Abstract
The constituents of highly active anti-retroviral therapy (HAART) include HIV-1 protease inhibitors (HPIs) and nucleoside reverse transcriptase inhibitors (NRTIs). Endothelial cell (EC) barriers, especially the blood-brain-barrier (BBB) suppresses the entry of HAART drugs to subendothelial HIV-1 reservoirs. The ATP binding cassette (ABC) transporter family members, multidrug resistant-1 (MDR-1) and multidrug resistance-associated proteins (MRPs) can efflux both HPIs and NRTIs from intracellular compartments. Using brain derived ECs from non-human sources, previous studies suggested a dominant role for MDR-1 in HAART efflux from the BBB. However, due to species variations in ABC-transporter expression, drug-efflux functions using human brain ECs need to be investigated. Furthermore, role of ABC-transporters in drug-efflux from systemic EC barriers need to be studied. We monitored the expression of ABC-transporters in primary human ECs obtained from brain (HBMVECs), aorta (HAECs), pulmonary-artery (HPAECs), dermal-microvessel (HDMVECs) and umbilical vein (HUVECs). Gene expression for MDR-1 and MRPs (MRP-1 to MRP-5) were analyzed by reverse transcriptase polymerase chain reaction (RT-PCR). Drug efflux functions were determined by calcein retention assays. Intracellular accumulation of both 3H-saquinavir (an HPI) and 3H-zidovudine (an NRTI) were also monitored in HAECs and HBMVECs. Both assays were carried out in presence of verapamil (20–60 µM) or MK-571 (12.5–50 µM) inhibitors of MDR-1 and MRPs, respectively in presence of verapamil or MK-571. The HBMVECs expressed higher levels of MRPs than MDR-1 and only MK-571 significantly (p<0.01) suppressed calcein efflux from these cells. However, both HAECs and HPAECs showed MDR-1 and MRP expression and calcein efflux was inhibited by both verapamil and MK-571. Both inhibitors suppressed 3H-saquinavir efflux from HAECs, but only MK-571 suppressed saquinavir efflux from HBMVECs. In both ECs, 3H-zidovudine efflux was only suppressed by MK-571. Thus, primary human ECs, especially brain derived ECs, predominantly express MRPs and their specific inhibition may enhance HAART efficacy in subendothelial HIV-1 reservoirs.
Keywords: HIV-1, Brain reservoirs, Anti-retroviral drugs, MDR-1, MRP, Human endothelial cells
Introduction
Human immunodeficiency virus type-1 (HIV-1) is the etiologic agent of acquired immune deficiency syndrome (AIDS). Characteristic of the lentivirus family, HIV-1 also displays a long latency period and a slow progressive disease, culminating in severe immune deficiencies, opportunistic infections and neoplasms.1 The virus primarily infects CD4-positive T-helper (Th) lymphocytes and cytopathic effects of the virus on Th-cells causes a precipitous drop in their immune effector functions. Long term persistence of HIV-1 primarily occurs in monocytes and macrophages which transmigrate out of blood vessels and establish subendothelial sanctuaries in the brain, lymph nodes, bone marrow and intestinal mucosa.2 The microglial cells within the central nervous system (CNS) are well known to harbor and replicate the virus.3 These anatomical viral reservoirs can escape the actions anti-retroviral drugs and unhindered viral propagation can result in the generation of drug resistant mutants of HIV-1.4 Hence, the ultimate goal of successful treatment should be to achieve sustained therapeutic levels of anti-retroviral drugs in subendothelial viral reservoirs, especially within the CNS.
The clinically approved anti-HIV drugs target different stages of the viral replication cycle. A combination of nucleoside and non-nucleoside reverse transcriptase inhibitors (NRTIs and NNRTIs, respectively) and HIV-1 protease inhibitors (HPIs), known as the highly active antiretroviral therapy (HAART), is currently the treatment of choice in HIV-1 positive individuals.5 Indeed, HAART has been very successful in decreasing plasma viral load, rapid immune reconstitution and significantly increases CD4 cell counts in HIV-positive patients. However, even after years of uninterrupted therapy, HAART fails to completely eradicate the virus and selection of drug resistance mandates frequent switching to a different drug. In addition, long term exposure to high doses of HAART drugs can manifest side effects such as cardiovascular disease, diabetes and lipodystrophy.6 These confounding problems have severely dampened the initial enthusiasm associated with the potency of HAART regimen. It is believed that the inability of HAART drugs to traverse vascular barriers and achieve therapeutic concentrations in subendothelial compartments. It is imperative that we elucidate the reasons for decreased therapeutic efficacy of HAART within HIV-1 reservoirs and the understanding of factors that regulate the pharmacokinetics of HAART are of crucial significance.
One crucial parameter dictating HAART levels in patients is their metabolism by the cytochrome p450 (Cyp450) system.7 Another important factor is the regulation of drug transport across endothelial and epithelial barriers to different tissues. This is especially important at the gastrointestinal barriers for intestinal uptake for orally administered agents and at the blood-brain-barriers (BBB) for therapeutic levels of drugs in the CNS.8
Drug transporters dictate the entry of drugs into different compartments and actively efflux drugs from intracellular compartments.9, 10 These transporters play a critical roles in drug pharmacokinetics; affecting absorption and tissue distribution, hepatic uptake and export, as well as renal and biliary elimination of a variety of drugs.11, 12 The adenosine triphosphate (ATP) binding cassette (ABC) transporters are of crucial significance in this respect.13 ABC-transporters expressed on cancer cells are well known to decrease the entry of chemotherapeutic agents and are directly implicated in the development of multidrug resistant (MDR) tumors.14, 15 Recent studies have also shown that several anti-HIV drugs, especially the HPIs and NRTIs, are also substrates of ABC-transporters and their expression on both lymphocytes and BBB endothelial cells (ECs) can suppress entry of HAART into the cellular and anatomical reservoirs of HIV-1. (16 – 18)
The ABC-transporters are highly widespread and transport drugs across the lipid membranes against their concentration gradients, by utilizing the energy generated from ATP hydrolysis.13 Expression of different ABC-transporters is seen on different cell types and on different tissue barriers of brain, liver and kidney and the gastrointestinal tract.(19–21) The best characterized ABC-transporter is MDR-1 (also known as P-gp or ABCB1).22 MDR-1 displays a broad substrate spectrum comprising of both neutral and cationic organic compounds. Another ABC-transporter, recently cloned from a drug resistant breast cancer line, is BCRP (a.k.a. ABCG2) and substrate specificities and tissue localization of BCRP have been found to be similar to that of MDR-1. BCRP is also referred to as a half transporter since it has one transmembrane domain and functions as a dimer to transport a variety of drugs.14, 19 The immunosuppressant cyclosporine-A and verapamil, a calcium channel antagonist, are competitive inhibitors of MDR-1 mediated efflux (also referred to as MDR-modulators) and have often been used in the laboratory to determine MDR-1 specific drug-efflux.23
A newly discovered group of ABC transporters is the MDR associated proteins (MRPs) also referred to as the ABCC family of transporters.24 MRPs are found to cotransport drugs along with glutathione (GSH) or transport GSH-drug conjugates and glucuronide-drug conjugates. Amongst the nine members of the MRP transporters family, the first five (MRP-1, MRP-2, MRP-3, MRP-4 and MRP-5) are frequently associated with the efflux of therapeutic agents. MRP-1, MRP-2 and MRP-3, transport hydrophilic anionic compounds, large molecules and peptidomimetics,25 however, both MRP-4 and MRP-5 transport small polar compounds such as nucleosides, cyclic nucleotides and nucleoside analogs.26 Unlike the MDR-1 and BCRP transporters which are both expressed on apical surfaces of membranes, specific patterns of expression, either on the apical or basolateral, are typically seen with the MRPs. This polarized expression of MRPs regulates the directional transport of drugs in and out of various tissue compartments. Chemotherapeutic drugs, e.g. paclitaxel, uricosuric agents, e.g. probenecid, and the leukotrienes (LT) receptor antagonist, e.g. MK-571, are known inhibitors of MRPs.(24–26) Recent studies also indicate that verapamil, previously believed to be a specific inhibitor of MDR-1, may inhibit both MDR-1 and MRPs.27
Clinically approved HPIs, such as saquinavir, ritonavir and lopinavir, are found to be good substrates of MDR-1,16, 28, 29 and of both MRP-1 and MRP-2.30, 31 Both MDR-1 and MRPs were shown to limit the brain uptake of saquinavir in mice.32 Enhanced brain penetration of saquinavir was observed in murine CNS following coadministration of mefloquine, an inhibitor of MDR-1.33 The nucleotide transporters MRP-4 and MRP-5 have also been shown to efflux several of the NRTIs, such as zidovudine (AZT) and didanosine (ddC).28, 34, 35 However, neither the HPIs or NRTIs are substrates of BCRP, but may inhibit BCRP function.36 In post-mortem brain tissues from humans,37 and monkeys,38 the presence of MDR-1 and MRP transporters at the BBB suggest their crucial function in suppressing HAART entry. In vivo uptake and efflux of anti-HIV agents have been demonstrated in murine brains,32 which clearly showed the evidence of both MDR-1 and MRP transporters.
Since species differences in the kinetics of ABC-transporter expression and inhibition have recently been shown to occur,39, 40 data generated using non-human cells may not be fully relied upon to extrapolate HAART entry into the CNS. Drug-efflux studies using primary human brain ECs would be of critical significance. In addition, EC barriers to different organs may also dictate efflux of HAART drugs from subvascular HIV-1 reservoirs. Indeed, systemic reservoirs of HIV-1 in different organs have often been implicated as subendothelial sanctuaries. Drug-efflux functions at different EC barriers may critically regulate HAART efficacy, however, the ABC-transporter expression profile and HAART drug-efflux from ECs isolated from different organs has also not been fully elucidated.41, 42
In this study, we have monitored the basal level of expression of MDR-1 and MRPs (−1 to −5) in primary human ECs, obtained from large arteries such as aorta and pulmonary artery, from microvessels, such as the brain and dermal foreskin, and from umbilical veins. In these ECs, we have also determined the efflux functions associated with either MDR-1 or MRPs and ascertained the role of specific ABC-transporters in effluxing the anti-HIV drugs, saquinavir and zidovudine. In contrast to previous observations, our findings indicate a predominant role played by the MRP transporters in both HPI and NRTI efflux from human ECs.
Materials and Methods
Reagents
The fluorescent dye calcein acetoxy-methyl ester (Calcein-AM) was purchased from Molecular Probes (Eugene, OR). Verapamil was purchased from Calbiochem (San Diego, CA) and MK-571 was purchased from Biomol International (Plymouth Meeting, PA) The radiolabeled anti-HIV-1 drugs, [3H]-saquinavir and [3H]-zidovudine were purchased from Moravek Biochemicals (Brea, CA). The trizol™ reagent for RNA isolation was purchased from Invitrogen (Carlsbad, CA) and reagents for reverse transcription (RT), e.g. M-MLV reverse transcriptase, oligo-deoxythymidine (oligo-dT) primers and RNAase inhibitor, were purchased from Promega (Madison, WI). For polymerase chain reaction (PCR), the Taq DNA polymerase, KCl, MgCl2 and 10X PCR buffer were obtained from Sigma Aldrich (St. Louis, MO). The PCR primers were synthesized by the Midland Certified Reagent Company (Midland, TX). Diethyl pyrocarbonate (DEPC) water was purchased from Ambion (Austin, TX) and the BCA protein assay kit was purchased from Pierce (Rockford, IL).
Cell Cultures
Primary human endothelial cells (HAECs, HPAECs, HDMVECs and HUVECs, were purchased from Cambrex (Walkersville, MD). These cells were grown in EGM-2 complete media obtained from the manufacturer. The human brain derived cells, HBMVECs were purchased from the Applied Cell Biology Research Institute (Kirkland, WA). These cells were cultured in CS-C complete medium (Cell Systems Corporation, Kirkland, WA). All of the ECs were grown according to the manufacturer specified guidelines, with minor modifications. Briefly, original vial of cells from the suppliers (passage 3–5) were thawed and grown to 80–90 percent confluency, followed by trypsinization and subculturing at a 1:3 dilution in three 75 cm2 flasks. Once confluent, cells were harvested and resuspended in complete medium (EGM-2 or CS-C) containing 10 % dimethyl sulfoxide (DMSO) at a density of 106 cells/ml and cryopreserved in liquid nitrogen. The ECs were thawed from these secondary stocks were used in experiments and cells subcultured between passages 7–10 (p7–p10) were only used. During these passages, all of the ECs displayed the characteristic cobblestone appearances and showed factor VIII (von willabrandt factor) positive staining. All of the above cell types were grown in an incubator set at 37°C and 5% CO2.
The adriamycin resistant line, NCI/ADR (previously known as MCF-7/ADR) which overexpresses MDR-1, were obtained from National Cancer Institute (Bethesda, MD).43, 44 The pancreatic carcinoma cells PANC-1, known to express several different MRPs,45 and the breast cancer line MCF-7, which expresses low levels of transporters, were obtained from the American Type Culture Collection (ATCC; Manassas, VA). The above three cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% heat-inactivated fetal bovine serum (FBS), 100 mM penicillin, 100 µg/ml streptomycin, 100 mM glutamine and 10 mM sodium-pyruvate. Cells were trypsinized at a 1:5 dilution at confluency and media was replenished every 3–4 days and grown in an incubator set at 37°C and 5% CO2. All tissue culture reagents and additives were obtained from Sigma (St. Louis, MO).
Calcein-AM Efflux Assays
Calcein-AM, a lipid soluble dye, is a known substrate for both MDR-1 and MRP transporters, 46, 47 was used to determine MDR-1 and MRP-mediated efflux functions (Molecular Probes; Eugene, OR). Upon entering cells, endogenous esterases cleave calcein-AM to form the hydrophobic fluorescent calcein. ABC-transporters cause rapid efflux of the dye prior to esterification, hence intracellular retention of the fluorochrome is inversely proportional to the extent of cellular efflux. In our experiments, cells (2 ×105) were cultured in Krystal 24-well opaque black plates (Labnet, NJ) till 85–90 % confluent. Cells were first exposed to either verapamil (20–60 µM) or MK-571 (12.5–50 µM) for 15 minutes, prior to the addition of calcein-AM at a final concentration of 0.25 µM. Cells were incubated with calcein-AM at 37°C for 15–30 min in the dark, following which cells were washed three times in ice-cold phosphate buffered saline (PBS). Intracellular calcein fluorescence (retention) was determined using a FL×800 fluorimeter (Bio-Tek instruments). The absorption and emission wavelengths were set at 485±10 nm and 528±10 nm, respectively. Subsequently, cells were lysed and the extracted proteins were quantified using the BCA protein assay kit. The fluorescence measurements obtained from each well were normalized to the protein contents in the respective samples and are represented as fluorescence/mg of protein.
RNA isolation and RT-PCR assays
Total RNA was isolated from cells using the Trizol RNA isolation reagent (Invitrogen; Carlsbad, CA) according to the manufacturer’s instructions. The RNA were quantified using a spectrophotometer and RNA aliquots were frozen at −80°C for later use in reverse transcriptase (RT) polymerase chain reaction (PCR) assays. All RT-PCR assay reagents were obtained from Promega. The cDNA were generated from RNA (0.5µg) by using the murine leukemia virus (MuLV) RT enzyme (0.5 U), Oligo-dT (2.4µg/ml) and dNTPs (100 mM) and incubation at 42°C for 60 min. For each PCR amplication reaction 10 µl of this RT-product was used. The PCR reactions were carried out by using a thermal cycler from Perkin Elmer (Boston, MA; Model 9600) in the presence of Taq DNA-polymerase (0.5 U), in RED-Taq PCR buffer containing KCl (500 mM), MgCl2 (11 mM), dNTPs (200 µM) and the gene specific primers. The RT-PCR primer sequences and amplification protocols for MDR-1 was according to a previous publication.48 The primer sequences for MRP-1, MRP-2, MRP-3, MRP-4, MRP-5 and β-actin primers were obtained from Kõnig et al, (2005).49 The cycling conditions for each PCR reactions were according to the above published protocols. The PCR products were electrophoresed on a 2.0 % agarose gel containing ethidium bromide (10 µg/ml). The generation of right size products was verified by using a molecular weight marker (Roche Diagnostic, IN). Band intensities for PCR-products were determined from scanned gel pictures, by using a GS-700 imaging densitometer (Bio-Rad). The intensity of amplified ABC-transporter mRNAs were normalized to the values obtained with β-actin mRNA levels, in the respective samples. The primer sequences and product sizes for each of the mRNAs are provided below:-
| Primer Name | Oligonucleotide Sequences | Product Sizes (bp) |
|---|---|---|
| MDR1-fwd | 5′- AGG CCA ACA TAC ATG CCT TC -3′ | 309 |
| MDR1-rev | 5′- GCT CCT TGA CTC TGC CAT TC -3′ | |
| MRP1-fwd | 5′-CTG ACA AGC TAG ACC ATG AAT GT-3′ | 353 |
| MRP1-rev | 5′-TCA CAC ACC AAG CCG GCG TCT TT-3′ | |
| MRP2-fwd | 5′-CTT CGG AAA TCC AAG ATC CTG G-3′ | 284 |
| MRP2-rev | 5′-TAG AAT TTT GTG CTG TTC ACA TTC T-3′ | |
| MRP3-fwd | 5′-GGA CCC TGC GCA TGA ACC TG-3′ | 450 |
| MRP3-rev | 5′-AGG CAA GTC CAG CAT CTC TGC-3′ | |
| MRP4-fwd | 5′-GGA TCC AAG AAC TGA TGA GTT AAT-3′ | 358 |
| MRP4-rev | 5′-TCA CAG TGC TGT CTC GAA AAT AG-3′ | |
| MRP5-fwd | 5′-GCT GTT CAG TGG CAC TGT CAG-3′ | 481 |
| MRP5-rev | 5′-TCA GCC CTT GAC AGC GAC CTT-3′ | |
| β-actin-fwd | 5′-TGACGGGGTCACCCACACTGTGCCCATCTA-3′ | 661 |
| β-actin-rev | 5′-CTAGAAGCATTTGCGGTGGAC-GATGGAGGG-3′ |
Saquinavir and Zidovudine efflux assays
Radiolabeled drugs were utilized to measure their intracellular accumulations and efflux rates in HBMVECs and HAECs. The ECs were grown in 24-well culture plates till confluency and fresh media was added. Cells were first incubated with either verapamil (20 – 60 µM) or MK-571 (12.5 – 50 µM), or both inhibitors, for 15–20 min, followed by a 2.0 hr exposure to either 3H-saquinavir (1.7 pM; specific activity: 1.0 Ci/mM) or 3H-zidovudine (AZT) (17 pM; specific activity: 12.0 Ci/mM). For the determination of intracellular loading concentrations in the presence or absence of inhibitors, cells were washed with cold PBS and cell extracts were harvested at this time point (0-time point). To measure temporal efflux of the drugs, cells were washed with PBS at 37° C and incubated with fresh medium in the presence or absence of the inhibitors. The intracellular retention of radiolabeled drugs was monitored at 15 min intervals till 1 hr (15, 30, 45 and 60 min). Cell extracts were obtained by lysing cells with 1.0 ml of NH4OH (1.0 M) for 5 min. Approximately 500 µl of extracts were transferred to scintillation vials containing 10 ml of scintillation cocktail (Ecolite, MP, USA) and 200 µl of extracts were used to measure protein levels using the BCA protein estimation kit. The counts per minute (CPM) values in cell extracts were determined with a Tri-Carb 2800TR Liquid Scintillation counter (Perkin Elmer, USA) which were then normalized to the protein contents in respective samples and are represented as CPM/ µg of protein. The radioactivity obtained in control cells (no inhibitors) at the 0-time point (peak loading) was designated as 100% retention and percent change in retention in the presence verapamil, MK-571, or both, was quantified at different times (15 –60 min) post washing of the cells.
Statistical analysis
All statistical analyses were carried out using the INSTAT-2 software (Graph Pad, San Diego, CA). Each treatment condition consisted of three to four replicates and each experiment was performed at least three to five times. Data obtained were used to calculate the standard error of means (± SEM). The significance of changes from control values were determined by using a two-tailed student’s t-test and comparison between three or more groups were carried out with one-way analysis of variance (ANOVA). Results were considered significant when P values were found to be < 0.05.
Results
ABC-transporter gene expression correlated with MDR-1 and MRP mediated drug-efflux functions in the MCF-7, NCI/ADR and PANC-1 cells
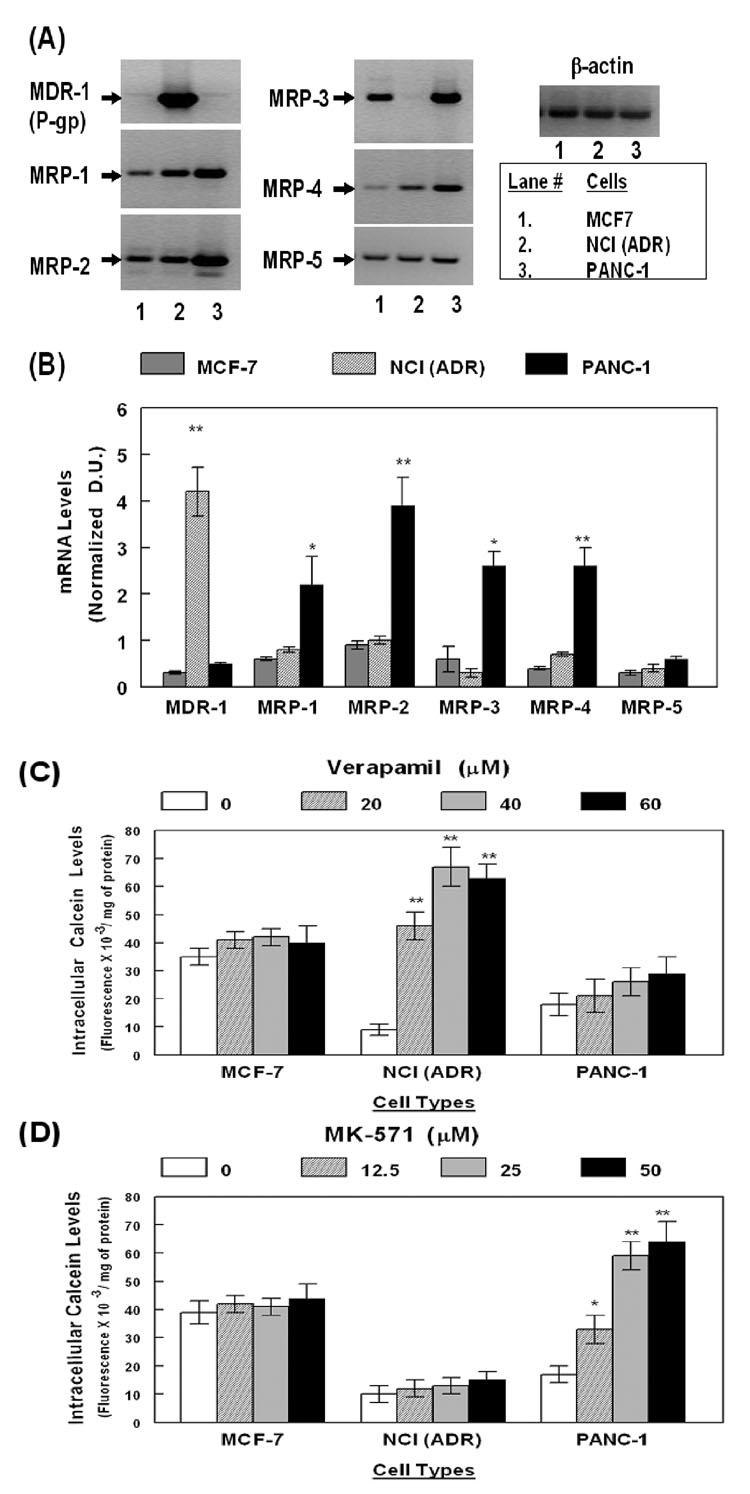
In order to optimize the RT-PCR assays and calcein-efflux assays, cell lines known to overexpress either MDR-1; e.g. NCI/ADR cells,43 or MRPs; e.g. PANC-1 cells,45 were used as positive controls, for high expression of ABC-transporters and for constitutive drug-efflux functions. The MCF-7 cells, which express low levels of ABC-transporters and have very little drug-efflux function, were used as negative controls. Total RNAs were obtained from each of these cell types and RT-PCR amplification of MDR-1, MRPs (−1 to −5) and β-actin, specific mRNAs were carried out as specified in Materials and Methods. The RT-PCR product intensities obtained for each of the ABC-transporters were normalized to β-actin levels in the respective samples. The representative gel picture shows the PCR products (Fig. 1A) and the bar graphs depict the normalized densitometric unit (D.U.) values (Fig. 1B). In the three transformed cell lines, RT-PCR amplification results demonstrated a clear difference in mRNA expression of MDR-1 and MRPs. MDR-1 expression was significantly (P<0.01) higher in the NCI/ADR cells, however, in both MCF-7 and PANC-1 cells, basal level of MDR-1 mRNA was much lower. In contrast to the MCF-7 and NCI/ADR lines, the expression of all five MRPs were significantly higher in PANC-1 cells (*, p<0.01; **, p<0.01). These data validated that our semi-quantitative RT-PCR assays corroborate the overexpression of ABC-transporters which have been previously documented in these cell lines. In the five primary ECs tested in our following experiments, similar RT-PCR amplification protocols were used in determining MDR-1 and MRP (−1 to −5) gene expression (Fig. 2).
Figure 1. ABC-transporter gene expression and transporter specific efflux function in MDR-1 and MRP overexpressing cell lines.
The MCF7, NCI(ADR) and PANC-1 cells were used as controls for MDR-1 (P-gp) and MRP (MRP-1, to MRP-5) gene expression and MDR-1 and MRP mediated efflux function. In (A), the RT-PCR amplified products are shown in a representative gel picture. The densitometric values of product intensities (n=4) were normalized to β-actin levels. In (B), the ratios are presented as empirical densitometric units (D.U.). Drug-efflux data from calcein assays are shown in (C) and (D). Fluorescence readings (Fluorescence/mg of protein) observed in the absence (clear bars) or presence of either verapamil (C) or MK-571 (D) are shown (n=5). Error bars represent the ±SEM of values and significant differences in mRNA expression and calcein-retention, are indicated by P values (*, p<0/05; **, p<0.02).
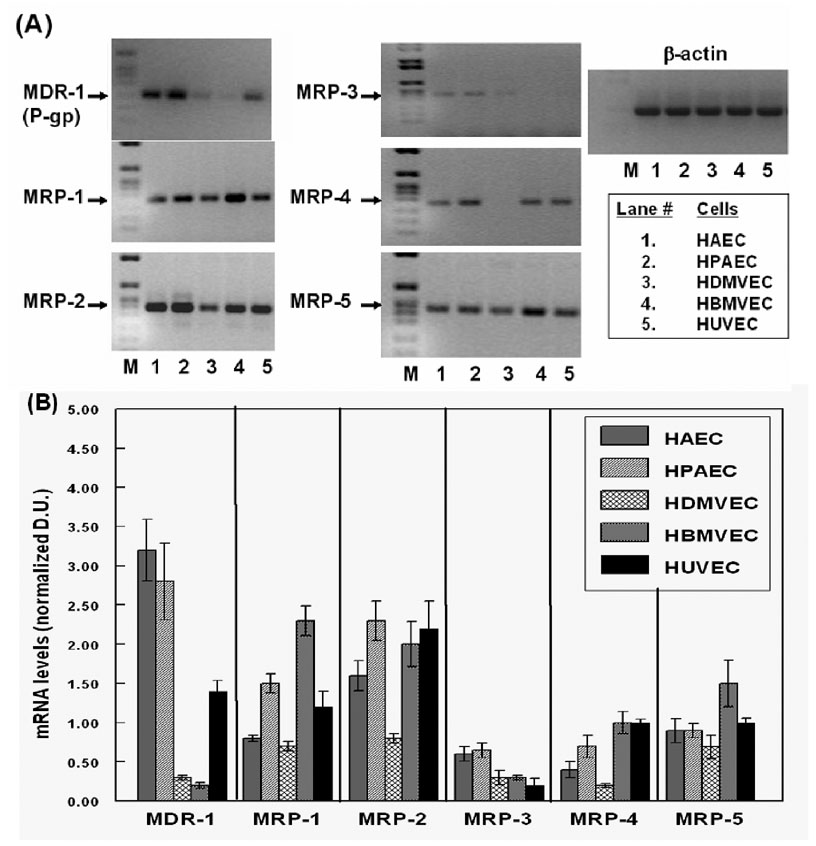
Figure 2. Differential expression of MDR-1 and MRPs genes in primary human endothelial cells.
Total RNAs from HAECs, HPAECs, HDMVECs, HBMVECs and HUVECs were analyzed for MDR-1, MRP-1, MRP-2, MRP-3, MRP-4 and MRP-5, gene expression by RT-PCR. In (A), the representative gel pictures show the amplified products obtained in each of the EC types examined (lanes 1–5) along with a molecular weight marker (lane-M). In (B), the bar graphs show the normalized densitometric ratios of the PCR products (n=5). The error bars represent ±SEM of values.
In vitro assays were carried out to monitor ABC-transporter specific drug-efflux functions in the MCF-7, NCI/ADR and PANC-1 cells (Fig. 1C and 1D). Calcein-AM, a substrate for both MDR-1 and MRP transporters,47 was used to measure drug efflux functions, as specified in Materials and Methods. Intracellular calcein fluorescence, observed in the presence or absence of the inhibitors, verapamil (20–60 uM) or MK-571 (12.5 – 50 µM), demonstrated transporter specific efflux in each cell type. Results showed that both NCI/ADR and PANC-1 has a significantly higher drug-efflux function, as compared to MCF-7 cells. This was evident from lower intracellular calcein fluorescence in the absence of the inhibitors and pre-exposure of cells to either verapamil or MK-571 showed much higher intracellular calcein in both NCI/ADR and PANC-1 cells. Verapamil pre-exposure resulted in a 7–10 fold increase (p<0.01) in fluorescence in the NCI/ADR cells but not in MCF-7 or PANC-1 cells and pretreatment with MK-571 resulted in a significant (P<0.01) increase (5–8 fold) in calcein retention in PANC-1 cells only, but not in the MCF-7 or NCI/ADR cells. Neither of these inhibitors significantly increased calcein retention in the MCF-7 cells. These findings established that the calcein efflux function of MDR-1 and MRP transporters correlate very well with their gene expression in each of these cell lines. Furthermore, data from PANC-1 cells showed that MK-571 is a highly specific inhibitor of MRPs and do not suppress MDR-1 mediated efflux. Verapamil, recently found to be a dual inhibitor,27, 45 was indeed able to suppress calcein efflux via either of these transporters. However, similar to previous findings, our experiments indicated that verapamil is a more potent inhibitor of MDR-1 than of MRPs. This was evident from a better inhibitory effect in NCI/ADR cells as compared to PANC-1 cells. In subsequent studies, the above calcein-retention assay protocols were utilized to measure ABC-transporter mediated drug-efflux in the primary human ECs (Fig. 3).
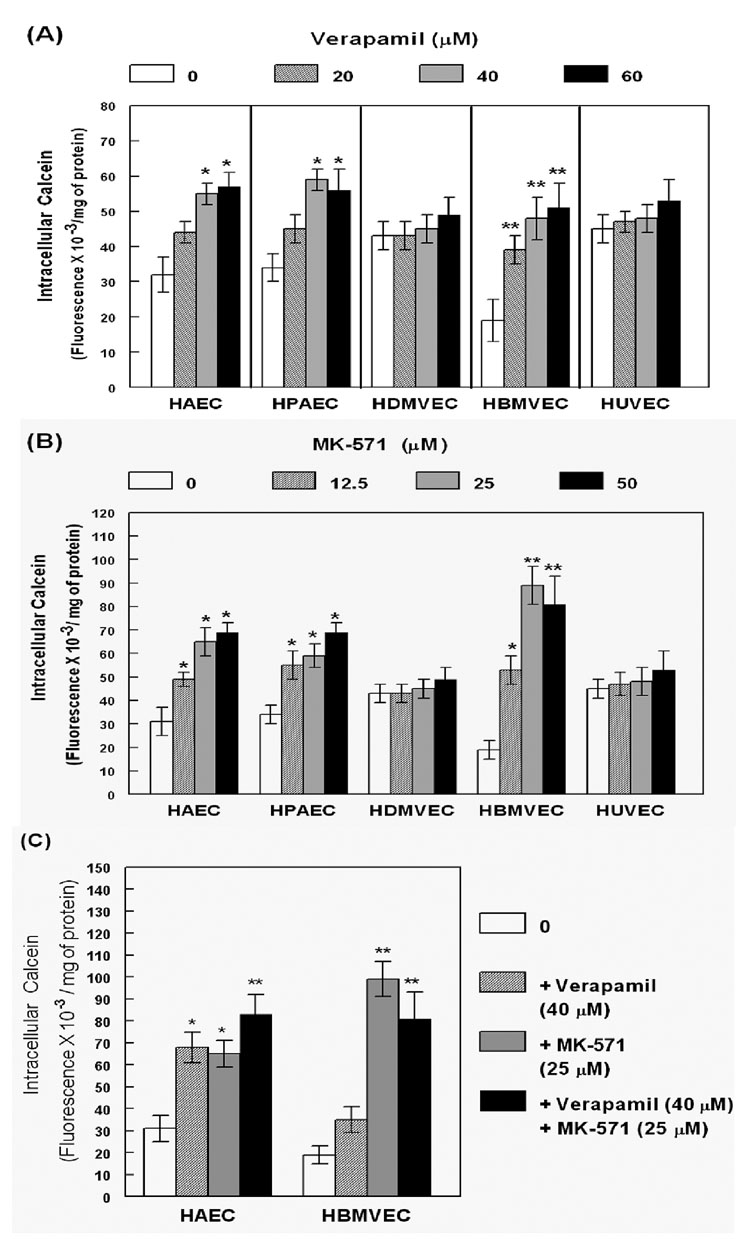
Figure 3. MDR-1 and MRP-mediated drug-efflux functions in primary human endothelial cells.
The HAECs, HPAECs, HDMVECs, HBMVECs and HUVECs, were incubated with calcein-AM in the absence (clear bars) or presence (shaded bars) of the inhibitors, (A). verapamil (20–60 µM), (B) MK-571 (12.5 – 50 µM), or (C) both (verapamil; 40 µM and MK-571; 25 µM). The fluorescence readings (fluorescence/ mg of protein) from five independent experiments (n=5) are presented in the bar graphs. Error bars representing the ±SEM of values. As compared to in controls (clear bars), significant differences in calcein-retention in presence of verapamil, MK-571, or both, are indicated by the P values (*, p<0.05; **, p<0.02).
Endothelial cells differentially express both MDR-1 and MRPs and show different rates of calcein efflux
Investigations on ABC-transporter expression and function in human brain derived ECs are of crucial significance to understand drug-efflux rates from the human CNS.2, 8, 22 Furthermore, the monitoring of functional ABC-transporter expression in ECs obtained from other vascular beds, would also have relevance in understanding drug transport into various subendothelial compartments and therapeutic efficacy of various other drugs.1, 31 Hence, we used ECs isolated from human brain microvessels (HBMVECs) and from aorta (HAEC), pulmonary artery (HPAECs), dermal microvessel (HDMVECs) and umbilical vein (HUVECs). Similar to our previous studies in transformed cells, in each of these primary ECs we monitored both ABC-transporter gene expression by RT-PCR (Fig. 2) and ABC-transporter specific efflux function by using calcein-AM (Fig. 3). Both HBMVECs and HAECS were also used to measure anti-HIV drug-efflux (Fig. 4 and Fig. 5).
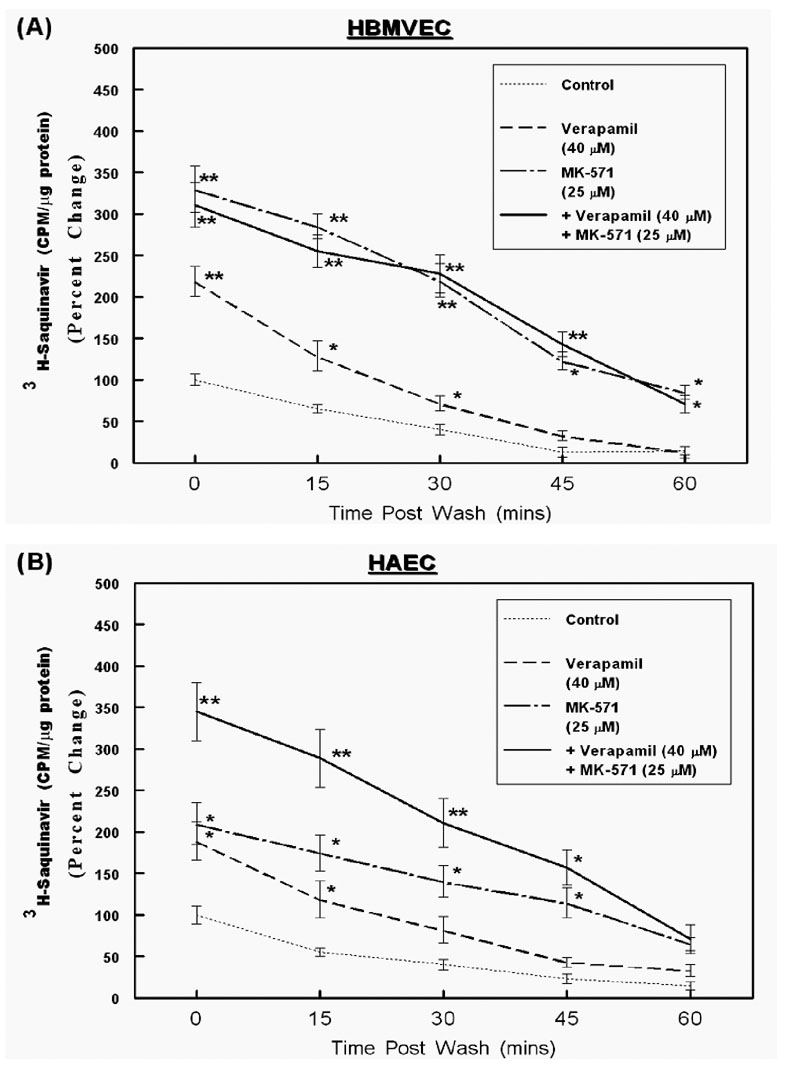
Figure 4. MDR-1 and MRP-mediated efflux of saquinavir from HBMVECs and HAECs.
Intracellular accumulation and temporal efflux of 3H-saquinavir was measured in both HBMVECs (A) and HPAECs (B). Cells were pre-incubated for 15 min with either verapamil (40 µM) or MK-571 (25 µM), or both, followed by exposure to 3H-saquinavir for 2 hrs. Cells extracts were obtained at 0-time point to determine drug accumulation and at 15 min intervals (15, 30, 45 and 60 min) to determine temporal efflux. The normalized values (CPM/ µg of protein) obtained in control samples (no inhibitor) at the 0-time point (loading) were designated a value of 100 and the percent changes are shown in the line graphs (n=3). The error bars represent ±SEM of values and significant differences in intracellular accumulation and temporal efflux of saquinavir are indicated by P values (* p< 0.05; ** p< 0.01).
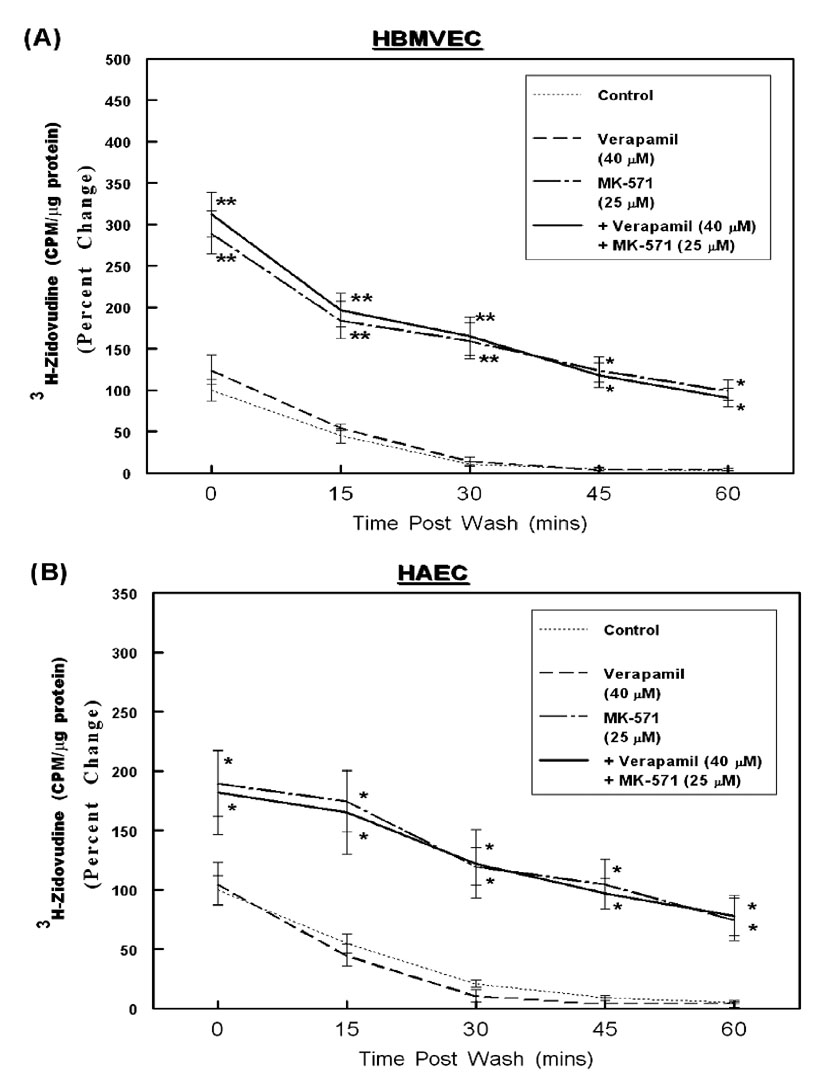
Figure 5. MDR-1 and MRP-mediated efflux of Zidovudine from HBMVECs and HAECs.
Intracellular accumulation and temporal efflux of 3H-zidovudine was measured in both HBMVECs (A) and HPAECs (B), similar to the saquinavir efflux studies. The percent changes in normalized values (CPM/ µg of protein) are depicted in the line graphs (n=3). Error bars represent ±SEM and significant differences in zidovudine accumulation and temporal efflux are indicated by P values (* p< 0.05; ** p< 0.01).
As evident from Fig. 2, all of the ECs expressed detectable levels all five of the MRPs (−1 to −5) and showed significant differences in MDR-1 expression. The bar graphs in Fig. 2B shows that both HAECs and HPAECs, express a 10–12 fold higher MDR-1 mRNA levels, as compared to that observed in either HDMVECs and HBMVECs. Interestingly, the brain derived ECs consistently showed the lowest expression of MDR-1. The expression of MRP-1 was relatively similar in all of the ECs, except in the brain derived ECs which showed a 2–3 fold higher MRP-1 expression than in the other ECs. All of the ECs also showed a constitutively high expression of MRP-2, except the HDMVECs which showed a 2-fold lower expression. The mRNA levels for MRP-3 were the lowest of all the MRPs analyzed and a slightly higher expression was observed in HAECs and HPAECs. The MRP-4 mRNAs were found in all of the ECs except for the dermal derived ECs and MRP-5 expression was the highest in HBMVECs. In summary, our RT-PCR data showed that the ECs obtained from large vessels, i.e. HAECs and HPAECs (lanes 1 & 2) expressed significantly higher levels of MDR-1 and constitutively expressed all five of the MRPs. The microvessel derived ECs, i.e. HDMVECs and HBMVECs (lanes 3 & 4) showed the lowest expression of MDR-1. Interestingly however, in contrast to previous findings in murine and bovine brain ECs, our studies showed low levels of MDR-1 in HBMVECs. Furthermore, the brain derived ECs consistently showed high levels of MRP-1, MRP-2, MRP-4 and MRP-5, expression.
In subsequent experiments, calcein-retention assays were carried out in the presence or absence of verapamil (Fig. 3A) or MK-571 (Fig. 3B) or both (Fig. 3C), in each of the ECs. The bar graphs show intracellular calcein fluorescence, normalized to the protein contents (Fluorescence/mg of protein). Calcein levels in the absence (clear bars) or presence (shaded bars) of the inhibitors, clearly demonstrated the differences in rate of efflux via each transporter. Furthermore, Fig. 3 shows that the level of calcein retention in presence and absence of increasing concentrations of verapamil and MK-571, also differed in each of the ECs. Results showed that the HBMVECs, HAECs and HPAECs, accumulated lower levels of calcein, as compared to the HDMVECs and HUVECs, suggesting that these cells have higher drug-efflux functions. Furthermore, pre-treatment with verapamil (20 – 60 µM) or MK-571 (12.5 – 50 µM), or both (Ver: 40 µM; MK: 35µM) significantly increased intracellular calcein fluorescence only in these three ECs and not in HDMVECs or HUVECs. This suggested functional drug-efflux mechanisms due to either or both MDR-1 and MRPs. Interestingly, in HBMVECs pre-exposure to MK-571 suppressed calcein-efflux more than that observed in cells pre-exposed to verapamil. No significant increase in calcein accumulation was observed upon incubation of HBMVECs with both inhibitors (Fig. 3C). This implied a predominant role of MRP transporters, but not of MDR-1, in drug efflux from the human brain derived ECs. Both verapamil and MK-571 showed relatively similar increase in calcein retention in HAECs and HPAECs and a higher retention was observed upon coexposure to both transporter inhibitors. These data suggested that both groups of transporters may be functional in the large vessel ECs, HAECs and HPAECs. However, even in large vessel ECs, MRP inhibition by MK-571 suppressed calcein efflux more robustly and at lower concentrations than verapamil. Even though both HUVECs and HDMVECs showed some MDR-1 and MRP expression, no detectable drug-efflux function was seen in these cells, suggesting that the expression and function of ABC-transporters may also be regulated at levels other than gene expression, as well. Our findings showed that the drug-efflux rates (Fig. 3) partially correlated with the ABC-transporter expression data (Fig. 2) in human ECs. Furthermore, the MRP transporters seemed to be the predominant drug efflux mechanism utilized in all of the human ECs.
MRP transporters are predominantly responsible for the efflux of saquinavir and zidovudine from both brain and aortic ECs
Although the drug-efflux assays using calcein suggested functional ABC-transporter expression in different ECs, substrate specificities of these transporters are known to differ significantly. Hence, their crucial roles in regulating HAART drug efflux can only be ascertained from studies monitoring the transport of the anti-HIV drugs themselves. The HBMVECs (A) and HAECs (B) which showed the most significant levels of calcein efflux, were utilized in our following studies to determine the intracellular retention and temporal efflux of tritium (3H) labeled saquinavir (Fig. 4) and zidovudine (Fig. 5). These two anti-HIV agents were chosen since a number of previous studies had shown that saquinavir is effluxed by both MDR-1 and MRP-1 and MRP-2,31 and zidovudine can be effluxed by both MRP-4 and MRP-5.34 For these drug-efflux studies, ECs were incubated with radiolabeled saquinavir (Fig.4, A & B) or zidovudine (Fig. 5, A & B). The intracellular accumulation of drugs was measured at two hours, in the presence or absence of verapamil (20 – 60 µM) or MK-571 (12.5 – 50 µM) or both (Ver: 40 µM + MK: 25 µM). The intracellular radioactivity (CPM) accumulated at this 0-time point designates the effect of ABC-transporter inhibition on peak loading of ECs with the anti-HIV drugs. The extracellular radioactivities were washed off and the rate of drug-efflux was monitored at 15 min intervals till one hour. Results showed that the intracellular accumulation of saquinavir was higher in both HBMVECs and HAECs when drug-efflux functions were inhibited by either verapamil or MK-571 (Fig. 4), however, only MK-571 was able to increase zidovudine accumulation in both cell types (Fig. 5). Interestingly, as compared to HAECs, in the HBMVECs both saquinavir and zidovudine uptake was atleast 5–7 fold and 2–3 fold lower, respectively. This suggested a higher membrane resistance to entry of lipophilic chemicals into the brain ECs and smaller molecules such as nucleoside analogs entered at a faster rate. However, in both ECs the intracellular accumulations (0-time) for both saquinavir and zidovudine were highest when the ECs were incubated with MK-571. In HBMVECs, MK-571 pre-incubation showed a 3.5–4 fold higher accumulation of saquinavir and only a 2-fold increase in presence of verapamil and no further increase in saquinavir accumulation were seen in the presence of both inhibitors. This data clearly suggested a dominant role of the MRP transporters in saquinavir efflux. Similarly, a 2.5–3 fold higher accumulation of zidovudine was seen in brain ECs only in presence of MK-571, but not with verapmil exposure, again underscoring the crucial importance of MRPs in the efflux of both HPIs and NRTIs in brain derived ECs. However, in HAECs the exposure to either verapamil or MK-571 alone increased saquinavir accumulation and suppressed saquinavir efflux. Furthermore, a higher intracellular accumulation and a longer retention were seen upon coexposure to both inhibitors, which suggested that both MDR-1 and MRPs are involved in saquinavir efflux from HAECs. In both cell types, a significant decrease in intracellular radioactivity for both saquinavir and zidovudine was evident within 15–30 min, which indicated active diffusion from these cells. Exposure to the LT-receptor antagonist, MK-571 was able to suppress the efflux of both saquinavir and zidovudine from brain and aorta derived endothelial cells.
Discussion
Our current findings demonstrate the importance of inhibiting drug-efflux transporters on vascular endothelial barriers, in order to enhance HAART efficacy in subendothelial reservoirs of HIV-1. By delineating the specific ABC-transporters involved in different human tissues, strategies may be developed to potentiate the actions of drugs which are substrates for these transporters. Drug efflux functions were observed in human brain derived ECs, which have been previously shown to express these transporters.11, 28, 29 Our calcein assay data showed that ABC-transporters are differentially expressed in ECs obtained from a number of different vasculatures. An especially robust expression was observed in both brain microvessels and large vessels of the cardiovascular system. The evidence of increased saquinavir and zidovudine efflux from human ECs (both HBMVECs and HAECs) clearly suggested that ABC-transporters can suppress HAART entry, not only in the CNS but also in other systemic organs. These findings clearly implicated the importance of ABC-transporters in systemic EC barriers.
The possible utility of concomitant treatment with ABC-transporter inhibitors, in order to augment the entry of both HPIs and NRTIs into the CNS, have been proposed by a number of previous studies.29, 23, 33, 40 However, the role of different transporters in drug-efflux from the human brain had been derived from indirect evidences, from in vitro cultured brain microvessel ECs from mice,32, 33 or rats.50, 51 These previous observations showed that several HPIs are effluxed from brain ECs predominantly by the MDR-1 transporter. Neither MRP expression nor MRP-associated efflux function was found to be significantly associated with HPI efflux from mouse brain.33 Hence, approaches to suppress the efflux of therapeutic agents from the human brain had been directed towards a specific blockade of the MDR-1, but not via inhibition of the MRP tranporters.32, 53 Salama et al, (2005),52 had monitored the impact of either pharmacologic inhibition of MDR-1 (P-gp) by GF120918 on nelfinavir levels in the brain and other tissues in mice. Treatment with GF120918 provided tissue-specific effects with enhanced drug accumulation in the brain (approximately 21-fold) and the heart (3.3-fold) of mice. However, our current findings suggest that extrapolations towards the role of MDR-1 in human brain ECs was made erroneously from findings in nonhuman ECs.
In previous studies using human brain specimen, the expression of MDR-1 was indeed shown by immuno-histochemical (IHC) localization in tissues from HIV-positive individuals,37 hippocampal sclerosis and brain tumors.(53–55) These studies had indicated high level of expression of both MDR-1 and MRPs in different regions of the brain and their expression was augmented in pathologic samples, but not in normal tissues. Interestingly, in contrast to the murine brain data, findings from human brain had suggested that several MRPs were specifically localized to the brain endothelium and the highest levels of MDR-1 (P-gp) were seen only in the astrocytic foot processes and neuronal cells. Furthermore, similar to our findings, a previous study using in vitro cultured human brain microvessel derived ECs had also indicated a lack of MDR-1 and a robust MRP-1 expression.56 However, drug-efflux functions by the human endothelium were not monitored in this previous study. A recent study had also used human brain ECs and monitored the entry of several anti-HIV agents and documented the influx and efflux of several anti-HIV drugs including saquinavir and zidovuvudine.57 These investigators showed that the entry of anti-HIV drugs into the brain could be facilitated by liposomal preparations of these agents. However, the role of ABC-transporters in intracellular drug accumulation by brain ECs, or the effects of different transporter inhibitors in regulating influx or efflux of drugs at the BBB, was not investigated. Inflammatory microenvironments in the brain were seen to increase drug-efflux transporter expression, but significant induction in MDR-1 was only associated with astrocytes, but not at the BBB.
Although the MRP transporters are expressed in normal human brain tissue and are upregulated in brain endothelium during CNS dysfunctions, the role of MRPs had been mostly overlooked. Our findings provide the first evidence that the expression of ABC-transporters in human brain ECs may be very different from those observed in other species. In ECs, several MRPs were constitutively expressed higher than the MDR-1 transporter and the MRP-dependent drug-efflux functions were greater in these cells (Fig. 2 and Fig. 3). Studies using MK-571 corroborated that MRPs, possibly both MRP-1 and MRP-2, are of paramount importance in extruding saquinavir from both HBMVECs and HAECs (Fig. 4). The higher expression of both MRP-4 and MRP-5 were also correlated with the rapid efflux of the nucleoside analog, zidovudine (Fig. 5). Incubation of cells with the leukotriene (LT) receptor antagonist MK-571 resulted in both a higher level and a longer duration of retention of both anti-HIV drugs. Together, our findings demonstrated that MRP inhibition can significantly increase the intracellular accumulation of both HPIs and NRTIs.
We have documented that only the large vessel derived ECs, such as HAECs and HPAECs show high expression of MDR-1 and a significant inhibition of drug efflux by verapamil alone and in combination with MK-571, was observed (Fig. 3, Fig. 4 and Fig. 5). Four of the five MRPs (−1, −2, −4 & −5) tested were found to be constitutively expressed in ECs from aorta, pulmonary artery, dermal microvessel and umbilical vein and incubation with MK-571 suppressed the drug-efflux function in all of these cells. In both aortic and brain derived ECs, the efflux of both of these agents were suppressed by MK-571 at a more significant rate than that observed with verapamil. The prevalence of cardiovascular disease (CVD) in HIV-1 infected patients suggests that cardiac EC barriers may also efflux HAART drugs which facilitate the persistence of these subvascular HIV-1 reservoirs. Our findings in HAECs and HPAECs may also be of importance in the understanding of the pharmacokinetics of a variety of other drugs which are substrates for these transporters and must cross the vascular barriers in order to be effective. In addition to anti-cancer and anti-HIV drugs, both MDR-1 and MRPs are also known to recognize several anti-hypertensive drugs,58 and cardiovascular agents,59, 60 as substrates and their presence on human vascular ECs may suggest decreased drug efficacy at their site of action. In addition to their role in drug efflux, the MDR-1 transporter has been implicated in regulating tissue cholesterol levels and may be linked to cardiovascular disease (CVD).61, 62 Since MRPs efflux GSH from cells, they may also increase oxidative stress and apoptosis by decreasing intracellular antioxidant levels.63, 64 Our findings of the functional expression of both MDR-1 and MRPs in different human ECs may suggest their crucial roles in vascular disease manifestations linked to endothelial dysfunctions and oxidative stress, as well.
We observed that the mRNAs for both MDR-1 and MRPs were detectable in both dermal and vein derived ECs, however, they did not show significant levels of drug-efflux via either MDR-1 or MRP transporters. This may implicate that the regulation of transporter functions may also occur at the post-transcriptional, post-translational levels, or their membrane localization. The regulation of transporter expression may also be dependent on cellular microenvironments. Previous studies have shown that expression of ABC-transporters can be regulated following chronic exposure to inflammatory cytokines.65 In HIV-1 infected micro-environments, inflammatory cytokines as well as HIV-1 proteins may increase transporter expression and function. The HIV-1 Tat protein has indeed been shown to increase MDR-1 expression in murine brain derived ECs, but effects of Tat on MRPs were not monitored in this study.66 A thorough understanding of the role of microenvironments on the endothelial ABC-transporter expression will be important in determining drug-efflux functions.
Although we did not monitor protein levels of the transporters in ECs, our RT-PCR and calcein assay data clearly showed their functional expression. Furthermore, data obtained from both of these assays were validated using the NCI/ADR and PANC-1 cell lines (Fig. 1 and Fig. 2). In both NCI/ADR and PANC-1 cells lines both western immunodetection and immunofluorescence microscopy (IFM) studies, using commercially available antibodies to different ABC-transporters, were able to successfully detect these proteins (data not shown). However, we were unable to detect a sharp band in extracts from ECs or observe a membrane specific fluorescent signal in our IFM analyses. This may be due to different glycosylation patterns on the ABC-transporter glycoproteins which may be expressed in primary cells, as compared to their homogeneous pattern in in transformed cell lines.
ABC-transporters are known to be expressed in a polarized fashion and specific subtypes are expressed on the apical and the basal membranes of EC barriers.22, 25, 54 Hence, another drawback of our studies is that we only monitored drug-efflux in one direction and have not ascertained their transport from the apical to the basolateral compartments. Our findings do not provide data on the transport of saquinavir and zidovudine to subendothelial compartments and the role that different MRPs may play in regulating this transport. However, since we have observed a higher expression of both MDR-1 and MRP-2, both found to be apically expressed in brain ECs, our findings suggest an increased efflux of HPIs back into the blood circulation. Interestingly however, the HBMVECs also showed expression of both MRP-1 and MRP-5, both known to be expressed on the basolateral membrane. This may suggest regulation of HPI and NRTI transport towards the luminal side, as well. Studies using trans-well culture chambers should aid in understanding the role of each transporter in ultimately regulating the directional transport of anti-HIV agents.
The observations that multiple ABC-transporters can efflux several of the HAART drugs from endothelial cells, present a significant challenge to attaining therapeutic efficacy in sequestered HIV-1 reservoirs. Furthermore, the evidences that the ABC-transporters play crucial roles in normal physiologic functions also raise concerns regarding their general and systemic inhibition to increase drug transport. Strategies towards tissue specific inhibition of ABC-transporters will need to be implemented and transporter specific inhibitors will need to be developed. Our results clearly showed that the MRP-specific inhibitor MK-571 can significantly suppress HAART drug efflux from human EC barriers. Interestingly, several LT receptor antagonists, structural analogs of MK-571, such as montelukast, zafirlukast and pranlukast, are prescribed medications against asthma. It would be of interest to see whether these clinically approved MRP substrates/inhibitors can also suppress MRP-mediated efflux of anti-HIV drugs.
In conclusion, our studies showed that human ECs express functional MDR-1 and MRPs (−1 to −5) which are capable of effluxing both saquinavir and zidovudine. The MRP transporters were found to be the predominant drug-efflux mechanism in all of these primary human ECs. Our findings implicated that systemic HIV-1 reservoirs in both the CNS and in other systemic tissues may persist even in the face of HAART treatment, due to active efflux of both HPIs and NRTIs from the EC barriers to different tissues. Concomitant inhibition of ABC-transporters, specifically the MRPs, may enhance both HPI and NRTI entry to HIV-1 reservoirs. Thus, our findings clearly implicate that strategies to target the MRP transporters will be beneficial in increasing drug efficacy in subendothelial compartments.
Acknowledgements
This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID) grant 5 R21-AI064048.
References
- 1.Pomerantz RJ. Reservoirs, sanctuaries and residual disease: the hiding spots of HIV-1. HIV Clin Trials. 2003;4(2):137–143. doi: 10.1310/80jh-148k-nadq-u927. Review. [DOI] [PubMed] [Google Scholar]
- 2.Lambotte O, Deiva K, Tardieu M. HIV-1 persistence, viral reservoir and the central nervous system in the HAART era. Brain Pathol. 2003;13(1):95–103. doi: 10.1111/j.1750-3639.2003.tb00010.x. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aquaro S, Svicher V, Schols D, Pollicita M, Antinori A, Balzarini J, Perno CF. Mechanisms underlying activity of antiretroviral drugs in HIV-1-infected macrophages: new therapeutic strategies. J Leukoc Biol. 2006;80(5):1103–1110. doi: 10.1189/jlb.0606376. [DOI] [PubMed] [Google Scholar]
- 4.Tobin NH, Learn GH, Holte SE, Wang Y, Melvin AJ, McKernan JL, Pawluk DM, Mohan KM, Lewis PF, Mullins JI, Frenkel LM. Evidence that low-level viremias during effective highly active antiretroviral therapy result from two processes: expression of archival virus and replication of virus. J Virol. 2005;79(15):9625–9634. doi: 10.1128/JVI.79.15.9625-9634.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Honda M, Oka S. Current therapy for human immunodeficiency virus infection and acquired immunodeficiency syndrome. Int J Hematol. 2006;84(1):18–22. doi: 10.1532/IJH97.06102. Review. [DOI] [PubMed] [Google Scholar]
- 6.Boyd M, Reiss P. The long-term consequences of antiretroviral therapy: a review. J HIV Ther. 2006;11(2):26–35. Review. [PubMed] [Google Scholar]
- 7.Walubo A. The role of cytochrome P450 in antiretroviral drug interactions. Expert Opin Drug Metab Toxicol. 2007;3(4):583–598. doi: 10.1517/17425225.3.4.583. Review. [DOI] [PubMed] [Google Scholar]
- 8.Thomas SA. Anti-HIV drug distribution to the central nervous system. Curr Pharm Des. 2004;10(12):1313–1324. doi: 10.2174/1381612043384835. [DOI] [PubMed] [Google Scholar]
- 9.Deeken JF, Löscher W. The blood-brain barrier and cancer: transporters, treatment and Trojan horses. Clin Cancer Res. 2007;13(6):1663–1674. doi: 10.1158/1078-0432.CCR-06-2854. Review. [DOI] [PubMed] [Google Scholar]
- 10.Oswald S, Grube M, Siegmund W, Kroemer HK. Transporter-mediated uptake into cellular compartments. Xenobiotica. 2007;37(10–11):1171–1195. doi: 10.1080/00498250701570251. [DOI] [PubMed] [Google Scholar]
- 11.Scherrmann JM. Expression and function of multidrug resistance transporters at the blood-brain barriers. Expert Opin Drug Metab Toxicol. 2005;1(2):233–246. doi: 10.1517/17425255.1.2.233. [DOI] [PubMed] [Google Scholar]
- 12.Girardin F. Membrane transporter proteins: a challenge for CNS drug development. Dialogues Clin Neurosci. 2006;8(3):311–321. doi: 10.31887/DCNS.2006.8.3/fgirardin. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hollenstein K, Dawson RJ, Locher KP. Structure and mechanism of ABC transporter proteins. Curr Opin Struct Biol. 2007;17(4):412–418. doi: 10.1016/j.sbi.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Xu J, Peng H, Zhang JT. Human multidrug transporter ABCG2, a target for sensitizing drug resistance in cancer chemotherapy. Curr Med Chem. 2007;14(6):689–701. doi: 10.2174/092986707780059580. [DOI] [PubMed] [Google Scholar]
- 15.Yasuhisa K, Shin-ya M, Michinori M, Kazumitsu U. Mechanism of multidrug recognition by MDR1/ABCB1. Cancer Sci. 2007;98(9):1303–1310. doi: 10.1111/j.1349-7006.2007.00538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janneh O, Jones E, Chandler B, Owen A, Khoo SH. Inhibition of P-glycoprotein and multidrug resistance-associated proteins modulates the intracellular concentration of lopinavir in cultured CD4 T cells and primary human lymphocytes. J Antimicrob Chemother. 2007;60(5):987–993. doi: 10.1093/jac/dkm353. [DOI] [PubMed] [Google Scholar]
- 17.Chaillou S, Durant J, Garraffo R, Georgenthum E, Roptin C, Clevenbergh P, Dunais B, Mondain V, Roger PM, Dellamonica P. Intracellular concentration of protease inhibitors in HIV-1-infected patients: correlation with MDR-1 gene expression and low dose of ritonavir. HIV Clin Trials. 2002;3(6):493–501. doi: 10.1310/0873-bvdp-akay-445u. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal S, Pal D, Mitra AK. Both P-gp and MRP2 mediate transport of Lopinavir, a protease inhibitor. Int J Pharm. 2007;339(1–2):139–147. doi: 10.1016/j.ijpharm.2007.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leslie EM, Deeley RG, Cole SP. Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2 and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol. 2005;204(3):216–237. doi: 10.1016/j.taap.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Maher JM, Slitt AL, Cherrington NJ, Cheng X, Klaassen CD. Tissue distribution and hepatic and renal ontogeny of the multidrug resistance-associated protein (Mrp) family in mice. Drug Metab Dispos. 2005;33(7):947–955. doi: 10.1124/dmd.105.003780. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka Y, Slitt AL, Leazer TM, Maher JM, Klaassen CD. Tissue distribution and hormonal regulation of the breast cancer resistance protein (Bcrp/Abcg2) in rats and mice. Biochem Biophys Res Commun. 2005;326(1):181–187. doi: 10.1016/j.bbrc.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Lee G, Bendayan R. Functional expression and localization of P-glycoprotein in the central nervous system: relevance to the pathogenesis and treatment of neurological disorders. Pharm Res. 2004;21(8):1313–1330. doi: 10.1023/b:pham.0000036905.82914.8e. [DOI] [PubMed] [Google Scholar]
- 23.Ponte-Sucre A. Availability and applications of ATP-binding cassette (ABC) transporter blockers. Appl Microbiol Biotechnol. 2007;76(2):279–286. doi: 10.1007/s00253-007-1017-6. Review. [DOI] [PubMed] [Google Scholar]
- 24.Kruh GD, Belinsky MG. The MRP family of drug efflux pumps. Oncogene. 2003;22(47):7537–7552. doi: 10.1038/sj.onc.1206953. Review. [DOI] [PubMed] [Google Scholar]
- 25.Dallas S, Miller DS, Bendayan R. Multidrug resistance-associated proteins: expression and function in the central nervous system. Pharmacol Rev. 2006;58(2):140–161. doi: 10.1124/pr.58.2.3. Review. [DOI] [PubMed] [Google Scholar]
- 26.Sampath J, Adachi M, Hatse S, Naesens L, Balzarini J, Flatley RM, Matherly LH, Schuetz JD. Role of MRP4 and MRP5 in biology and chemotherapy. AAPS PharmSci. 2002;4(3):E14. doi: 10.1208/ps040314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biscardi M, Teodori E, Caporale R, Budriesi R, Balestri F, Scappini B, Gavazzi S, Grossi A. Multidrug reverting activity toward leukemia cells in a group of new verapamil analogues with low cardiovascular activity. Leuk Res. 2006;30(1):1–8. doi: 10.1016/j.leukres.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Ronaldson PT, Lee G, Dallas S, Bendayan R. Involvement of P-glycoprotein in the transport of saquinavir and indinavir in rat brain microvessel endothelial and microglia cell lines. Pharm Res. 2004;21(5):811–818. doi: 10.1023/b:pham.0000026433.27773.47. [DOI] [PubMed] [Google Scholar]
- 29.Bachmeier CJ, Spitzenberger TJ, Elmquist WF, Miller DW. Quantitative assessment of HIV-1 protease inhibitor interactions with drug efflux transporters in the blood-brain barrier. Pharm Res. 2005;22(8):1259–1268. doi: 10.1007/s11095-005-5271-y. [DOI] [PubMed] [Google Scholar]
- 30.Janneh O, Owen A, Chandler B, Hartkoorn RC, Hart CA, Bray PG, Ward SA, Back DJ, Khoo SH. Modulation of the intracellular accumulation of saquinavir in peripheral blood mononuclear cells by inhibitors of MRP1, MRP2, P-gp and BCRP. AIDS. 2005;19(18):2097–2102. doi: 10.1097/01.aids.0000194793.36175.40. [DOI] [PubMed] [Google Scholar]
- 31.Meaden ER, Hoggard PG, Newton P, Tjia JF, Aldam D, Cornforth D, Lloyd J, Williams I, Back DJ, Khoo SH. P-glycoprotein and MRP1 expression and reduced ritonavir and saquinavir accumulation in HIV-infected individuals. J Antimicrob Chemother. 2002;50(4):583–588. doi: 10.1093/jac/dkf161. [DOI] [PubMed] [Google Scholar]
- 32.Park S, Sinko PJ. P-glycoprotein and mutlidrug resistance-associated proteins limit the brain uptake of saquinavir in mice. J Pharmacol Exp Ther. 2005;312(3):1249–1256. doi: 10.1124/jpet.104.076216. [DOI] [PubMed] [Google Scholar]
- 33.Owen A, Janneh O, Hartkoorn RC, Chandler B, Bray PG, Martin P, Ward SA, Hart CA, Khoo SH, Back DJ. In vitro synergy and enhanced murine brain penetration of saquinavir coadministered with mefloquine. J Pharmacol Exp Ther. 2005;314(3):1202–1209. doi: 10.1124/jpet.105.086272. [DOI] [PubMed] [Google Scholar]
- 34.Jorajuria S, Dereuddre-Bosquet N, Becher F, Martin S, Porcheray F, Garrigues A, Mabondzo A, Benech H, Grassi J, Orlowski S, Dormont D, Clayette P. ATP binding cassette multidrug transporters limit the anti-HIV activity of zidovudine and indinavir in infected human macrophages. Antivir Ther. 2004;9(4):519–528. [PubMed] [Google Scholar]
- 35.Wijnholds J, Mol CA, van Deemter L, de Haas M, Scheffer GL, Baas F, Beijnen JH, Scheper RJ, Hatse S, De Clercq E, Balzarini J, Borst P. Multidrug-resistance protein 5 is a multispecific organic anion transporter able to transport nucleotide analogs. Proc Natl Acad Sci U S A. 2000;97(13):7476–7481. doi: 10.1073/pnas.120159197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta A, Zhang Y, Unadkat JD, Mao Q. HIV protease inhibitors are inhibitors but not substrates of the human breast cancer resistance protein (BCRP/ABCG2) J Pharmacol Exp Ther. 2004;310(1):334–341. doi: 10.1124/jpet.104.065342. [DOI] [PubMed] [Google Scholar]
- 37.Boska MD, Mosley RL, Nawab M, Nelson JA, Zelivyanskaya M, Poluektova L, Uberti M, Dou H, Lewis TB, Gendelman HE. Advances in neuroimaging for HIV-1 associated neurological dysfunction: clues to the diagnosis, pathogenesis and therapeutic monitoring. Curr HIV Res. 2004;2(1):61–78. doi: 10.2174/1570162043485095. [DOI] [PubMed] [Google Scholar]
- 38.Marcondes MC, Burudi EM, Huitron-Resendiz S, Sanchez-Alavez M, Watry D, Zandonatti M, Henriksen SJ, Fox HS. Highly activated CD8(+) T cells in the brain correlate with early central nervous system dysfunction in simian immunodeficiency virus infection. J Immunol. 2001;167(9):5429–5438. doi: 10.4049/jimmunol.167.9.5429. [DOI] [PubMed] [Google Scholar]
- 39.Katoh M, Suzuyama N, Takeuchi T, Yoshitomi S, Asahi S, Yokoi T. Kinetic analyses for species differences in P-glycoprotein-mediated drug transport. J Pharm Sci. 2006;95(12):2673–2683. doi: 10.1002/jps.20686. [DOI] [PubMed] [Google Scholar]
- 40.Suzuyama N, Katoh M, Takeuchi T, Yoshitomi S, Higuchi T, Asashi S, Yokoi T. Species differences of inhibitory effects on P-glycoprotein-mediated drug transport. J Pharm Sci. 2007;96(6):1609–1618. doi: 10.1002/jps.20787. [DOI] [PubMed] [Google Scholar]
- 41.Hauser IA, Koziolek M, Hopfer U, Thévenod F. Therapeutic concentrations of cyclosporine A, but not FK506, increase P-glycoprotein expression in endothelial and renal tubule cells. Kidney Int. 1998;54(4):1139–1149. doi: 10.1046/j.1523-1755.1998.00095.x. [DOI] [PubMed] [Google Scholar]
- 42.Iwahana M, Utoguchi N, Mayumi T, Goryo M, Okada K. Drug resistance and P-glycoprotein expression in endothelial cells of newly formed capillaries induced by tumors. Anticancer Res. 1998;18(4C):2977–2980. [PubMed] [Google Scholar]
- 43.Liscovitch M, Ravid D. A case study in misidentification of cancer cell lines: MCF-7/AdrR cells (re-designated NCI/ADR-RES) are derived from OVCAR-8 human ovarian carcinoma cells. Cancer Lett. 2007 1–2;245:350–352. doi: 10.1016/j.canlet.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 44.Sarver JG, Klis WA, Byers JP, Erhardt PW. Microplate screening of the differential effects of test agents on Hoechst 33342, rhodamine 123 and rhodamine 6G accumulation in breast cancer cells that overexpress P-glycoprotein. J Biomol Screen. 2002;7(1):29–34. doi: 10.1177/108705710200700105. [DOI] [PubMed] [Google Scholar]
- 45.Rosati A, Candussio L, Crivellato E, Klugmann FB, Giraldi T, Damiani D, Michelutti A, Decorti G. Bodipy-FL-verapamil: a fluorescent probe for the study of multidrug resistance proteins. Cell Oncol. 2004;26(1–2):3–11. doi: 10.1155/2004/576173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sulová Z, Macejová D, Seres M, Sedlák J, Brtko J, Breier A. Combined treatment of P-gp-positive L1210/VCR cells by verapamil and all-trans retinoic acid induces down-regulation of P-glycoprotein expression and transport activity. Toxicol In Vitro. 2008;22(1):96–105. doi: 10.1016/j.tiv.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 47.Limtrakul P, Chearwae W, Shukla S, Phisalphong C, Ambudkar SV. Modulation of function of three ABC drug transporters, P-glycoprotein (ABCB1), mitoxantrone resistance protein (ABCG2) and multidrug resistance protein 1 (ABCC1) by tetrahydrocurcumin, a major metabolite of curcumin. Mol Cell Biochem. 2007;296(1–2):85–95. doi: 10.1007/s11010-006-9302-8. [DOI] [PubMed] [Google Scholar]
- 48.Hua J, Mutch DG, Herzog TJ. Stable suppression of MDR-1 gene using siRNA expression vector to reverse drug resistance in a human uterine sarcoma cell line. Gynecol Oncol. 2005;98(1):31–38. doi: 10.1016/j.ygyno.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 49.König J, Hartel M, Nies AT, Martignoni ME, Guo J, Büchler MW, Friess H, Keppler D. Expression and localization of human multidrug resistance protein (ABCC) family members in pancreatic carcinoma. Int J Cancer. 2005;115(3):359–367. doi: 10.1002/ijc.20831. [DOI] [PubMed] [Google Scholar]
- 50.Volk H, Potschka H, Löscher W. Immunohistochemical localization of P-glycoprotein in rat brain and detection of its increased expression by seizures are sensitive to fixation and staining variables. J Histochem Cytochem. 2005;53(4):517–531. doi: 10.1369/jhc.4A6451.2005. [DOI] [PubMed] [Google Scholar]
- 51.Demeule M, Labelle M, Régina A, Berthelet F, Béliveau R. Isolation of endothelial cells from brain, lung and kidney: expression of the multidrug resistance P-glycoprotein isoforms. Biochem Biophys Res Commun. 2001;281(3):827–834. doi: 10.1006/bbrc.2001.4312. [DOI] [PubMed] [Google Scholar]
- 52.Salama NN, Kelly EJ, Bui T, Ho RJ. The impact of pharmacologic and genetic knockout of P-glycoprotein on nelfinavir levels in the brain and other tissues in mice. J Pharm Sci. 2005;94(6):1216–1225. doi: 10.1002/jps.20344. [DOI] [PubMed] [Google Scholar]
- 53.Kubota H, Ishihara H, Langmann T, Schmitz G, Stieger B, Wieser HG, Yonekawa Y, Frei K. Distribution and functional activity of P-glycoprotein and multidrug resistance-associated proteins in human brain microvascular endothelial cells in hippocampal sclerosis. Epilepsy Res. 2006;68(3):213–228. doi: 10.1016/j.eplepsyres.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 54.Nies AT, Jedlitschky G, König J, Herold-Mende C, Steiner HH, Schmitt HP, Keppler D. Expression and immunolocalization of the multidrug resistance proteins, MRP1–MRP6 (ABCC1–ABCC6), in human brain. Neuroscience. 2004;129(2):349–360. doi: 10.1016/j.neuroscience.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 55.Bronger H, König J, Kopplow K, Steiner HH, Ahmadi R, Herold-Mende C, Keppler D, Nies AT. ABCC drug efflux pumps and organic anion uptake transporters in human gliomas and the blood-tumor barrier. Cancer Res. 2005;65(24):11419–11428. doi: 10.1158/0008-5472.CAN-05-1271. [DOI] [PubMed] [Google Scholar]
- 56.Umeki N, Fukasawa Y, Ohtsuki S, Hori S, Watanabe Y, Kohno Y, Terasaki T. mRNA expression and amino acid transport characteristics of cultured human brain microvascular endothelial cells (hBME) Drug Metab Pharmacokinet. 2002;17(4):367–373. doi: 10.2133/dmpk.17.367. [DOI] [PubMed] [Google Scholar]
- 56.Kuo YC, Su FL. Transport of stavudine, delavirdine and saquinavir across the blood-brain barrier by polybutylcyanoacrylate, methylmethacrylate-sulfopropylmethacrylate and solid lipid nanoparticles. Int J Pharm. 2007;340(1–2):143–152. doi: 10.1016/j.ijpharm.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 57.Bachmakov I, Werner U, Endress B, Auge D, Fromm MF. Characterization of beta-adrenoceptor antagonists as substrates and inhibitors of the drug transporter P-glycoprotein. Fundam Clin Pharmacol. 2006;20(3):273–182. doi: 10.1111/j.1472-8206.2006.00408.x. [DOI] [PubMed] [Google Scholar]
- 58.Kurzawski M, Bartnicka L, Florczak M, Górnik W, Droździk M. Impact of ABCB1 (MDR1) gene polymorphism and P-glycoprotein inhibitors on digoxin serum concentration in congestive heart failure patients. Pharmacol Rep. 2007;59(1):107–111. [PubMed] [Google Scholar]
- 59.Couture L, Nash JA, Turgeon J. The ATP-binding cassette transporters and their implication in drug disposition: a special look at the heart. Pharmacol Rev. 2006;58(2):244–258. doi: 10.1124/pr.58.2.7. Review. [DOI] [PubMed] [Google Scholar]
- 60.Yunomae K, Arima H, Hirayama F, Uekama K. Involvement of cholesterol in the inhibitory effect of dimethyl-beta-cyclodextrin on P-glycoprotein and MRP2 function in Caco-2 cells. FEBS Lett. 2003;536(1–3):225–231. doi: 10.1016/s0014-5793(03)00059-0. [DOI] [PubMed] [Google Scholar]
- 61.Rothnie A, Theron D, Soceneantu L, Martin C, Traikia M, Berridge G, Higgins CF, Devaux PF, Callaghan R. The importance of cholesterol in maintenance of P-glycoprotein activity and its membrane perturbing influence. Eur Biophys J. 2001;30(6):430–442. doi: 10.1007/s002490100156. [DOI] [PubMed] [Google Scholar]
- 62.Krause MS, Oliveira LP, Jr, Silveira EM, Vianna DR, Rossato JS, Almeida BS, Rodrigues MF, Fernandes AJ, Costa JA, Curi R, de Bittencourt PI., Jr MRP1/GS-X pump ATPase expression: is this the explanation for the cytoprotection of the heart against oxidative stress-induced redox imbalance in comparison to skeletal muscle cells? Cell Biochem Funct. 2007;25(1):23–32. doi: 10.1002/cbf.1343. [DOI] [PubMed] [Google Scholar]
- 63.Hammond CL, Marchan R, Krance SM, Ballatori N. Glutathione export during apoptosis requires functional multidrug resistance-associated proteins. J Biol Chem. 2007;282(19):14337–14347. doi: 10.1074/jbc.M611019200. [DOI] [PubMed] [Google Scholar]
- 64.Petrovic V, Teng S, Piquette-Miller M. Regulation of drug transporters during infection and inflammation. Mol Interv. 2007;7(2):99–111. doi: 10.1124/mi.7.2.10. [DOI] [PubMed] [Google Scholar]
- 65.Hayashi K, Pu H, Tian Jandras IE, Lee YW, Hennig B, Toborek M. HIV-Tat protein induces P-glycoprotein expression in brain microvascular endothelial cells. J Neurochem. 2005;93(5):1231–1241. doi: 10.1111/j.1471-4159.2005.03114.x. [DOI] [PubMed] [Google Scholar]