Abstract
Her-2/neu (ErbB2) is a transmembrane tyrosine kinase and acts as a co-receptor for the other EGFR family members. It is well known that high expression of Her-2/neu is associated with a poor prognosis in breast cancer. Quercetin, a flavonoid present in many vegetables and fruits, has been studied extensively as a chemoprevention agent in several cancer models. In this study, we observed that quercetin decreased the level of Her-2/neu protein in time- and dose-dependent manners and also inhibited the downstream survival PI3K-Akt signaling pathway in Her-2/neu-overexpressing breast cancer SK-Br3 cells. We also observed that quercetin induced polyubiquitination of Her-2/neu. When the proteasome pathway was blocked by MG-132 during quercetin treatment, accumulation of the NP-40 insoluble form of Her-2/neu occurred. Interestingly, data from immunocomplex studies revealed that quercetin promoted interaction between Her-2/neu and Hsp90 which is a molecular chaperone involved in stabilization of Her-2/neu. In this condition, inhibition of Hsp90 activity by a specific inhibitor, geldanamycin (GA), or intracellular ATP depletion caused dissociation of Hsp90 from Her-2/neu and promoted ubiquitination and down-regulation of Her-2/neu protein. In addition, the carboxyl terminus of Hsc70-interacting protein (CHIP), a chaperone-dependent E3 ubiquitin ligase, played a crucial role in the quercetin-induced ubiquitination of Her-2/neu. Inhibition of tyrosine kinase activity of Her-2/neu by quercetin could indicate an lateration in the Her-2/neu structure which promotes CHIP recruitments and down-regulation of Her-2/neu. We believe that by using quercetin, new therapeutic strategies can be developed to treat Her-2/neu overexpressing cancers.
Keywords: Her-2/neu, quercetin, ubiquitin, chaperone, Hsp90
Breast cancer has become the most frequently diagnosed cancer among women in the United States and the second most frequent cause of cancer death [Jemal et al., 2006]. Even though early diagnosis of breast cancer with mammography has improved over the last ten years, the optimal management of this disease remains undefined. Her-2/neu overexpression is observed in around 30% of all breast cancer patients and is directly linked to deregulated activation of intracellular mitogenic signaling, leading to aggressive tumor behavior and resistance to cancer chemotherapy [Slamon et al., 1987; 1989]. An increase in Her-2/neu expression also has been found to enhance malignant phenotypes of cancer cells, including those with metastatic potential [Niehans et al., 1993]. The association of Her-2/neu overexpression in cancer cells with chemoresistance and metastasis provides a plausible interpretation for the poor clinical outcome of patients with Her-2/neu-overexpressing cancers; it suggests that the enhanced tyrosine kinase activity of Her-2/neu might play a critical role in the initiation, progression, and outcome of human tumors. So Her-2/neu has become an important therapeutic target in breast cancer [Nahta and Esteva, 2003].
Her-2/neu is a member of the subclass I of the receptor tyrosine kinase (RTK) superfamily, which comprises four members: Her-1/epidermal growth factor receptor (EGFR)/ErbB1, Her-2/neu/ErbB2, Her-3/ErbB3, and Her-4/ErbB4. These four receptors have an extracellular ligand-binding region, a single membrane-spanning region and a cytoplasmic tyrosine kinase-containing domain [Hynes and Lane, 2005]. They are essential mediators of cell proliferation and differentiation. Their aberrant activation is associated with the development and severity of many cancers. Ligand binding to these receptors induces the formation of receptor homo- and hetero-dimers and activation of the intrinsic kinase domain, resulting in phosphorylation on specific tyrosine residues within the cytoplasmic tail. These phosphorylated residues serve as docking sites for a range of proteins and the recruitment of proteins leads to the activation of intracellular signaling pathways. Homo- and hetero-dimerization of these receptors result in signal amplification and diversification to extracellular ligands [Olayioye et al., 2000]. For example, Her-2/neu heterodimerization with EGFR antagonizes EGFR/c-Cbl association and promotes receptor longevity and recycling to the cell surface. Dimerization of Her-2/neu and Her-3 occurs frequently and is a preferred heterodimer. Although Her-3 is a kinase-defective protein, heterodimerization with Her-2/neu enables Her-3 response to extracellular ligand and the ability to directly couple to the PI3K (phosphatidylinositol 3-kinase)-Akt cell survival pathway.
Studies performed in animal model have shown that down-regulation of Her-2/neu can suppress tumor growth and dissemination [Drebin et al., 1986; Katsumata et al., 1995]. Treatment with trastuzumab (Herceptin), the first approved monoclonal antibody treatment for breast cancer, results in significant improvement in patient survival when used in combination with chemotherapy in patients with metastatic Her-2/neu positive tumors [Baselga et al., 1996; Slamon et al., 2001]. Trastuzumab inhibits signaling of MAPK and PI3K pathways, promotes cell cycle arrest, and induces apoptosis, possibly by mediating the internalization and degradation of the Her-2/neu receptor and consequently diminishing its intracellular signaling [Baselga et al., 2001; Sliwkowski et al., 1999]. Although trastuzumab proved to be an effective therapeutic agent, patients treated with trastuzumab were found to be at an increased risk for cardiac dysfunction, characterized by symptoms of congestive heart failure (CHF). Recent reports that Her-2/neu plays an essential role in cardiac development during embryogenesis and as a survival factor in adult myocardium suggest an explanation of the cytotoxic side effect of trastuzumab [Lee et al., 1995; Meyer and Birchmeier, 1995; Crone et al., 2002; Ozcelik et al., 2002].
An alternative therapeutic agent may be the natural product quercetin (3,5,7,3′,4′-pentahydroxyflavone), which is orally bioavailable, and is a flavonoid found in many fruits and vegetables. Epidemiological studies have shown that the consumption of vegetables, fruits, and tea is associated with a low risk of cancer [Block et al., 1992]. Quercetin and its metabolites are potent antioxidants which have oxygen radical scavenging properties and inhibit xanthine oxidase and lipid peroxidation in vitro [Bors et al., 1994; da Silva et al., 1998; Vulcain et al., 2005]. Previous research has also shown that quercetin has anti-tumor, anti-inflammatory, anti-allergic, and anti-viral activities [Middleton and Kandaswami, 1992; Kandaswami and Middleton, 1994; Wang, 2000]. Quercetin has been shown to be protective against breast cancer in animal model [Verma et al., 1988].
In this report we further explored the anti-tumor activity of quercetin by investigating its effect on the ubiquitinylation and down-regulation of Her-2/neu in SK-Br3 breast cancer cells. In spite of the previous reports that quercetin decreased the expression of Her-2/neu proteins in HT-29 and PC-3 cell lines [Kim et al., 2005; Huynh et al., 2003], detailed mechanisms of quercetin induced-down-regulation of Her-2/neu protein are largely unknown. Interestingly, although molecular chaperone Hsp90 is recruited to Her-2/neu during this process, quercetin treatment eventually leads to the down-regulation of Her-2/neu protein. This effect makes quercetin a promising candidate for treatment and prevention of breast cancer.
Materials and Methods
Cells, antibodies, and plasmids
Human breast adenocarcinoma SK-Br3 and MCF-7, human ovarian adenocarcinoma SK-Ov3, and COS7 (African Green Monkey SV40-transfected kidney fibroblast cell line) cells were obtained from American Type Culture Collection (Manassas, VA). Anti-Her-2/neu, anti-EGFR, anti-phospho-PI3K, anti-ubiquitin antibodies and normal mouse IgG were from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse Her-2/neu monoclonal antibody and rabbit anti-CHIP polyclonal antibody were from Calbiochem (San Diego, CA). Rat anti-Hsp90 monoclonal antibody (SPA-840) was from Stressgen Biotech Corp. (Victoria, BC, Canada). Mouse anti-HIS monoclonal antibody was from Invitrogen (Carlsbad, CA). Mouse anti-actin monoclonal antibody was from ICN (Costa Mesa, CA). Mouse phospho-tyrosine monoclonal antibody was from Upstate (Lake Placid, NY). pCMV-Her-2/neu expressing plasmid was a kind gift of Dr. M.C. Hung (M.D. Anderson Cancer Center, Houston, TX). pcDNA3.1/myc-HIS-CHIP expressing plasmid was a kind gift of Dr. C. Patterson (University of North Carolina, Chapel Hill, NC).
Reagents
Quercetin (2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-4H-1-benzopyran-4-one dehydrate), ammonium chloride, geldanamycin, and 2-deoxyglucose were purchased from Sigma Chemical Co. (St. Louis, MO). Adriamycin and MG-132 was purchased from Calbiochem (San Diego, CA).
Cell culture and transfection
SK-Br3 and COS7 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (HyClone, Logan, UT) and 100 units of penicillin and streptomycin (Invitrogen). The cells were maintained in a humidified atmosphere containing 5% CO2 and air at 37 °C. One day prior to the experiment, cells were plated. For transient transfections, each plasmid was premixed with Lipofectamine (Invitrogen) and added to COS7 cells at 50-70% confluency. Cells were continually cultured in the same medium for 24 h before the appropriate treatment.
Protein analysis
Cells were washed once with cold phosphate-buffered saline (pH 7.0) and collected by scraping. Harvested cells were lysed by resuspending in lysis buffer (50 mM Tris-HCl (pH 7.5), 0.5 mM EDTA, 150 mM NaCl, and 0.5% Nonidet P-40 (NP-40)) supplemented with protease inhibitors and phosphatase inhibitors (Calbiochem). Cell lysates were clarified by centrifugation at 13,000 rpm (4°C) for 15 min, and protein concentration was determined by the Bradford method (Bio-Rad). NP-40-insoluble fractions were lysed in 1× SDS sample buffer (50 mM Tris-HCl (pH 6.8), 2% SDS, 10% glycerol, and 0.01% bromophenol blue containing 1.28 M β-mercaptoethanol), and boiled for 15 min. For immunoprecipitation, 0.5–1 mg of lysate was incubated with 1.5 μg of mouse monoclonal antibodies at 4°C for 2 h, followed by the addition of protein A-agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA) and rotation at 4°C overnight. The beads were washed three times with lysis buffer, resuspended in SDS sample buffer and boiled for 5 min. Immunoprecipitated proteins or equal amounts of cell lysate were separated by 10% to 15% SDS-polyacrylamide gels, transferred to nitrocellulose, immunoblotted, and detected by chemiluminescence using ECL detection reagents (Amersham Biosciences).
Flow cytometry
Cell surface expression of Her-2/neu protein in SK-Br3 cells was determined by flow cytometry using a mouse anti–Her-2/neu antibody directed against the extracellular domain (clone Ab-5; Oncogene Research Products, San Diego, CA). In brief, after quercetin (200 μM) or control treatment for 24 h, cells were washed, harvested, and resuspended in phosphate-buffered saline (PBS) containing 1% FBS, and then incubated with 5 μg/mL Ab-5 antibody for 1 h at 4°C. The cells were washed again and then incubated with a fluorescein isothiocyanate (FITC)–conjugated anti-mouse IgG secondary antibody diluted 1:200 in cold PBS containing 1% FBS for 45 min at 4°C. The cells were washed once more in cold PBS, and flow cytometric analysis was performed with an EPICS XL flow cytometer (Beckman-Coulter, Fullerton, CA).
Northern blot analysis
For Northern blot hybridization, total RNAs (10 μg) isolated from cells were fractionated by electrophoresis in 1.2% formaldehyde agarose gels, blotted onto Nytran Plus (Schleicher & Schuell), and hybridized with the 32P-labeled Her-2/neu or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA probe.
Real-time RT-PCR
Total RNA was prepared using RNeasy-mini kit (Qiagen) including on-column DNase digestion to eliminate genomic DNA contamination. cDNA was generated from 2ug of RNA using SuperScript III™ reverse transcriptase (Invitrogen). Real-time RT-PCR was carried out using Platinum®SYBR® Green (Invitrogen) with ABI 7300 Real time PCR system. All samples were duplicated (or triplicated). Primer sequences are as follows: Her-2/neu forward, 5′-GGGAAGAATGGGGTCGTCAAA-3′; Her-2/neu reverse, 5′-CTCCTCCCTGGGGTGTCAAGT-3′ (RTPrimerDB database, http://medgen.UGent.be/rtprimerdb/); 18S-rRNA forward, 5′-GTAACCCGTTGAACCCCATT-3′; 18S-rRNA reverse, 5′-CCATCCAATCGGTAGTAGCG-3′ [Yogev-Falach et al., 2006].
RNA interference
siRNA targeting human CHIP gene was purchased from Santa Cruz Biotechnology. Control siRNA or CHIP siRNA was premixed with Her-2/neu overexpressing plasmid and then transfected with Lipofectamine (Invitrogen) to COS7 cells according to manufacturer's recommendations. After 24 h, 100 μM of quercetin was applied for 2 h and then cells were collected for immunoprecipitation and Western blot analysis.
In vitro Her-2/neu tyrosine kinase assay
Immunocomplex was precipitated from lysate of SK-Br3 cells as described above and then washed three times with lysis buffer and once in kinase assay buffer (25 mM Tris-HCl (pH 7.5), 5 mM beta-glycerophosphate, 2 mM dithiothreitol (DTT), 0.1 mM Na3VO4, 10 mM MgCl2, and 10 mM MnCl2). For autophosphorylation assay, radiolabeled ATP (10 μCi of [γ-32P]ATP, ICN, Irvine, CA) and 100 μM of cold ATP were added to the beads, followed by incubation for 30 min at 30°C. The reaction product was separated by SDS-PAGE and then transferred to nitrocellulose membrane. The autophosphorylation of Her-2/neu was visualized by autoradiography and the Her-2/neu protein in reaction mixture was shown by Western-blot analysis. Trans-tyrosine kinase activity of Her-2/neu was measured using non-radioactive Tyrosine Kinase Activity Assay Kit (CHEMICON International) according to manufacturers' instructions. Quercetin was preincubated with the reaction mixture including immunoprecipitated Her-2/neu protein and synthetic biotinylated peptide substrate at room temperature for 10 min and then reaction was started by adding ATP, followed by incubation for 1 h at 30°C. After quenching the enzyme reaction with EDTA, the peptide substrates were immobilized by binding to the streptavidin-coated plate. The fraction of phosphorylated substrate was visualized using a phosphotyrosine monoclonal antibody conjugated to HRP and an ensuing chromagenic substrate reaction.
Results
Quercetin down-regulates Her-2/neu and dephosphorylates Akt
To investigate the chemopreventive and therapeutic effects of quercetin on Her-2/neu overexpressing cells, human breast carcinoma SK-Br3 cells were treated with quercetin and examined for Her-2/neu protein level. As shown in Figs. 1A-C, Her-2/neu protein level was decreased with quercetin treatment in time- and dose-dependent manners. The level of Her-2/neu protein began to decrease after 8 h of quercetin treatment (Fig. 1B). Quercetin treatment also dephosphorylated PI3K and Akt (Figs. 1A and 1C). Our previous studies from in vitro kinase assay demonstrated that dephosphorylation of Akt corresponds to the loss of its kinase activity [Kim and Lee, 2007]. Since the PI3K and Akt signal transduction pathway is one of the important survival signaling mechanisms activated in Her-2/neu-overexpressed cells [Hynes and Lane, 2005], it is likely that inhibition of PI3K and Akt activity by treatment with quercetin contributes to enhancement of apoptotic death [Kim and Lee, 2007]. In accordance with the decrease of total Her-2/neu level, membrane bound Her-2/neu protein was also significantly decreased during treatment with quercetin. Flow cytometry analysis using a mouse anti–Her-2/neu antibody directed against the extracellular domain of Her-2/neu showed that quercetin treatment significantly reduced the cell surface Her-2/neu (Fig. 1D). Quercetin down-regulated Her-2/neu protein level in Her-2/neu overexpressing ovarian cancer cell line SK-Ov3 (Fig. 1E) and in another breast cancer cell line MCF-7 (Fig. 1F). These results confirmed that down-regulation of Her-2/neu by quercetin is not a cell line specific effect.
Fig. 1.
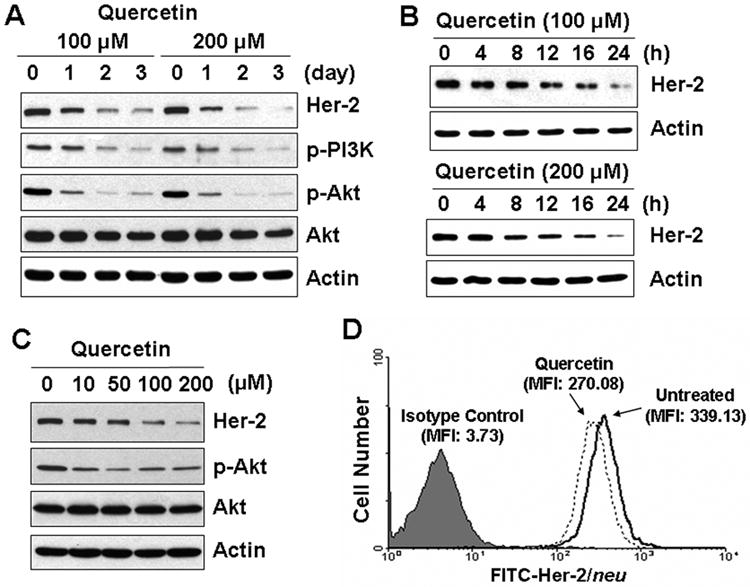
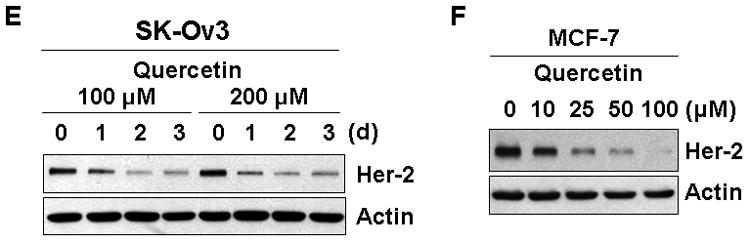
Quercetin down-regulates Her-2/neu in SK-Br3. A: SK-Br3 cells were treated with 100 μM or 200 μM of quercetin for the indicated days. B: SK-Br3 cells were treated with 100 μM or 200 μM of quercetin for the indicated h. C: SK-Br3 cells were treated with indicated amount of quercetin for 24 h. Cellular levels of Her-2/neu protein and phosphorylation of the downstream kinases, phosphoinositide-3 kinase (PI3K) and Akt, were compared from whole cell lysate. D: Cell surface expression of Her-2/neu protein in SK-Br3 cells was determined by flow cytometry using a mouse anti–Her-2/neu antibody directed against the extracellular domain of Her-2/neu. SK-Br3 cells were incubated in the absence or presence of 200 μM of quercetin for 24 h. E: SK-Ov3 cells were treated with 100 μM or 200 μM of quercetin for the indicated days. F: MCF-7 cells were treated with indicated amount of quercetin for 24 h. Cellular levels of Her-2/neu protein was compared from whole cell lysate.
Quercetin-induced Her-2/neu down-regulation is not due to transcription inhibition
To examine whether Her-2/neu gene expression is affected by quercetin treatment, Northern blot analysis was performed (Fig. 2A). SK-Br3 cells were treated with 100 μM quercetin for the indicated time period and then the mRNA level of Her-2/neu was determined. Unlike the level of Her-2/neu protein, there was no remarkable reduction of Her-2/neu mRNA during treatment with quercetin. Quantitative anaylsis using real-time RT-PCR of Her-2/neu transcription also supported the Northern blot analysis result (Fig. 2B). These results suggest that quercetin affects the translational machinery or protein stability of Her-2/neu protein rather than transcription.
Fig. 2.
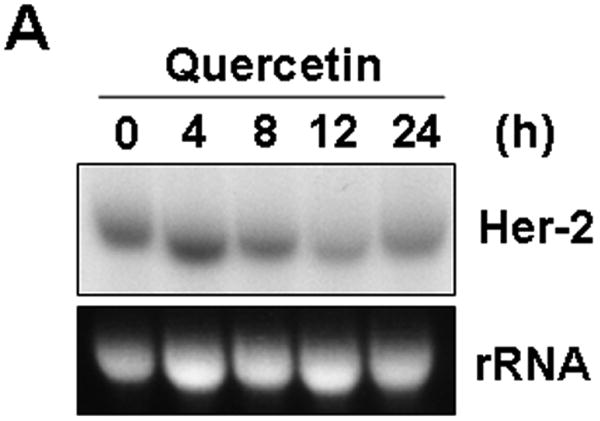
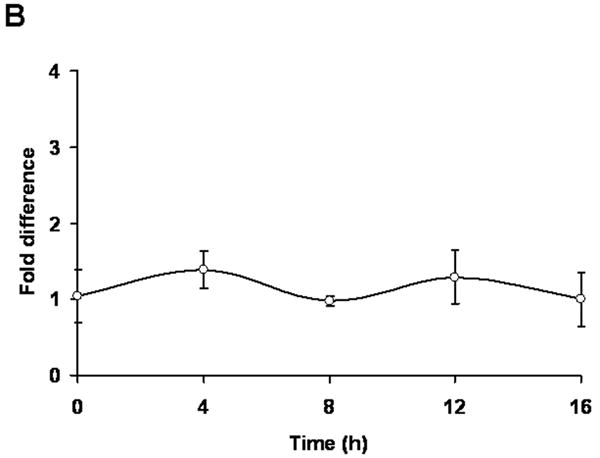
Her-2/neu down-regulation by quercetin is not regulated at transcription level. A: SK-Br3 cells were treated with 100 μM of quercetin for the indicated times and then total RNA was extracted. Her-2/neu mRNA levels were compared by Northern blot analysis. Ethidium bromide stained rRNA bands are shown as a loading control. B: Her-2/neu mRNA levels were compared by real-time RT-PCR analysis using 18S rRNA as an internal control. Error bars represent standard error from the mean (SEM) for three independent experiments.
Quercetin not only induces ubiquitination of Her-2/neu but also enhances the recruitment of Hsp90 to Her-2/neu
To investigate the molecular mechanism of quercetin-induced Her-2/neu down-regulation, a representative proteasome inhibitor (MG-132) or lysosomal inhibitor (NH4Cl) was simultaneously applied with quercetin. Co-treatment with the proteasome inhibitor MG-132 resulted in accumulation of insoluble (aggregated) forms of Her-2/neu protein in cell lysates (Fig. 3A). Unlike MG-132, the lysosomal inhibitor NH4Cl did not prevent the down-regulation of Her-2/neu protein during treatment with quercetin. Since Figure 3A indicates that quercetin probably induces proteasome-dependent degradation of Her-2/neu protein, we examined the ubiquitination of Her-2/neu proteins during quercetin treatment. Figure 3B and 3C show that quercetin induced ubiquitination of Her-2/neu protein in time- and dose-dependent manners. These results suggest that quercetin induces down-regulation of Her-2/neu protein through the ubiquitin-dependent proteasome degradation pathway. It is well known that chaperone protein Hsp90 plays an essential role in maturation, stabilization and activation of Her-2/neu protein [Neckers et al., 1999]. It has also been shown that a specific Hsp90 inhibitor geldanamycin (GA) dissociates Hsp90 from the Her-2/neu protein complex and destabilizes Her-2/neu protein via ubiquitination and subsequent proteasomal degradation [Xu et al., 2001]. To investigate whether quercetin functions in a similar way to GA, the association between Her-2/neu and Hsp90 was examined after quercetin treatment. Unlike treatment with GA, treatment with quercetin increased the association of Hsp90 to Her-2/neu protein (Figs. 3B, C). Thus, unexpectedly, ubiquitination and Hsp90 recruitment to Her-2/neu occurred at the same time during quercetin treatment. However, quercetin treatment neither caused polyubiquitination of EGFR nor increased its association to Hsp90 (Fig. 3D), suggesting that regulatory machinery which is specific to Her-2/neu turnover is involved in quercetin-mediated Her-2/neu down-regulation.
Fig. 3.
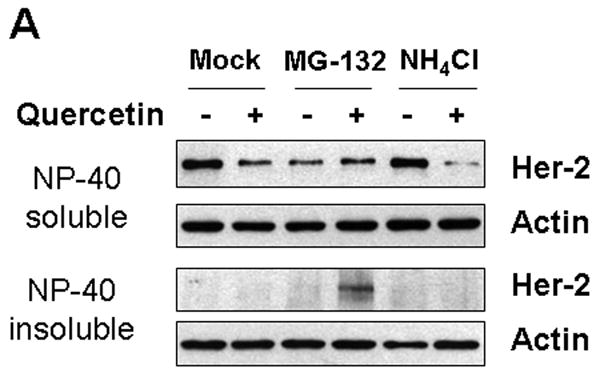
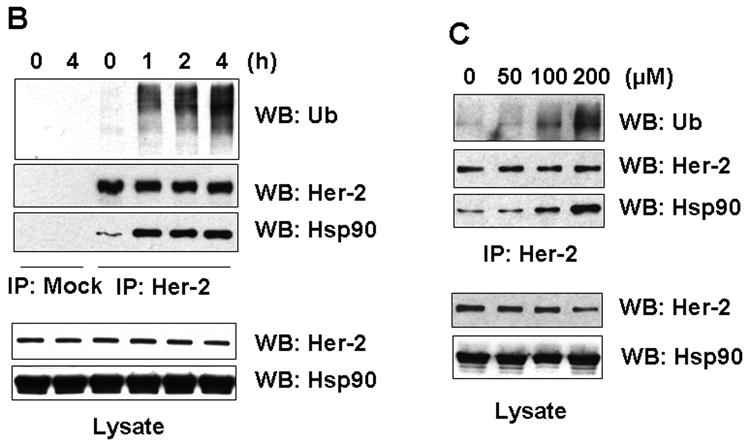
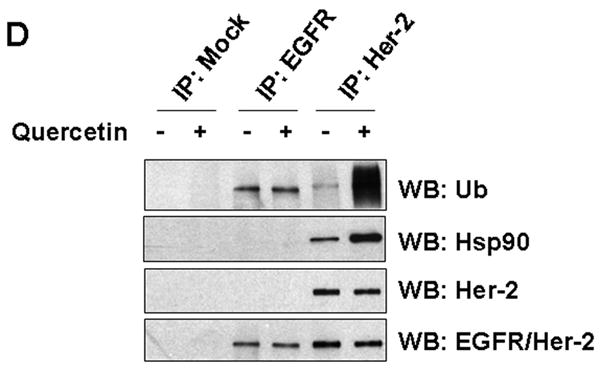
Quercetin induces ubiquitination of Her-2/neu and concomitantly enhances the recruitment of Hsp90 to Her-2/neu. A: SK-Br3 cells were cotreated with 200 μM of quercetin and MG-132 (5 μM) or NH4Cl (10 mM) for 24 hr. Cell extract was prepared by resuspending in lysis buffer (NP-40 soluble) and the insoluble proteins were extracted with SDS sample buffer and then boiled (NP-40 insoluble). Her-2/neu protein level was compared by Western blot analysis. B: SK-Br3 cells were treated with 200 μM of quercetin for the indicated times After Her-2/neu proteins were immunoprecipitated, ubiquitination level and interacting amount of Hsp90 were compared by Western blot analysis. C: SK-Br3 cells were treated with increased amounts of quercetin for 4 h and then analysed as above. D: SK-Br3 cells were either left untreated or treated with 200 μM of quercetin for 4 h and then whole cell lysate was prepared. After immunoprecipitation with normal mouse serum, anti-EGFR, or anti-Her-2/neu antibody, the ubiquitination level and interacting amount of Hsp90 were compared by Western blot analysis.
The iron chelator function of quercetin is not involved in the down-regulation of Her-2/neu protein
Recently, several researchers reported that quercetin acts as an iron chelator [Morel et al., 1994] and its iron chelating effect causes the accumulation of HIF-1α in normoxia [Triantafyllou et al., 2007; Jeon et al., 2007]. To examine whether the iron chelating effect of quercetin leads to Her-2/neu down-regulation, cells were pretreated with an excess amount of iron prior to quercetin treatment. Figure 4A showed that pretreatment with an excess amount of iron prevented quercetin-induced Her-2/neu down-regulation. In order to verify the importance of the iron chelator function, desferrioxamine (DFX), a well-known iron-specific chelator, was applied. Unlike quercetin, DFX induced HIF-1α accumulation in normoxia, but did not cause Her-2/neu down-regulation (Fig. 4B). These data suggest that quercetin-induced Her-2/neu down-regulation is not simply due to iron chelation. We further examined the effect of iron on ubiquitination and interaction with Hsp90 of Her-2/neu protein induced by querecetin. Cells were treated with quercetin for 4 h and iron was added 30 min before or 30 min after quercetin treatment. In both cases, addition of exogenous iron prevented the ubiquitination of Her-2/neu and the interaction between Her-2/neu and Hsp90 (Fig. 4C). In contrast, treatment with the iron chelator DFX did not show any effect on ubiquitination of Her-2/neu and Hsp90 recruitment (Fig. 4D). From these results, although quercetin's iron chelating effect is not the main cause of Her-2/neu down-regulation, we can speculate that the moiety of quercetin which interacts with iron might be essential for Her-2/neu down-regulation.
Fig. 4.
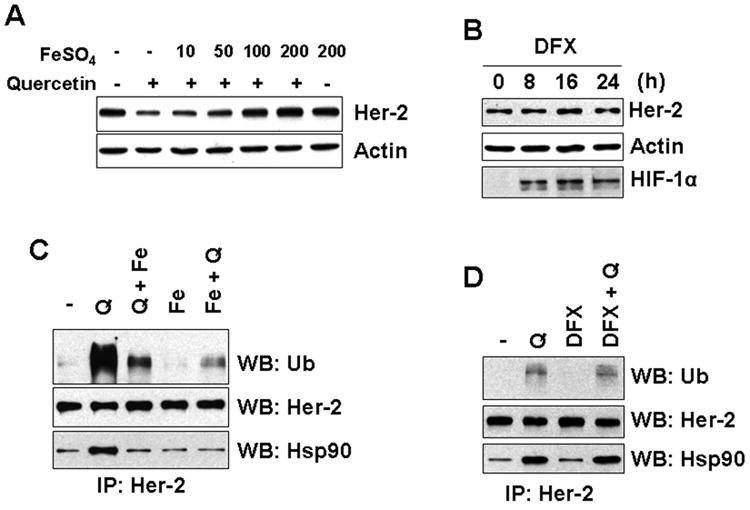
Iron chelation effect of quercetin is not involved in Her-2/neu down-regulation. A: SK-Br3 cells were pretreated with various amount of FeSO4 for 30 min and then 200 μM of quercetin was applied for additional 24 h. Cellular Her-2/neu protein level was compared by Western blot analysis. B: SK-Br3 cells were treated with 200 μM of desferrioxamine (DFX) for the indicated time. Cellular Her-2/neu protein level was compared by Western blot analysis. Accumulation of HIF-1α is shown as confirmation of the biological effect of DFX. C: SK-Br3 cells were treated with 200 μM of quercetin or 200 μM of FeSO4 for 4 h. For the samples with combined treatment, FeSO4 was applied 30 min before (Fe + Q) or after (Q + Fe) the treatment with quercetin. D: SK-Br3 cells were treated with quercetin (200 μM), DFX (200 μM), or both for 4 h. After Her-2/neu protein was immunoprecipitated, ubiquitination level and interacting amount of Hsp90 was compared by Western blot analysis.
Role of Hsp90 in Her-2/neu ubiquitination and down-regulation by Quercetin
Hsp90 inhibitor GA is known to exert its destabilizing effects by dissociating Her-2/neu from Hsp90-containing multiprotein complexes, thereby targeting Her-2/neu for proteolytic degradation [Xu et al., 2001]. To examine the biological meaning of the increased association of Hsp90 to Her-2/neu protein in quercetin-mediated down-regulation, different amounts of GA was treated in combination with quercetin. As shown in Fig. 5A, pretreatment with quercetin facilitated the effect of GA on Her-2/neu down-regulation. GA after pretreatment with quercetin still caused Hsp90 dissociation and also increased ubiquitination of Her-2/neu, resulting in enhanced degradation of Her-2/neu protein (Fig. 5B). It is also known that the chaperone activity of Hsp90 requires ATP and that lowering ATP concentration triggers dissociation of Hsp90 from Her-2/neu and promotes degradation of Her-2/neu protein [Peng et al., 2005]. In combination with Quercetin, when the chaperone function of Hsp90 was perturbed by removing glucose from the medium (Fig. 5C) or by the addition of 2-deoxyglucose (2-DG), a non-metabolizable glucose analog (Fig. 5D), Hsp90 dissociated from Her-2/neu protein complex and rapid induction of ubiquitination of Her-2/neu was observed. These results were coinciding with the effect of the pharmacological Hsp90 inhibitor GA. The dual function of quercetin in the regulation of Her-2/neu protein discussed later.
Fig. 5.
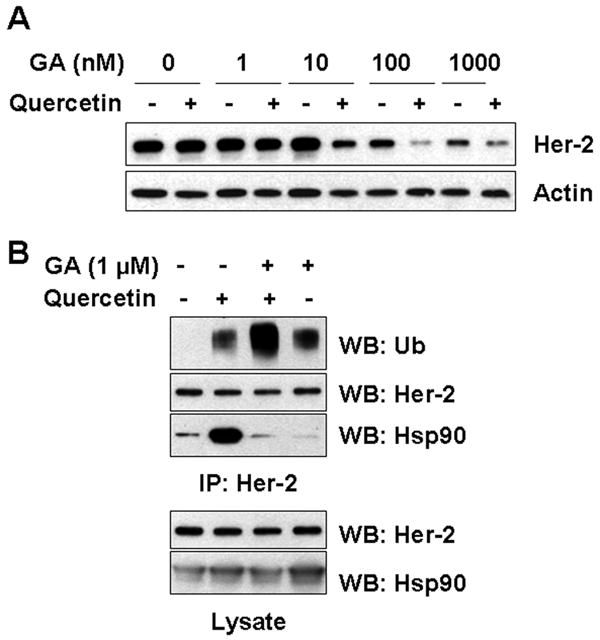
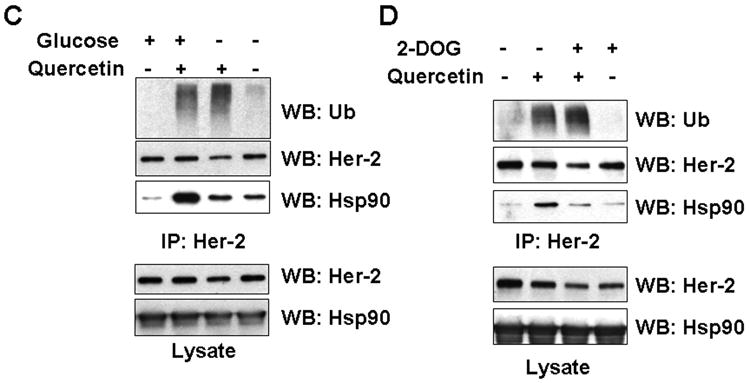
Effect of Hsp90 inhibition on Her-2/neu protein level and ubiquitination during treatment with quercetin. A: SK-Br3 cells were pretreated with 200 μM of quercetin for 30 min, and then indicated amount of specific Hsp90 inhibitor, GA, was added for additional 4 h. Cellular levels of Her-2/neu protein were compared from whole cell lysate. B: GA was applied in combination with 200 μM of quercetin for 1.5 h. After Her-2/neu proteins were immunoprecipitated, ubiquitination and Hsp90 level was compared by Western blot analysis. In order to inhibit Hsp90 activity, intracellular ATP level was reduced by glucose deprivation for 4 h (C) or by addition of non-metabolizable glucose analog, 2-deoxyglucose (5 mM), in the culture medium for an h in advance (D). After treatment of quercetin (200 μM) for additional 4 h, Her-2/neu protein was immunoprecipitated and then ubiquitination and Hsp90 level was compared by Western blot analysis.
CHIP participates in quercetin-induced ubiquitination of Her-2/neu
CHIP, the carboxyl terminus of Hsc70-interacting protein, a chaperone-dependent E3 ubiquitin ligase, plays a role in both GA-enhanced and steady-state ubiquitination of Her-2/neu protein [Xu et al., 2002]. We hypothesized that CHIP serves as a ubiquitin ligase during quercetin-induced ubiquitination of Her-2/neu protein. To test the hypothesis, we first investigated whether quercetin treatment induces association between Her-2/neu and CHIP. COS7 cells were cotransfected with pCMV-Her-2/neu and pcDNA3.1-HIS-CHIP. Cells were treated with quercetin for 4 h and cell lysates were immunoprecipitated with anti-Her-2/neu antibody, and then the coimmunoprecipitated CHIP amount was determined by Western blot. As shown in Fig. 6A, quercetin treatment markedly increased the interaction between Her-2/neu and CHIP. We next examined the effect of knock-down of CHIP expression on the quercetin-induced ubiquitination of Her-2/neu. COS7 cells were cotransfected with pCMV-Her-2/neu plasmid and siRNA targeting CHIP and then ubiquitination of Her-2/neu was analyzed after immunoprecipitation. Knock-down of CHIP inhibited quercetin-induced ubiquitination of Her-2/neu as well as the basal level of ubiquitination (Fig. 6B). These results suggest that quercetin-enhanced ubiquitination of Her-2/neu is mediated through an increase in binding activity of CHIP.
Fig. 6.
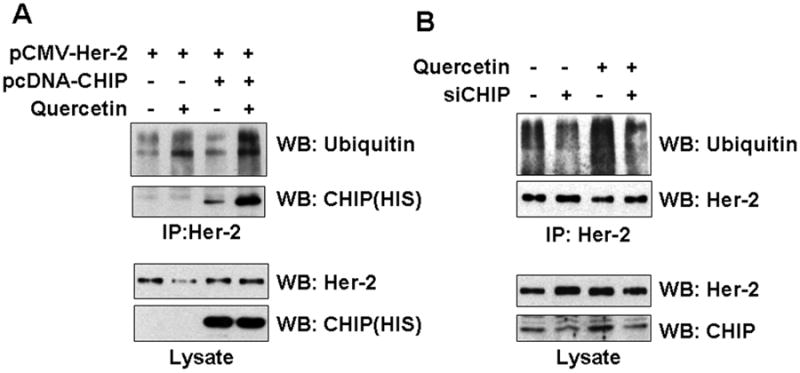
CHIP is involved in quercetin-induced ubiquitination of Her-2/neu. A: COS7 cells were cotransfected with pCMV-Her-2/neu and pcDNA-HIS-CHIP. After 24 h, cells were either untreated or treated with quercetin for 4 h. Her-2/neu protein was immunoprecipitated and then ubiquitination of Her-2/neu and the level of interaction between Her-2/neu and CHIP were compared. B: COS7 cells were cotransfected with pCMV-Her-2/neu and CHIP siRNA. After 24 h, cells were either untreated or treated with quercetin for 4 h. Her-2/neu protein was immunoprecipitated and then ubiquitination level was compared.
Quercetin inhibited Her-2/neu tyrosine kinase activity
We hypothesized that quercetin might interact with Her-2/neu protein and cause inhibition of receptor function and/or structural destabilization, which eventually lead to deagradation of Her-2/neu. To verify this possibility, we first examined the tyrosine phosphorylation level of Her-2/neu during quercetin treatment. As shown in Fig. 7A, quercetin treatment decreased the tyrosine phosphorylation level of endogeneous Her-2/neu, which might be caused by inhibition of Her-2/neu tyrosine kinase activity. To check this possibility, in vitro assays were performed using immunoprecipitated Her-2/neu protein. As shown in Fig. 7B, in vitro IP-kinase assay results showed that 2 μM of quercetin was enough to inhibit autophosphorylation activity of Her-2/neu completely. Trans-tyrosine kinase activity of Her-2/neu was also measured using a synthetic peptide as a tyrosine kinase substrate (Fig. 7C). In the presence of quercetin, trans-tyrosine kinase activity was reduced to 20% compared to the control. These results suggest that quercetin can bind to Her-2/neu protein and inhibit the tyrosine kinase function, which might cause an alteration of Her-2/neu protein structure and eventually lead to degradation.
Fig. 7.
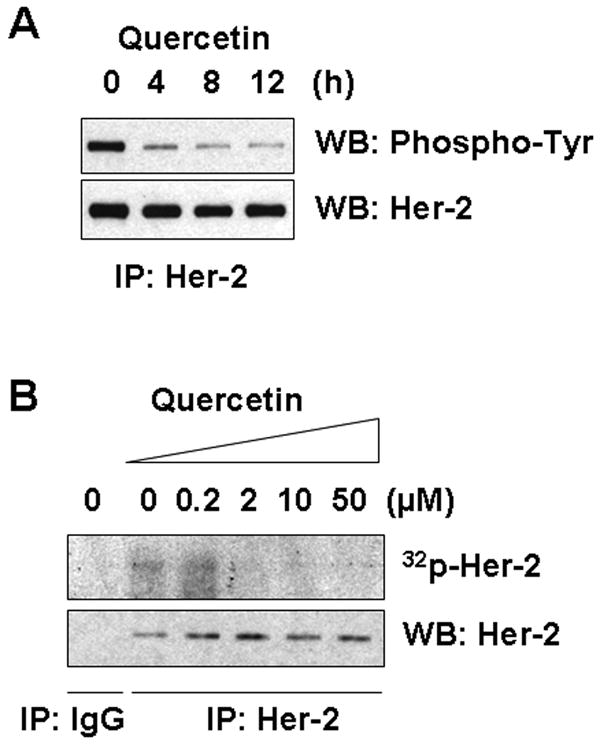
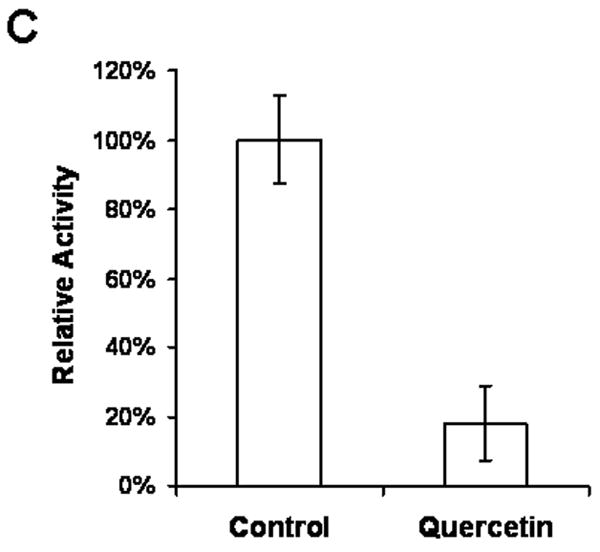
Quercetin inhibited Her-2/neu tyrosine kinase activity. A: SK-Br3 cells were treated with 200 μM of quercetin for the indicated time. Her-2/neu protein was immunoprecipitated and then tyrosine phosphorylation was detected with anti-phospho-tyrosine monoclonal antibody. B: Her-2/neu protein was isolated by immunoprecipitation and then autophosphorylation activity was compared by incubating with [γ-32p]ATP in the presence of different amounts of quercetin. C: Her-2/neu protein was isolated by immunoprecipitation and then tyrosine kinase activity was measured in the presence of DMSO or 200 μM of quercetin using a synthetic peptide as a substrate.
Quercetin enhanced adriamycin-induced cell death
Adriamycin is a DNA-interacting drug widely used in chemotherapy of breast cancer patient. In order to examine the biological effect of down regulation of Her-2/neu protein mediated by quercetin, adriamycin was applied to SK-Br3 cells in combination with 100 μM of quercetin. After 2 days of incubation, cell viability was determined by the trypan blue exclusion assay. As shown in Fig. 8, combined treatment of adriamycin with quercetin enhanced SK-Br3 cell death synergistically. Adriamycin alone induced cell death in a concentration-dependent manner; 79% and 63% of the cells survived after exposure to 10 and 15 μM of adriamycin, respectively. When adriamycin were applied in combination with quercetin, the viability of cells was decreased to 63% and 42%, respectively. This result suggested that quercetin could enhance the chemotherapeutic efficacy of adriamycin by down-regulating Her-2/neu.
Fig. 8.
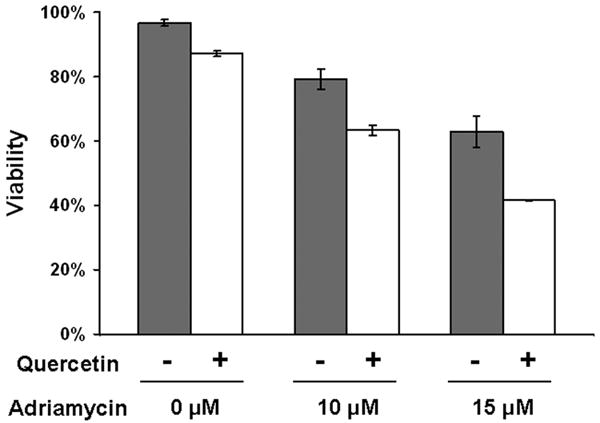
Quercetin enhanced adriamycin-induced cell death. SK-Br3 cells were treated with 100 μM of quercetin in combination with indicated amount of adriamycin for 2 days. Cell viability was determined by the trypan blue exclusion assay. Error bars represent standard error from the mean (SEM) for three separate experiments.
Discussion
The major observation reported here is that the natural product quercetin effectively down-regulates Her-2/neu protein in Her-2/neu overexpressing human breast cancer SK-Br3 cells. Quercetin inhibits tyrosine kinase activity of Her-2/neu and induces poly-ubiquitination by recruiting the E3-ubiquitin ligase CHIP. The ubiquitinated Her-2/neu protein is then degraded by the proteasome; this is shown when the inhibition of protein degradation by MG-132 leads to accumulation of the NP-40 insoluble form of Her-2/neu (Fig. 3A). Other kinases such as Akt and Raf-1 are reported to show a similar accumulation of their insoluble form when their proteasome degradation pathway is blocked [Basso et al., 2002; Schulte et al., 1997]. Unlike proteasome inhibitors, lysosomal inhibitors such as NH4Cl and chloroquine, which alkalinizes the lysosome, fail to prevent quercetin-induced Her-2/neu down-regulation (Fig. 3A, data not shown). Although Her-2/neu is known as a target of caspases [Tikhomirov and Carpenter, 2001], pan-caspase inhibitor z-VAD-FMK cannot prevent Her-2/neu protein down-regulation during treatment with quercetin (Data not shown).
Several researchers reported that the Hsp90 inhibitor GA promotes Her-2/neu ubiquitination concomitant with dissociation of Hsp90 from client proteins [Xu et al., 2001]. Unlike GA, quercetin induces ubiquitination of Her-2/neu as well as an increase in interaction between Hsp90 and Her-2/neu. We speculate that this increase in interaction between Hsp90 and Her-2/neu may result in preventing immediate proteolytic degradation of the ubiquitinated form of Her-2/neu. This perspective is supported by the observations that the combined treatment of specific Hsp90 inhibitor GA and quercetin synergistically enhances the ubiquitination (Fig. 5B) and down-regulation (Fig. 5A) of Her-2/neu. Alternatively, lowering the intracellular ATP level prevents Hsp90 recruitment and rapidly enhances ubiquitination of Her-2/neu protein during treatment with quercetin (Figs. 5C, D). Quercetin may directly bind to Her-2/neu, probably at an ATP-binding site in the kinase domain, and induce a structural modification of Her-2/neu. This postulation is supported by the observations that quercetin treatment inhibits Her-2/neu tyrosine kinase activity (Figs. 7A-C). As this quercetin-bound form of Her-2/neu is unstable, it can be speculated that this form is maintained either by recruiting more chaperone Hsp90 or preventing release from Hsp90. Another possibility is that quercetin may act on Hsp90 chaperone activity by affecting interaction with co-chaperones. We are currently in the process of investigating these possibilities.
Recently it was reported that the chaperone-dependent E3 ubiquitin ligase CHIP is involved in GA-induced Her-2/neu degradation [Peng et al., 2005]. GA promotes CHIP association with Her-2/neu and subsequently induces ubiquitination and degradation via the proteasome. These observations suggest that pharmacological enhancement of the CHIP–Her-2/neu interaction may be therapeutically useful for down-regulating Her-2/neu activity. Quercetin also enhances the interaction between Her-2/neu and CHIP (Fig. 6A) and plays a major role in ubiquitination of Her-2/neu both in normal status and quercetin-treated conditions (Fig. 6B). Nonetheless, previous studies have shown that CHIP is not the only E3 ubiquitin ligase capable of mediating GA-induced down-regulation of Her-2/neu [Peng et al., 2005]. Thus, involvement of other E3 ligases in the degradation of Her-2/neu needs to be further studied.
Besides quercetin, several other natural products are known to have the effect of down-regulating Her-2/neu protein. Among plant flavonoids, apigenin has a potent growth-inhibitory effect and induces apoptosis in Her-2/neu overexpressing breast cancer cells. Apigenin dissociates Her-2/neu from GRP94 and produces reduction of the intracellular level of Her-2/neu through the ubiquitin-proteasome dependent protein degradation pathway [Way et al., 2004]. Curcumin, a major pigment extracted from the plant Curcuma longa Lin, covalently modifies Her-2/neu and induces CHIP-dependent ubiquitination, but curcumin induces Her-2/neu depletion other than by the proteasomal degradation pathway [Jung et al., 2007]. Oleic acid (OA; 18:1n-9), the main monounsaturated fatty acid of olive oil, is known to suppress the overexpression of Her-2/neu. In contrast to quercetin, OA exposure specifically represses the transcriptional activity of the Her-2/neu gene promoter; this is due to the up-regulation of the Ets protein PEA3 (a transcriptional repressor of the Her-2/neu gene promoter) by OA [Menendez et al., 2005]. Compared to these other natural compounds, quercetin induces Her-2/neu down-regulation in a unique manner.
In the treatment of Her-2/neu overexpressing breast cancers, there are two major approaches to targeting Her-2/neu: down-regulation of Her-2/neu protein level using ectodomain-binding antibodies (mAbs) and inhibition of tyrosine kinase activity by small molecule inhibitors that compete with ATP in the tyrosine kinase domain [Imai and Takaoka, 2006]. In this report, we revealed that quercetin inhibits Her-2/neu tyrosine kinase activity (Figs. 7A-C) and down-regulates Her-2/neu protein level (Fig. 1). It is interesting that the manner in which quercetin down-regulates Her-2/neu protein is very similar to that of the CI-1033 tyrosine kinase inhibitor [Citri et al., 2002]. Along with inhibition of tyrosine phosphorylation, CI-1033 enhances ubiquitylation and accelerates endocytosis and subsequent intracellular degradation of Her-2/neu protein. The degradative pathway stimulated by tyrosine kinase inhibitors appears to be chaperone mediated. Similar to quercetin and GA, CI-1033 and GA additively inhibit tumor cell growth [Citri et al., 2002]. We believe that, as shown in the study of CI-1033, an understanding of the dual inhibitory function of quercetin advances clinical application, allowing effective therapeutic and preventive studies to be initiated.
Acknowledgments
Grant sponsor: NCI; Grant numbers: CA95191, CA96989, CA121395; Grant sponsor: DOD prostate program funds; Grant numbers: PC020530, PC040833; Grant sponsor: Susan G. Komen Breast Cancer Foundation fund; Grant number: BCTR60306.
Abbreviations used
- GA
Geldanamycin
- PI3K
phosphatidylinositol 3-kinase
- DFX
desferrioxamine
- RTK
receptor tyrosine kinase
- CHIP
carboxyl terminus of Hsc70-interacting protein
- EGFR
epidermal growth factor receptor
- DMEM
Dulbecco's modified Eagle's medium
- PBS
phosphate-buffered saline
- FITC
fluorescein isothiocyanate
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- DTT
dithiothreitol
- EDTA
ethylenediaminetetraacetate
- HRP
horse radish peroxidase
References
- Baselga J, Albanell J, Molina MA, Arribas J. Mechanism of action of trastuzumab and scientific update. Semin Oncol. 2001;28:4–11. doi: 10.1016/s0093-7754(01)90276-3. [DOI] [PubMed] [Google Scholar]
- Baselga J, Tripathy D, Mendelsohn J, Baughman S, Benz CC, Dantis L, Sklarin NT, Seidman AD, Hudis CA, Moore J, Rosen PP, Twaddell T, Henderson IC, Norton L. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J Clin Oncol. 1996;14:737–44. doi: 10.1200/JCO.1996.14.3.737. [DOI] [PubMed] [Google Scholar]
- Basso AD, Solit DB, Chiosis G, Giri B, Tsichlis P, Rosen N. Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 and is destabilized by inhibitors of Hsp90 function. J Biol Chem. 2002;277:39858–66. doi: 10.1074/jbc.M206322200. [DOI] [PubMed] [Google Scholar]
- Block G, Patterson B, Subar A. Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutr Cancer. 1992;18:1–29. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- Bors W, Michel C, Saran M. Flavonoid antioxidants: rate constants for reactions with oxygen radicals. Methods Enzymol. 1994;234:420–9. doi: 10.1016/0076-6879(94)34112-5. [DOI] [PubMed] [Google Scholar]
- Citri A, Alroy I, Lavi S, Rubin C, Xu W, Grammatikakis N, Patterson C, Neckers L, Fry DW, Yarden Y. Drug-induced ubiquitylation and degradation of ErbB receptor tyrosine kinases: implications for cancer therapy. Embo J. 2002;21:2407–17. doi: 10.1093/emboj/21.10.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone SA, Zhao YY, Fan L, Gu Y, Minamisawa S, Liu Y, Peterson KL, Chen J, Kahn R, Condorelli G, Ross J, Jr, Chien KR, Lee KF. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med. 2002;8:459–65. doi: 10.1038/nm0502-459. [DOI] [PubMed] [Google Scholar]
- da Silva EL, Piskula MK, Yamamoto N, Moon JH, Terao J. Quercetin metabolites inhibit copper ion-induced lipid peroxidation in rat plasma. FEBS Lett. 1998;430:405–8. doi: 10.1016/s0014-5793(98)00709-1. [DOI] [PubMed] [Google Scholar]
- Drebin JA, Link VC, Weinberg RA, Greene MI. Inhibition of tumor growth by a monoclonal antibody reactive with an oncogene-encoded tumor antigen. Proc Natl Acad Sci U S A. 1986;83:9129–33. doi: 10.1073/pnas.83.23.9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh H, Nguyen TT, Chan E, Tran E. Inhibition of ErbB-2 and ErbB-3 expression by quercetin prevents transforming growth factor alpha (TGF-alpha)- and epidermal growth factor (EGF)-induced human PC-3 prostate cancer cell proliferation. Int J Oncol. 2003;23:821–9. [PubMed] [Google Scholar]
- Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–54. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- Imai K, Takaoka A. Comparing antibody and small-molecule therapies for cancer. Nat Rev Cancer. 2006;6:714–27. doi: 10.1038/nrc1913. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- Jeon H, Kim H, Choi D, Kim D, Park SY, Kim YJ, Kim YM, Jung Y. Quercetin activates an angiogenic pathway, hypoxia inducible factor (HIF)-1-vascular endothelial growth factor, by inhibiting HIF-prolyl hydroxylase: a structural analysis of quercetin for inhibiting HIF-prolyl hydroxylase. Mol Pharmacol. 2007;71:1676–84. doi: 10.1124/mol.107.034041. [DOI] [PubMed] [Google Scholar]
- Jung Y, Xu W, Kim H, Ha N, Neckers L. Curcumin-induced degradation of ErbB2: A role for the E3 ubiquitin ligase CHIP and the Michael reaction acceptor activity of curcumin. Biochim Biophys Acta. 2007;1773:383–90. doi: 10.1016/j.bbamcr.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Kandaswami C, Middleton E., Jr Free radical scavenging and antioxidant activity of plant flavonoids. Adv Exp Med Biol. 1994;366:351–76. doi: 10.1007/978-1-4615-1833-4_25. [DOI] [PubMed] [Google Scholar]
- Katsumata M, Okudaira T, Samanta A, Clark DP, Drebin JA, Jolicoeur P, Greene MI. Prevention of breast tumour development in vivo by downregulation of the p185neu receptor. Nat Med. 1995;1:644–8. doi: 10.1038/nm0795-644. [DOI] [PubMed] [Google Scholar]
- Kim WK, Bang MH, Kim ES, Kang NE, Jung KC, Cho HJ, Park JH. Quercetin decreases the expression of ErbB2 and ErbB3 proteins in HT-29 human colon cancer cells. J Nutr Biochem. 2005;16:155–62. doi: 10.1016/j.jnutbio.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Kim YH, Lee YJ. TRAIL apoptosis is enhanced by quercetin through Akt dephosphorylation. J Cell Biochem. 2007;100:998–1009. doi: 10.1002/jcb.21098. [DOI] [PubMed] [Google Scholar]
- Lee KF, Simon H, Chen H, Bates B, Hung MC, Hauser C. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature. 1995;378:394–8. doi: 10.1038/378394a0. [DOI] [PubMed] [Google Scholar]
- Menendez JA, Vellon L, Colomer R, Lupu R. Effect of gamma-linolenic acid on the transcriptional activity of the Her-2/neu (erbB-2) oncogene. J Natl Cancer Inst. 2005;97:1611–5. doi: 10.1093/jnci/dji343. [DOI] [PubMed] [Google Scholar]
- Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378:386–90. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- Middleton E, Jr, Kandaswami C. Effects of flavonoids on immune and inflammatory cell functions. Biochem Pharmacol. 1992;43:1167–79. doi: 10.1016/0006-2952(92)90489-6. [DOI] [PubMed] [Google Scholar]
- Mimnaugh EG, Chavany C, Neckers L. Polyubiquitination and proteasomal degradation of the p185c-erbB-2 receptor protein-tyrosine kinase induced by geldanamycin. J Biol Chem. 1996;271:22796–801. doi: 10.1074/jbc.271.37.22796. [DOI] [PubMed] [Google Scholar]
- Morel I, Lescoat G, Cillard P, Cillard J. Role of flavonoids and iron chelation in antioxidant action. Methods Enzymol. 1994;234:437–43. doi: 10.1016/0076-6879(94)34114-1. [DOI] [PubMed] [Google Scholar]
- Nahta R, Esteva FJ. HER-2-targeted therapy: lessons learned and future directions. Clin Cancer Res. 2003;9:5078–84. [PubMed] [Google Scholar]
- Neckers L, Schulte TW, Mimnaugh E. Geldanamycin as a potential anti-cancer agent: its molecular target and biochemical activity. Invest New Drugs. 1999;17:361–73. doi: 10.1023/a:1006382320697. [DOI] [PubMed] [Google Scholar]
- Niehans GA, Singleton TP, Dykoski D, Kiang DT. Stability of HER-2/neu expression over time and at multiple metastatic sites. J Natl Cancer Inst. 1993;85:1230–5. doi: 10.1093/jnci/85.15.1230. [DOI] [PubMed] [Google Scholar]
- Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. Embo J. 2000;19:3159–67. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcelik C, Erdmann B, Pilz B, Wettschureck N, Britsch S, Hubner N, Chien KR, Birchmeier C, Garratt AN. Conditional mutation of the ErbB2 (HER2) receptor in cardiomyocytes leads to dilated cardiomyopathy. Proc Natl Acad Sci U S A. 2002;99:8880–5. doi: 10.1073/pnas.122249299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Guo X, Borkan SC, Bharti A, Kuramochi Y, Calderwood S, Sawyer DB. Heat shock protein 90 stabilization of ErbB2 expression is disrupted by ATP depletion in myocytes. J Biol Chem. 2005;280:13148–52. doi: 10.1074/jbc.M410838200. [DOI] [PubMed] [Google Scholar]
- Schulte TW, An WG, Neckers LM. Geldanamycin-induced destabilization of Raf-1 involves the proteasome. Biochem Biophys Res Commun. 1997;239:655–9. doi: 10.1006/bbrc.1997.7527. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–12. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- Sliwkowski MX, Lofgren JA, Lewis GD, Hotaling TE, Fendly BM, Fox JA. Nonclinical studies addressing the mechanism of action of trastuzumab (Herceptin) Semin Oncol. 1999;26:60–70. [PubMed] [Google Scholar]
- Tikhomirov O, Carpenter G. Caspase-dependent cleavage of ErbB-2 by geldanamycin and staurosporin. J Biol Chem. 2001;276:33675–80. doi: 10.1074/jbc.M101394200. [DOI] [PubMed] [Google Scholar]
- Triantafyllou A, Liakos P, Tsakalof A, Chachami G, Paraskeva E, Molyvdas PA, Georgatsou E, Simos G, Bonanou S. The flavonoid quercetin induces hypoxia-inducible factor-1alpha (HIF-1alpha) and inhibits cell proliferation by depleting intracellular iron. Free Radic Res. 2007;41:342–56. doi: 10.1080/10715760601055324. [DOI] [PubMed] [Google Scholar]
- Verma AK, Johnson JA, Gould MN, Tanner MA. Inhibition of 7,12-dimethylbenz(a)anthracene- and N-nitrosomethylurea-induced rat mammary cancer by dietary flavonol quercetin. Cancer Res. 1988;48:5754–8. [PubMed] [Google Scholar]
- Vulcain E, Goupy P, Caris-Veyrat C, Dangles O. Inhibition of the metmyoglobin-induced peroxidation of linoleic acid by dietary antioxidants: Action in the aqueous vs. lipid phase. Free Radic Res. 2005;39:547–63. doi: 10.1080/10715760500073865. [DOI] [PubMed] [Google Scholar]
- Wang HK. The therapeutic potential of flavonoids. Expert Opin Investig Drugs. 2000;9:2103–19. doi: 10.1517/13543784.9.9.2103. [DOI] [PubMed] [Google Scholar]
- Way TD, Kao MC, Lin JK. Apigenin induces apoptosis through proteasomal degradation of HER2/neu in HER2/neu-overexpressing breast cancer cells via the phosphatidylinositol 3-kinase/Akt-dependent pathway. J Biol Chem. 2004;279:4479–89. doi: 10.1074/jbc.M305529200. [DOI] [PubMed] [Google Scholar]
- Xu W, Marcu M, Yuan X, Mimnaugh E, Patterson C, Neckers L. Chaperone-dependent E3 ubiquitin ligase CHIP mediates a degradative pathway for c-ErbB2/Neu. Proc Natl Acad Sci U S A. 2002;99:12847–52. doi: 10.1073/pnas.202365899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Mimnaugh E, Rosser MF, Nicchitta C, Marcu M, Yarden Y, Neckers L. Sensitivity of mature Erbb2 to geldanamycin is conferred by its kinase domain and is mediated by the chaperone protein Hsp90. J Biol Chem. 2001;276:3702–8. doi: 10.1074/jbc.M006864200. [DOI] [PubMed] [Google Scholar]
- Yogev-Falach M, Bar-Am O, Amit T, Weinreb O, Youdim MB. A multifunctional, neuroprotective drug, ladostigil (TV3326), regulates holo-APP translation and processing. Faseb J. 2006;20:2177–9. doi: 10.1096/fj.05-4910fje. [DOI] [PubMed] [Google Scholar]


