Abstract
Oxidative stress is implicated in the cognitive deterioration associated with normal aging as well as neurodegenerative disorders such as Alzheimer’s and Parkinson's diseases. We investigated the effect of ascorbic acid (vitamin C) on oxidative stress, cognition and motor abilities in mice null for gulono-γ-lactone oxidase (Gulo). Gulo−/− mice are unable to synthesize ascorbic acid and depend on dietary ascorbic acid for survival. Gulo−/− mice were given supplements that provided them either with ascorbic acid levels equal to- or slightly higher than wild-type mice (Gulo-sufficient), or with lower-than-physiological levels (Gulo-low) that were just enough to prevent scurvy. Ascorbic acid is a major antioxidant in mice and any reduction in ascorbic acid level is therefore likely to result in increased oxidative stress. Ascorbic acid levels in the brain and liver were higher in Gulo-sufficient mice than in Gulo-low mice. F4-neuroprostanes were elevated in cortex and cerebellum in Gulo-low mice and in the cortex of Gulo-sufficient mice. All Gulo−/− mice were cognitively normal but had a strength and agility deficit that was worse in Gulo-low mice. This suggests that low levels of ascorbic acid and elevated oxidative stress as measured by F4-neuroprostanes alone are insufficient to impair memory in the knockouts but may be responsible for the exacerbated motor deficits in Gulo-low mice, and that ascorbic acid may have a vital role in maintaining motor abilities.
Keywords: Oxidative stress, Cognition, Ascorbic acid, Motor deficits, Mice, Alzheimer’s disease
Introduction
Oxidative stress arises from an imbalance between the production of free radicals and physiological antioxidant capability (Andersen, 2004), and has been implicated in the cognitive impairments associated with normal aging and disorders such as Alzheimer’s disease, frontotemporal dementia, and HIV-related dementia (Andersen, 2004; Pratico, 2002; Munoz et al. 2006). The relationship among antioxidants, oxidative stress, and cognition has been explored in humans (Kang et al., 2006, Engelhart et al., 2002, Luchsinger et al., 2003) and mouse models (Tchantchou et al., 2005), but is not yet fully understood. Antioxidants such as ascorbic acid (Vitamin C) attenuate the amnestic effects of age, lesion, and the muscarinic receptor antagonist scopolamine in mice (Arzi et al. 2004; Parle and Dhingra, 2003; DeAngelis & Furlan, 1995). The noötropic effects of ascorbic acid may be directly related to its antioxidant properties, but it may also affect cognitive function by altering neurotransmission, neuromodulation, or second messenger signaling (Subramanian, 1977; Coffey & Hadden, 1985; Debler et al., 1991; Rice et al., 2002). For example, ascorbic acid has been shown to affect the number and binding affinity of cholinergic receptors in vitro, and induce changes in acetylcholinesterase levels in vivo (Ghosh et al., 1993; Dhingra et al. 2006; Knaack & Podleski, 1985; Knaack et al. 1986).
Unlike humans and other primates, rodents are able to synthesize ascorbic acid from glucose in situ. Because of this, studies of ascorbic acid deficiency in mice have been difficult to conduct. Recently, a mouse line has been generated with a targeted deletion of the gene that codes for the enzyme L-gulono-γ-lactone oxidase (Gulo; EC 1.1.3.8), which catalyzes the final step of ascorbic acid biosynthesis (Maeda et al. 2000). Gulo−/− mice are unable to synthesize ascorbic acid, and without dietary ascorbic acid supplements become scorbutic, lose weight, and eventually die. Ascorbic acid deficiency in Gulo−/− mice also leads to decreased plasma antioxidant capability, suggesting that these animals may be susceptible to increased levels of oxidative stress in the brain. The present study was designed specifically to address this question. Gulo−/− mice were supplemented with ascorbic acid in their drinking water, either at a concentration sufficient to produce wild-type organ ascorbic acid levels, or at a low concentration that was just enough to prevent scurvy. To determine whether increased oxidative stress in these mice would affect learning and memory, a battery of cognitive and sensorimotor tasks was conducted. The sensorimotor tasks served as direct measures of motor ability and also as control tasks to ascertain whether there were any differences between groups that could influence interpretation of data from the tests of learning and memory. Following behavioral testing, ascorbic acid levels were measured in the cortex, hippocampus, cerebellum, striatum and liver. Oxidative stress in the brain was measured by quantification of F4-neuroprostanes, a brain-specific marker of lipid peroxidation (Montuschi et al., 2007). We predicted that Gulo−/− mice receiving low levels of ascorbic acid would show increased oxidative stress in the brain and poorer cognitive functioning.
Materials and Methods
Animals
Gulo−/− mice were bred in-house from heterozygous Gulo+/− mice obtained from Mutant Mouse Regional Resource Centers (www.mmrrc.org, stock # 000015-UCD) and were maintained on a C57BL/6J background (Jackson laboratories, Bar Harbor, ME, USA stock #000664). Gulo−/− mice were mated to obtain litters of Gulo−/− mice for the present study. Wild-type Gulo+/+ (C57BL/6J) mice were used as controls for physiological ascorbic acid and oxidative stress levels. Approximately equal numbers of male and female mice were used in each experimental group in the present study.
Animals were housed by genotype in tub cages containing two-to-five mice per cage in a temperature- and humidity-controlled vivarium in the same suite of rooms in which the behavioral testing was conducted. Mice were kept on a 12:12-hour light:dark cycle with lights on at 6 am. Mice had free access to food and water for the duration of the experiment. All procedures were approved by the Vanderbilt University Institutional Animal Care and Use Committee and were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Ascorbic acid treatments
Gulo−/− mice require dietary supplements of ascorbic acid to maintain levels of ascorbic acid high enough to prevent scurvy. Data from previous studies with these mice (Maeda et al. 2000, Parsons et al. 2006) suggest that supplements of 0.33 g/l of drinking water provide enough ascorbic acid to maintain weight and health in these mice. A low level supplement was chosen following pilot testing in our lab designed to provide a level of ascorbic acid that was very low but did not result in scurvy. The mice were monitored carefully for any changes in weight and checked daily for other signs of sickness that could indicate the onset of scurvy (hunched posture, severe lassitude, hair changes). Therefore, drinking water was supplemented with either 0.33 g/l (sufficient) or 0.033 g/l (low) ascorbic acid. Treatments were given in deionized water with 20 µl 0.5 M EDTA/l to increase the stability of the ascorbic acid in solution. Water bottles were filled with fresh ascorbic acid-supplemented water twice each week. Breeding pairs, and all mice up to 6 weeks of age, were maintained on the sufficient treatment level. Mouse chow (Purina #5001) was available ad libitum throughout the course of the experiment and contained a very small amount of ascorbic acid (100 mg/kg diet). At 6 weeks of age mice were randomly assigned to treatment groups and some of the Gulo−/− mice were switched to the low concentration of ascorbic acid supplementation for the remainder of the study. Wild-type controls received deionized water with no additional ascorbic acid so we could determine whether brain ascorbic acid and other factors reached normal levels in the Gulo-sufficient group. Group composition was as follows: Gulo-sufficient 6 male, 7 female; Gulo-low 9 male, 12 female; wild-type control 16 male, 7 female.
Neurochemistry
Mice were briefly anaesthetized with isoflurane and killed by decapitation. The brain was quickly removed from the skull and divided in half at the midline. Hemispheres were carefully dissected and the hippocampus, striatum, cerebellum and frontal and medial cortex removed as previously described (McDonald et al., 1997, 1998a). Samples of liver were also taken and all dissected tissue was frozen in liquid nitrogen and stored at −80° C until needed.
Ascorbic acid
Ascorbic acid measurements were made in cerebellum, hippocampus, striatum, frontal cortex and liver. The liver is the site of ascorbic acid synthesis in the mouse and as such is an organ that should reflect a large change in ascorbic acid levels in Gulo−/− mice that do not receive adequate supplementation. Concentrations were measured by ion pair HPLC (Pachla & Kissinger, 1979) and electrochemical detection as previously described (May et al. 1998), except that tetrapentyl ammonium bromide was used as the ion pair reagent. Tissue ascorbic acid was extracted as follows. Tissue samples were weighed and wet tissue was homogenized in a 1.5 ml microfuge tube with a combination of two solutions, 25% (w/v) aqueous metaphosphoric acid and 100 mM sodium phosphate buffer containing 5 mM EDTA (pH 8.0), mixed together in a ratio of 2:7. A total of 10 µl of buffer solutions was used for each mg of tissue. The samples were then centrifuged at 13,600 g for 4 min at 3° C, and aliquots of the clear supernatant were taken for assay of ascorbic acid as described above following appropriate dilution with HPLC mobile phase.
Lipid peroxidation
Neuroprostanes were assessed to monitor brain lipid peroxidation. Neuroprostanes are F-ring isoprostane-like compounds derived from non-enzymatic peroxidation of docosahexaenoic acid that have been shown to be excellent markers of brain oxidant stress (Roberts et al. 1998). Neuroprostane levels were measured in tissue from the medial cortex from 23 animals with a minimum of 6 animals per group, and in cerebellum in 12 animals with 4 per group, using methods previously described by Roberts et al. (1998). Briefly, following tissue extraction, esterified F4-neuroprostanes were quantified by stable isotope dilution negative ion chemical ionization gas chromatography mass spectrometry (GC/MS) using [2H4]-15-F2t-isoprostane as an internal standard.
Behavioral Procedures
Behavioral testing schedule
Body weights were recorded at 6, 10, 14 and 18 weeks of age. Four locomotor activity sessions were conducted on two consecutive days at 6 and at 10 weeks of age. Further behavioral testing took place between 10 and 18 weeks of age. The sensorimotor tests for gait, horizontal beam, and rotarod were conducted on separate days at 11 weeks of age, followed by the elevated plus maze test for anxiety the following week. Cognitive function tests were conducted beginning at 14 weeks of age with a single Y-maze trial conducted the day before commencement of water maze testing. The inverted screen task for grip strength was run after the water maze. Mice were sacrificed at 18 weeks of age following the conclusion of behavioral testing, and tissues were collected and stored for later analysis as described above. Behavioral testing and sacrifice of mice were conducted between 12:00 and 17:00.
Sensorimotor function
Gait
Gait was measured by coating the hind paws of each mouse with non-toxic black ink (Carters Brand Neat-Flo Inker for Felt/Foam Stamp Pads; Hill et al. 2004). Each mouse received a single trial in which it was placed at the beginning of a 40 × 10 cm runway and permitted to run freely to the end. The middle toe print was used for gait measurement, recorded as the mean distance (cm) of three consecutive right hind paw prints.
Horizontal beam
Balance was assessed using a horizontal beam task (Hill et al. 2004). The apparatus consisted of a cloth-wrapped rod (diam. 1.0 cm, length 33 cm) suspended horizontally, 45 cm from the bench top between two platforms. At the beginning of each trial the mouse was placed in the centre of the apparatus so that the rod passed between front and back legs. Each trial was scored as follows by an experienced observer: 0 = the mouse moved to either platform within 30 s; 0.5 = the mouse moved to either platform within 60 s; 1 = the mouse moved from the centre third into either the left or the right third but did not climb onto the platform; 2 = the mouse moved from original position on the rod but did not leave the centre third; 4 = the mouse fell from rod. Mice received three trials in a single session, with an intertrial interval (ITI) of 60 s.
Inverted Screen
Grip strength was assessed using an inverted screen apparatus, as previously described (McDonald et al. 2001; Lijam et al. 1997). The mouse was placed onto an area of wire mesh measuring approximately 15 × 15 cm. The apparatus was slowly inverted such that the mouse hung upside down beneath the wire mesh, approximately 60 cm above a tub cage containing soft bedding material. Each mouse received three identical trials with an ITI of approximately 2 min. Latency to fall was recorded on each trial with a maximum of 300 s. The mean latency to fall was calculated for each mouse.
Rotarod
Motor coordination and balance were tested using a commercially-available accelerating rotarod (Ugo Basile model 7650, Stoelting Co., Wood Dale, IL), as previously described (Hill et al. 2004; McDonald et al. 2001). The rotarod began at 4 rotations per minute (rpm) and accelerated to 40 rpm at a smooth rate across the course of the 300-s trial. Mice were placed on the rod while it was rotating slowly at 4 rpm. The time taken for the mouse to fall from the rotating rod was recorded with a maximal trial duration of 300 s. Occasionally mice clung to the rod and the whole animal rotated along with it. This behavior was classified as a “rotation,” and the time at which this occurred for the first time on each trial was also recorded for each mouse. Thus the rotarod score was defined as latency to fall or to the first rotation, whichever occurred first. Three sessions were conducted on consecutive days, with three trials per session.
Cognition
Exploratory locomotor activity
Locomotor activity was assessed in commercially-available activity monitors (ENV-510; MED Associates, Georgia, VT), as previously described (McDonald et al. 1998b; Siesser et al. 2005, 2006). Activity was automatically recorded by the breaking of infrared beams as the mouse explored the chamber, and analyzed using a Windows-based computer. Each session lasted 10 min, and the chambers were cleaned with a 10% alcohol solution between each mouse. Four activity sessions were conducted to assess memory for the testing environment, and each mouse was put in the same activity monitor on every trial. Two sessions were conducted on two consecutive days when mice were six weeks old, followed by two sessions on consecutive days at ten weeks of age.
Implicit memory can be measured by comparing performance between sequential test sessions using methods modified from the original work on memory savings by Ebbinghaus (1885). Memory savings for context and procedure, sometimes referred to as cognitive savings, have previously been measured in operant response chambers between trials conducted within a single day (Delcasso et al. 2007), and also in a radial arm water maze task to compare test sessions that were conducted several weeks apart (Austin et al. 2003). In the present study, the change in activity over time (habituation) was used to infer memory of the environment. Typically, activity will decrease from one test session to the next until a baseline level of activity is reached. Initial day-to-day habituation was measured by taking the difference in distance (d) traveled on sessions one and two as a percentage of the initial session activity level [100 · (d1-d2) / d1]. Using this formula the change in activity across test session can be compared between groups regardless of initial activity levels. Memory savings between the second and third test sessions, which were conducted four weeks apart, were calculated in the following way: [100 · (d1- d3) / (d1- d2)]. An increase in activity between the second and third sessions indicates a ‘loss’ of the initial habituation and is reflected in a savings score of <100%. Inversely, equivalent or reduced activity on the third session (savings score of ≥100%) indicates that all of the habituation processes have been ‘saved’ and the habituation process is either continuing or baseline activity levels have been reached. Pilot tests in several mouse models showed that deficits in memory savings can be observed with a 4-week interval between test sessions in mice with memory impairments (unpublished data).
Y-maze spontaneous alternation
Spontaneous alternation was tested during a single trial in a standard Y-maze made of clear acrylic tubing, as previously described (Reiserer et al. 2007). The maze had three identical arms 32 cm long, radiating symmetrically from the center of the maze. At the beginning of the trial the mouse was placed at the end of one of the arms, designated arm A, and prevented from escaping from the maze by means of a clear guillotine door. The number and sequence of arm entries made during a 5-min. session were recorded. Mice were required to enter an arm with all four paws in order for it to be counted as an entry. An experimenter scored the behavior from behind a screen using a remote video monitor. Alternations were defined as an entry into each arm within three consecutive arm choices (i.e. no repetitions, e.g. ABC or BAC). Percent alternation was calculated as the number of alternations divided by the number of total arm entries minus two.
Morris water maze
Hidden-platform testing was conducted in a 107-cm diam. pool with a circular acrylic platform (10 cm diam.) submerged 1 cm below the surface of the water, as previously described (Bernardo et al. 2007). Mice were given four acquisition trials per day for seven days in a spaced fashion, i.e., each mouse completed its first trial before the first mouse began its second trial. ITIs ranged from 10 to 15 minutes. The water maze was located in the centre of a room with distinct, visual cues fixed to the walls that were clearly visible from the pool. These extra-maze cues remained stationary throughout acquisition and probe test sessions. Sessions were captured by an overhead camera and analyzed in real time using an NIH Image macro on a Macintosh computer written specifically for the water maze task (Miyakawa et al. 2001a, 2001b). Latency and path length to reach the hidden platform were the variables of interest during task acquisition. Twenty-four hours following target acquisition a 60-s probe trial was conducted. The time spent in the target and non-target quadrants and the average distance from the platform location (search error) were the two primary dependent measures derived from the probe trial. Search error is a more sensitive measure of selective search on the probe trial than time in quadrant (Gallagher et al. 1993, Bernardo et al. 2007). Swim speed and peripheral swimming were also assessed in the water maze, to determine whether differences in performance could be attributed to non-cognitive factors. Peripheral swimming was defined as the percentage of time the mouse spent within 8 cm of the wall of the pool.
Anxiety
Locomotor activity chambers
Anxiety levels can significantly confound cognitive tests if one group of animals differs systematically from another. Anxiety was first assessed using the locomotor activity chambers described above. Mice with higher anxiety tend to stay closer to the walls of the apparatus rather than in the open centre. The periphery was defined as the area within 3.6 cm of each of the side walls, and the centre area the remaining 20.8 × 20.8 cm square. Data from the first 10-min trial conducted in the apparatus were reanalyzed to calculate the percent of time that mice spent close to the periphery of the apparatus.
Elevated plus maze
A standard elevated plus maze was used to assess anxiety via differential exploratory tendencies in enclosed versus open arms, as previously described (Reiserer et al. 2007). At the beginning of a session, mice were gently placed in one of the open arms facing the central area, and allowed to explore freely for 5 min. Sessions were recorded and analyzed using NIH Image software on a Macintosh computer, running a macro specially written to collect data about the mouse’s position on the maze throughout the trial (Miyakawa et al. 2001a). The time spent in closed arms was calculated as the percent of total time on arms, excluding time in the central area. The number of entries into arms and total distance traveled were also recorded.
Statistical analyses
All data were examined for gender differences. There were no main effects of gender, or Group × Gender interactions, and data were collapsed into treatment groups. Behavioral and neurochemical assays with single dependent variables (e.g. gait, horizontal beam task, spontaneous alternation) were analyzed using univariate ANOVA. Planned follow-up comparisons were conducted following significant omnibus ANOVA effects using Bonferroni LSD. To protect against a Type I error, follow-up tests were restricted to comparison of the groups of interest, i.e., the Gulo-sufficient vs. the Gulo-low to assess the effects of ascorbic acid deficiency, and Gulo-sufficient vs. wild-type to verify that ascorbic acid supplementation “normalized” the Gulo−/− mice. When indicated by the data, post-hoc comparisons were also made between Gulo-low and wild-type mice using Bonferroni-corrected t-tests. All other behavioral data were analyzed using Group × Session repeated-measures ANOVA. Savings was measured as a percent change from baseline, the meaning of which differs depending on whether it is an increase or a decrease. Because this renders the data susceptible to skewing by a small number of subjects, analyses were conducted using log10-transformed data. Mice that recorded higher activity levels on the second locomotor activity session than the first were excluded from habituation analyses as this behavior suggests an abnormal habituation process that could skew the data (two Wild-type control, two Gulo-low). One wild-type mouse died following water maze training and tissue samples were not kept for analysis. Cortical samples with neuroprostane levels that lay three standard deviations above or below the group mean were excluded from analysis of oxidative stress to minimize risk of Type II error (one Gulo-low, one Gulo-sufficient and two wild-type control for cortex only).
Results
Body Weight
The weights of all Gulo−/− and wild-type mice increased with age as expected [F3,150 = 149.53, P <.001]. Males were heavier than females [F1, 50 = 138.19, P <.001] but there were no differences according to treatment group [F2, 50 = .064, P <.94, data not shown].
Neurochemistry
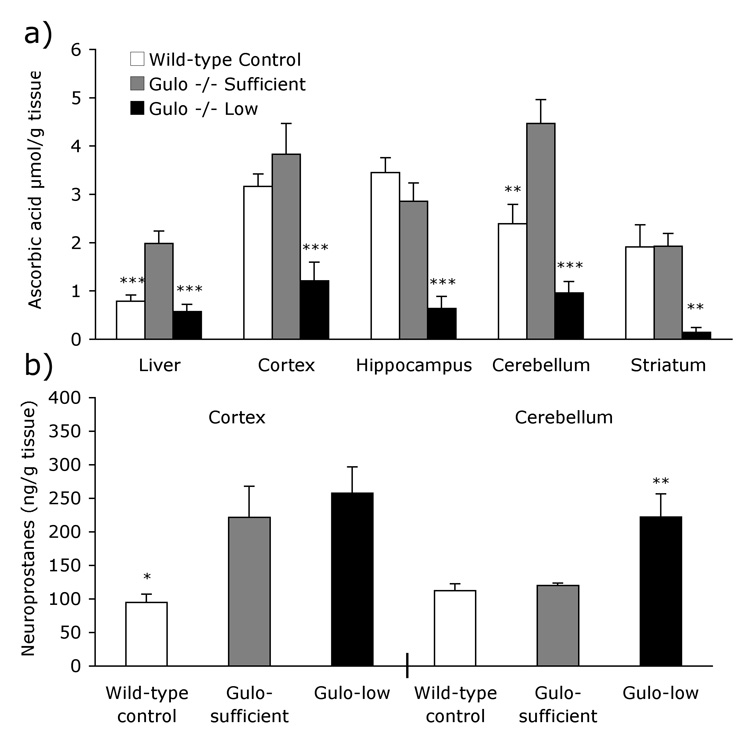
Ascorbic acid levels were measured in liver, cortex, hippocampus, striatum and cerebellum [Fig. 1a]. As expected there were clear differences in ascorbic acid levels among the three groups in all tissues assayed [Fs >5.97, Ps <.05]. Gulo-low mice had significantly lower ascorbic acid levels than Gulo-sufficient mice in all areas assayed (Ps <.05). Interestingly, Gulo-sufficient mice had higher levels of ascorbic acid than wild-type mice in liver and cerebellum (Ps <.05), but ascorbic acid levels in the cortex, hippocampus and striatum did not differ between the two groups.
Figure 1. Neurochemical differences in Gulo−/− mice.
(a) The low (0.033g/l) dose of ascorbic acid was enough for Gulo-low mice to maintain wild-type levels in liver but not in brain. Greater ascorbic acid supplementation (0.33g/l) in the Gulo-sufficient group resulted in higher levels of ascorbic acid than wild-type mice in liver and cerebellum. Gulo-sufficient mice had significantly higher ascorbic acid levels than Gulo-low mice in all areas assayed. (b) F4-Neuroprostanes were elevated in the cortex in both groups of Gulo knockouts despite high ascorbic acid levels in the Gulo-sufficient group. In the cerebellum only Gulo-low mice had elevated neuroprostane levels. Bars represent group mean ± S.E.M. Group differences are depicted as follows: Gulo-low or wild-type control from Gulo-sufficient * P <.05, ** P <.01, *** P <.001.
Oxidative stress was assessed in the cortex and cerebellum of four to eight mice per group. As shown in Figure 1b, cortical neuroprostane levels differed significantly across the three groups [F2, 18 = 5.83, P <.05]. Surprisingly, Gulo-sufficient mice had significantly increased oxidative stress compared to wild-type controls (P <.05). In fact cortical neuroprostane levels of Gulo-sufficient mice did not differ from those in the Gulo-low treatment group (P =1.0). In the cerebellum neuroprostane levels were also different among the groups [F2, 9 = 8.65, P <.01]. As expected, oxidative stress was elevated in the Gulo-low group, relative to that of Gulo-sufficient mice (P <.05). Cerebellar neuroprostane levels in Gulo-sufficient mice were no different than those of wild-type controls (P =1.0).
Neurological and muscular function
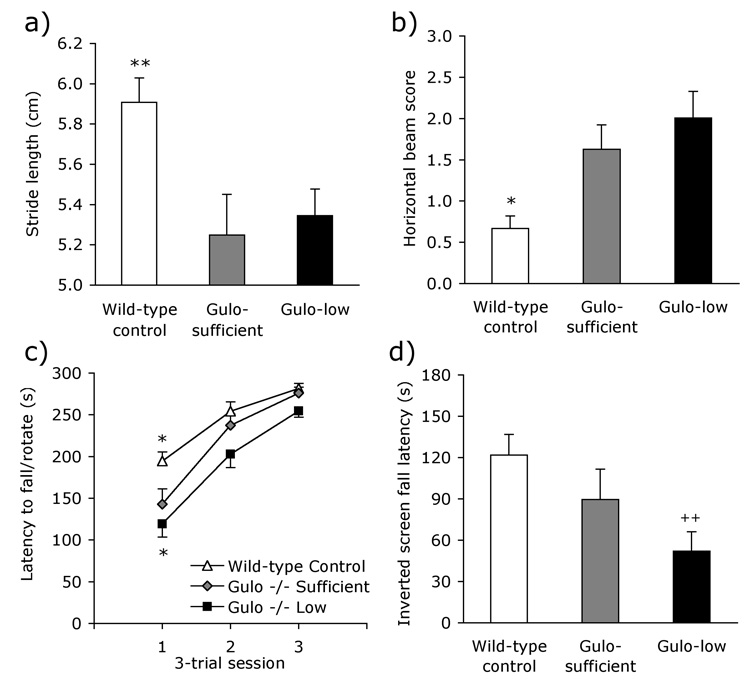
On the hindpaw footprint assessment of gait, there were significant differences in stride length across the groups [Fig. 2a; F2, 54 = 6.36, P <0.01]. Follow-up analyses showed that wild-type control mice had a longer gait than Gulo-sufficient mice (P <.05); the two Gulo−/− groups did not differ (P >.66). A similar pattern of group differences was evident on the horizontal beam task [Fig. 2b; F2, 54 = 8.18, P <0.001]. Follow-up tests confirmed that wild-type mice performed better than Gulo−/− mice on the sufficient (P <.05) supplementation regimen. Performance of the two Gulo−/− groups was similar in terms of overall score (P > 1.0); however qualitative differences in performance were evident that are not reflected in the numerical score. Specifically, Gulo-low mice were more likely than Gulo-sufficient mice to fall from the horizontal beam [χ2 = 4.23, P < .05]. Fourteen of the 21 Gulo-low mice fell from the apparatus on at least one of the three trials, compared to only four of 13 for the Gulo-sufficient group. The incidence of falling among the Gulo-sufficient mice did not differ significantly from that of wild-type mice [3 of 21; χ2 = 1.30, P = .25].
Figure 2. Neurological and motor dysfunction in Gulo−/− mice.
Gulo-sufficient mice had (a) a shorter stride length than wild-type control mice and (b) exhibited poorer grip strength on the horizontal beam, but did not differ from Gulo-low mice on these tasks. Impaired agility in the Gulo-sufficient group compared to wild-type mice was seen on the (c) rotarod and (d) inverted screen tasks. These deficits were exacerbated by subsistence levels of ascorbic acid supplementation in the Gulo-low group. Data shown are group means ± S.E.M. Group differences are depicted as follows: Gulo-low or wild-type control from Gulo-sufficient * P <.05, Gulo-low from wild-type control mice ++ P <.01.
All mice improved across the three rotarod test sessions [Fig. 2c; F2, 108 = 66.96, P <0.001], and all groups improved at similar rates across the three days of testing [Group X Session F4, 108 = 1.41, P =.24]. However, there was a significant main effect of group [F2, 54 = 15.29, P <0.001]. Follow-up analyses showed that performance of wild-type mice exceeded that of Gulo-sufficient mice (P <.05), and that Gulo-sufficient mice performed significantly better than the Gulo-low group (P <.05). A similar performance pattern was evident on the inverted-screen task [Fig. 2d; F2, 54 = 5.23, P <.01]. However, the group differences were not significant on follow-up comparison. Because of the lack of group differences in the presence of large effect sizes, a post-hoc comparison was conducted between wild-type and Gulo-low mice. Wild-type mice were able to hang upside down on the on the wire mesh significantly longer than the Gulo-low treatment mice (P <.01).
Cognitive function
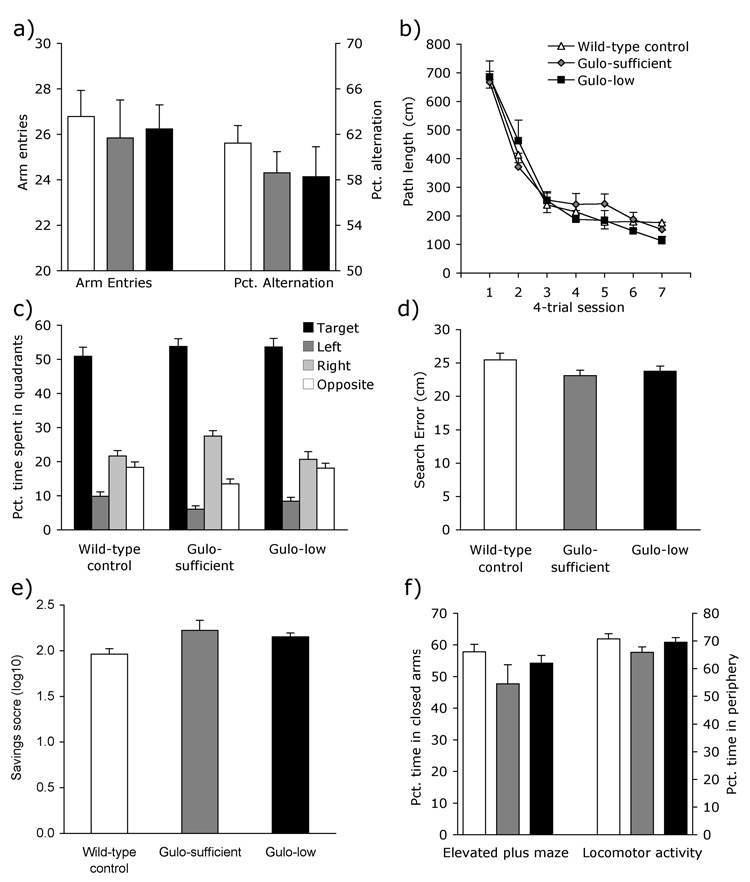
Despite impaired motor skills, Gulo−/− mice performed normally on all cognitive tests. All three groups made similar numbers of arm entries and alternated at equivalent rates in the Y-maze [Fig. 3a; Fs2, 54 < 0.81, Ps > .45]. Similarly, the groups of mice did not differ in their ability to locate a hidden platform in the Morris water maze. Escape latency, path length, and peripheral swimming all improved across test sessions and were similar across groups [session Fs6, 324 > 84.60, Ps <.001; group: Fs2, 54 < 1.17, Ps >.32; Group × Session: Fs12, 324 < 0.79, Ps >.66; path length data shown in Figure 3b]. Twenty-four hours following the final training session, a 60-s no-platform probe trial was administered to assess memory for the platform location. All groups showed a strong preference for the target quadrant, spending approximately 50 percent of the trial swimming in the vicinity of the former platform location [Fig. 3c; Wild-type F3, 66 = 70.35, P<.001; Gulo-sufficient F3, 36 = 94.40, P <.001; Gulo-low F3, 60 = 79.64, P <.001]. Mean search error during the probe trial also did not differ across groups [Fig. 3d; F2, 54 = 1.39, P =.26]. During the probe trial there were significant group differences in swim speed [Wild-type 18.1 ± .35 cm/s, Gulo-sufficient 16.8 ± .47, Gulo-low 16.4 ± .37; F2, 54 = 5.72, P <.01]. Follow-up analyses showed that although wild-type mice swam faster than Gulo knockouts treated with sufficient amounts of ascorbic acid, this difference was not significant (P =.099). Swim speeds of the two Gulo−/− groups did not differ (P =1.0). Consistent with the deficits observed on the other sensorimotor tasks, Gulo-low mice swam significantly slower than wild-type controls (P<.01). Although we did not conduct a cued-platform version of the water-maze task, it is unlikely that the Gulo−/− mice had impaired vision given their excellent performance on the hidden-platform visuospatial version of the task.
Figure 3. Normal cognitive function and anxiety in Gulo−/− mice.
Gulo−/− and wild-type mice performed similarly on measures of cognitive function including (a) exploration (arm-entries) and alternation in the Y-maze, (b) hidden-platform learning during water maze acquisition, and (c–d) spatial memory for the location of the platform during a 60-s probe trial measured by (c) time spent in each quadrant and (d) search error. (e) Gulo mice showed no impairment in long-term contextual memory. (f) Gulo−/− and wild-type mice also performed similarly on the measures of anxiety in the elevated plus maze and locomotor activity chambers. Bars represent group mean ± S.E.M.
Distance traveled in the locomotor activity chambers did not differ among groups during the first two sessions, indicating normal short-term habituation [F2, 50 = 2.97, P =.06]. Poorer long-term memory for the context of the testing chamber is reflected in lower levels of habituation of activity ‘saved’ during the third compared to the second session. All three groups habituated at or above 100%, indicating a retained memory for the testing context and maximal habituation on the third session. Saved memory levels did not differ among the groups [Fig. 3e; F2, 48 =2.37, P =.11].
Anxiety
All three groups of mice spent 65–70% of the time exploring the peripheral areas of the locomotor activity chambers [Fig. 3f; F2, 54 = 1.50, P =.23], indicative of normal anxiety levels. There were also no group differences when anxiety was assessed in the elevated plus maze [Fig 3f; Fs2, 54 < 2.13, Ps >.13].
Discussion
Ascorbic acid treatments led to large differences in tissue levels between the two Gulo−/− groups, but not between ascorbic acid-sufficient and wild-type mice. Ascorbic acid levels were significantly lower in Gulo-low mice than Gulo-sufficient mice in every area assayed, whereas in the liver and cerebellum more ascorbic acid was detected in Gulo-sufficient mice than in wild-types. Wild-type ascorbic acid levels in brain and liver were similar to previously reported data in mice and rats (Maeda et al., 2000, Rice & Russo-Menna, 1998; Milby et al. 1982). Although 0.33 g/l ascorbic acid in the drinking water of Gulo −/− mice in the original studies of Maeda et al. resulted in plasma ascorbic acid concentrations that were about 60% of those in wild-type mice, our studies suggest that this level of supplementation is sufficient to match or exceed wild-type ascorbic acid levels in several brain areas and in liver. As expected, neuroprostane levels were elevated in Gulo-low mice in both cortex and cerebellum; however, they were also elevated in the cortex of the Gulo-sufficient group. Despite increased oxidative stress, cognitive abilities were completely normal in both groups of Gulo−/− mice. Instead, they exhibited widespread sensorimotor deficits that were worse in the mice maintained through adulthood on the low level of ascorbic acid.
Despite normal ascorbic acid levels in the cortex of Gulo-sufficient mice, both groups of knockout mice had significantly elevated cortical neuroprostane levels. There are three possible explanations for this. The first is that ascorbic acid acted as a pro-oxidant in the Gulo-sufficient group, although this is unlikely given the similar cortical ascorbic acid levels between wild-type and Gulo-sufficient mice. Nevertheless, ascorbic acid has been shown to have a pro-oxidant role in some conditions in vivo, especially in the presence of iron and other free transition metals (De Vriese et al. 2008, Poljsak & Raspor, 2008, Martin et al., 2006). Given the elevated ascorbic acid levels in the cerebellum and liver of Gulo-sufficient mice, this possibility cannot be excluded without further investigation. Second, defects in oxidant defenses could have been induced by the Gulo gene knockout beyond those due to simply preventing the synthesis of ascorbic acid. The Gulo enzyme is not known to play a part in any other biological pathway; nevertheless it is possible that as the capability to synthesize ascorbic acid was lost through evolutionary pathways, other antioxidant mechanisms adapted to compensate for this loss. Thus the Gulo−/− mice would be disadvantaged compared to wild-type mice despite the provision of high levels of ascorbic acid in drinking water. Third, oxidative stress and subsequent tissue damage resulting from continued lipid peroxidation may have occurred to pups at some time during fetal development due to inability of the Gulo homozygote dams to provide adequate ascorbic acid. In this case, the concentration of ascorbic acid that provided adult Gulo−/− mice with greater than or equal to wild-type ascorbic acid levels (0.33 g/l drinking water) may have been insufficient when given to pregnant dams to prevent oxidant stress in pups.
Despite elevated F4-neuroprostanes in the cortex, the Gulo−/− mice performed normally on the tests of cognitive function regardless of ascorbic acid supplementation regimen. Oxidative stress was not measured in the hippocampus; however, hippocampal and cortical ascorbic acid levels were similar in both Gulo groups, suggesting that F4-neuroprostane levels may have been elevated in the hippocampus as well. Nevertheless, spatial working memory in the Y-maze, spatial reference memory in the water maze, day-to-day habituation, and long-term memory for the testing context in the activity monitors were all equivalent among the three groups. This suggests that neither the increased oxidative stress observed nor the differences in brain ascorbic acid levels were determinants of cognitive function. Lipid peroxidation is thought to be an important determinant of cognitive abilities via damage to cell membranes, as this can lead to damage to membrane fluidity and impaired synaptic function (Hong et al., 2004; Omoi et al., 2006). Consistent with this, considerable evidence shows that lipid peroxidation is associated with memory impairment (Vienbergs et al., 2000; Farr et al., 2003; Baydas et al., 2005; Kiray et al., 2006). However, cognitive abilities are also associated with oxidative damage to proteins and nucleic acids (Liu et al., 2002; Ogonovszky et al., 2005), which were not measured in the present study. It is possible that these may be more important mediators of cognitive ability in the present context, and if unchanged in Gulo−/− mice may better explain their intact learning and memory.
In contrast to the normal ascorbic acid and elevated neuroprostanes observed in the cortex of Gulo−/− mice, cerebellar ascorbic acid was significantly higher in the sufficient-treated knockouts, and cerebellar oxidative stress levels were normal. This suggests that in the brain, greater antioxidant levels are required to neutralize oxidative stress in mice lacking Gulo. Ironically, the sensorimotor tasks were conducted as controls, to ensure that the mice had the fundamental abilities required to complete the cognitive tests. Instead, both groups of Gulo−/− mice exhibited normal learning and memory but a distinct motor dysfunction on tasks involving strength and agility. Swim speed was also slower in Gulo−/− mice during the water maze probe trial when all mice had to swim for the full 60 s. These results were obtained despite equivalent body weights among the groups. The cerebellum mediates motor learning, and oxidative damage in the cerebellum is associated with motor deficits in aged and ethanol-exposed rats (Bickford et al., 2000; Zima et al., 2001; Cui et al., 2007). Furthermore, low striatal ascorbic acid levels impair motor function and are associated with the abnormal motor behaviors exhibited by the R6/2 mutant mouse model of Huntington’s disease (Rebec & Wang, 2001; Rebec et al., 2002). Ascorbic acid levels in the striatum did not differ between Gulo-sufficient and control mice in the present study, and therefore cannot explain the motor abnormalities in these mice. However, the very low levels of ascorbic acid in the striatum and cerebellum of Gulo-low mice may have contributed to the behavioral impairments found in these mice. As with the elevated cortical neuroprostanes, it is also possible that the motor deficits in the Gulo−/− mice are the sequelae of a maternally-mediated developmental ascorbic acid deficiency. In this case the deficits may reflect a Gulo−/− phenotype that is modest and exacerbated by ascorbic acid deficiency in the Gulo-low group, or more severe and partially ameliorated by ascorbic acid supplementation in the Gulo-sufficient group. Further study is required to determine which of these is the case.
Another possibility that must be considered is that the motor deficits are related to the abnormal cerebellar ascorbic acid levels in the Gulo−/− groups, independent of oxidative stress. Many biological systems work efficiently only under physiological conditions, and significant aberrations from the norm in either direction can result in dysfunction. It is not clear why ascorbic acid was elevated to such an extent in the Gulo-sufficient mice, when levels are normally under such tight homeostatic control. It may be the result of a heretofore unknown function of Gulo, or perhaps a property of the cerebellum to store up excess nutrients when available, or a high concentration of vitamin C transporters (SVCT2) compared to other brain areas (Mun et al. 2006). Oelrichs et al. (1988) reported transporter-mediated intracellular uptake of excess ascorbic acid in rat cerebellar tissue that increased linearly with the concentration in the medium. Meydani et al. (1986) reported a similar phenomenon with vitamin E in vivo. They showed that α-tocopherol levels were highest in cortical tissue, and lowest in cerebellum, in rats fed the minimal requirements of vitamin E. Supplementation with excess vitamin E significantly increased α-tocopherol in cerebellum only, resulting in levels higher than those in either cortex or hippocampus. Although different mechanisms mediate synthesis and metabolism of vitamins C and E, the pattern reported by Meydani et al. is identical to the one exhibited for ascorbic acid by the Gulo-sufficient group (Figure 1a). Further research is needed to determine the precise reason for the increased cerebellar ascorbic acid in the Gulo-sufficient mice, but we must recognize the possibility that abnormal ascorbic acid levels may have contributed to the observed motor deficits. We must also consider the possibility that other abnormalities in this mutant mouse line contributed to their behavioral deficits. Maeda et al. (2000) reported high cholesterol and damaged aortic walls in Gulo−/− mice maintained under sub-optimal ascorbic acid supplementation. However, because the impairments reported here were detected in adequately-supplemented as well as low ascorbic acid-supplemented Gulo groups, and because the knockout mice were impaired on the low-physical-demand tasks (e.g. gait analysis) as well as the highly physically demanding tasks (e.g. water maze), it is unlikely that the cardiovascular phenotypes reported by Maeda et al. significantly affected performance of the Gulo−/− mice.
In contrast to our findings of elevated lipid peroxidation in the brains of Gulo −/− mice, Maeda et al. (2000) reported that malondialdehyde levels, a relatively non-specific marker of lipid peroxidation, were unchanged in the livers of Gulo−/− mice. Although this result may seem at odds with our finding of elevated F4-neuroprostanes in the brains of Gulo −/− mice, two points are relevant. First, the levels of polyunsaturated fats in the brain are higher than those in the liver (Artmann et al., 2008, Suarez et al. 1996), which might make the brain more susceptible to free radical-initiated lipid peroxidation than the liver. Second, F4-neuroprostanes are a specific and highly sensitive marker for oxidative damage to lipids in the brain (Montuschi et al. 2007) and thus may reveal differences not apparent in the data reported by Maeda et al.
We have shown that Gulo−/− mice exhibit impaired motor function and increased oxidative stress, even when ascorbic acid levels are equivalent to those of wild-type controls. Despite elevated oxidative stress, cognition was normal in both Gulo−/− groups. Brain ascorbic acid levels are high during normal human gestation, especially during the final trimester, and are thought to reflect the importance of the vitamin during brain development (Zalani et al. 1989, Kratzing and Kelly, 1982). Our data also suggest an important role for ascorbic acid in fetal development, but one that is not yet understood. Indeed, our results indicate that ascorbic acid may play a greater role in development than has previously been thought, and deficiency may induce permanent behavioral impairments that are not reversible by dietary supplementation.
Acknowledgements
This work was supported by grants from the National Institute of Health (AG023138 to James May & AG022439 to Mike McDonald). We thank Dr. Sean Davies and Dr. Ginger Milne for help with neuroprostane measurements.
References
- 1.Andersen JK. Oxidative stress in neurodegeneration: cause or consequence? Nat Med. 2004;(10 Suppl):S18–S25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- 2.Artmann A, Petersen G, Hellgren LI, Boberg J, Skonberg C, Nellemann C, Hansen SH, Hansen HS. Influence of dietary fatty acids on endocannabinoid and N-acylethanolamine levels in rat brain, liver and small intestine. Biochimica et biophysica acta. 2008 doi: 10.1016/j.bbalip.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Arzi A, Hemmati AA, Razian A. Effect of vitamins C and E on cognitive function in mouse. Pharmacol Res. 2004;49(3):249–252. doi: 10.1016/j.phrs.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Austin L, Arendash GW, Gordon MN, Diamond DM, DiCarlo G, Dickey C, Ugen K, Morgan D. Short-term beta-amyloid vaccinations do not improve cognitive performance in cognitively impaired APP + PS1 mice. Behavioral neuroscience. 2003;117:478–484. doi: 10.1037/0735-7044.117.3.478. [DOI] [PubMed] [Google Scholar]
- 5.Baydas G, Yasar A, Tuzcu M. Comparison of the impact of melatonin on chronic ethanol-induced learning and memory impairment between young and aged rats. Journal of pineal research. 2005;39:346–352. doi: 10.1111/j.1600-079X.2005.00257.x. [DOI] [PubMed] [Google Scholar]
- 6.Bernardo A, McCord M, Troen AM, Allison JD, McDonald MP. Impaired spatial memory in APP-overexpressing mice on a homocysteinemia-inducing diet. Neurobiol Aging. 2007;28(8):1195–1205. doi: 10.1016/j.neurobiolaging.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 7.Bickford PC, Gould T, Briederick L, Chadman K, Pollock A, Young D, Shukitt-Hale B, Joseph J. Antioxidant-rich diets improve cerebellar physiology and motor learning in aged rats. Brain research. 2000;866:211–217. doi: 10.1016/s0006-8993(00)02280-0. [DOI] [PubMed] [Google Scholar]
- 8.Coffey RG, Hadden JW. Neurotransmitters, hormones, and cyclic nucleotides in lymphocyte regulation. Fed Proc. 1985;44(1 Pt 1):112–117. [PubMed] [Google Scholar]
- 9.Cui L, Hofer T, Rani A, Leeuwenburgh C, Foster TC. Comparison of lifelong and late life exercise on oxidative stress in the cerebellum. Neurobiology of aging. 2007 doi: 10.1016/j.neurobiolaging.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Angelis L, Furlan C. The effects of ascorbic acid and oxiracetam on scopolamine-induced amnesia in a habituation test in aged mice. Neurobiol Learn Mem. 1995;64(2):119–124. doi: 10.1006/nlme.1995.1050. [DOI] [PubMed] [Google Scholar]
- 11.Debler EA, Sershen H, Hashim A, Lajtha A, Reith ME. Carrier-mediated efflux of [3H]dopamine and [3H]1-methyl-4-phenylpyridine: effect of ascorbic acid. Synapse. 1991;7(2):99–105. doi: 10.1002/syn.890070203. [DOI] [PubMed] [Google Scholar]
- 12.Delcasso S, Jeantet Y, Cho YH. A new test for long-term spatial memory using an operant chamber in mice. Behav Brain Res. 2007;178(2):200–207. doi: 10.1016/j.bbr.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 13.De Vriese AS, Borrey D, Mahieu E, Claeys I, Stevens L, Vanhaeverbeke A, Roelens M, Langlois MR. Oral vitamin C administration increases lipid peroxidation in hemodialysis patients. Nephron. 2008;108:c28–c34. doi: 10.1159/000112526. [DOI] [PubMed] [Google Scholar]
- 14.Dhingra D, Parle M, Kulkarni SK. Comparative brain cholinesterase-inhibiting activity of Glycyrrhiza glabra, Myristica fragrans, ascorbic acid, and metrifonate in mice. J Med Food. 2006;9(2):281–283. doi: 10.1089/jmf.2006.9.281. [DOI] [PubMed] [Google Scholar]
- 15.Ebbinghaus H. In: Memory: A Contribution to Experimental Psychology. Ruger Henry A, Bussenius Clara E., translators. New York: Teachers College; 1885. (1913). [Google Scholar]
- 16.Engelhart MJ, Geerlings MI, Ruitenberg A, van Swieten JC, Hofman A, Witteman JC, Breteler MM. Dietary intake of antioxidants and risk of Alzheimer disease. Jama. 2002;287:3223–3229. doi: 10.1001/jama.287.24.3223. [DOI] [PubMed] [Google Scholar]
- 17.Farr SA, Poon HF, Dogrukol-Ak D, Drake J, Banks WA, Eyerman E, Butterfield DA, Morley JE. The antioxidants alpha-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. Journal of neurochemistry. 2003;84:1173–1183. doi: 10.1046/j.1471-4159.2003.01580.x. [DOI] [PubMed] [Google Scholar]
- 18.Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107(4):618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh C, Dick RM, Ali SF. Iron/ascorbate-induced lipid peroxidation changes membrane fluidity and muscarinic cholinergic receptor binding in rat frontal cortex. Neurochem Int. 1993;23(5):479–484. doi: 10.1016/0197-0186(93)90133-p. [DOI] [PubMed] [Google Scholar]
- 20.Hill KE, Zhou J, McMahan WJ, Motley AK, Burk RF. Neurological dysfunction occurs in mice with targeted deletion of the selenoprotein P gene. J Nutr. 2004;134(1):157–161. doi: 10.1093/jn/134.1.157. [DOI] [PubMed] [Google Scholar]
- 21.Hong JH, Kim MJ, Park MR, Kwag OG, Lee IS, Byun BH, Lee SC, Lee KB, Rhee SJ. Effects of vitamin E on oxidative stress and membrane fluidity in brain of streptozotocin-induced diabetic rats. Clinica chimica acta; international journal of clinical chemistry. 2004;340:107–115. doi: 10.1016/j.cccn.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Kang JH, Cook N, Manson J, Buring JE, Grodstein F. A randomized trial of vitamin E supplementation and cognitive function in women. Arch Intern Med. 2006;166(22):2462–2468. doi: 10.1001/archinte.166.22.2462. [DOI] [PubMed] [Google Scholar]
- 23.Kiray M, Bagriyanik HA, Pekcetin C, Ergur BU, Uysal N, Ozyurt D, Buldan Z. Deprenyl and the relationship between its effects on spatial memory, oxidant stress and hippocampal neurons in aged male rats. Physiological research / Academia Scientiarum Bohemoslovaca. 2006;55:205–212. doi: 10.33549/physiolres.930742. [DOI] [PubMed] [Google Scholar]
- 24.Knaack D, Podleski T. Ascorbic acid mediates acetylcholine receptor increase induced by brain extract on myogenic cells. Proc Natl Acad Sci U S A. 1985;82(2):575–579. doi: 10.1073/pnas.82.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knaack D, Shen I, Salpeter MM, Podleski TR. Selective effects of ascorbic acid on acetylcholine receptor number and distribution. J Cell Biol. 1986;102(3):795–802. doi: 10.1083/jcb.102.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kratzing CC, Kelly JD. Tissue levels of ascorbic acid during rat gestation. Int J Vitam Nutr Res. 1982;52(3):326–332. [PubMed] [Google Scholar]
- 27.Lijam N, Paylor R, McDonald MP, Crawley JN, Deng CX, Herrup K, et al. Social interaction and sensorimotor gating abnormalities in mice lacking Dvl1. Cell. 1997;90(5):895–905. doi: 10.1016/s0092-8674(00)80354-2. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Head E, Gharib AM, Yuan W, Ingersoll RT, Hagen TM, Cotman CW, Ames BN. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl-L-carnitine and/or R-alpha -lipoic acid. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:2356–2361. doi: 10.1073/pnas.261709299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luchsinger JA, Tang MX, Shea S, Mayeux R. Antioxidant vitamin intake and risk of Alzheimer disease. Arch Neurol. 2003;60(2):203–208. doi: 10.1001/archneur.60.2.203. [DOI] [PubMed] [Google Scholar]
- 30.Maeda N, Hagihara H, Nakata Y, Hiller S, Wilder J, Reddick R. Aortic wall damage in mice unable to synthesize ascorbic acid. Proc Natl Acad Sci U S A. 2000;97(2):841–846. doi: 10.1073/pnas.97.2.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin BD, Schoenhard JA, Hwang JM, Sugden KD. Ascorbate is a pro-oxidant in chromium-treated human lung cells. Mutation research. 2006;610:74–84. doi: 10.1016/j.mrgentox.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 32.May JM, Qu ZC, Mendiratta S. Protection and recycling of alpha-tocopherol in human erythrocytes by intracellular ascorbic acid. Arch Biochem Biophys. 1998;349(2):281–289. doi: 10.1006/abbi.1997.0473. [DOI] [PubMed] [Google Scholar]
- 33.McDonald MP, Miller KM, Li C, Deng C, Crawley JN. Motor deficits in fibroblast growth factor receptor-3 null mutant mice. Behav Pharmacol. 2001;12(6–7):477–486. doi: 10.1097/00008877-200111000-00009. [DOI] [PubMed] [Google Scholar]
- 34.McDonald MP, Wenk GL, Crawley JN. Analysis of galanin and the galanin antagonist M40 on delayed non-matching-to-position performance in rats lesioned with the cholinergic immunotoxin 192 IgG-saporin. Behav Neurosci. 1997;111(3):552–563. doi: 10.1037//0735-7044.111.3.552. [DOI] [PubMed] [Google Scholar]
- 35.McDonald MP, Willard LB, Wenk GL, Crawley JN. Coadministration of galanin antagonist M40 with a muscarinic M1 agonist improves delayed nonmatching to position choice accuracy in rats with cholinergic lesions. J Neurosci. 1998a;18(13):5078–5085. doi: 10.1523/JNEUROSCI.18-13-05078.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDonald MP, Wong R, Goldstein G, Weintraub B, Cheng SY, Crawley JN. Hyperactivity and learning deficits in transgenic mice bearing a human mutant thyroid hormone beta1 receptor gene. Learn Mem. 1998b;5(4–5):289–301. [PMC free article] [PubMed] [Google Scholar]
- 37.Meydani M, Macauley JB, Blumberg JB. Influence of dietary vitamin E, selenium and age on regional distribution of alpha-tocopherol in the rat brain. Lipids. 1986;21(12):786–791. doi: 10.1007/BF02535413. [DOI] [PubMed] [Google Scholar]
- 38.Milby K, Oke A, Adams RN. Detailed mapping of ascorbate distribution in rat brain. Neuroscience letters. 1982;28:169–174. doi: 10.1016/0304-3940(82)90147-1. [DOI] [PubMed] [Google Scholar]
- 39.Miyakawa T, Yamada M, Duttaroy A, Wess J. Hyperactivity and intact hippocampus-dependent learning in mice lacking the M1 muscarinic acetylcholine receptor. J Neurosci. 2001a;21(14):5239–5250. doi: 10.1523/JNEUROSCI.21-14-05239.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miyakawa T, Yared E, Pak JH, Huang FL, Huang KP, Crawley JN. Neurogranin null mutant mice display performance deficits on spatial learning tasks with anxiety related components. Hippocampus. 2001b;11(6):763–775. doi: 10.1002/hipo.1092. [DOI] [PubMed] [Google Scholar]
- 41.Montuschi P, Barnes P, Roberts LJ., 2nd Insights into oxidative stress: the isoprostanes. Current medicinal chemistry. 2007;14:703–717. doi: 10.2174/092986707780059607. [DOI] [PubMed] [Google Scholar]
- 42.Mun GH, Kim MJ, Lee JH, Kim HJ, Chung YH, Chung YB, Kang JS, Hwang YI, Oh SH, Kim JG, Hwang DH, Shin DH, Lee WJ. Immunohistochemical study of the distribution of sodium-dependent vitamin C transporters in adult rat brain. Journal of neuroscience research. 2006;83:919–928. doi: 10.1002/jnr.20751. [DOI] [PubMed] [Google Scholar]
- 43.Munoz A, Rey P, Guerra MJ, Mendez-Alvarez E, Soto-Otero R, Labandeira-Garcia JL. Reduction of dopaminergic degeneration and oxidative stress by inhibition of angiotensin converting enzyme in a MPTP model of parkinsonism. Neuropharmacology. 2006;51(1):112–120. doi: 10.1016/j.neuropharm.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 44.Oelrichs BA, Kelly JD, Kratzing CC, Winzor DJ. Accumulation of ascorbate in rat cerebellum. Int J Vitam Nutr Res. 1988;58(2):213–217. [PubMed] [Google Scholar]
- 45.Ogonovszky H, Berkes I, Kumagai S, Kaneko T, Tahara S, Goto S, Radak Z. The effects of moderate-, strenuous- and over-training on oxidative stress markers, DNA repair, and memory, in rat brain. Neurochemistry international. 2005;46:635–640. doi: 10.1016/j.neuint.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 46.Omoi NO, Arai M, Saito M, Takatsu H, Shibata A, Fukuzawa K, Sato K, Abe K, Fukui K, Urano S. Influence of oxidative stress on fusion of pre-synaptic plasma membranes of the rat brain with phosphatidyl choline liposomes, and protective effect of vitamin E. Journal of nutritional science and vitaminology. 2006;52:248–255. doi: 10.3177/jnsv.52.248. [DOI] [PubMed] [Google Scholar]
- 47.Pachla LA, Kissinger PT. Analysis of ascorbic acid by liquid chromatography with amperometric detection. Methods Enzymol. 1979;62:15–24. doi: 10.1016/0076-6879(79)62183-3. [DOI] [PubMed] [Google Scholar]
- 48.Parle M, Dhingra D. Ascorbic Acid: a promising memory-enhancer in mice. J Pharmacol Sci. 2003;93(2):129–135. doi: 10.1254/jphs.93.129. [DOI] [PubMed] [Google Scholar]
- 49.Parsons KK, Maeda N, Yamauchi M, Banes AJ, Koller BH. Ascorbic acid-independent synthesis of collagen in mice. American journal of physiology. 2006;290:E1131–E1139. doi: 10.1152/ajpendo.00339.2005. [DOI] [PubMed] [Google Scholar]
- 50.Poljsak B, Raspor P. The antioxidant and pro-oxidant activity of vitamin C and trolox in vitro: a comparative study. J Appl Toxicol. 2008;28:183–188. doi: 10.1002/jat.1264. [DOI] [PubMed] [Google Scholar]
- 51.Pratico D. Alzheimer's disease and oxygen radicals: new insights. Biochem Pharmacol. 2002;63(4):563–567. doi: 10.1016/s0006-2952(01)00919-4. [DOI] [PubMed] [Google Scholar]
- 52.Reiserer RS, Harrison FE, Syverud DC, McDonald MP. Impaired spatial learning in the APPSwe + PSEN1DeltaE9 bigenic mouse model of Alzheimer's disease. Genes Brain Behav. 2007;6(1):54–65. doi: 10.1111/j.1601-183X.2006.00221.x. [DOI] [PubMed] [Google Scholar]
- 53.Rice ME, Forman RE, Chen BT, Avshalumov MV, Cragg SJ, Drew KL. Brain antioxidant regulation in mammals and anoxia-tolerant reptiles: balanced for neuroprotection and neuromodulation. Comp Biochem Physiol C Toxicol Pharmacol. 2002;133(4):515–525. doi: 10.1016/s1532-0456(02)00116-3. [DOI] [PubMed] [Google Scholar]
- 54.Rice ME, Russo-Menna I. Differential compartmentalization of brain ascorbate and glutathione between neurons and glia. Neuroscience. 1998;82:1213–1223. doi: 10.1016/s0306-4522(97)00347-3. [DOI] [PubMed] [Google Scholar]
- 55.Roberts LJ, 2nd, Montine TJ, Markesbery WR, Tapper AR, Hardy P, Chemtob S, Dettbarn WD, Morrow JD. Formation of isoprostane-like compounds (neuroprostanes) in vivo from docosahexaenoic acid. The Journal of biological chemistry. 1998;273:13605–13612. doi: 10.1074/jbc.273.22.13605. (Poljsak and Raspor 2008) [DOI] [PubMed] [Google Scholar]
- 56.Siesser WB, Cheng SY, McDonald MP. Hyperactivity, impaired learning on a vigilance task, and a differential response to methylphenidate in the TRbetaPV knock-in mouse. Psychopharmacology (Berl) 2005;181(4):653–663. doi: 10.1007/s00213-005-0024-5. [DOI] [PubMed] [Google Scholar]
- 57.Siesser WB, Zhao J, Miller LR, Cheng SY, McDonald MP. Transgenic mice expressing a human mutant beta1 thyroid receptor are hyperactive, impulsive, and inattentive. Genes Brain Behav. 2006;5(3):282–297. doi: 10.1111/j.1601-183X.2005.00161.x. [DOI] [PubMed] [Google Scholar]
- 58.Subramanian N. On the brain ascorbic acid and its importance in metabolism of biogenic amines. Life Sci. 1977;20(9):1479–1484. doi: 10.1016/0024-3205(77)90438-6. [DOI] [PubMed] [Google Scholar]
- 59.Tchantchou F, Chan A, Kifle L, Ortiz D, Shea TB. Apple juice concentrate prevents oxidative damage and impaired maze performance in aged mice. J Alzheimers Dis. 2005;8(3):283–287. doi: 10.3233/jad-2005-8306. [DOI] [PubMed] [Google Scholar]; Veinbergs I, Mallory M, Sagara Y, Masliah E. Vitamin E supplementation prevents spatial learning deficits and dendritic alterations in aged apolipoprotein E-deficient mice. The European journal of neuroscience. 2000;12:4541–4546. [PubMed] [Google Scholar]
- 60.Veinbergs I, Mallory M, Sagara Y, Masliah E. Vitamin E supplementation prevents spatial learning deficits and dendritic alterations in aged apolipoprotein E-deficient mice. The European journal of neuroscience. 2000;12:4541–4546. [PubMed] [Google Scholar]
- 61.Zalani S, Rajalakshmi R, Parekh LJ. Ascorbic acid concentration of human fetal tissues in relation to fetal size and gestational age. Br J Nutr. 1989;61(3):601–606. doi: 10.1079/bjn19890147. [DOI] [PubMed] [Google Scholar]
- 62.Zima T, Fialova L, Mestek O, Janebova M, Crkovska J, Malbohan I, Stipek S, Mikulikova L, Popov P. Oxidative stress, metabolism of ethanol and alcohol-related diseases. Journal of biomedical science. 2001;8:59–70. doi: 10.1007/BF02255972. [DOI] [PubMed] [Google Scholar]