Abstract
Multipotent neural stem/progenitor cells (NSCs) from the embryonic hippocampus are potentially useful as donor cells to repopulate the degenerated regions of the aged hippocampus after stroke, epilepsy or Alzheimer’s disease. However, the efficacy of NSC grafting strategy for repairing the injured aged hippocampus is unknown. To address this issue, we expanded FGF-2 responsive NSCs from the hippocampus of embryonic day 14 green fluorescent protein expressing transgenic mice as neurospheres in vitro and grafted into the hippocampus of 24-months old F344 rats at 4 days after CA3 region injury. Engraftment, migration and neuronal/glial differentiation of cells derived from NSCs were analyzed at one-month post-grafting. Differentiation of neurospheres in culture dishes or after placement onto organotypic hippocampal slice cultures demonstrated the ability of these cells to generate considerable amounts of neurons, astrocytes and oligodendrocytes. Following grafting into the injured aged hippocampus, cells derived from neurospheres survived and dispersed, but exhibited no directed migration into the degenerated or intact hippocampal cell layers. Phenotypic analyses of graft-derived cells revealed neuronal differentiation in 3-5% of cells, astrocytic differentiation in 28% of cells, and oligodendrocytic differentiation in 6-10% cells. The results demonstrate for the first time that NSCs derived from the fetal hippocampus survive and give rise to all three CNS phenotypes following transplantation into the injured aged hippocampus. However, grafted NSCs do not exhibit directed migration into lesioned areas or widespread neuronal differentiation, suggesting that direct grafting of primitive NSCs is not adequate for repair of the injured aged brain without priming the microenvironment.
Keywords: Stem cell grafts, neural stem cells, aged hippocampus, stem cell differentiation, dentate neurogenesis
Introduction
Neural stem/progenitor cells (NSCs) competent for creating new neurons endure in both adult and aged brain especially in neurogenic regions such as the anterior subventricular zone of the forebrain (Reynolds and Weiss, 1992; Luskin, 1993; Alvarez-Buylla and Lois, 1995; Palmer et al., 1999) and subgranular zone of the dentate gyrus in the hippocampus (Kaplan and Hinds, 1977; Kaplan and Bell, 1984; Kuhn et al., 1996; Bernal and Peterson, 2004; Hattiangady and Shetty, 2008). These NSCs seem to help in partial self-repair of the adult brain after injury or disease (Magavi et al., 2000; Nakatomi et al., 2002; Lindvall et al., 2004; Emsley et al., 2005; Dietrich and Kempermann, 2006; Lindvall and Kokaia, 2006; Macas et al., 2006). Nevertheless, there is no proof so far for wide-ranging functional recovery with spontaneous replacement of degenerated neurons by new neurons produced by endogenous NSCs. Consequently, disease or injury related neurodegeneration in both adult and aged brain is not followed by adequate self repair (Lichtenwalner and Parent, 2006; Sohur et al., 2006), and synaptic reorganization of surviving neurons after injury is aberrant in many instances (Martino, 2004; Shetty et al., 2005a; Sutula and Dudek, 2007). Grafting of fetal neural cells committed to specific neuronal phenotypes into appropriate sites within the damaged young or aged brain has been found to be useful for both facilitating the repair of disrupted circuitry and preventing the formation of abnormal synaptic reorganization (Shetty and Turner, 1996; Isacson and Deacon, 1997; Sanberg et al., 1997; Kordower et al., 1998; Whittemore, 1999; Bjorklund and Lindvall, 2000; Zaman and Shetty, 2002; Shetty et al., 2005a). Nonetheless, problems associated with obtaining considerable amounts of fetal tissue and ethical concerns preclude routine use of fetal cells as donor cells for grafting in human neurodegenerative disorders (Turner and Shetty, 2003). As a result, alternative sources of neural cells that allow maintenance and expansion in vitro for prolonged periods and exhibit characteristics of primary fetal neurons with regard to neuronal differentiation, and structural and functional integration into the host following grafting are essential (Dunnett and Rosser, 2007; Master et al., 2007; Shetty and Hattiangady, 2007).
Multipotent NSCs from the developing and adult brain, which could be expanded in culture as neurospheres using mitogens such as fibroblast growth factor-2 (FGF-2) and epidermal growth factor (EGF), are one of the potential alternatives to fresh fetal cells for neural grafting in neurodegenerative disorders (Reynolds and Weiss, 1992; Reynolds et al., 1992; Shetty and Turner, 1998, 1999; Palmer et al., 1999; Shetty, 2004; Scheffler et al., 2006). As a result, there is great interest in ascertaining the differentiation and integration of multiple types of NSCs in the young adult brain using distinct animal models of neurological disorders (Pluchino et al., 2003; Chu et al., 2004; Silani and Corbo, 2004; Hofstetter et al., 2005; Oliveira and Hodges, 2005; Conti et al., 2006; Vazey et al., 2006; Yasuhara et al., 2006a,b, 2007; Lee et al., 2007a,b; Shetty and Hattiangady, 2007).
However, the behavior of the progeny of NSCs after transplantation into the injured aged brain is unknown though a few studies have examined the fate of grafted NSCs in the intact aged brain (Hodges et al., 2000; Qu et al., 2001). As clinical application of neural grafting will comprise mostly elderly people afflicted with neurodegeneration, analyses of the potential of different NSCs to reinstate neurons that are lost due to disease or injury in the aged brain are of significance. Furthermore, as aging is associated with multiple changes in the brain microenvironment, including elevated oxidative stress and accumulation of protein and lipid by-products (Limke and Rao, 2002, 2003; Brazel and Rao, 2004; Shetty et al., 2004, 2005b), assessments made solely from NSC grafts into the younger brain may not be adequate for treating the injured or diseased aged brain. Moreover, NSC grafting studies in hippocampal injury models using aged animals have importance for developing ideal NSC grafting strategies for repair of the injured aged hippocampus in neurodegenerative diseases, such as stroke, temporal lobe epilepsy (TLE) and Alzheimer’s disease. Additionally, it is important to examine whether NSC grafts have propensity for tumor formation in the injured aged brain (Dihne et al., 2006; Carpentino et al., 2007). Considering these, we examined the behavior of FGF-2-responsive NSCs isolated from the fetal hippocampus following grafting into the injured aged hippocampus. Particularly, their capability for survival, directed migration into lesioned areas, and differentiation into different central nervous system phenotypes were examined.
Hippocampal NSCs isolated from embryonic day (E) 14 transgenic mice (129-Gt(ROSA)26Sortm1(EGFP)Luo/J) encoding green fluorescent protein (GFP) were expanded as neurospheres in vitro. Ability of neurosphere cells for robust neuronal differentiation was assessed through direct culturing of neurospheres in substrate-coated petri plates containing differentiation medium as well as via implantation of neurospheres onto organotypic hippocampal slice cultures. For in vivo studies, grafting of neurospheres was performed into the hippocampus of aged (24-months old) Fischer 344 (F344) rats at 4 days after an intracerebroventricular kainic acid (ICV KA) administration, a model of focal hippocampal injury and hyperexcitability. Transplants were examined at one month post-grafting, using immunofluorescence for a variety of markers that visualize neurons, astrocytes, oligodendrocytes and progenitor cells.
Materials and Methods
Preparation of neurosphere cultures
Pregnant green fluorescent protein (GFP) expressing mice (129-Gt(ROSA)26Sortm1(EGFP)Luo/J; Jackson Laboratory) were anesthetized on embryonic day (E) 14, and fetuses were removed via cesarean section and collected in a petri plate containing calcium and magnesium free Hank’s Balanced Salt Solution (HBSS, Sigma) with 0.6% glucose, 10 mM HEPES and 1% penicillin-streptomycin. Fetuses that are positive for GFP were identified using an inverted fluorescent microscope, the heads of these fetuses were collected through decapitation, and the brains were removed and stored in the HBSS solution. Following this, hippocampi were carefully dissected using a dissection microscope and hippocampal tissues were processed for dissociation and preparation of single cell suspension using mechanical trituration, as described in our previous study (Shetty, 2004). The density of cells was then adjusted to 0.5 X 106 viable cells/10 ml of culture medium containing Dulbecco’s modified Eagle’s medium (DMEM)/F12 (Life Technologies, Gaithersburg, MD) (3:1 mixture), B-27 supplement (Gibco) (1ml/50 ml of medium), FGF-2 (Peprotech) (20ng/ml), penicillin (Sigma) (100U/ml) and streptomycin (Sigma) (100 μg/ml). The cell suspension was plated onto 50 ml (25-cm2) Falcon tissue culture flasks with canted necks (10ml/flask: Becton Dickinson). Cultures were maintained at 370C in a humidified atmosphere of 5% CO2 and 19.8% O2. Free-floating primary neurospheres from each flask were passaged at 7 DIV through mechanical trituration and re-plating of dissociated cells. Both single isolated cells and smaller clumps of cells obtained after trituration of primary neurospheres proliferated and yielded secondary neurospheres. Different cell types within secondary neurospheres were analyzed immunocytochemically using markers of undifferentiated cells such as nestin (a marker of primitive neurofilament protein), Sox-2 (a transcription factor expressed in stem/progenitor cells), and glial fibrillary acidic protein (GFAP; a marker of both NSCs and astrocytes).
Differentiation cultures
The FGF-2 generated secondary neurospheres were rinsed, resuspended in a defined differentiation medium (Svendsen et al., 1995) containing DMEM (95.5 ml/100ml), B-27 (2ml/100ml), L-glutamine (1.25 ml/100 ml), and penicillin (100 units/ml) and streptomycin (100 μg/ml). Neurospheres were then transferred onto sterile and coated (poly-L-lysine 0.05 mg/ml) 35-mm Falcon dishes, and cultured in 1.5 ml of differentiation medium per dish. Differentiation was examined at 7 DIV by immunostaining using markers of neurons (β-III tubulin identifying TuJ-1 antibody], astrocytes (GFAP) and oligodendrocytes (O1 and O4).
Organotypic hippocampal slices and grafting
The hippocampal slice preparation was performed using the sterile procedure described by Stoppini et al., (1991). Following deep halothane anesthesia, postnatal day 8 rat pups (n=12) were decapitated, and the brains were rapidly removed and rinsed in ice-cold Gey’s balanced salt solution (GBSS; GIBCO) supplemented with 0.65% glucose. Hippocampi were dissected and 400 μm thick slices were cut perpendicular to the septo-temporal axis of the hippocampus using a McIllwain tissue chopper (Marty et al., 1996). Hippocampal slices were then transferred into the culture medium and individual slices were transferred onto Millicell-CM membranes of millicell inserts (Millipore Corporation, Marlborough, MA). Four to five slices were placed on each millicell insert in these experiments. The millicell inserts were kept in 6-well plates (one millicell/well) above 1.2 ml of Stoppini medium (pH = 7.2, (Stoppini et al., 1991). The plates were incubated at 370C with CO25% and O2 19.8%, and cultures were maintained as described in our previous study (Shetty and Turner, 1999).
Transplantation of hippocampal neurospheres into organotypic slices
After 7 days of incubation, intact and healthy hippocampal slice cultures were selected and used as host tissues for transplantation of individual secondary neurospheres of hippocampal stem cells. For this, neurospheres were thoroughly washed in HBSS by repeated re-suspension and centrifugation. Then, a single secondary neurosphere was dropped onto the surface of every slice near the end of the hippocampal fissure using a 20 μl micropipette. The neurosphere was then gently pushed to the CA3 region using a fine tungsten needle without causing any injury to the slice. The health of organotypic slice cultures following implantation of neurospheres was examined through daily observation using a Nikon inverted phase contrast microscope. Only the cultures that appeared healthy (i.e. with apparent retention of hippocampal cytoarchitecture) were used for phenotypic analyses of implanted NSCs. All slice cultures were terminated at 7 days after grafting by fixing in 4% paraformaldehyde solution in 0.1M phosphate buffered saline (PBS) for 30 minutes.
Kainic acid lesions
A unilateral ICV KA administration was performed on 24-months old male Fischer 344 rats (Harlan-Sprague-Dawley, n = 8). Rats were anesthetized with a mixture of ketamine (50 mg/ml), xylazine (6 mg/ml) and acepromazine (0.5 mg/ml) at a dose of 1.25-ml/kg body weight and fixed into a stereotaxic apparatus. In each of these rats, 0.4 μg of KA in 1μl of physiological saline was injected into the right lateral ventricle over a period of 20 minutes using the following stereotaxic coordinates: antero-posterior, 3.7 mm caudal to bregma; lateral, 4.1 mm right lateral to mid line; and ventral, 4.5 mm ventral to the surface of the brain. The incisor bars were set at 3.7 mm below the interaural line. All experiments were performed as per the animal protocol approved by the animal studies subcommittee of the Durham Veterans Affairs Medical Center and the Institutional Animal Care and Use Committee of the Duke University Medical Center.
Grafting of neurospheres into the injured aged hippocampus
Secondary neurospheres from mice expressing GFP were washed in DMEM and collected in a differentiation medium containing B-27 nutrient medium. At 4 days post-KA administration, aged rats with hippocampal lesions were anesthetized and fixed into a stereotaxic apparatus. For grafting, the plane of the incisor bar was set at 3.3 ± 0.3 mm below the interaural line. The skull was exposed through a midline incision and three burr holes (i.e. 3 sites ipsilateral to the KA administration) were drilled using the following stereotaxic coordinates: (1) anterior-posterior (AP) = 3.2 mm posterior to the bregma, lateral (L) = 2.0 mm right lateral to the midline, and ventral (V) = 3.5 mm from the surface of brain; (2) AP = -3.8 mm, L = 2.6 mm, V = 3.5 mm; (3) AP = -4.5 mm, L = 3.3 mm, V = 3.5 mm. These co-ordinates were selected to place grafts in the CA3 region just above the degenerated pyramidal cell layer. Two-three neurospheres in 1-2 μl of the cell suspension were drawn into a glass capillary needle fitted to a 10 μl Hamilton syringe and inserted through each of the above three burr holes to a depth of 3.5 mm from the surface of the brain. Neurospheres were then injected and the needle was left in place for an additional 8-10 minutes before withdrawal. Transplants were placed into 3 sites in the injured hippocampus to cover most of the septo-temporal extent of the hippocampus. The skin over the skull was stapled and animals were returned to their cages. Animals receiving grafts were immunosuppressed via daily subcutaneous injections of cyclosporine A (Biomol, 12 mg/Kg bw), beginning on the day of grafting and ending on the day of perfusion.
Antibodies
Antibodies from various sources were used for immunocytochemical characterization of NSCs and their progeny, and host tissues. This comprised antibodies for markers of NSCs (nestin, Sox-2), neurons (MAP-2ab, MAP-5, NeuN, GABA, β-III tubulin), astrocytes (GFAP, S-100 β), oligodendrocytes (O1, O4, Rip), oligodendrocyte progenitors (NG2), reactive astrocytes (vimentin) and activated microglia (ED-1). The antibodies to nestin (Rat 401; 1:10) and Rip (1:100) were obtained from Developmental Studies Hybridoma Bank (Department of Biological Sciences, University of Iowa, Iowa city, IA). The antibody to MAP-2ab (1:500) was from Roche (Indianapolis, IN) whereas, the antibodies to MAP-5 (1:1000), NeuN (1:1000), NG2 (1:100), O1 (1:100), O4 (1:100), Sox-2 (1:1000), S-100 β (1:1000) and vimentin (1:1000) were from Chemicon (Tamecula, CA). The antiserum to gamma amino butyric acid (GABA; 1:5000) was from Sigma (St. Louis, MO), the antiserum to glial fibrillary acidic protein (GFAP; 1:1000) was from Dako (Carpinteria, CA), the antibody to β-III tubulin (TuJ-1 antibody; 1:1000) was from Covance, and the antibody to ED-1 was from Serotech (1:1000; Raleigh, NC). The affinity purified and biotinylated secondary antibodies (horse anti-mouse IgG and goat anti-rabbit IgG; 1:200), and avidin-biotin reagents (elite ABC kit) were obtained from Vector (Burlinghame, CA). The other secondary antibodies (Goat anti-mouse FITC, goat anti-rabbit FITC, Alexa Fluor conjugated goat anti-mouse IgG and goat anti-rabbit IgG [1:200]) and streptavidin Texas red were from Molecular Probes (Eugene, OR).
Analyses of differentiation of neurosphere cells in vitro
Cultures were fixed with 4% paraformaldehyde in 0.1 M phosphate buffer (pH = 7.4) for 20 minutes, washed thrice in 0.1 M phosphate buffered saline (PBS) and processed for immunocytochemical staining of various antigens using avidin biotin complex (ABC) or indirect immunofluorescence methods (Shetty, 2004). For dual-labeling experiments using immunofluorescence, cultures were first treated with a mixture of two primary antibodies raised respectively in mouse and rabbit (e.g. TuJ-1 and GFAP), washed in PBS, treated with a mixture of appropriate secondary antibodies conjugated to Alexa Fluor 594 and Alexa Fluor 350. Following a thorough wash in PBS, cultures were covered with a slow fade solution (Molecular Probes, Eugene, OR), and observed under a Nikon fluorescence microscope using appropriate filters. Negative controls were processed similarly except that the primary antibody step was omitted. Cells positive for the above antigens were not observed in negative control cultures.
Analyses of differentiation of neurospheres implanted onto organotypic hippocampal slices
For immunostaining, slice cultures with transplants were fixed with 4% paraformaldehyde in 0.1 M phosphate buffer (pH = 7.4) for 30 minutes at 7 days after implantation of neurospheres. Organotypic hippocampal slices containing transplants were then peeled off from the millicell membrane, washed thrice in 0.1M phosphate buffered saline (PBS), and processed for immunofluorescence analyses, using primary antibodies against antigens specific for neurons (β-III tubulin, and MAP-2), astrocytes (S-100β) and oligodendrocytes (Rip). The secondary antibodies comprised either anti-mouse IgG conjugated to Alexa Fluor 594 or anti-rabbit IgG conjugated to Alexa Fluor 594. Using a fluorescence microscope, percentages of GFP+ cells (i.e. cells derived from neurospheres of NSCs) expressing neuronal, astrocytic, oligodendrocytic, and progenitor-specific antigens were quantified in 4-6 organotypic slice cultures receiving neurosphere implants.
Analyses of hippocampal cytoarchitecture and inflammation after ICV KA administration
The extent of hippocampal injury and inflammation was examined in a subgroup of aged rats receiving ICV KA administration. The rats were perfused transcardially with 4% paraformaldehyde solution at 4 or 16 days post-KA administration. The brains were cryoprotected in sucrose and sliced coronally (30-μm-thick sections) through the hippocampus using a cryocut, and collected serially in 24-well plates filled with phosphate buffer. Every 20th section through the hippocampus was processed for Nissl staining to ascertain the cytoarchitecture. To determine the extent of injury induced inflammation, additional series of sections (every 20th) were processed for visualization of the activated microglial cells using ED-1 immunohistochemistry and reactive astrocytes using vimentin immunostaining.
Analyses of differentiation of neurospheres grafted into the injured aged hippocampus
Thirty days following grafting, transplanted rats were perfused transcardially with 4% paraformaldehyde solution. Brains were cryoprotected in sucrose and sliced coronally (30-μm-thick sections) through the hippocampus using a cryocut, and collected serially in 24-well plates filled with phosphate buffer. Every 10th section through the grafted hippocampus was scanned using fluorescence microscope for the presence of GFP positive grafted cells. Sections containing transplants were processed for various immunofluorescence analyses, using primary antibodies against antigens specific for neurons (β-III tubulin, NeuN and MAP-2), astrocytes (GFAP, S-100β), oligodendrocytes (Rip, O1) and oligodendrocyte progenitors (NG2). The secondary antibodies comprised either anti-mouse IgG conjugated to Alexa Fluor 594 or anti-rabbit IgG conjugated to Alexa Fluor 594. Using a fluorescence microscope, percentages of GFP+ cells (i.e. cells derived from neurospheres of NSCs) expressing neuronal, astrocytic, oligodendrocytic, and oligodendrocyte progenitor-specific antigens were quantified in the injured aged hippocampus of 6 animals receiving neurosphere implants. Negative control sections were processed similarly except that the primary antibody step was omitted. Cells positive for the above antigens were not observed in negative control sections.
Results
Formation of neurospheres from the hippocampus of E14 transgenic mice expressing GFP
A fraction of cells isolated from the hippocampus of E14 transgenic mice expressing GFP exhibited hypertrophy, underwent proliferation, and gave rise to neurospheres when incubated in a culture medium containing FGF-2. Passaging of cells from primary neurospheres examined the ability of these cells for further expansion. As in primary cultures, single isolated cells and small clumps of cells obtained after trituration of primary neurospheres proliferated and yielded secondary neurospheres.
Cell types within neurospheres
Immunocytochemical analyses of neurospheres revealed the presence of the primitive neurofilament protein nestin (Hockfield and McKay, 1985) in a vast majority of neurosphere cells. Typically, some cells in the periphery of neurospheres exhibited short processes and were intensely immunoreactive for nestin in comparison to cells in the core of the neurospheres exhibiting no processes (Fig. 1 [A1-A3]). Evaluation of neurospheres with GFAP immunostaining also showed similar trend. Most of the GFAP positive cells were located in the peripheral region of the neurospheres and GFAP positive processes could be seen clearly in this region (Fig. 1 [B1-B3]). Thus, it is possible that cells with processes in the periphery of neurospheres represent cells that are partially committed to radial glial or astrocytic lineages. To substantiate the above possibility, we examined neurosphere cells with immunostaining for Sox-2, which is a member of the Sox (sex determining region of Y-chromosome) gene family and encodes transcription factors regulating crucial developmental decisions in different systems (Kamachi et al., 2000). The choice of Sox-2 to identify putative NSCs within neurospheres in this study is based on earlier findings that Sox-2 is a useful marker of NSCs both in vitro and in vivo (Avilion et al., 2003; Brazel and Rao, 2004; Komitova and Eriksson, 2004; Hattiangady and Shetty, 2008). We found that a large number of Sox-2 positive cells are located within the core of neurospheres in this study (Fig. 1 [C1-C3]). These cells were smaller and exhibited immature features such as the absence of processes. All neurosphere cells (including those with short processes in the periphery) were negative for markers of neurons (MAP-2ab, NeuN, β-III tubulin, MAP-5, and GABA), and oligodendrocytes (galactocerebroside, Rip, and O4).
Figure 1.

Secondary neurospheres generated from hippocampi of embryonic day 14 mice expressing green fluorescent protein (GFP) comprise cells immunopositive for the primitive neurofilament protein nestin, the intermediate filament protein glial fibrillary acidic protein (GFAP), and the transcription factor Sox-2. Note that vast majority of neurosphere cells express nestin (A1-A3), and substantial fractions of neurosphere cells express GFAP (B1-B3) and Sox-2 (C1-C3). The GFAP+ cells are distributed throughout the neurosphere whereas the Sox-2+ cells appear to be abundant mostly in the core of neurosphere. Scale bar, 100μm.
Differentiation of neurosphere cells in vitro
The differentiation potential of cells within secondary neurospheres was characterized by plating them onto poly-L-lysine-coated culture dishes containing differentiation medium for 7 days. Immunostaining for markers of neurons (β-III tubulin), astrocytes (GFAP), and oligodendrocytes (O1 and O4) clearly showed the presence of all three CNS cell types among differentiated cells derived from neurosphere cells (Figs. 2 and 3). By 7 days, a large number of cells migrated away from the core of neurospheres and 3 distinct zones could be visualized, which comprised core, intermediate and peripheral zones. The core of neurospheres at this stage mostly contained tightly packed undifferentiated cells that are not positive for mature neuronal or glial markers (data not illustrated). The intermediate zone comprised a glial bed made up of GFAP positive thick astrocytic processes and β-III tubulin positive neurons over this glial bed (Fig. 2 [A1-A4]). Virtually all neurons expressed GFP though the intensity of GFP varied from neuron to neuron (Fig. 2 [A1-A4]). The peripheral zone comprised cells that have differentiated into β-III tubulin positive neurons and O1 or O4 positive oligodendrocytes. Neurons in the intermediate and/or peripheral zones of neurospheres are arranged either in packed clusters (Fig. 2 [B1]) or dispersed with processes contacting each other (Fig. 2 [B2]). On the other hand, oligodendrocytes are mostly dispersed with each cell exhibiting extensive processes (Fig. 3 [A1-B3]). All oligodendrocytes immunoreactive for O1 or O4 also displayed intense GFP expression in the soma (Fig. 3 [A1-B3]). Thus, NSCs expanded from the hippocampus of E14 transgenic mice retain GFP expression even after the differentiation of the progeny of NSCs into neurons and glia. Quantification of β-III tubulin positive neurons among GFP+ cells in the intermediate zone revealed that ∼32% (Mean ± S.E.M = 31.6 ± 5.9%; n=4 independent cultures) of neurosphere cells differentiate into neurons. Whereas, quantification of GFAP+ cells among GFP+ cells demonstrated that ∼53% (Mean ± S.E.M = 53.2 ± 3.5%; n=4 independent cultures) of neurosphere cells differentiate into astrocytes. Thus, a vast majority of neurosphere cells differentiate into neurons and astrocytes. However, neurosphere cells also gave rise to O1 or O4 positive oligodendrocytes but they were mostly restricted to the peripheral zone and minimal in density, in comparison to the density of neurons and astrocytes.
Figure 2.

Differentiation of neurons and astrocytes from neurosphere cells in vitro. The neurons among green fluorescent protein (GFP) positive neurosphere cells (A1) were visualized with immunofluorescence for β-III tubulin (cells indicated by arrows in A1-A4). The astrocytes among GFP+ cells were identified through immunofluorescence for glial fibrillary acidic protein (GFAP; blue colored cells with thick processes in A4). B1 and B2 demonstrate examples of clusters of β-III tubulin+ neurons derived from neurospheres after 7 days incubation in the differentiation medium. Note that the core (C) of the neurosphere does not have any neurons. Scale bar, 50μm.
Figure 3.

Differentiation of neurosphere cells into oligodendrocytes in vitro. The oligodendrocytes among green fluorescent protein (GFP) positive neurosphere cells (A1) were visualized with immunofluorescence for O1 and O4 (markers of oligodendrocytes). The cells with characteristic oligodendrocytic morphology were commonly observed in the periphery of neurosphere cultures after 7 days incubation in the differentiation medium. Scale bar, 50μm.
Behavior of neurosphere cells following implantation into the organotypic hippocampal slices
Hippocampal slice cultures showed good survival and retained their organotypic organization. When fixed and stained with NeuN at 1-2 weeks after plating, slice cultures demonstrated conspicuous delineation of hippocampal cell layers and intact neurons (Fig. 4 [A1]). Examination of individual subfields of the hippocampus (CA1 and CA3, and dentate gyrus) showed that different strata in every hippocampal subfield were well preserved (data not illustrated), consistent with previous studies (Marty et al., 1996; Shetty and Turner, 1999). Neurospheres placed on organotypic slice cultures rapidly exhibited loss of their spherical shape with dispersion of cells away from the core of the neurosphere into the CA3 and dentate regions (Fig. 4 [A1-A3]). Migration of smaller clusters or individual cells away from the core of neurosphere is illustrated in Fig. 4 [A3]). A fraction of the migrated cells expressed the neuronal marker β-III tubulin with well defined neurites (Fig. 4 [B1-C3]). Another fraction of migrated neurosphere cells differentiated into S-100β+ mature astrocytes (Fig. 4 [D1-E3]). Generally, neurons immunopositive for β-III tubulin and MAP-2 were scattered over astrocyte-like cells in slice cultures. The astrocyte-like cells exhibited mostly flat soma with thicker processes (Fig. 4 [E1-E3]). Quantification of β-III tubulin positive neurons among GFP+ cells in slice cultures receiving neurosphere implants revealed that ∼21% (Mean ± S.E.M = 20.9 ± 2.6%; n=4 cultures) of neurosphere cells differentiate into neurons. Whereas, quantification of S-100β+ cells among GFP+ cells demonstrated that ∼38% (Mean ± S.E.M = 37.95 ± 1.7%; n=4 cultures) of neurosphere cells differentiate into astrocytes. However, mature oligodendrocytes immunopositive for Rip were absent among neurosphere cells at 1 week after implantation into organotypic slice cultures. Thus, differentiation of fractions of neurosphere cells into neurons and astrocytes occurs rapidly following their implantation into organotypic slice cultures. However, ∼40% of neurosphere cells do not express any of the neuronal or glial markers tested at one week post-implantation, suggesting that the overall differentiation of neurosphere cells proceeds slowly after their implantation into organotypic slice cultures, in comparison to differentiation of neurosphere cells in substrate-coated dishes.
Figure 4.
Neurospheres placed onto organotypic slice cultures exhibit rapid dispersion of cells and differentiate into neurons and glia. Figure A1 shows an example of the dispersion of green fluorescent protein (GFP) positive neurosphere cells in an organotypic slice immunostained for neuron-specific nuclear antigen (NeuN). A2 illustrates an example of the migration of green fluorescent protein positive neurosphere cells in an organotypic slice immunostained for β-III tubulin at 7 days after the implantation of a neurosphere. A3 shows magnified view of a region from A2 and demonstrates both individual and smaller clusters of GFP+ neurosphere cells that are migrating away from the core of neurosphere. Figures B1-C3 demonstrates differentiation of neurosphere cells in organotypic hippocampal slice cultures into β-III tubulin+ neurons (arrows), visualized via immunostaining with TuJ-1 antibody. Note the expression of GFP in both soma and dendrites of these neurons. Figures D1-E3 illustrates differentiation of neurosphere cells into mature astrocytes (arrows), identified through immunostaining with an antibody to S-100β. Scale bar, A1 and A2 = 500μm; A3 = 200μm; B1-E3 = 50μm.
Extent of hippocampal lesion after ICV KA administration
Histological evaluation of the hippocampus ipsilateral to the ICV KA administration at 4 days post-injection (i.e. equivalent to the time at which grafting was performed) revealed degeneration of substantial number of dentate hilar neurons, most of pyramidal neurons in CA3b and CA3c sub regions, and a fraction of pyramidal neurons in the CA3a sub region (Fig. 5 [B1, B2]). However, CA1 pyramidal and dentate granule cell layers were spared. In hippocampus contralateral to the KA administration, all hippocampal cell layers were intact, which is consistent with our previous observations in ICV KA treated aged animals (Shetty et al., 2004; Zaman and Shetty, 2002). Moreover, an extensive neurodegeneration in the hippocampus ipsilateral to ICV KA administration was also confirmed by the occurrence of a large number of ED-1 immunopositive activated microglial cells (Fig. 5 [C1, C2]).
Figure 5.

Pattern of hippocampal neurodegeneration in aged rats at 4 days after a unilateral intracerebroventricular (ICV) kainic acid (KA) administration, visualized by Nissl staining. Figure A1 illustrates an example of the hippocampus from a naïve aged rat. Figure B1 shows an example of the hippocampus ipsilateral to the ICV KA administration in an aged rat. Note the loss of CA3 pyramidal neurons (indicated by asterisks). A2 and B2 are magnified views of regions from A1 and B1. Note an extensive neurodegeneration in the dentate hilus and the CA3c sub region after ICV KA administration (B2), in comparison to the age-matched intact rat. Figure C1 shows distribution of ED-1 immunopositive activated microglial cells in the hippocampus ipsilateral to KA administration. Figure C2 is the magnified view of a region from C1 demonstrating the morphology of ED-1 immunopositive activated microglial cells. Scale bar, A1, B1, C1 = 500 μm; A2, B2, C2 = 200 μm.
Survival and differentiation of neurospheres following grafting into the lesioned aged hippocampus
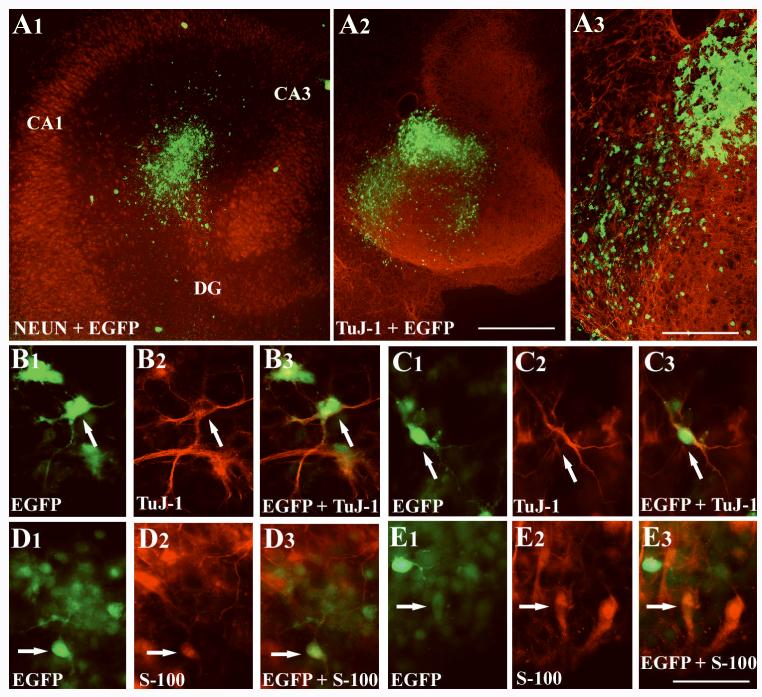
Surviving cells (i.e. GFP+ cells) derived from grafted neurospheres could be observed in the injured hippocampus of all aged animals receiving neurosphere transplants. The overall survival appeared to be good, as cellular debris at one-month post-grafting in the graft core was negligible and large numbers of GFP+ grafted cells could be observed in and around the graft core (Figs. 6 [A1, B1] and 7 [A1]). Although a majority of neurosphere cells dispersed away from the core of neurosphere into the surrounding regions, long-distance migration or directed migration of graft-derived cells into the degenerated CA3 region or into intact dentate or CA1 cell layers was not observed (Figs. 6 [A1-B2] and 7 [A1]). Additionally, active migration was not seen even when NSC grafts were engrafted in close vicinity of the neurogenic subgranular zone (Fig. 7 [A1]). The morphology of graft-derived cells comprised cells with unipolar, bipolar or multipolar soma and a few thin processes (Figs. 6 [C-H] and 7 [A2]). Lack of active migration of grafted cells may be related to reactive glia in the injured hippocampus, as vimentin immunostaining revealed a large number of reactive astrocytes and their processes around the periphery of graft (Fig. 7 [B]). The origin of reactive astrocytes appeared to be from the host hippocampus, as similar reactive astrocytes could be observed in the hippocampus of kainate-treated rats receiving no grafts (data not illustrated), suggesting that kainate-induced CA3 region injury induces the generation of reactive astrocytes. Lack of GFP expression (the donor cell marker) in reactive astrocytes also supports the host origin of these cells.
Figure 6.

Neurospheres survive transplantation into the lesioned aged hippocampus but cells derived from neurospheres stay clustered close to the core of neurospheres. A1 and B1 illustrate examples of green fluorescent protein (GFP) positive neurosphere transplants in the injured aged hippocampus at 30 days post-grafting. A2 and B2 show magnified view of regions of transplants in A1 and B1 and demonstrate the morphology of cells derived from neurosphere grafts. Figures C-H illustrates the morphology of individual cells derived from neurosphere grafts. Note that the morphology of some of these cells resemble neurons (F and G), astrocytes (C and H), and oligodendrocytes (D and E). Scale bar, A1 and B1 = 200μm; A2 and B2 = 100μm; C-H = 50μm.
Figure 7.
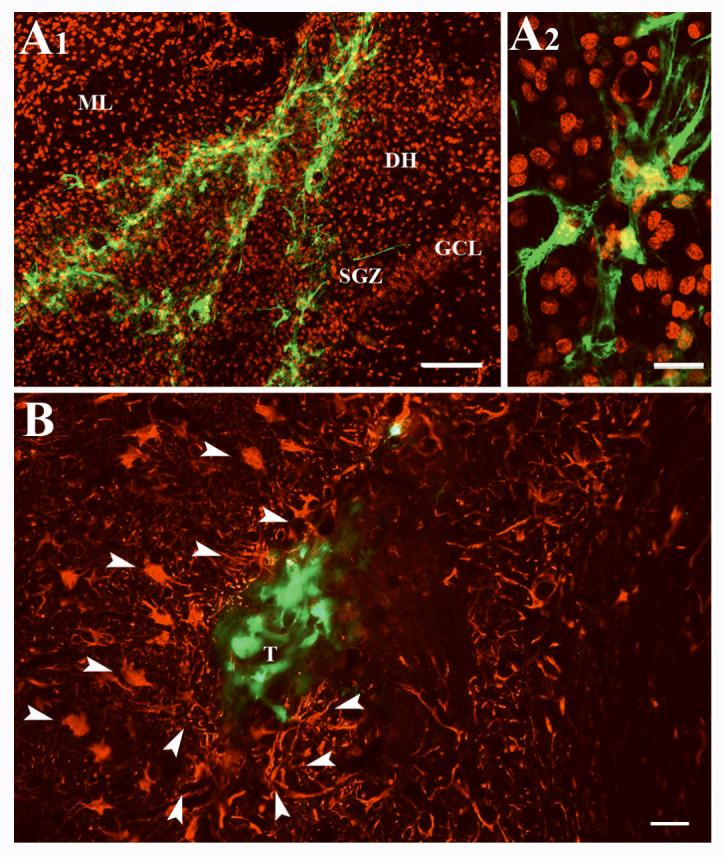
An example of neurosphere transplant placed in the dentate gyrus. A1 shows distribution of green fluorescent protein (GFP) positive cells derived from neurosphere grafts in the dentate gyrus. The nuclei of hippocampal cells are visualized with DAPI staining and DAPI+ nuclei were given a pseudo red color for highlighting the location of GFP+ grafted cells. Note that neurosphere cells do not migrate into the neurogenic subgranular zone (SGZ) despite their placement in close vicinity of the SGZ. A2 shows magnified view of a region of transplant from A1 demonstrating the morphology of some of the graft-derived cells. Figure B shows vimentin positive reactive astrocytes and their processes (indicated by arrowheads) around the periphery of a neurosphere transplant. DH, dentate hilus; GCL, granule cell layer; ML, molecular layer; T, transplant. Scale bar, A1 = 100μm. A2 and B = 20μm.
Analysis of the differentiation of neurospheres cells at 30 days post-grafting suggested that a small fraction of neurosphere cells differentiate into neurons following grafting into the injured aged hippocampus, as confirmed by dual immunofluorescence for GFP with markers of neurons such as TuJ-1, NeuN and MAP-2 (Fig. 8 [A1-D3]). Quantification of β-III tubulin positive neurons among GFP+ cells in neurosphere transplants placed into the injured aged hippocampus revealed that ∼5% (Mean ± S.E.M = 5.32 ± 0.4%; n=6) of neurosphere cells differentiate into neurons. Analyses of NeuN+ neurons among GFP+ cells showed that ∼3% (2.7 ± 0.5%; n=4) of neurosphere cells differentiate into mature neurons at one-month post-grafting. Interestingly, significant fractions of neurosphere cells expressed markers of astrocytes such as GFAP or S-100β (Fig. 8 [E1-F3]). Measurement of S-100β+ cells among GFP+ cells showed that ∼28% (27.7 ± 8.2%; n=6) of neurosphere cells differentiate into mature astrocytes. In addition, though infrequent, smaller fractions of neurosphere cells expressed markers of oligodendrocytes such as O1 and rip (Fig. 8 [G1, G3]) or the oligodendrocytic progenitor cell marker NG2 (Fig. 8 [H1-H3]). Quantification revealed that differentiation of neurosphere cells into O1+ oligodendrocytes is ∼10% (10 ±1.3%; n=5), Rip+ mature oligodendrocytes is ∼6% (5.9 ± 0.4%; n=4) and NG2+ oligodendrocyte progenitors is ∼9% (9.3 ± 0.9%; n=3). From the above, it is clear that ∼53% of cells derived from neurosphere grafts differentiate into neurons, astrocytes, oligodendrocytes or oligodendrocytic progenitors at one-month post-grafting in the injured aged hippocampus.
Figure 8.
Differentiation of cells derived from green fluorescent protein (GFP) positive neurosphere transplants into neurons, astrocytes and oligodendrocytes at one-month post-grafting in the injured aged hippocampus. The upper panel shows differentiation of GFP+ neurosphere cells into neurons, as visualized through immunostaining using antibodies to TuJ-1 (A1-B3), neuron-specific nuclear antigen (NeuN; C1-C3), and microtubule associated protein-2 (MAP-2; D1-D3). The middle panel shows differentiation of GFP+ neurosphere cells into astrocytes, as visualized through immunostaining using antibodies to glial fibrillary acidic protein (GFAP; E1-E3) and S-100β (F1-F3). The lower panel demonstrates differentiation of individual GFP+ neurosphere cells into oligodendrocytes and oligodendrocytic progenitors, as identified via immunostaining using antibodies to Rip (G1-G3) and neuron-glia proteoglycan 2 (NG2; H1-H3). Scale bar, A1 and B3 = 50μm; C1-D3 = 25μm; E1-H3 = 25μm.
Discussion
This investigation represents the first study to examine the behavior of grafted NSCs in the injured aged brain. The results provide evidence that NSCs derived from the fetal hippocampus survive and give rise to all three CNS phenotypes following transplantation into the injured aged hippocampus. However, apart from a simple dispersion from the graft core, active or directed migration of grafted NSCs away from the grafted site into neurogenic or degenerated non-neurogenic regions of the host hippocampus was not observed. Furthermore, differentiation of grafted NSCs into neurons was limited, as evidenced by only 3-5% of graft-derived cells expressing markers of neurons (β-III tubulin, NeuN or MAP-2). Differentiation of grafted NSCs into glia was however more robust as ∼28% of cells derived from grafted neurospheres differentiated into GFAP+ astrocytes, 6-10% into Rip+/ O1+ oligodendrocytes (Rip) and ∼9% into NG2+ oligodendrocytic progenitors. Thus, direct grafting of primitive NSCs does not appear to be adequate for repair of the injured aged brain through neuronal replacement. It may be necessary to either prime the host microenvironment for improved neuronal differentiation from grafted NSCs or transplant pre-differentiated neurons derived from NSCs in culture (Rosser et al., 2007). Nevertheless, because of their capability for survival and differentiation into a large number of astrocytes under adverse conditions, NSCs appear ideal for delivering neurotrophic factors or other neuroprotective compounds into the injured or diseased aged brain (Capowski et al., 2007).
Significance of stem cell grafting studies in the injured aged hippocampus
It is well known that age-related neurodegenerative disorders such as Alzheimer’s disease, dementia and increased incidence of stroke in elderly individuals are typified by considerable degeneration of neurons in different regions of the brain. Recently, NSC grafting approach has been perceived as a potential therapy for alleviating age-related neurodegenerative disorders (Limke and Rao, 2003; Bernal and Peterson, 2004; Brazel and Rao, 2004; Borlongan et al., 2005). The goals of this strategy include either replacement of the lost or diseased neurons and glia by cells derived from the grafted NSCs or the use of genetically engineered NSCs as delivery vehicles to dispense factors such as neurotrophic factors and other proteins that are known to alleviate neuronal dysfunction, restrain inflammation or progression of the disease on a long-term basis. However, for these strategies to be successful there are some requirements. First, it is vital that grafted NSCs must survive transplantation into the injured aged host brain which is typically not conducive for survival of transplanted cells unless the donor cells are pre-treated and grafted with specific neurotrophic factors (Zaman and Shetty, 2002; Rao et al., 2006). Second, it would be highly useful if they migrate into the site of injury or sites where cell replacements are needed. Alternatively, it would be helpful for delivering factors if they grow processes into injured regions. Furthermore, if the goal is reconstruction of the damaged circuitry, then, grafted NSCs need to be able to differentiate into cell types that are specific to the injured/diseased region and make appropriate functional connections with the host tissue. Stem cell therapy in the injured aged brain is however complicated because of availability of lower levels neurotrophic factors that support NSC proliferation and differentiation (Zaman and Shetty, 2002; Shetty et al., 2004; Rao et al., 2006) and increases in proinflammatory cytokines (Gemma et al., 2007). Nevertheless, direct NSC grafting studies in injury or disease models of aged animals are critically needed for developing this approach for future, in addition to studies in similar models of young or adult animals (Hallbergson et al., 2003; Limke and Rao, 2003; Bernal and Peterson, 2004; Brazel and Rao, 2004). Therefore, as a first step in this direction, we examined the ability of the injured aged hippocampus to support survival, migration and differentiation of NSCs expanded from the fetal hippocampus.
Potential reasons for impaired migration of grafted NSCs in the injured aged hippocampus
In this study, NSCs survived grafting into the injured aged hippocampus and large numbers of GFP+ grafted cells could be observed in and around the graft core. However, the overall survival of grafted cells could not be quantified as NSCs were transplanted as neurospheres. Virtually all grafted cells remained in smaller clusters without showing any affinity for migrating away from the core into either the neurogenic region or the degenerated CA3 cell layer in the injured aged hippocampus. Interestingly, active migration was not apparent even when NSC grafts were placed in close vicinity of the neurogenic subgranular zone. Precise reasons for lack of migration of grafted NSCs in the injured aged brain are not clear. Lack of migration does not appear to be an intrinsic feature of the chosen NSCs in this study. This is because similar NSCs exhibited dispersion as well as active migration when implanted into organotypic slice cultures as neurospheres. Furthermore, previous studies show that NSCs grafted into the intact adult or middle-aged hippocampus exhibit migration into both neurogenic and non-neurogenic regions (Richardson et al., 2005; Hattiangady et al., 2007). Additionally, it has been reported that NSCs grafted into the lateral ventricle of intact aged rats exhibit extensive and positional incorporation into the host brain with improvement of cognitive score assessed by the Morris water maze in aged rats exhibiting memory impairment (Qu et al., 2001). Therefore, absence of migration of grafted NSCs in the injured aged brain in this study might be due to the presence of inflammation typified by a large number of reactive astrocytes and activated microglial cells (Shetty et al., 2004). This inflammation mostly appeared to be a result of kainate induced CA3 region injury existing at the time of grafting. This is because, both reactive astrocytes and activated microglial cells could be observed in kainate treated rats receiving no grafts. However, the trauma inflicted at the time of grafting might have contributed towards accumulation of reactive astrocytes around the periphery of grafts. Additional reasons for absence of migration of grafted NSCs likely include the cross-species grafting paradigm (i.e. mouse NSCs into the rat brain with cyclosporine mediated immune suppression) employed or grafting of NSCs as neurospheres (instead of single cell suspension). Long-term studies using allografts of NSCs prepared as single cell suspension in the injured aged brain are necessary to determine whether lack of migration observed in this study is linked to the above mentioned technical issues.
Role of microenvironment in limited neuronal differentiation of grafted NSCs in the injured aged hippocampus
Analyses of the fate of grafted NSCs revealed limited neuronal differentiation, robust astrocytic differentiation and moderate oligodendrocyte differentiation in the injured aged hippocampus. In comparison to neuronal differentiation of these NSCs in simple two-dimensional cultures on poly-L-lysine coated dishes (32% of all cells) or following implantation into organotypic slice cultures (21% of all cells), neuronal differentiation was drastically diminished following grafting into the injured aged hippocampus. This was evidenced by an occasional occurrence of GFP+ neurosphere cells positive for neuronal markers such as β-III tubulin (5% of GFP+ cells) or NeuN (3% of GFP+ cells). The overall trend was slightly better for differentiation of grafted NSCs into Rip+/O1+ oligodendrocytes (6-10% of GFP+ cells) or NG2+ oligodendrocytic progenitors (9% of GFP+ cells). However, S-100β+ or GFAP+ astrocytes were considerably more frequent among GFP+ grafted NSCs (28% of GFP+ cells). Considering all cell types, ∼53% of cells derived from neurosphere grafts differentiated into neurons, astrocytes, oligodendrocytes or oligodendrocytic progenitors at one-month post-grafting in the injured aged hippocampus. Approximately 47% of remaining cells derived from neurosphere grafts however did not express any of the markers of neurons, astrocytes, oligodendrocytes or oligodendrocyte progenitors, suggesting that a significant fraction of grafted neurosphere cells remain undifferentiated at 30 days post-grafting in the injured aged hippocampus. Limited differentiation of grafted NSCs into neurons is not an intrinsic feature of these cells as significantly greater fractions of NSCs differentiated into neurons in culture and following implantation into the organotypic slice cultures in this study. Considering this, one might question the conduciveness of the microenvironment of the injured aged hippocampus for widespread differentiation of grafted NSCs into neurons and other CNS cell types. Fetal cell grafting studies into the injured aged hippocampus on the other hand imply that the extent of neuronal differentiation of grafted fetal cells is similar between grafts placed into the injured young hippocampus and the injured aged hippocampus (Zaman and Shetty, 2002; Rao et al., 2006). However, NSCs are primitive, proliferating and uncommitted cells, in comparison to fetal cells that are mostly committed to a neuronal lineage and are post-mitotic at the time of grafting. Analyses of cell types within neurospheres in this study indeed demonstrated the presence of cells that expressed the primitive intermediate filament protein nestin, the transcription factor Sox-2 or the astrocytic/stem cell marker GFAP but complete absence of cells expressing NeuN, β-III tubulin or Rip, suggesting that the donor cells used in our grafting studies contained a mixture of stem/progenitor cells and precursor cells of neurons and glia but not mature neurons and astrocytes.
Considering the above, it is possible that signaling proteins that direct differentiation of primitive NSCs into different mature CNS cell types are diminished in the injured aged hippocampus. Indeed, the levels of several neurotrophic factors that are known to aid neuronal differentiation of hippocampal cells are diminished in the intact aged hippocampus and injury to hippocampus does not up-regulate these factors to levels that are typically found in the injured young hippocampus (Shetty et al., 2004; Hattiangady et al., 2005; Shetty et al., 2005b). Moreover, studies show that NSCs persist in the hippocampus of Alzheimer’s disease brains (Lovell et al., 2006). Although these NSCs do not seem to produce significant numbers of neurons in vivo, culturing and incubation of NSCs isolated from Alzheimer’s disease brains in differentiation medium leads to generation of β-III tubulin positive neurons and GFAP-positive astrocytes (Lovell et al., 2006). Thus, it is likely that the microenvironment of the injured aged host is not conducive for widespread differentiation of grafted NSCs into neurons. However, it remains to be examined whether the minimal neuronal differentiation of grafted NSCs observed in this study is a phenomenon specific to the injured aged brain or generally applicable to the injured adult brain. Comparative analyses of the differentiation of NSCs grafted into the injured adult brain and injured aged brain are needed in future studies to address this issue. Furthermore, it should be noted that the investigation of cell types derived from grafted NSCs was performed at a relatively shorter post-transplantation delay (i.e. one month after grafting) in this study. Therefore, it is plausible that a vast majority of NSCs grafted into the injured aged hippocampus may just be exhibiting a delay in their differentiation rather than a loss of differentiation potential. Analyses at extended time-points after grafting are needed in future to determine this possibility. Additionally, because of the perceived unfavorable milieu in the injured aged brain for differentiation of neurons, it is important to examine in future the differentiation pattern of NSCs that are grafted with different concentrations of distinct neurotrophic factors or NSCs that are genetically engineered to exclusively differentiate into neurons.
Links between neuronal differentiation and NSC graft-mediated recovery from injury or disease
Although the ability to replace lost neurons in injured brain regions via grafting of NSCs or neural cell lines is attractive for reconstruction of the damaged or disrupted circuitry (Hara et al., 2007), recovery from injury or disease related functional deficits may also be achievable through neuroprotection and repair mediated by grafted NSCs and their glial progeny particularly astrocytes (Ourednik et al., 2002; Yasuhara et al., 2006b; Hara et al., 2007; Sanberg, 2007; Yasuhara et al., 2007). For instance, a recent study by Redmond and colleagues (Redmond et al., 2007) demonstrated that undifferentiated human NSCs placed into the substantia nigra and caudate nuclei of monkeys exhibiting Parkinson’s disease survived transplantation and induced significant behavioral recovery even though only a very small fraction of grafted NSCs differentiated into dopamine producing cells. However, a large number of grafted NSCs differentiated into astrocytes and expressed neurotrophic factors such as glial-derived neurotrophic factor (GDNF), suggesting that homeostatic adjustments to the microenvironment protected the existing dopaminergic neurons in the host and enhanced their function (Redmond et al., 2007; Sanberg, 2007). This suggests that neuronal replacement is not a critical pre-requisite for achieving functional recovery through NSC transplantation if the grafted NSCs are capable of providing considerable neuroprotection and promoting the plasticity (including the sprouting of axons to appropriate denervated regions) of remaining host neurons through release of compounds that facilitate neuroprotection and/or brain repair. In this context, it is interesting to note that grafting of NSCs into the hippocampus of adult rats with transient global ischemia lead to partial improvement in impaired spatial learning though only 3-9% of grafted NSCs differentiated into neurons (Toda et al., 2001). Furthermore, grafting of NSCs engineered to secrete trophic factors such as GDNF or IGF-1 was found to be promising for functional recovery in animal models of Parkinson’s disease (Akerud et al., 2001; Ebert et al., 2008). Nonetheless, these issues need to be tested rigorously in future through long-term NSC grafting studies in models of diseases afflicting the elderly (such as Alzheimer’s disease) using aged animals.
Acknowledgments
This research was supported by grants from National Institutes Health (NIA RO1 AG20924, NINDS RO1 NS43507, and NINDS RO1 NS54780 to A.K.S) and Department of Veterans Affairs (Merit Review Award to A.K.S.).
References
- Akerud P, Canals JM, Snyder EY, Arenas E. Neuroprotection through delivery of glial cell line-derived neurotrophic factor by neural stem cells in a mouse model of Parkinson’s disease. J Neurosci. 2001;21:8108–8118. doi: 10.1523/JNEUROSCI.21-20-08108.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Lois C. Neuronal stem cells in the brain of adult vertebrates. Stem Cells. 1995. 1995;13(May):263–272. doi: 10.1002/stem.5530130307. [DOI] [PubMed] [Google Scholar]
- Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17:126–140. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal GM, Peterson DA. Neural stem cells as therapeutic agents for age-related brain repair. Aging Cell. 2004;3:345–351. doi: 10.1111/j.1474-9728.2004.00132.x. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Lindvall O. Cell replacement therapies for central nervous system disorders. Nat Neurosci. 2000;3:537–544. doi: 10.1038/75705. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Yu G, Matsukawa N, Yasuhara T, Hara K, Xu L. Cell transplantation: stem cells in the spotlight. Cell Transplant. 2005;14:519–526. [PubMed] [Google Scholar]
- Brazel CY, Rao MS. Aging and neuronal replacement. Ageing Res Rev. 2004;3:465–483. doi: 10.1016/j.arr.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Capowski EE, Schneider BL, Ebert AD, Seehus CR, Szulc J, Zufferey R, Aebischer P, Svendsen CN. Lentiviral vector-mediated genetic modification of human neural progenitor cells for ex vivo gene therapy. J Neurosci Methods. 2007;163:338–349. doi: 10.1016/j.jneumeth.2007.02.022. [DOI] [PubMed] [Google Scholar]
- Carpentino JE, Hartman NW, Grabel LB, Naegele JR. Region-specific differentiation of embryonic stem cell-derived neural progenitor transplants into the adult mouse hippocampus following seizures. J Neurosci Res. 2007 doi: 10.1002/jnr.21514. [Epub ahead of print] PMID: 17918739.
- Chu K, Kim M, Chae SH, Jeong SW, Kang KS, Jung KH, Kim J, Kim YJ, Kang L, Kim SU, Yoon BW. Distribution and in situ proliferation patterns of intravenously injected immortalized human neural stem-like cells in rats with focal cerebral ischemia. Neurosci Res. 2004;50:459–465. doi: 10.1016/j.neures.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Conti L, Reitano E, Cattaneo E. Neural stem cell systems: diversities and properties after transplantation in animal models of diseases. Brain Pathol. 2006;16:143–154. doi: 10.1111/j.1750-3639.2006.00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich J, Kempermann G. Role of endogenous neural stem cells in neurological disease and brain repair. Adv Exp Med Biol. 2006;557:191–220. doi: 10.1007/0-387-30128-3_12. [DOI] [PubMed] [Google Scholar]; Emsley JG, Mitchell BD, Kempermann G, Macklis JD. Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Prog Neurobiol. 2005;75:321–341. doi: 10.1016/j.pneurobio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Dihné M, Bernreuther C, Hagel C, Wesche KO, Schachner M. Embryonic stem cell-derived neuronally committed precursor cells with reduced teratoma formation after transplantation into the lesioned adult mouse brain. Stem Cells. 2006;24:1458–1466. doi: 10.1634/stemcells.2005-0413. [DOI] [PubMed] [Google Scholar]
- Dunnett SB, Rosser AE. Stem cell transplantation for Huntington’s disease. Exp Neurol. 2007;203:279–292. doi: 10.1016/j.expneurol.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Ebert AD, Beres AJ, Barber AE, Svendsen CN. Human neural progenitor cells over-expressing IGF-1 protect dopamine neurons and restore function in a rat model of Parkinson’s disease. Exp Neurol. 2008;209:213–223. doi: 10.1016/j.expneurol.2007.09.022. [DOI] [PubMed] [Google Scholar]
- Gemma C, Bachstetter AD, Cole MJ, Fister M, Hudson C, Bickford PC. Blockade of caspase-1 increases neurogenesis in the aged hippocampus. Eur J Neurosci. 2007;26:2795–2803. doi: 10.1111/j.1460-9568.2007.05875.x. [DOI] [PubMed] [Google Scholar]
- Hallbergson AF, Gnatenco C, Peterson DA. Neurogenesis and brain injury: managing a renewable resource for repair. J Clin Invest. 2003;112:1128–1133. doi: 10.1172/JCI20098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Matsukawa N, Yasuhara T, Xu L, Yu G, Maki M, Kawase T, Hess DC, Kim SU, Borlongan CV. Transplantation of post-mitotic human neuroteratocarcinoma-overexpressing Nurr1 cells provides therapeutic benefits in experimental stroke: in vitro evidence of expedited neuronal differentiation and GDNF secretion. J Neurosci Res. 2007;85:1240–1251. doi: 10.1002/jnr.21234. [DOI] [PubMed] [Google Scholar]
- Hattiangady B, Shetty AK. Aging does not alter the number or phenotype of putative stem/progenitor cells in the neurogenic region of the hippocampus. Neurobiol Aging. 2008;29:129–147. doi: 10.1016/j.neurobiolaging.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattiangady B, Rao MS, Shetty GA, Shetty AK. Brain-derived neurotrophic factor, phosphorylated cyclic AMP response element binding protein and neuropeptide Y decline as early as middle age in the dentate gyrus and CA1 and CA3 subfields of the hippocampus. Exp Neurol. 2005;195:353–371. doi: 10.1016/j.expneurol.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Hattiangady B, Shuai B, Cai J, Coksaygan T, Rao MS, Shetty AK. Increased dentate neurogenesis after grafting of glial restricted progenitors or neural stem cells in the aging hippocampus. Stem Cells. 2007;25:2104–2117. doi: 10.1634/stemcells.2006-0726. [DOI] [PubMed] [Google Scholar]
- Hockfield S, McKay RD. Identification of major cell classes in the developing mammalian nervous system. J Neurosci. 1985;12:3310–3328. doi: 10.1523/JNEUROSCI.05-12-03310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges H, Veizovic T, Bray N, French SJ, Rashid TP, Chadwick A, Patel S, Gray JA. Conditionally immortal neuroepithelial stem cell grafts reverse age-associated memory impairments in rats. Neuroscience. 2000;101:945–955. doi: 10.1016/s0306-4522(00)00408-5. [DOI] [PubMed] [Google Scholar]
- Hofstetter CP, Holmstrom NA, Lilja JA, Schweinhardt P, Hao J, Spenger C, Wiesenfeld-Hallin Z, Kurpad SN, Frisen J, Olson L. Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat Neurosci. 2005;8:346–353. doi: 10.1038/nn1405. [DOI] [PubMed] [Google Scholar]
- Isacson O, Deacon T. Neural transplantation studies reveal the brain’s capacity for continuous reconstruction. Trends Neurosci. 1997;20:477–482. doi: 10.1016/s0166-2236(97)01081-3. [DOI] [PubMed] [Google Scholar]
- Kamachi Y, Uchikawa M, Kondoh H. Pairing SOX off: with partners in the regulation of embryonic development. Trends Genet. 2000;16:182–187. doi: 10.1016/s0168-9525(99)01955-1. [DOI] [PubMed] [Google Scholar]
- Kaplan MS, Bell DH. Mitotic neuroblasts in the 9-day-old and 11-month-old rodent hippocampus. J Neurosci. 1984;4:1429–1241. doi: 10.1523/JNEUROSCI.04-06-01429.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;97:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- Komitova M, Eriksson PS. Sox-2 is expressed by neural progenitors and astroglia in the adult rat brain. Neurosci Lett. 2004;369:24–27. doi: 10.1016/j.neulet.2004.07.035. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Freeman TB, Chen EY, Mufson EJ, Sanberg PR, Hauser RA, Snow B, Olanow CW. Fetal nigral grafts survive and mediate clinical benefit in a patient with Parkinson’s disease. Mov Disord. 1998;13:383–393. doi: 10.1002/mds.870130303. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996 Mar 15. 1996;16(6):2027–33. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Al Shamy G, Elkabetz Y, Schoefield CM, Harrsion NL, Panagiotakos G, Socci ND, Tabar V, Studer L. Directed Differentiation And Transplantation of Human Embryonic Stem Cell Derived Motoneurons. Stem Cells. May 3. 2007a doi: 10.1634/stemcells.2007-0097.; [Epub ahead of print] PMID: 17478583.
- Lee HJ, Kim KS, Kim EJ, Choi HB, Lee KH, Park IH, Ko Y, Jeong SW, Kim SU. Brain transplantation of immortalized human neural stem cells promotes functional recovery in mouse intracerebral hemorrhage stroke model. Stem Cells. 2007b;25:1204–1212. doi: 10.1634/stemcells.2006-0409. [DOI] [PubMed] [Google Scholar]
- Lichtenwalner RJ, Parent JM. Adult neurogenesis and the ischemic forebrain. J Cereb Blood Flow Metab. 2006;26:1–20. doi: 10.1038/sj.jcbfm.9600170. [DOI] [PubMed] [Google Scholar]
- Limke TL, Rao MS. Neural stem cells in aging and disease. J Cell Mol Med. 2002;6:475–496. doi: 10.1111/j.1582-4934.2002.tb00451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limke TL, Rao MS. Neural stem cell therapy in the aging brain: pitfalls and possibilities. J Hematother Stem Cell Res. 2003;12:615–623. doi: 10.1089/15258160360732641. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z. Stem cells for the treatment of neurological disorders. Nature. 2006;441:1094–1096. doi: 10.1038/nature04960. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z, Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders-how to make it work. Nat Med. 2004;10(Suppl):S42–50. doi: 10.1038/nm1064. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Geiger H, Van Zant GE, Lynn BC, Markesbery WR. Isolation of neural precursor cells from Alzheimer’s disease and aged control postmortem brain. Neurobiol Aging. 2006;27:909–917. doi: 10.1016/j.neurobiolaging.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993 Jul. 2003;11(1):173–89. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Macas J, Nern C, Plate KH, Momma S. Increased generation of neuronal progenitors after ischemic injury in the aged adult human forebrain. J Neurosci. 2006;26:13114–3119. doi: 10.1523/JNEUROSCI.4667-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405:951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- Martino G. How the brain repairs itself: new therapeutic strategies in inflammatory and degenerative CNS disorders. Lancet Neurol. 2004;3:372–378. doi: 10.1016/S1474-4422(04)00771-9. [DOI] [PubMed] [Google Scholar]
- Marty S, Carroll P, Cellerino A, Castren E, Staiger V, Thoenen H, Lindholm D. Brain-derived neurotrophic factor promotes the differentiation of various hippocampal nonpyramidal neurons, including Cajal-Retzius cells, in organotypic slice cultures. J Neurosci. 1996;16:675–687. doi: 10.1523/JNEUROSCI.16-02-00675.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Master Z, McLeod M, Mendez I. Benefits, risks and ethical considerations in translation of stem cell research to clinical applications in Parkinson’s disease. J Med Ethics. 2007;33:169–73. doi: 10.1136/jme.2005.013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110:429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- Oliveira AA, Jr., Hodges HM. Alzheimer’s disease and neural transplantation as prospective cell therapy. Curr Alzheimer Res. 2005;2:79–95. doi: 10.2174/1567205052772759. [DOI] [PubMed] [Google Scholar]
- Ourednik J, Ourednik V, Lynch WP, Schachner M, Snyder EY. Neural stem cells display an inherent mechanism for rescuing dysfunctional neurons. Nat Biotechnol. 2002;20:1103–1110. doi: 10.1038/nbt750. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Markakis EA, Willhoite AR, Safar F, Gage FH. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J Neurosci. 1999;19:8487–8497. doi: 10.1523/JNEUROSCI.19-19-08487.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, Galli R, Del Carro U, Amadio S, Bergami A, Furlan R, Comi G, Vescovi AL, Martino G. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422:688–694. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- Qu T, Brannen CL, Kim HM, Sugaya K. Human neural stem cells improve cognitive function of aged brain. Neuroreport. 2001;12:1127–1132. doi: 10.1097/00001756-200105080-00016. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Shetty AK. Fetal hippocampal CA3 cell grafts enriched with FGF-2 and BDNF exhibit robust long-term survival and integration and suppress aberrant mossy fiber sprouting in the injured middle-aged hippocampus. Neurobiol Dis. 2006;21:276–290. doi: 10.1016/j.nbd.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Redmond DE, Jr., Bjugstad KB, Teng YD, Ourednik V, Ourednik J, Wakeman DR, Parsons XH, Gonzalez R, Blanchard BC, Kim SU, Gu Z, Lipton SA, Markakis EA, Roth RH, Elsworth JD, Sladek JR, Jr., Sidman RL, Snyder EY. From the Cover: Behavioral improvement in a primate Parkinson’s model is associated with multiple homeostatic effects of human neural stem cells. Proc Natl Acad Sci USA. 2007;104:12175–12180. doi: 10.1073/pnas.0704091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Tetzlaff W, Weiss S. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J Neurosci. 1992;12:4565–4574. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RM, Broaddus WC, Holloway KL, Sun D, Bullock MR, Fillmore HL. Heterotypic neuronal differentiation of adult subependymal zone neuronal progenitor cells transplanted to the adult hippocampus. Mol Cell Neurosci. 2005;28:674–682. doi: 10.1016/j.mcn.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Rosser AE, Zietlow R, Dunnett SB. Stem cell transplantation for neurodegenerative diseases. Curr Opin Neurol. 2007;20:688–692. doi: 10.1097/WCO.0b013e3282f132fc. [DOI] [PubMed] [Google Scholar]
- Sanberg PR. Neural stem cells for Parkinson’s disease: To protect and repair. Proc Natl Acad Sci USA. 2007;104:11869–11870. doi: 10.1073/pnas.0704704104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanberg PR, Borlongan CV, Koutouzis TK, Norgren RB, Jr., Cahill DW, Freeman TB. Human fetal striatal transplantation in an excitotoxic lesioned model of Huntington’s disease. Ann N Y Acad Sci. 1997;831:452–460. doi: 10.1111/j.1749-6632.1997.tb52217.x. [DOI] [PubMed] [Google Scholar]
- Scheffler B, Edenhofer F, Brüstle O. Merging fields: stem cells in neurogenesis, transplantation, and disease modeling. Brain Pathol. 2006;16:155–168. doi: 10.1111/j.1750-3639.2006.00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty AK. Progenitor cells from the CA3 region of the embryonic day 19 rat hippocampus generate region-specific neuronal phenotypes in vitro. Hippocampus. 2004;14:595–614. doi: 10.1002/hipo.10206. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Hattiangady B. Prospects of stem cell therapy for temporal lobe epilepsy. Stem Cells. 2007;25:2396–407. doi: 10.1634/stemcells.2007-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty AK, Turner DA. Development of fetal hippocampal grafts in intact and lesioned hippocampus. Prog Neurobiol. 1996;50:597–653. doi: 10.1016/s0301-0082(96)00048-2. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Turner DA. In vitro survival and differentiation of neurons derived from epidermal growth factor-responsive postnatal hippocampal stem cells: inducing effects of brain-derived neurotrophic factor. J Neurobiol. 1998;35:395–425. doi: 10.1002/(sici)1097-4695(19980615)35:4<395::aid-neu7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Turner DA. Neurite outgrowth from progeny of epidermal growth factor-responsive hippocampal stem cells is significantly less robust than from fetal hippocampal cells following grafting onto organotypic hippocampal slice cultures: effect of brain-derived neurotrophic factor. J Neurobiol. 1999;38:391–413. doi: 10.1002/(sici)1097-4695(19990215)38:3<391::aid-neu8>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Rao MS, Hattiangady B, Zaman V, Shetty GA. Hippocampal neurotrophin levels after injury: Relationship to the age of the hippocampus at the time of injury. J Neurosci Res. 2004;78:520–532. doi: 10.1002/jnr.20302. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Zaman V, Hattiangady B. Repair of the injured adult hippocampus through graft-mediated modulation of the plasticity of the dentate gyrus in a rat model of temporal lobe epilepsy. J Neurosci. 2005a;25:8391–8401. doi: 10.1523/JNEUROSCI.1538-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty AK, Hattiangady B, Shetty GA. Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: role of astrocytes. Glia. 2005b;51:173–186. doi: 10.1002/glia.20187. [DOI] [PubMed] [Google Scholar]
- Silani V, Corbo M. Cell-replacement therapy with stem cells in neurodegenerative diseases. Curr Neurovasc Res. 2004;1:283–289. doi: 10.2174/1567202043362243. [DOI] [PubMed] [Google Scholar]
- Sohur US, Emsley JG, Mitchell BD, Macklis JD. Adult neurogenesis and cellular brain repair with neural progenitors, precursors and stem cells. Philos Trans R Soc Lond B Biol Sci. 2006;361:1477–1497. doi: 10.1098/rstb.2006.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Sutula TP, Dudek FE. Unmasking recurrent excitation generated by mossy fiber sprouting in the epileptic dentate gyrus: an emergent property of a complex system. Prog Brain Res. 2007;163:541–63. doi: 10.1016/S0079-6123(07)63029-5. [DOI] [PubMed] [Google Scholar]
- Svendsen CN, Fawcett JW, Bentlage C, Dunnett SB. Increased survival of rat EGF-generated CNS precursor cells using B27 supplemented medium. Exp Brain Res. 1995;102:407–414. doi: 10.1007/BF00230645. [DOI] [PubMed] [Google Scholar]
- Toda H, Takahashi J, Iwakami N, Kimura T, Hoki S, Mozumi-Kitamura K, Ono S, Hashimoto N. Grafting neural stem cells improved the impaired spatial recognition in ischemic rats. Neurosci Lett. 2001;316:9–12. doi: 10.1016/s0304-3940(01)02331-x. [DOI] [PubMed] [Google Scholar]
- Turner DA, Shetty AK. Clinical prospects for neural grafting therapy for hippocampal lesions and epilepsy. Neurosurgery. 2003;52:632–644. doi: 10.1227/01.neu.0000047825.91205.e6. [DOI] [PubMed] [Google Scholar]
- Vazey EM, Chen K, Hughes SM, Connor B. Transplanted adult neural progenitor cells survive, differentiate and reduce motor function impairment in a rodent model of Huntington’s disease. Exp Neurol. 2006;199:384–396. doi: 10.1016/j.expneurol.2006.01.034. [DOI] [PubMed] [Google Scholar]
- Whittemore SR. Neuronal replacement strategies for spinal cord injury. J Neurotrauma. 1999;16:667–673. doi: 10.1089/neu.1999.16.667. [DOI] [PubMed] [Google Scholar]
- Yasuhara N, Shibazaki N, Tanaka S, Nagai M, Kamikawa Y, Oe S, Asally M, Kamachi Y, Kondoh H, Yoneda Y. Triggering neural differentiation of ES cells by subtype switching of importin-alpha. Nat Cell Biol. 2007;9:72–79. doi: 10.1038/ncb1521. [DOI] [PubMed] [Google Scholar]
- Yasuhara T, Borlongan CV, Date I. Ex vivo gene therapy: transplantation of neurotrophic factor-secreting cells for cerebral ischemia. Front Biosci. 2006a;11:760–775. doi: 10.2741/1834. [DOI] [PubMed] [Google Scholar]
- Yasuhara T, Matsukawa N, Hara K, Yu G, Xu L, Maki M, Kim SU, Borlongan CV. Transplantation of human neural stem cells exerts neuroprotection in a rat model of Parkinson’s disease. J Neurosci. 2006b;26:12497–12511. doi: 10.1523/JNEUROSCI.3719-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman V, Shetty AK. Combined neurotrophic supplementation and caspase inhibition enhances survival of fetal hippocampal CA3 cell grafts in lesioned CA3 region of the aging hippocampus. Neuroscience. 2002;109:537–553. doi: 10.1016/s0306-4522(01)00478-x. [DOI] [PubMed] [Google Scholar]