizona VA Health Care System (SAVAHCS) in Tucson, Arizona. The study team held weekly conference calls to update data collection, review progress, and solve problems as a team. Survey packets included scannable forms programmed with Teleforms 6.2, (Copyright 2007 Cardiff) and developed at the SAVAHCS site, which were sent to potential respondents by their respective KP site. All returned survey instruments were copied at the respective sites, deidentified, and sent to SAVAHCS for scanning and data management. The Teleforms verification process has nearly 100% accuracy for fixed-choice questions, far exceeding keypunching strategies. Focus groups were held at the KPNC and KPNW sites only, due to budgetary and recruitment restrictions. KPNW's secure website allowed sites to exchange deidentified data and ensure privacy at all times.
Kaiser Permanente is the largest and oldest nonprofit health plan with headquarters in the US. Although only KPNC, KPNW, and KPHI were sites for this study, KP serves the health care needs of 8.4 million members in nine states (California, Colorado, Georgia, Hawaii, Maryland, Ohio, Oregon, Virginia, and Washington) and Washington, D.C. Approximately 77% of KP members are age 18 and older, and about 0.06% are diagnosed with CRC each year. Enrollees are generally representative of the adult employed population in KP's service areas. While KP is often seen as one unified health care system because of its national electronic medical record system, each region is independent and maintains unique procedures.
Human Subjects Approval
KP institutional review boards (IRBs) at each site (KPNC, KPNW, and KPHI) and the SAVAHCS coordinating site's IRB, located at the University of Arizona, reviewed all IRB documents, with the University of Arizona maintaining overall regulatory oversight. There was no formal consent for the study's survey portion of the study. Each survey packet had a cover letter with all the elements of informed consent, including clear explanations of the study and risks, which were mainly related to potential breach of confidentiality. The process was fully compliant with all applicable Health Insurance Portability and Accountability Act (HIPAA) requirements. Therefore, if the patient completed the survey instrument, he or she provided implied consent to participate in the study. Each focus group participant signed informed consent and HIPAA documents prior to participation. Participants were provided a $5 gift certificate for participation in the survey and a $25 gift certificate for participation in the focus groups.
Phase I Cross-Sectional Survey
Eligibility and Identification of Subjects
Persons eligible for participation in this study had to meet the following inclusion criteria: (1) current KP member; (2) age 18 or older; (3) diagnosed with CRC at least five years prior to survey; and (4) history of a major gastrointestinal procedure including major resections of the colon, rectum, or anus that did (cases) or did not (controls) result in a stoma. In addition, the following exclusion criteria were applied: (1) CRC patients in whom the stoma was reversed; (2) severe mental illness or cognitive impairment that would impede ability to complete the survey; and (3) KP member who had requested no contact (0.01%).
Potential participants were identified through an electronic search of each site's computerized tumor registry. In order to meet their recruitment objectives, additional eligible subjects at KPNW and KPHI were identified by searching (1) electronic medical records for CRC diagnoses and ostomy procedures, (2) pharmacy records for ostomy supplies, and (3) stoma nurse logs for active cases with colorectal cancers. CRC patients identified through these three steps had enrolled in KPNW or KPHI after their cancers had been diagnosed and treated elsewhere. This was not necessary at the KPNC site. A comparison of subject recruitment by each method showed no differences in socio-demographic or clinical variables.
For the purposes of this study, “cases” were CRC survivors with an intestinal stoma. “Controls” were CRC survivors who had surgical procedures that did not result in a permanent stoma. Controls were matched to cases by the following hierarchy: 1) tumor site (rectum versus colon); 2) gender; 3) age group; and 4) years since diagnosis (in five-year groups), or closest age if unable to match within plus or minus five years. Gastrointestinal tract level of surgical procedure is critical because surgery in the area of the rectum, whether leading to an ostomy or not, entails a high risk of injuring the nerves controlling sexual and bladder function. The control group was used to assess whether general HRQOL problems were related specifically to the stoma or to CRC-related health conditions and/or history of surgery.
Measures
Health-related quality of life measures included the modified City of Hope Quality of Life (mCOH-QOL)-Ostomy questionnaire18 and the SF-36v226. Both the mCOH-QOL-Ostomy and the SF-36v2 have demonstrated high reliability and validity in numerous patient samples18-20,26-28. Using both measures allowed the assessment of both general and ostomy-specific aspects of HRQOL. The questionnaires were converted into “scannable” forms, formatted by a graphic artist, and pilot tested for acceptability and ease of self-administration.
The mCOH-QOL-Ostomy questionnaire consists of socio-demographic items and scaled and non-scaled HRQOL-related items. Utilizing the City of Hope four-dimensional framework18, the mCOH-QOL-Ostomy questionnaire provides scale scores for the following domains: physical well-being (11 items), psychological well-being (13 items), social well-being (12 items), and spiritual well-being (6 items). In addition, an overall HRQOL score can be calculated. Due to the inclusion of ostomy-specific items in the mCOH-QOL-Ostomy, an abridged version of the questionnaire was used in this study for the control subjects. The six items in the spiritual well-being scale remained unchanged, but the physical (8 items), psychological (12 items), and social well-being (8 items) scales were reduced by one or more items. For both versions of the mCOH-QOL-Ostomy, the items, subscales, and overall score are on an 11-point scale where 0 = poorest and 10 = best functioning and/or well-being. The mCOH-QOL-Ostomy also includes several optional open-ended questions related to ostomy equipment problems, ostomy location issues, irrigation practices, and the “greatest challenge encountered in having an ostomy.” These questions were included in the version for the cases but not in the abridged version for the controls.
The SF-36v2 is an improved version of the SF-36, one of the most utilized measures of functional health and well-being in the world today27,28. The SF-36v2 produces scores for the following eight multi-item scales: physical functioning, role limitations due to physical health problems, pain, general health perceptions, mental health, role limitations due to emotional problems, social functioning, and energy/fatigue. The scale scores can range from 0 to 100, with higher scores reflecting better functioning or well-being. In addition, physical (PCS) and mental (MCS) component summary scores can be calculated from the SF-36v2 based on a population norm-based scoring function26. SF-36v2 responses were also scored with a preference-weighting system to obtain an overall HRQOL (SF-6D) score for each respondent. Scale and summary scores were calculated with QualityMetric Health Outcomes Scoring Software 2.0 (copyright QualityMetric, Lincoln, Rhode Island, USA 2004−2007).
Survey Recruitment and Processing
The mail survey packet comprised a cover letter, a composite questionnaire that included the mCOH-QOL-Ostomy and the SF-36v2, and a postage-paid return envelope. The institutional PI mailed each packet. Survey materials were mailed out over a two-year period as subjects were identified and/or became eligible for participation. Two weeks after each survey packet was mailed, potential subjects who had not yet returned the study questionnaire were contacted by phone. If they were interested in participating, individuals were asked to complete the questionnaire and return it in the postage-paid envelope, or if they preferred they were given the option to answer the questionnaire items over the phone. Patients refusing participation were no longer contacted.
Sample Size
Previous studies of persons with stomas identified seven variables (appearance, anxiety, being “in control,” travel, sports/recreation, intimacy and “feelings about the future”) as key issues affecting HRQOL in this population29. Based on means and standard deviations of these items and mCOH-QOL-Ostomy scales measured in ostomates (i.e., persons with ostomies) previously, a sample size of 300 cases and 300 controls would provide 85% power or greater to detect a 0.5-point difference in the total HRQOL score and subscales, with the exception of the spiritual well-being subscale (72% power), and provide 90% power or greater to detect a 1.0-point difference in the key items of concern, with the exception of intimacy (85% power) 18.
Computerized Clinical and Administrative Data
A comprehensive socio-demographic and medical history was obtained for study participants from each site's automated information system. Medical history items included type of stoma, reason for stoma, length of time since surgery, stage of disease, type of operation, operative findings, and other medical problems. The Charlson-Deyo comorbidity index was constructed from ICD-9-CM codes from outpatient and inpatient encounters during the year prior to survey completion, which provided a view of the co-morbidities and health care utilization at the time leading up to the survey30-33. We also constructed a separate Charlson-Deyo index based on ICD-9-CM codes during the 12 months after the date of CRC diagnosis. In addition, we collected information on cancer-related symptoms, complications, and treatments during each of these 12-month periods. Since patients ranged from five years to more than 20 years past their initial cancer diagnosis, time since initial diagnosis was a critical variable to define “cohorts” of CRC survivors. We collected information on each patient's sequence of primary tumors and metastases and where the index CRC tumor fits within this sequence.
Statistical Analysis of Survey and Clinical/Administrative Data
We compared demographic and clinical characteristics between cases and controls using the Student's t-test for continuous measures and the chi-square test for categorical measures. Reliability was assessed using Cronbach's alpha for mCOH-QOL-Ostomy and SF-36v2 scales in cases and controls. Differences between cases and controls on measures from the mCOH-QOL-Ostomy and SF-36v2 were determined with multiple linear regression, adjusting for potential confounding variables, in Stata version 10 (Copyright StataCorp LP 1996−2007, College Station, Texas, USA) . Variables that had been hypothesized a priori as potential confounders based on clinical literature and experience included age, time since index surgery (or time since CRC diagnosis), the Charlson-Deyo comorbidity index for year prior to the survey, and married/partnered status. Other potential confounders included race/ethnicity, income, and education. We also evaluated gender as a potential effect modifier, as suggested by our previous work in veterans with stomas. Comparisons of individual measures within an instrument were subjected to a Bonferroni adjustment.
Survey Text Data
Qualitative analysis was conducted on open-ended responses in the mCOH-QOL-Ostomy questionnaire relating to stoma equipment, location, irrigation practices, and greatest challenges. The investigators individually reviewed the responses using content analysis to quantify and analyze the presence, meanings, and relationships of words and concepts on the verbatim written transcripts using four linked processes: processing the raw data, data reduction, data display, and conclusion drawing/verification34. We employed an a priori four-dimensional mCOH-QOL-Ostomy framework (i.e., physical, psychological, social, and spiritual well-being)18.
Original data were de-identified and assigned a subject ID. Investigators, trained in qualitative content analysis, read and examined all data to identify units of analysis, which were defined as a paragraph, sentence, verb phrase, or single word that conveyed a single meaning, idea, or concept35. Responses frequently had several themes within an individual essay. While some responses were divided into two or more themes, individual statements were not double coded. At least three investigators coded each essay. Differences were discussed with the entire investigative team, which decided final coding. Data within each theme were compared using thematic analysis. All categories (representing themes) were identified and validated through discussion, review, verification, and consensus. This review occurred during weekly conference calls. Text that corresponded to a domain in the mCOH-QOL-Ostomy questionnaire were categorized and displayed in a table organized according to the a priori domains. Comments that did not correspond to a category within a domain, but were deemed significant based on repetition and emphases throughout the data, were categorized under a miscellaneous domain. Using constant comparative analysis of these comments, two additional domains were identified: ostomy-specific content, and health care issues. Two investigators completed a final validation review to ensure consistency and clarity across all data.
Phase II Focus Group Study
Focus Group Selection and Recruitment
Focus groups comprised of cases further delineated barriers and concerns, in addition to adaptation methods and skills. We identified the break variables, by which segregation into different focus groups should occur, to determine the number of focus groups required. Based on the mCOH-QOL-Ostomy questionnaire overall score, our goal was to include subjects who successfully adapted (in the highest HRQOL quartile), as well as those who were extremely challenged with stomal issues (in the lowest HRQOL quartile). Gender was the second break variable, as our experience indicated that issues of body image, role adjustment, and sexuality commonly differ between genders. Focus groups were conducted at two sites—Oakland, California and Portland, Oregon—to account for regional treatment variation. Our goal was to recruit four to eight same-gender, high-quartile and low-quartile participants at each site to provide adequate “saturation” (the point at which the moderator is hearing the same issues repeated with no new ideas arising).
Focus Group Format
Focus groups were recorded with digital audio-recorders for later transcription. A discussion guide, with a series of overarching questions, was presented in an open-ended fashion. Open-ended questions included:
- After you had the surgery, which medical staff (nurses, doctors, social workers, anyone else?) was least helpful to you in learning to live with your stoma?
- ○ What else would you have liked them to have done for you/told you?
- ○ How has your medical system not helped with your ostomy care?
- Tell me about the daily care of your ostomy.
- ○ Do you irrigate your ostomy? If so, why?
- ○ Have you ever tried irrigating your ostomy?
- ○ If you stopped irrigating your ostomy, why did you?
- If you have other medical conditions, how do those conditions affect your daily life in addition to having a stoma?
- ○ Do you need more help in caring for your ostomy because of your other medical problems?
- ○ Do other medical problems interfere with your ostomy care?
If you had a close friend or family member who was going to have an ostomy, what advice, based on your experiences, would you give him/her about learning to live with it?
Prompts were listed for each major question to help remind the moderator of topics to be discussed under that question, and for the moderator to use if the topic did not arise spontaneously. The group facilitator (MG) for each session was experienced in this role. In addition, there was a silent recorder (RK), who observed and took notes throughout each focus group in order to record participant's statements to help clarify transcriptions and document field observations regarding participant behavior (e.g., early or late arrival time; demeanor in responding to focus group topics) and unobtrusive measures (e.g., manner of dress for the focus groups, race/ethnicity). These data assisted us in developing and enhancing themes relevant to the worldview of individuals with ostomies and the concomitant impact on their lives. Each focus group lasted approximately two hours, which enabled the moderator to cover most or all topics while allowing for continued elaboration of some points. Focus group audiotapes were transcribed verbatim for qualitative analysis, with the exception that names were replaced by focus group ID number.
Focus Group Transcripts Review and Analysis
Two qualitatively trained clinical investigators independently reviewed all focus-group transcripts to illuminate potential categories, using a deductive a priori City of Hope four-dimensional framework17-19 comprised of four domains: physical well-being, psychological well-being, social well-being, and spiritual well-being. The audio recorded narrative was transcribed as rich text format, and content analyzed using HyperRESEARCH (Copyright 1997−2007, ResearchWare, Inc., Randolph, MA, USA). We developed categories within these domains, coding relevant selections within the transcripts. Two new domains emerged: ostomy-specific content and health-care issues, and were added to our conceptual framework. Emergent themes were compared to determine similarities and differences by cut points and sites. Two investigators completed a final validation review to ensure consistency and clarity across all data. Selections that were discordantly coded (10−15%) were discussed in order to further refine and specify the coding.
RESULTS: STUDY ACCRUAL AND PSYCHOMETRIC PROPERTIES
Phase I- Survey
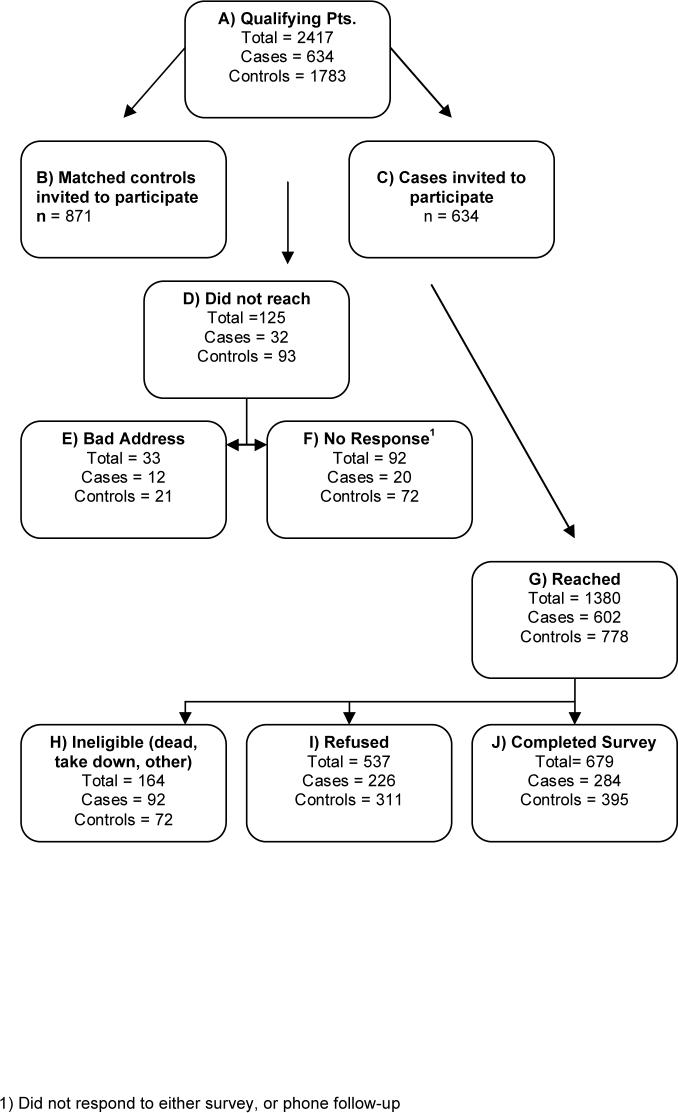
Participant Flow and Recruitment. Completed survey instruments were obtained from 284 cases and 395 controls across the three sites, representing a 52% (679/1308) response rate. (Based on Figure 1, the denominator [1308] for the calculation of the response rate is the sum of J [679], I [537], and F [92].) Just under 20% (n=133) of the survey instruments were completed via a telephone interview, which took approximately 30 minutes on average. The flow of potential subjects from identification through survey completion is shown in Figure 1. The non-response rate was higher among controls than cases in KPNC (p<0.001), but not in the other two sites. The number of study packets mailed from each site and the reasons for non-participation, including refusal, are provided in Table 1.
Figure 1.
All Sites KP Ostomy Survey Response Diagram
Table 1.
Number of mailings per site and reasons for non-participation
| Sites | KPNC | KPNW | KPHI | p-value |
|---|---|---|---|---|
| Mailings | 980 | 383 | 142 | |
| Incorrect address | 2.8% | 1.6%, | 0% | (p=0.054)* |
| No response** | 8.8% | 0.8% | 2.1% | (p<0.001)* |
| Refusal | 44% | 35% | 61% | (p=0.001)*** |
KPNC = Kaiser Permanente Northern California
KPNW = Kaiser Permanente Northwest
KPHI = Kaiser Permanente Hawaii
Fisher's exact test
Did not respond to survey mailing or follow-up phone calls
Chi-Squared test
Overall response (i.e., recruitment) rates were 51% (KPNC), 62% (KPNW), and 38% (KPHI) (p<0.001) and were higher among cases than controls in KPNW and KPHI, but not in KPNC. Of the case respondents, 81.7% (232/284) responded to one or more of the open-ended questions. Response to open-ended questions among the 284 cases included 176 (62%) to the “greatest challenge living with ostomy,” 124 (44%) to pouch problems, and 81 (29%) to ostomy location problems. Responses varied in length from one to 909 words.
Survey Respondent Socio-Demographic and Clinical Characteristics
No differences were noted in the socio-demographic characteristics of cases or controls (Table 2). Cases and controls had comparable median time elapsed since surgery, which had skewed right distributions for both groups (9.7 vs. 10.0, p=0.68). A difference in the mean Charlson-Deyo score was noted; this was statistically significant (p=0.002) in our large study sample but not likely to be clinically significant.
Table 2.
Socio-demographic and clinical characteristics for cases and controls
| Characteristic |
Cases (n = 284) |
Controls (n = 395) |
p-value |
|---|---|---|---|
| Mean age (SD) |
72.4 (10.3) |
71.1 (11.2) |
0.13 |
| Male gender (%) |
58.7 |
59.0 |
0.95 |
| Race/Ethnicity (%) | |||
| White, non-Hispanic | 74.8 | 78.9 | 0.22 |
| Black, non-Hispanic | 3.6 | 3.6 | |
| Hispanic/Latino | 7.5 | 3.6 | |
| Asian | 9.6 | 8.4 | |
| Other/Unknown/Mixed |
4.6 |
5.6 |
|
| Education (%) | |||
| Not a high school graduate | 12.5 | 9.9 | 0.77 |
| High school graduate | 26.7 | 22.9 | |
| Vocational school degree | 5.3 | 6.8 | |
| Some college, no degree | 22.4 | 25.2 | |
| College graduate | 16.4 | 17.1 | |
| Some graduate school, no degree | 6.1 | 7.0 | |
| Graduate school degree |
10.7 |
11.2 |
|
| Annual Household Income (%) | |||
| $15,000 or less | 10.1 | 7.1 | 0.22 |
| $15,001 to $30,000 | 25.9 | 21.5 | |
| $30,001 to $50,000 | 25.5 | 27.1 | |
| $50,001 to $75,000 | 15.7 | 16.5 | |
| $75,001 to $100,000 | 9.4 | 7.9 | |
| More than $100,000 | 4.9 | 7.9 | |
| Unknown/no answer |
8.4 |
12.2 |
|
| Married/Partnered Prior to Surgery (%) |
74.7 |
77.4 |
0.41 |
| Married/Partnered Currently (%) |
62.8 |
65.1 |
0.54 |
| Employment (%) | |||
| Full-time | 11.9 | 15.7 | 0.48 |
| Part-time | 8.7 | 7.1 | |
| Retired | 75.2 | 72.7 | |
| Unemployed/unknown |
4.2 |
4.6 |
|
| Years since surgery | |||
| Median | 9.6 | 10.0 | 0.74* |
| Inter-quartile range (25th to 75th) |
6.2 − 15.2 |
6.8 − 14.0 |
|
| Charlson-Deyo Comorbidity Score (SD) |
1.8 (2.0) |
1.4 (1.7) |
0.002 |
| Tumor Location (%) | |||
| Colon | 11.8 | 37.5 | <0.001 |
| Rectum | 88.2 | 62.5 |
Mann-Whitney test
Psychometric Analysis
Cronbach's alpha was used to test the internal consistency reliability of the mCOH-QOL-Ostomy and SF-36v2 questionnaires in our study sample. According to Nunnally and Bernstein, the minimum standard for an acceptable level of reliability is 0.70 for group comparisons36. As described above, the mCOH-COL-Ostomy questionnaire used for the controls had 8 fewer scaled items than the standard version used for the cases; hence, the reliability of the versions was evaluated separately. The scales for both versions of the mCOH-QOL-Ostomy questionnaire demonstrated nearly identical and quite acceptable levels of reliability (Table 3). In addition, the SF-36v2 scales also proved to be highly reliable in our study sample.
Table 3.
| mCOH-QOL-Ostomy | ||||||||
|---|---|---|---|---|---|---|---|---|
| Group | Physical Well-being | Psychological Well-being | Social Well-being | Spiritual Well-being | Overall | |||
| Cases | 0.85 | 0.85 | 0.90 | 0.80 | 0.94 | |||
| Controls | 0.85 | 0.84 | 0.90 | 0.80 | 0.93 | |||
| SF-36v2 | ||||||||
| PF | RP | BP | GH | VT | SF | RE | MH | |
| Total | 0.94 | 0.95 | 0.89 | 0.85 | 0.88 | 0.81 | 0.93 | 0.88 |
City of Hope Quality of Life-Ostomy questionnaire
SF-36 Version 2
PF = physical functioning; RP = role limitations due to physical problems; BP = bodily pain; GH = general health perceptions; VT = vitality; SF = social functioning; RE = role limitations due to emotional problems; MH = mental health
Since the mCOH-QOL-Ostomy questionnaire was abridged for use in control subjects, we tested the construct validity of the abridged version relative to that of the full version that was used in the cases. Convergent evidence for its construct validity was demonstrated by correlating the three mCOH-QOL-Ostomy scale scores (physical, psychological, and social well-being) for each version with the scores from corresponding scales on the SF-36v2 (physical functioning, mental health, and social functioning). The results demonstrated relatively similar Pearson product-moment correlations in terms of both direction and magnitude for the two versions. The correlations were 0.34 vs. 0.46, 0.71 vs. 0.67, and 0.43 vs. 0.38 for cases and controls in the physical, psychological, and social scales, respectively. All of the correlations were significant (p<0.0001). These data provide evidence that the removal of the ostomy-specific items from the mCOH-QOL-Ostomy questionnaire did not meaningfully diminish the construct validity of the measure in the control subjects. In fact, the convergent evidence for construct validity in the abridged physical well-being scale in the control subjects is even stronger than that for the original version in the cases. Table 4 provides additional evidence of very similar measurement properties for the two versions of the mCOH-QOL-Ostomy questionnaire. Although the abridged version had more respondents at the ceiling of the scales, neither version had substantial floor or ceiling effects in our study subjects.
Table 4.
Measurement properties of the two versions* of the mCOH-QOL-Ostomy questionnaire
| Scale | n | Number of items | Mean** | SD | SEM | % at Floor | % at Ceiling |
|---|---|---|---|---|---|---|---|
| Cases | |||||||
| Total QOL | 284 | 43 | 7.21 | 1.58 | 0.39 | 0.0 | 0.0 |
| Physical well-being | 284 | 11 | 7.34 | 1.80 | 0.69 | 0.0 | 3.2 |
| Psychological well-being | 284 | 13 | 7.21 | 1.76 | 0.68 | 0.0 | 1.8 |
| Social well-being | 284 | 12 | 7.23 | 2.15 | 0.69 | 0.0 | 6.0 |
| Spiritual well-being | 282 | 6 | 6.86 | 2.27 | 1.02 | 0.0 | 4.6 |
| Controls | |||||||
| Total QOL | 395 | 35 | 7.67 | 1.46 | 0.39 | 0.0 | 0.3 |
| Physical well-being | 395 | 8 | 7.48 | 2.00 | 0.77 | 0.0 | 11.4 |
| Psychological well-being | 395 | 12 | 7.66 | 1.55 | 0.61 | 0.0 | 1.8 |
| Social well-being | 395 | 8 | 8.34 | 1.96 | 0.63 | 0.0 | 18.2 |
| Spiritual well-being | 394 | 6 | 7.01 | 2.21 | 0.99 | 0.0 | 8.4 |
Cases completed the standard version of the City of Hope Quality of Life (mCOH-QOL)-Ostomy questionnaire. Controls completed the abridged version of the mCOH-QOL-Ostomy Questionnaire.
Based on an 11-point scale where 0 = poorest and 10 = best functioning and/or well-being.
SD= standard deviation
SEM = standard error of measurement
Phase II- Focus Groups
Patient Participation
Focus group participation was greater at KPNC (Table 5), although meaningful discussions occurred within all groups. Participants were overwhelmingly non-Hispanic white, although we did have one African American, one Asian, and one multi-racial participant. Groups of low-HRQOL subjects had more no-shows. Reasons contributing to low participation rates included living a great distance from the centers, other medical problems, and forgetting to attend the focus group. Inter-rater reliability in focus group coding was high, with less than 10% disagreement. Where disagreement was present, the entire research team made coding decisions.
Table 5.
Focus group participants
| Focus Group | KPNW | KPNC | Total |
|---|---|---|---|
| High* Males | 4 | 8 | 12 |
| High Females | 5 | 4 | 10 |
| Low* Males | 3 | 2 | 5 |
| Low Females | 3 | 4 | 7 |
| Totals | 15 | 18 | 34 |
KPNW = Kaiser Permanente Northwest
KPNC = Kaiser Permanente Northern California
“High” and “Low” indicate that the participant was in the high (better) or low (worse) quartile in the distribution of overall quality of life scores on the mCOH-QOL-Ostomy, respectively.
DISCUSSION
For CRC survivors with a permanent ostomy, the literature demonstrates that the ostomy has a significant impact on their day-to-day lives37-52. There are approximately 153,760 new cases of CRC each year in the US53 and an estimated 1.1 million colorectal cancer survivors.3 Colorectal cancer screening has decreased the incidence of CRC overall and the severity of CRC at diagnosis. Despite a decrease in the frequency of stoma formation in incident CRC, there will continue to be large numbers of patients requiring a stoma related to CRC. The medical community must not neglect these patients’ HRQOL concerns. In addition, as clearly articulated in the Institute of Medicine's report titled “From Cancer Patient to Cancer Survivor: Lost in Transition,” knowledge of the medical, functional, and psychosocial consequences of cancer and its treatment is essential if the best possible outcomes for cancer survivors are to be achieved54.
One of the strengths of this study is that we focused on the HRQOL of an understudied population (i.e., community-dwelling long-term CRC survivors). While this population is well-defined, little is known about long-term management and self-care issues55. We were able to accrue a large sample of survey respondents, providing relatively high power for quantitative analysis of patient-reported outcomes and clinical factors between cases and controls. Our control group was well-matched on multiple characteristics, except site of tumor (rectal vs. colon), for which we will control in subsequent analyses.
While we did not specifically query survey respondents regarding the time it took to complete the questionnaires, there was likely a moderate response burden. Importantly, many subjects indicated in their open-ended responses that they welcomed the opportunity to speak about and impart their experience and knowledge regarding this important issue.
In addition, there are several limitations that arose due to the study's design complexity and objectives. The mail survey component introduced the potential for response bias based on systematic differences between responders and non-responders. Our overall response rate was over 50%, which is a common target for community surveys56. However, although we had hoped to compare the socio-demographic characteristics of non-responders to responders, privacy regulations prohibited this. In addition, while our control subjects appeared to be similar to our cases, there were different distributions for site of tumors. While this is an important matching criterion, we felt it was important to be inclusive of all response data and will control for site in our subsequent analyses.
We conducted focus groups that included multiple sessions limited to a small number of participants. Optimal group size can be determined by four critical factors: 1) the number of questions asked; 2) the allotted time for each question; 3) the format of the focus group session; and 4) the duration of the session. While larger groups may be more resource efficient, smaller groups can enhance the free exchange of ideas and personal experiences57. However, a limitation of our study is that we had few minority focus group participants, despite our considerable attempts to identify and include them in these groups using minority recruitment methods58.
SUMMARY AND CONCLUSIONS
The objective of this paper was to describe our study objectives, complex mixed-methods design, and patient sample. Subsequent manuscripts will present our study findings. Mixed-methods designs, such as the one described here, are especially applicable in HRQOL studies, since these designs can incorporate the collection of quantitative data to allow comparison between or among groups as well as qualitative data, for which the subject is the authority and issues can emerge which might otherwise be missed. Findings of this study may help to identify and further elucidate common problems and coping strategies employed by long-term CRC survivors with ostomies and more credibly inform the development of interventions aimed at meeting this population's educational, medical, psychosocial, and self-care needs.
Acknowledgements
The authors gratefully acknowledge the assistance of the following people in the conduct of this research, data analysis, and manuscript preparation: Eric Matayoshi, MD, Site PI, Kaiser Permanente Hawaii, Honolulu, HI; Carmit McMullen, PhD, Co-Investigator and Kaiser Permanente Northwest Project Coordinator, Portland, OR; Carol Baldwin, PhD, RN, Co-Investigator, Arizona State University; Sylvan Green, MD, Co-Investigator, University of Arizona; Andrea Altschuler, PhD, Co-Investigator, Kaiser Permanente Division of Research, Oakland, CA; Deborah Harrison, Primary Study Coordinator and Site Study Coordinator, Southern Arizona Veterans Affairs Health Care System, Tucson, AZ; Bernie Collins, PhD, Project Coordinator, Kaiser Permanente Division of Research, Oakland, CA; Cyndee Yonahara-Lau, Project Coordinator, Kaiser Permanente Moanalua Medical Center, Honolulu, HI; Mary Wagner, Administrative Assistant, Southern Arizona Veterans Affairs Health Care System, Tucson, AZ.
Footnotes
Research Support:
This research was performed by the SAVAHCS/Kaiser Permanente Collaborative Research Group and was made possible by Grant Number R01 CA106912, HR-QOL in Colorectal Cancer Survivors with Stomas from the National Cancer Institute, National Institutes of Health in collaboration with resources and the use of facilities provided at the Southern Arizona Veterans Affairs Health Care System, Tucson, Arizona.
References
- 1.Ries LAG, Harkins D, Krapcho M, et al. SEER Cancer Statistics Review, 1975−2003. National Cancer Institute; Bethesda, MD: 2006. http://seer.cancer.gov/csr/1975_2003/, based on November 2005 SEER data submission, posted to the SEER web site 2006). Retrieved December 6, 2006. [Google Scholar]
- 2.Platell CF, Semmens JB. Review of survival curves for colorectal cancer. Dis Colon Rectum. 2004;47:2070–5. doi: 10.1007/s10350-004-0743-4. [DOI] [PubMed] [Google Scholar]
- 3.Mariotto AB, Yabroff KR, Feuer EJ, et al. Projecting the number of patients with colorectal carcinoma by phases of care in the US: 2000−2020. Cancer Causes Control. 2006;17:1215–26. doi: 10.1007/s10552-006-0072-0. [DOI] [PubMed] [Google Scholar]
- 4.Solomon MJ, Pager CK, Keshava A, et al. What do patients want? Patient preferences and surrogate decision making in the treatment of colorectal cancer. Dis Colon Rectum. 2003;46:1351–7. doi: 10.1097/01.DCR.0000084432.45536.83. [DOI] [PubMed] [Google Scholar]
- 5.Pachler J, Wille-JørgensenJorgensen P. Quality of life after rectal resection for cancer, with or without permanent colostomy. The Cochrane Database of Systematic Reviews. 2005;(2) doi: 10.1002/14651858.CD004323.pub3. Art. No.: Sys Rev. 2004; (3):CD004323.pub3. DOI: 10.1002/14651858.CD004323.pub3. [DOI] [PubMed] [Google Scholar]
- 6.Sutherland AM, Orbach CE, Dyk RB, Bard M. The psychological impact of cancer and cancer surgery: I. Adaptation to the dry colostomy: preliminary report and summary of findings. Cancer. 1952;5:857–72. doi: 10.1002/1097-0142(195209)5:5<857::aid-cncr2820050503>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 7.Wirsching M, Druner HU, Herrmann G. Results of psychosocial adjustment to long-term colostomy. Psychother Psychosom. 1975;26:245–56. doi: 10.1159/000286938. [DOI] [PubMed] [Google Scholar]
- 8.Fazio VW, Fletcher J, Montague D. Prospective study of the effect of resection of the rectum on male sexual function. World J Surg. 1980;4:149–51. doi: 10.1007/BF02393562. [DOI] [PubMed] [Google Scholar]
- 9.Hurny C, Holland JC. Psychosocial sequelae of ostomies in cancer patients. CA Cancer J Clin. 1985;36:170–83. doi: 10.3322/canjclin.35.3.170. [DOI] [PubMed] [Google Scholar]
- 10.Grunberg KJ. Sexual rehabilitation of the cancer patient undergoing ostomy surgery. J Enterostomal Ther. 1986;13:148–52. doi: 10.1097/00152192-198607000-00039. [DOI] [PubMed] [Google Scholar]
- 11.Thomas C, Madden F, Jehu D. Psychological effects of stomas- I. Psychosocial morbidity one year after surgery. J Psychosom Res. 1987;31:311–6. doi: 10.1016/0022-3999(87)90050-x. [DOI] [PubMed] [Google Scholar]
- 12.Keyes K, Bisno B, Richardson J, Marston A. Age differences in coping, behavioral dysfunction and depression following colostomy surgery. Gerontologist. 1987;27:182–4. doi: 10.1093/geront/27.2.182. [DOI] [PubMed] [Google Scholar]
- 13.Klopp AL. Body image and self-concept among individuals with stomas. J Enterostomal Ther. 1990;17:98–105. doi: 10.1097/00152192-199005000-00017. [DOI] [PubMed] [Google Scholar]
- 14.Hojo K, Vernava AM, III, Sugihara K, Katumata K. Preservation of urine voiding and sexual function after rectal cancer surgery. Dis Colon Rectum. 1991;34:532–9. doi: 10.1007/BF02049890. [DOI] [PubMed] [Google Scholar]
- 15.Sprangers MAG, Taal BG, Aaronson NK, te Velde A. Quality of life in colorectal cancer: Stoma vs. non stoma patients. Dis Colon Rectum. 1995;38:361–9. doi: 10.1007/BF02054222. [DOI] [PubMed] [Google Scholar]
- 16.Nugent KP, Daniels P, Stewart B, Patankar R, Johnson CD. Quality of life in stoma patients. Dis Colon Rectum. 1999;42:1569–74. doi: 10.1007/BF02236209. [DOI] [PubMed] [Google Scholar]
- 17.Grant M. Quality of life issues in colorectal cancer. Developments in Supportive Cancer Care. 1999;3:4–9. [Google Scholar]
- 18.Grant M, Ferrell B, Dean G, et al. Revision and psychometric testing of the City of Hope Quality of Life-Ostomy Questionnaire. Qual Life Res. 2004;13:1445–57. doi: 10.1023/B:QURE.0000040784.65830.9f. [DOI] [PubMed] [Google Scholar]
- 19.Krouse R, Grant M, Ferrell B, et al. Quality of life outcomes in 599 cancer and non-cancer patients with colostomies. J Surg Res. 2007;138:79–87. doi: 10.1016/j.jss.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 20.Krouse RS, Mohler MJ, Wendel CS, et al. The VA Ostomy Health-Related Quality of Life Study: objectives, methods, and patient sample. Curr Med Res Opin. 2006;22:781–91. doi: 10.1185/030079906X96380. [DOI] [PubMed] [Google Scholar]
- 21.Krouse RS, Grant M, Wendel CS, et al. A mixed-methods evaluation of health-related quality of life for male veterans with and without intestinal stomas. Dis Colon Rectum. 2007;50(12):2054–66. doi: 10.1007/s10350-007-9004-7. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell KA, Rawl SM, Schmidt CM, et al. Demographic, clinical, and quality of life variables related to embarrassment in veterans living with an intestinal stoma. J Wound Ostomy Continence Nurs. 2007;34(5):524–32. doi: 10.1097/01.WON.0000290732.15947.9e. [DOI] [PubMed] [Google Scholar]
- 23.Jain S, McGory ML, Ko CY, et al. Comorbidities play a larger role in predicting quality of life compared to having an ostomy. Am J Surg. 2007;194(6):774–9. doi: 10.1016/j.amjsurg.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 24.Coons SJ, Chongpison Y, Wendel CS, et al. Overall quality of life and difficulty paying for ostomy supplies in the VA Ostomy Health-Related Quality of Life Study: an exploratory analysis. Med Care. 2007;45:891–5. doi: 10.1097/MLR.0b013e318074ce9b. [DOI] [PubMed] [Google Scholar]
- 25.Symms MR, Rawl SM, Grant M, et al. Sexual health and quality of life among male veterans with intestinal ostomies. Clin Nurs Spec. 2008;22(1):30–40. doi: 10.1097/01.NUR.0000304181.36568.a7. [DOI] [PubMed] [Google Scholar]
- 26.Ware JE, Kosinski M, Dewey JE. How to Score Version Two of the SF-36 Health Survey. QualityMetric Incorporated; Lincoln, RI: 2000. [Google Scholar]
- 27.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 28.Ware JE. SF-36 Health Survey update. In: Maruish ME, editor. The use of psychological testing for treatment planning and outcomes assessment. 3rd Edition Vol. 3. Lawrence Erlbaum Associates; Mahwah (NJ): 2004. pp. 693–718. [Google Scholar]
- 29.Andresen EM, Meyers AR. Health-related quality of life outcomes measures. Arch Phys Med Rehabil. 2000;81(12 Suppl 2):S30–45. doi: 10.1053/apmr.2000.20621. [DOI] [PubMed] [Google Scholar]
- 30.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 31.Needham DM, Scales DC, Laupacis A, Pronovost PJ. A systematic review of the Charlson comorbidity index using Canadian administrative databases: a perspective on risk adjustment in critical care research. J Crit Care. 2005;20:12–9. doi: 10.1016/j.jcrc.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Nuttall M, van der Meulen J, Emberton M. Charlson scores based on ICD-10 administrative data were valid in assessing comorbidity in patients undergoing urological cancer surgery. J Clin Epidemiol. 2006;59:265–73. doi: 10.1016/j.jclinepi.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 33.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–9. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 34.Miles MB, Huberman AM. Qualitative data analysis. Sage Publications; Thousand Oaks (CA): 1994. [Google Scholar]
- 35.Krippendorf K. Content analysis: An introduction to its methodology. Sage Publications; Beverly Hills: 1980. [Google Scholar]
- 36.Nunnally JC, Bernstein IH. Psychometric theory. 3rd ed McGraw-Hill; New York: 1994. [Google Scholar]
- 37.Lertsithichai P, Rattanapichart P. Temporary ileostomy versus temporary colostomy: a meta-analysis of complications. Asian J Surg. 2004;27:202–210. doi: 10.1016/S1015-9584(09)60033-6. discussion 211−2. [DOI] [PubMed] [Google Scholar]
- 38.Brown H, Randle J. Living with a stoma: a review of the literature. J Clin Nurs. 2005;14:74–81. doi: 10.1111/j.1365-2702.2004.00945.x. [DOI] [PubMed] [Google Scholar]
- 39.Burch J. Psychological problems and stomas: a rough guide for community nurses. Br J Community Nurs. 2005;10:224–7. doi: 10.12968/bjcn.2005.10.5.18051. [DOI] [PubMed] [Google Scholar]
- 40.Colwell JC. Dealing with ostomies: good care, good devices, good quality of life. J Support Oncol. 2005;3:72–4. [PubMed] [Google Scholar]
- 41.McKenzie F, White CA, Kendall S, et al. Psychological impact of colostomy pouch change and disposal. Br J Nurs. 2006;15:308–16. doi: 10.12968/bjon.2006.15.6.20678. [DOI] [PubMed] [Google Scholar]
- 42.O'Brien B, Baddi L, Benson A. Ostomy care: the added considerations for cancer patients. J Support Oncol. (3rd) 2005;3:71–2. [PubMed] [Google Scholar]
- 43.Wasserberg N, Kaiser AM, Nunoo-Mensah JW, et al. Preservation of bowel and urinary continence in the management of locally recurrent rectal cancer. J Surg Oncol. 2005;92:76–81. doi: 10.1002/jso.20371. [DOI] [PubMed] [Google Scholar]
- 44.Black PK. Psychological, sexual and cultural issues for patients with a stoma. Br J Nurs. 2004;13:692–7. doi: 10.12968/bjon.2004.13.12.13254. [DOI] [PubMed] [Google Scholar]
- 45.Borwell B. The psychosexual needs of stoma patients. Prof Nurse. 1997;12:250–5. [PubMed] [Google Scholar]
- 46.Caldwell K. Homosexuality: a neglected issue in stoma care. Br J Nurs. 1995;4:1009–12. doi: 10.12968/bjon.1995.4.17.1009. [DOI] [PubMed] [Google Scholar]
- 47.de Freitas MR, Pela NT. Contribution to the understanding of the sexuality of partners of people with permanent colostomy. Rev Lat Am Enfermagem. 2000;8:28–33. doi: 10.1590/s0104-11692000000500005. [DOI] [PubMed] [Google Scholar]
- 48.Golis AM. Sexual issues for the person with an ostomy. J Wound Ostomy Continence Nurs. 1996;23:33–7. doi: 10.1016/s1071-5754(96)90114-x. [DOI] [PubMed] [Google Scholar]
- 49.Junkin J, Beitz JM. Sexuality and the person with a stoma: implications for comprehensive WOC nursing practice. J Wound Ostomy Continence Nurs. 2005;32:121–8. doi: 10.1097/00152192-200503000-00009. [DOI] [PubMed] [Google Scholar]
- 50.Schover LR. Counseling cancer patients about changes in sexual function. Oncology (Williston Park) 1999;13:1585–91. [PubMed] [Google Scholar]
- 51.Sprunk E, Alteneder RR. The impact of an ostomy on sexuality. Clin J Oncol Nurs. 2000;4:85–8. [PubMed] [Google Scholar]
- 52.Tseng HC, Wang HH, Hsu YY, Weng WC. Factors related to stress in outpatients with permanent colostomies. Kaohsiung J Med Sci. 2004;20:70–7. doi: 10.1016/S1607-551X(09)70087-7. [DOI] [PubMed] [Google Scholar]
- 53.Norgaard M, Iversen LH, Sorensen HT. Colorectal cancer. Incidence and risk factors. Ugeskr Laeger. 2005;167:4157–9. [PubMed] [Google Scholar]
- 54.Hewitt M, Greenfield S, Stovall E, editors. From cancer patient to cancer survivor: lost in transition. National Academies Press; Washington, DC: 2005. [Google Scholar]
- 55.Aziz NM. Cancer survivorship research: State of knowledge, challenges and opportunities. Acta Oncol. 2007;46:417–32. doi: 10.1080/02841860701367878. [DOI] [PubMed] [Google Scholar]
- 56.Aday L. Designing and conducting health surveys. 2nd ed Jossey-Bass; San Francisco: 1996. [Google Scholar]
- 57.Trilling JS. Selections from current literature: focus group technique in chronic illness. Fam Pract. 1999;16:539–41. doi: 10.1093/fampra/16.5.539. [DOI] [PubMed] [Google Scholar]
- 58.Yancey AK, Ortega AN, Kumanyika SK. Effective recruitment and retention of minority research participants. Annu Rev Public Health. 2006;27:1–28. doi: 10.1146/annurev.publhealth.27.021405.102113. [DOI] [PubMed] [Google Scholar]