Abstract
Increased activity alleles (rscS1 and rscS2) of the symbiosis regulator RscS induced both syp transcription and biofilm formation in Vibrio fischeri. Neither allele encodes a protein variant; instead they carry mutations near the putative ribosome binding site (RBS) and, in the case of rscS1, an additional silent mutation at codon Leu25. In this study, we found that endogenous levels of RscS are very low under the culture conditions examined and that the increased activity alleles dramatically increased the levels of protein. Of the two mutations present in rscS1, the Leu25 mutation, which replaces a rare with a more common Leu codon, appeared to make the greater contribution to increased activity. Our results suggest that RscS levels are maintained at low levels in the cell by the presence of a weak promoter, possible inefficient ribosome binding and the presence of rare codons in the 5′ end of the gene. Restriction of RscS levels may be important to prevent spurious signaling by this sensor kinase in the absence of a squid host.
Keywords: Sensor kinase, histidine kinase, RscS, polysaccharide, symbiosis
Introduction
Two component regulatory systems permit bacteria to rapidly sense and respond to changes in their environment [reviewed in (Stock et al., 2000)]. Thus, they are commonly employed during bacterial colonization of a host, whether the ultimate outcome of colonization is symbiosis or pathogenesis. One example of a symbiotic association that utilizes two-component regulators during colonization is that between the marine bioluminescent bacterium Vibrio fischeri and the Hawaiian bobtail squid Euprymna scolopes [reviewed in (Nyholm and McFall-Ngai, 2004; Stabb, 2006; Visick and Ruby, 2006)]. Newly hatched squid are aposymbiotic and must acquire their bacterial symbionts from their seawater environment. Symbiotic initiation by V. fischeri requires the orphan sensor kinase RscS: a strain in which rscS has been disrupted fails to colonize squid, or does so only after a severe delay (Visick and Skoufos, 2001). In contrast, colonization is substantially enhanced when RscS is overexpressed (Yip et al., 2006). These data support the importance of this two component regulator in symbiotic colonization.
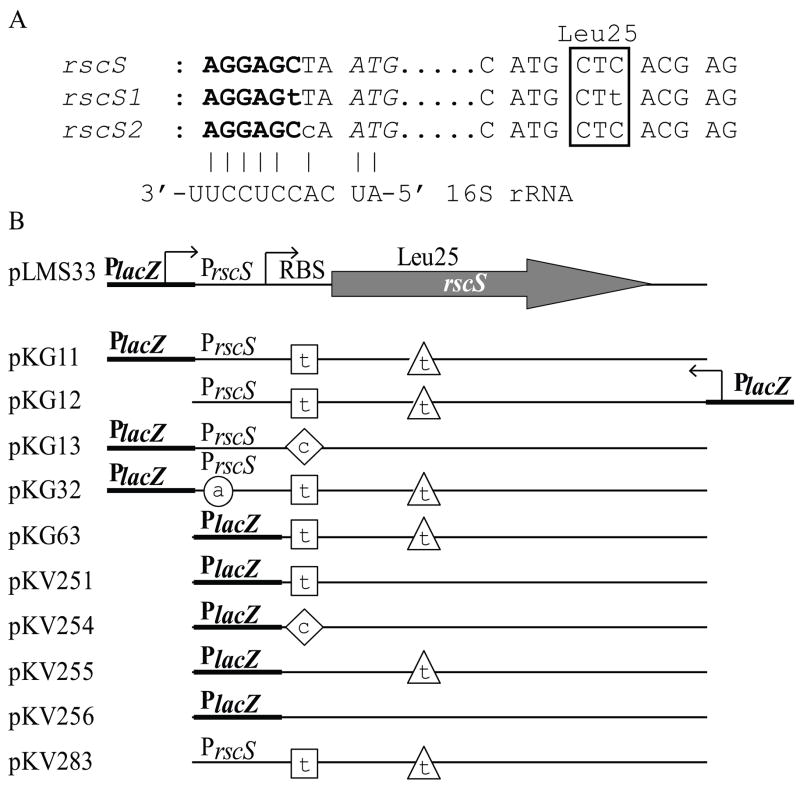
Our recent efforts to understand the role of RscS in symbiotic colonization led to the discovery that RscS regulates transcription of the symbiosis polysaccharide (syp) cluster, a group of 18 genes also required for initiation of the symbiosis (Yip et al., 2006). However, this regulation was not uncovered until we isolated two increased activity alleles of rscS [rscS1 and rscS2 (Yip et al., 2006)]. Plasmids bearing either of these two alleles (pKG11 and pKG13, respectively) dramatically increased syp transcription and induced a number of biofilm-associated phenotypes. Specifically, pKG11 induced wrinkled colony morphology and pellicle formation, two phenotypes not observed for the wild-type and vector control strains. pKG13 also induced wrinkled colony morphology, although to a lesser extent than pKG11 (Geszvain and Visick, unpublished data). Surprisingly, we found that both alleles encoded wild type RscS protein, but contained a mutation within or adjacent to the putative ribosome binding site (RBS, Fig. 1A). Additionally, pKG11 carried a silent mutation within the codon for Leu25 (Fig. 1A). The lack of a protein change suggested that these alleles increased activity by increasing levels of RscS protein, either through increasing the levels of transcript or increasing the efficiency of translation of the rscS transcript. However, semi-quantitative RT-PCR showed no detectable difference in rscS transcript steady state levels in cells carrying pKG11 (Yip et al., 2006). Here, we test the hypothesis that the mutations in pKG11 and/or pKG13 caused an increase in the levels of RscS protein and dissect the relative importance of the two mutations present in pKG11.
Figure 1.
Plasmids and mutations described in this study. A. Mutations present in rscS1 and rscS2. The putative ribosome binding site (RBS), is shown in bold. The start codon is in italics while the 25th codon, encoding Leu, is boxed. Also shown is the 3′ end of the 16S rRNA of V. fischeri (http://www.ergo-light.com/ERGO/) aligned to the putative RBS. B. A linear representation of the plasmids used in this study. The location of mutations is indicated by a square for the RBS mutation present in rscS1, a triangle for the Leu25 mutation, a diamond for the RBS change present in rscS2 and a circle for the promoter mutation present in pKG32.
Materials and Methods
Media
E. coli strains were grown in LB. V. fischeri strains were grown in the rich medium LBS (Graf et al., 1994; Stabb et al., 2001), or the minimal medium HMM-CAA-glu (Yip et al., 2005). The following antibiotics were added, as needed, to the final concentrations indicated: chloramphenicol (Cm), 5 μg ml−1; and tetracycline (Tet), 5 μg ml−1 in LBS, 30 μg ml−1 in HMM.
Strains
V. fischeri strain ES114 (Boettcher and Ruby, 1990) was the wild-type strain used in these experiments. Two derivatives of ES114 also were used. To identify the RscS protein, a strain which lacks the rscS gene was constructed: KV3378 (ES114 ΔrscS). This strain was generated via recombination with pKG9 (Table 1) as follows. We used conjugation to deliver the unstable plasmid pKG9 into V. fischeri strain ES114, using standard techniques (DeLoney et al., 2002; Visick and Skoufos, 2001). Following antibiotic selection for single recombinants in which pKG9 was integrated into the chromosome, we screened for cells that had undergone a second recombination event (identified by the loss of antibiotic resistance) to replace the wild-type copy of rscS with the deletion derivative (identified using PCR and confirmed by Southern analysis). To assess transcription of the syp locus, KV2566 (ES114 att Tn7::PsypA-lacZ) was constructed. To generate this strain, we introduced the suicide vector pEAH47 (Table 1) carrying a lacZ fusion to the sypA promoter into ES114 via a tetra-parental mating as described previously (O’Shea et al., 2006). Following selection for the insertion of Tn7 bearing the PsypA-lacZ reporter at the attTn7 site, we screened for the loss of antibiotic resistance markers associated with the delivery and helper plasmids. In addition to the V. fischeri strains, the following strains of Escherichia coli were used for the purposes of cloning and conjugation: DH5α (Woodcock et al., 1989), TOP10 F′ (Invitrogen, Carlsbad, CA) and Tam 1 (Active Motif, Carlsbad, CA).
Table 1.
Plasmids used in this study
| Plasmid | Description | Reference |
|---|---|---|
| pEAH47 | pEVS107 with 1.6 kb VFA1019 - sypA (VFA1020) intergenic region fused to lacZ | This work |
| pEVS107 | Delivery vector for Tn7::erm | (McCann et al., 2003) |
| pKG9 | pEVS79 (Stabb and Ruby, 2002) with the rscS locus from ~1 kb upstream to ~1 kb downstream of the gene but with an internal EcoRI/ClaI fragment carrying rscS removed | This work |
| pKG11 | pKV69 containing PrscS-rscS1 | (Yip et al., 2006) |
| pKG12 | pKV69 containing PrscS-rscS1, opposite orientation relative to PlacZ | This work |
| pKG13 | pKV69 containing PrscS-rscS2 | (Yip et al., 2006) |
| pKG32 | pKG11 with PrscS -35 element mutation | This work |
| pKG63 | pKV69 with rscS1 under control of PlacZ | This work |
| pKV69 | Mobilizable vector, CmR, TetR | (Visick and Skoufos, 2001) |
| pKV251 | pKV69 with RBS1 rscS under control of PlacZ | This work |
| pKV254 | pKV69 with rscS2 under control of PlacZ | This work |
| pKV255 | pKV69 with Leu25 rscS under control of PlacZ | This work |
| pKV256 | pKV69 with wild type rscS under control of PlacZ | This work |
| pKV283 | pKG11 deleted for the lac promoter | This work |
| pLMS33 | pKV69 containing rscS coding sequences plus 389 bp of sequence upstream of the ATG start codon, including PrscS- rscS (located 44–70 bp upstream of the ATG start codon) | (Visick and Skoufos, 2001) |
| Strains | Description | Reference |
| ES114 | V. fischeri wild type | (Boettcher and Ruby, 1990) |
| KV2566 | ES114 att Tn7::PsypA::lacZ | This work |
| KV3378 | ES114 ΔrscS | This work |
Plasmids
Plasmids used in this study are listed in Table 1. Most plasmids were constructed using standard molecular biology techniques with restriction enzymes or polymerase chain reaction. To facilitate subcloning, a silent Xho I site was incorporated into rscS at L385 in both the pLMS33 and pKG11 plasmids via PCR amplification. To separate the two mutations present in rscS1 as well as remove the PrscS promoter, the rscS gene was amplified from either pLMS33-Xho I or pKG11-Xho I using primers specific for the RBS region (rscS-RBS-F, ggatccTTTATAGGAGCTAATGCAATGAAA; rscS1-RBS-F, ggatccTTTATAGGAGtTAATGCAATGAAA; or rscS2-RBS-F, ggatccTTTATAGGAGCcAATGCAATGAAA) (Where the letters in bold denote the putative RBS) and the M13-forward primer, which annealed to the opposite strand downstream of the gene. The resulting PCR product was cloned into pCR2.1-TOPO (Invitrogen, Carlsbad, CA) and subsequently subcloned into pKV69.
Generation of anti-RscS antibody
To generate anti-RscS antibody, we first identified a 15 amino acid peptide near the carboxy terminus of RscS (C797-ELKRPSKYKLSDDA-810) that is predicted to be antigenic using the following Website: http://bio.dfci.harvard.edu/Tools/antigenic.pl. A search of the V. fischeri ES114 genome database at Integrated Genomics [http://www.ergo-light.com/ERGO/] revealed no significant matches, suggesting that this sequence is unique to RscS and thus would be specific for this protein. This peptide was generated and used to inject rabbits (Sigma Genosys, St. Louis, MO); the resulting anti-serum was subsequently purified with an affinity column (Alpha Diagnostic International, San Antonio, TX).
Western immunoblot analysis
Cells were grown in HMM-CAA-Glu with Tet at 22°C overnight. Lysates were separated on 8% SDS-PAGE, transferred to PVDF membrane and probed with 1:500 dilution of affinity purified rabbit anti-RscS. Bands were visualized using a horseradish peroxidase-conjugated donkey anti-rabbit secondary antibody and ECL reagents. A Lowry assay was performed to ensure equal loading (Lowry et al., 1951).
β-galactosidase assay
Cultures of the reporter strain KV2566 (PsypA::lacZ) carrying rscS or vector control plasmids were grown in LBS at 22°C for 6 – 48 hrs. Samples were concentrated and cells were lysed using chloroform. syp transcription then was assessed by a β-galactosidase assay (Miller, 1972) and standardized to the amount of protein as determined by a Lowry assay (Lowry et al., 1951).
Results
The RscS protein naturally exists in limited quantities
The lack of protein substitutions present in the increased activity alleles rscS1 and rscS2 suggested that the mutations present in these alleles increased protein levels. However, strains bearing plasmids carrying these alleles (pKG11 and pKG13) did not produce sufficient amounts of RscS protein to detect by Coomassie staining (data not shown), and thus a more sensitive technique such as western immunoblotting was required to analyze their levels. We therefore generated an anti-RscS antibody as described in Materials and methods. To evaluate the specificity of the purified antibodies, we performed a western blot experiment using extracts from V. fischeri carrying pKG11. The RscS antibody detected three species in this lysate, with the upper band (Fig. 2, arrow) roughly the correct size for RscS (predicted to be 106 kDa). To determine which of these bands represents RscS, we generated a V. fischeri strain in which rscS had been deleted (see Materials and methods) and introduced empty vector (pKV69) in order to treat the cells with the same antibiotics as those carrying rscS plasmids. We subsequently probed lysate derived from the ΔrscS – pKV69 strain with the RscS antibody. The middle of the three bands was still present in the rscS deletion, and thus we conclude that it represents a cross-reactive species (Fig. 2). However, both the top and the bottom bands were specific to RscS-containing strains. The faster migrating species may represent a proteolytic fragment of RscS.
Figure 2.
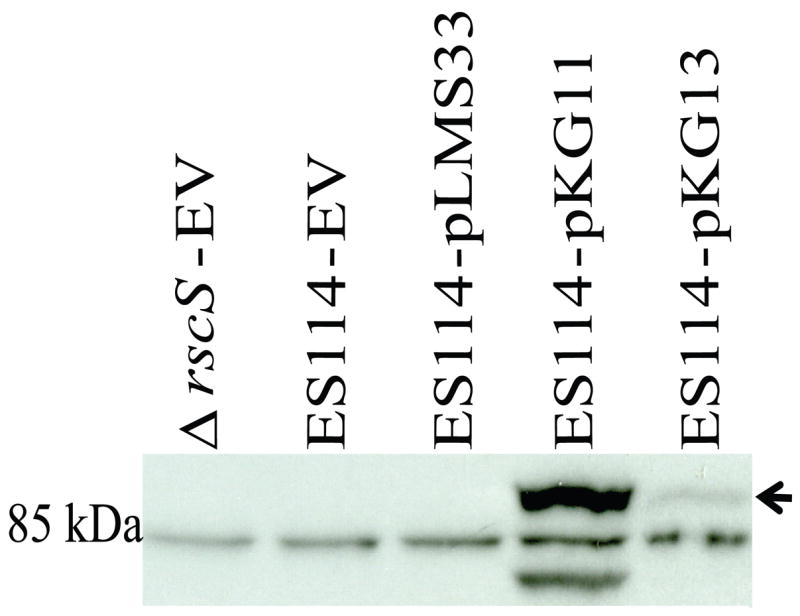
Western immunoblot analysis of RscS expressed from different constructs. The putative full-length RscS is indicated by an arrow. The approximate location of the 85 kDa protein marker is indicated. EV = the empty vector, pKV69. Shown is a representative blot from three independent experiments.
Using this antibody, we then evaluated the relative levels of RscS produced by different rscS alleles. RscS was not detected in wild-type strains carrying the empty vector (pKV69, Fig. 2), suggesting that the endogenous rscS gene produces very little protein under the culture conditions we used [HMM-CAA-Glu with 30 μg/ml tetracycline (Ruby and Nealson, 1977; Yip et al., 2005)]. RscS was also not detected in a strain carrying the wildtype rscS gene on a multi-copy plasmid (pLMS33, Fig. 2). In fact, RscS was only detected in strains carrying the increased activity alleles (pKG11 and pKG13, Fig. 2). Consistent with their relative effects on syp cluster transcription (Yip et al., 2006), pKG11 produced substantially more protein than pKG13 (Fig. 2). From this result, we conclude that the increased activity phenotypes associated with pKG11and pKG13 can be attributed to increased levels of RscS protein.
Transcription from the rscS promoter is weak
Each of the above plasmids, pLMS33 (wild-type rscS), pKG11 (rscS1), and pKG13 (rscS2), contain the rscS coding sequence and 389 bp of upstream sequence, including two predicted σ70-binding rscS promoters [a major and a minor promoter (Visick and Skoufos, 2001), referred to collectively as PrscS, Fig. 1B]. In each case, the rscS gene is also oriented correctly for transcription from the vector-derived lacZ promoter (PlacZ). Therefore, transcription of rscS from these plasmids may be driven by either PrscS or PlacZ. Our data suggest that rscS transcription from these plasmids depends heavily on PlacZ. First, pKG12, a subcloned plasmid variant in which rscS1 was present in the opposite orientation relative to PlacZ (and thus transcription would be dependent on the PrscS promoters, Fig. 1B) exhibited substantially less RscS activity than pKG11 as measured by induction of syp transcription (Fig. 3) and failed to induce wrinkled colony morphology (data not shown). Second, in a screen for increased activity derivatives of pKG11 performed as described in (Yip et al., 2006) but with pKG11 as the target for mutagenesis, we identified an allele with a mutation within the −35 hexamer of the major rscS promoter (TTGTAA to TTATAA, pKG32) that decreased the similarity of the −35 element to the E. coli consensus (TTGACA) and thus may decrease binding of RNA polymerase (RNAP) to PrscS (Busby and Ebright, 1994). Nonetheless, pKG32 results in ~2-fold increased RscS activity relative to pKG11 (Fig. 3). Third, deletion of PlacZ from pKG11 to generate plasmid pKV283 resulted in a substantial decrease in rscS activity (data not shown). Together, these results indicate that PrscS is a weak promoter; furthermore, they suggest that RNAP bound at this promoter may block transcription from PlacZ. Therefore, we predicted that removal of PrscS would result in increased rscS transcription and increased RscS activity. This was in fact the case: cloning the wild-type rscS gene directly downstream of PlacZ (pKV256, Fig. 1B) resulted in greatly increased RscS activity as shown by syp transcription (a 45-fold increase relative to pLMS33, Fig. 3). pKV256 also produced a substantial amount of RscS protein (data not shown) and, while pLMS33 has been shown to induce a slightly wrinkled colony morphology (Yip et al., 2006), pKV256 induced dramatic wrinkling (data not shown).
Figure 3.
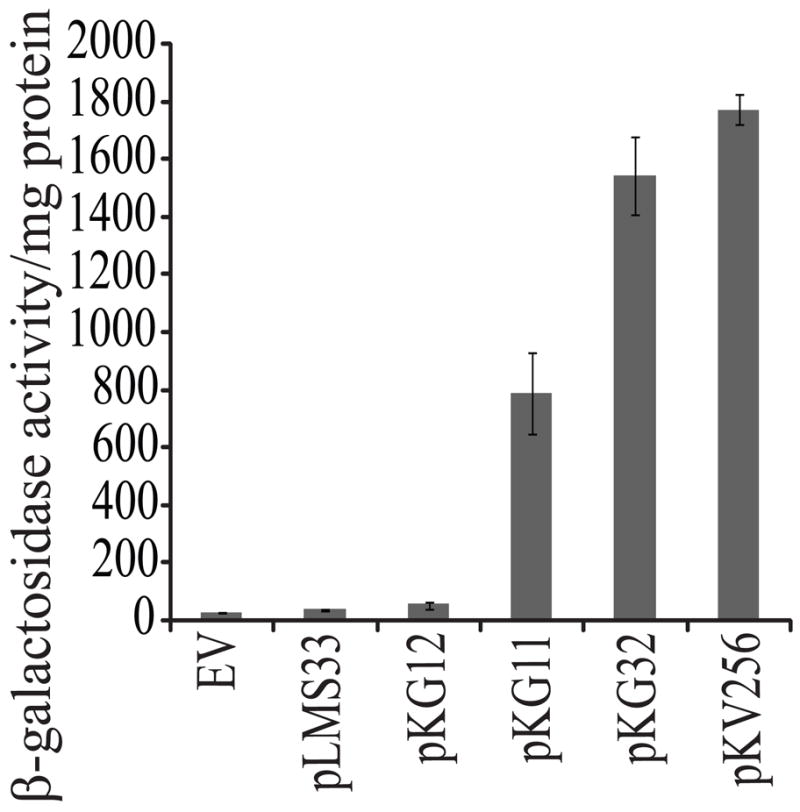
The effect of different rscS alleles on syp transcription. Cultures of the reporter strain KV2566 (PsypA::lacZ) carrying the indicated plasmids were grown in LBS (Graf et al., 1994; Stabb et al., 2001) at 22°C for 6 hrs, then syp transcription was assessed by a β-galactosidase assay (see Materials and methods). The assay was performed in triplicate, with error bars representing standard deviation. EV = the empty vector, pKV69.
The Leu25 mutation is primarily responsible for the rscS1 phenotype
To determine which of the two mutations present on pKG11 (RBS1 and Leu25) were responsible for its phenotypes, we generated derivatives analogous to pKV256 but containing each individual mutation as well as the two together (Fig. 1B, Table 1) and screened their effect on RscS activity by assessing syp transcription. Plasmid pKG63, which carries the two mutations present in rscS1, increased syp transcription 1.2 fold relative to the wild type (Fig. 4). This moderate effect, compared to that seen for the double mutant plasmid pKG11, may be due to the high baseline activity of the wild-type allele in pKV256. pKV251 (RBS1) had a slight effect on activity (1.13 fold increased over wild type, Fig. 4), while pKV255 (Leu25) increased activity 1.4 fold. In fact, the Leu25 mutation alone increased activity to a greater extent than the two mutations present together in pKG63 (Fig. 4). From these data we conclude that the Leu25 mutation was primarily responsible for the observed rscS1 phenotypes.
Figure 4.
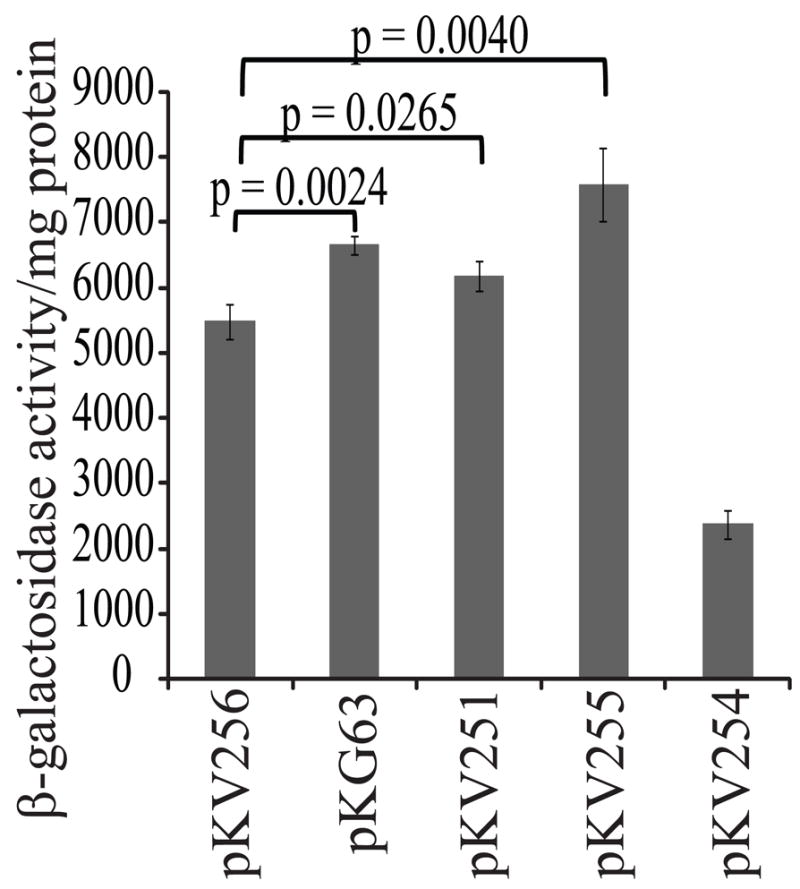
Contribution of RBS1, RBS2 and Leu25 mutations to the syp transcription phenotype. Assays were performed as described in Fig. 3, except cultures were incubated at 22°C for 48 hrs before removing samples. The p values refer to the variation between the two plasmids indicated by the brackets.
We similarly asked whether the RBS2 mutation (Fig. 1B) would increase RscS activity in a construct lacking the rscS promoter (pKV254). Surprisingly, we found that it did not (Fig. 4). These data suggest that the context of this mutation is critical for its activity.
Discussion
In this study, we have generated antibodies specific for RscS and shown, using these antibodies, that pKG11 and pKG13 both increase protein levels above that of the wild type allele. Of the two mutations present on pKG11, the ribosome binding site mutation and the silent mutation at Leu25, Leu25 appears to make the greater contribution to the increased activity phenotype. Furthermore, our western analysis revealed that endogenous RscS levels are undetectable under the culture conditions we examined.
Together, our data suggest that RscS may be restricted to low levels in the cell by as many as three possible mechanisms. First, the natural promoter PrscS appears to be weak. This is clear in the case of plasmids pKG12 and pKV283 (Fig 1B), which contain the rscS1 mutations but under control of PrscS; unlike rscS1 expressed from PlacZ-PrscS (pKG11) these constructs increase activity only slightly above that of empty vector (Fig. 3 and data not shown). Furthermore, the presence of PrscS on the plasmid decreased RscS activity and a mutation within the major promoter predicted to weaken RNAP binding increased RscS activity (Fig. 3). Finally, semi-quantitative RT-PCR revealed very low levels of endogenous rscS transcript (data not shown). It remains to be seen if transcription of rscS is up-regulated under a specific environmental condition, such as in response to the presence of a squid host. The promoter region of rscS appears to contain binding sites for the transcriptional regulators CRP and GlpR (O’Shea and Visick, unpublished data), suggesting that RscS levels may be regulated in response to the nutritional state of the cell. Conversely, the low levels of rscS message detected may be due to transcript instability, as has been seen with other sensor kinase gene transcripts (Aiso and Ohki, 2003).
A second mechanism by which RscS levels are maintained at a low level may be through inefficient ribosome binding (Barrick et al., 1994). This mechanism is suggested by the fact that both pKG11 and pKG13 contain changes within or adjacent to the RBS. However, these mutations do not increase complementarity between the RBS and the 16S rRNA; in fact the mutation present on pKG13 (RBS2) decreases complementarity to the 16S rRNA (Fig. 1A). Therefore, these mutations may not directly alter ribosome binding but instead may alter formation of a secondary structure in the rscS message that occludes the RBS (de Smit and van Duin, 1990); the mutations in pKG11 and pKG13 may disrupt this structure and facilitate translation initiation. In support of an indirect effect on ribosome binding, we found that RBS2 increased RscS activity in the PlacZ-PrscS plasmid (Yip et al., 2006) but decreased activity in the PlacZ plasmid (Fig. 4; compare pKV254 to pKV256). The different 5′ UTRs of the two plasmids may form alternative secondary structures, resulting in differential effects of RBS2 on RscS activity.
A third mechanism keeping RscS levels low in the cell may be the use of rare codons. Our results show that the Leu25 mutation contributes significantly to the increased activity of rscS1. This mutation changes the 25th codon from CTC to CTT. The wild type CTC codon is the rarest Leu codon, used in just 4% of V. fischeri Leu codons. The mutant CTT codon is found 5 times more frequently; in fact, it is the second most common Leu codon in V. fischeri (Nakamura et al., 2000). It has been proposed that the presence of rare codons within the first 25 amino acids results in decreased translation efficiency as ribosomes stalled close to the initiation codon may block ribosome entry (Chen and Inouye, 1994). The Leu25 codon is located within a stretch of six amino acids (L23ML25TRN28) of which all but the Met are encoded by rare codons, supporting the hypothesis that RscS is maintained at a low level through the use of rare codons in the message. Therefore, translation efficiency may be improved by the Leu25 mutation by increasing the efficiency of amino acid incorporation.
In summary, this work shows that increasing RscS protein levels is sufficient to increase RscS activity and that RscS protein levels are maintained at a low level in culture conditions. Because expression of RscS from pKG11 clearly enhances symbiosis (Yip et al., 2006), it seems likely that high levels of RscS must be detrimental to some aspect of the V. fischeri life cycle, perhaps during long-term symbiotic maintenance or during survival in the seawater. Future studies will address these possibilities.
Acknowledgments
The authors thank Elizabeth A. Hussa for construction of the reporter strain KV2566, Cindy Darnell for technical assistance and members of the lab for critical reading of the manuscript and helpful suggestions. This work was supported by NIH grant GM59690 awarded to K.L.V. and by the NIH under the Ruth L. Kirschstein National Research Service Award 1 F32 G073523 from the NIGMS awarded to K.G.
References
- Aiso T, Ohki R. Instability of sensory histidine kinase mRNAs in Escherichia coli. Genes Cells. 2003;8:179–187. doi: 10.1046/j.1365-2443.2003.00624.x. [DOI] [PubMed] [Google Scholar]
- Barrick D, Villanueba K, Childs J, Kalil R, Schneider TD, Lawrence CE, Gold L, Stormo GD. Quantitative analysis of ribosome binding sites in E.coli. Nucleic Acids Res. 1994;22:1287–1295. doi: 10.1093/nar/22.7.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettcher KJ, Ruby EG. Depressed light emission by symbiotic Vibrio fischeri of the sepiolid squid Euprymna scolopes. J Bacteriol. 1990;172:3701–3706. doi: 10.1128/jb.172.7.3701-3706.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busby S, Ebright RH. Promoter structure, promoter recognition, and transcription activation in prokaryotes. Cell. 1994;79:743–746. doi: 10.1016/0092-8674(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Chen GT, Inouye M. Role of the AGA/AGG codons, the rarest codons in global gene expression in Escherichia coli. Genes Dev. 1994;8:2641–2652. doi: 10.1101/gad.8.21.2641. [DOI] [PubMed] [Google Scholar]
- DeLoney CR, Bartley TM, Visick KL. Role for phosphoglucomutase in Vibrio fischeri-Euprymna scolopes symbiosis. J Bacteriol. 2002;184:5121–5129. doi: 10.1128/JB.184.18.5121-5129.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Smit MH, van Duin J. Secondary structure of the ribosome binding site determines translational efficiency: a quantitative analysis. Proc Natl Acad Sci U S A. 1990;87:7668–7672. doi: 10.1073/pnas.87.19.7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf J, Dunlap PV, Ruby EG. Effect of transposon-induced motility mutations on colonization of the host light organ by Vibrio fischeri. J Bacteriol. 1994;176:6986–6991. doi: 10.1128/jb.176.22.6986-6991.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- McCann J, Stabb EV, Millikan DS, Ruby EG. Population dynamics of Vibrio fischeri during infection of Euprymna scolopes. Appl Environ Microbiol. 2003;69:5928–5934. doi: 10.1128/AEM.69.10.5928-5934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- Nakamura Y, Gojobori T, Ikemura T. Codon usage tabulated from international DNA sequence databases: status for the year 2000. Nucleic Acids Res. 2000;28:292. doi: 10.1093/nar/28.1.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyholm SV, McFall-Ngai MJ. The winnowing: establishing the squid-vibrio symbiosis. Nat Rev Microbiol. 2004;2:632–642. doi: 10.1038/nrmicro957. [DOI] [PubMed] [Google Scholar]
- O’Shea TM, Klein AH, Geszvain K, Wolfe AJ, Visick KL. Diguanylate cyclases control magnesium-dependent motility of Vibrio fischeri. J Bacteriol. 2006;188:8196–8205. doi: 10.1128/JB.00728-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby EG, Nealson KH. Pyruvate production and excretion by the luminous marine bacteria. Appl Environ Microbiol. 1977;34:164–169. doi: 10.1128/aem.34.2.164-169.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabb EV. The Vibrio fischeri-Euprymna scolopes Light Organ Symbiosis. In: Thompson FL, Austin B, Swings J, editors. The Biology of Vibrios. Washington, D.C.: ASM Press; 2006. [Google Scholar]
- Stabb EV, Reich KA, Ruby EG. Vibrio fischeri genes hvnA and hvnB encode secreted NAD(+)-glycohydrolases. J Bacteriol. 2001;183:309–317. doi: 10.1128/JB.183.1.309-317.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabb EV, Ruby EG. RP4-based plasmids for conjugation between Escherichia coli and members of the Vibrionaceae. Methods Enzymol. 2002;358:413–426. doi: 10.1016/s0076-6879(02)58106-4. [DOI] [PubMed] [Google Scholar]
- Stock AM, Robinson VL, Goudreau PN. Two-component signal transduction. Annu Rev Biochem. 2000;69:183–215. doi: 10.1146/annurev.biochem.69.1.183. [DOI] [PubMed] [Google Scholar]
- Visick KL, Ruby EG. Vibrio fischeri and its host: it takes two to tango. Curr Opin Microbiol. 2006;9:632–638. doi: 10.1016/j.mib.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Visick KL, Skoufos LM. Two-component sensor required for normal symbiotic colonization of Euprymna scolopes by Vibrio fischeri. J Bacteriol. 2001;183:835–842. doi: 10.1128/JB.183.3.835-842.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock DM, Crowther PJ, Doherty J, Jefferson S, DeCruz E, Noyer-Weidner M, Smith SS, Michael MZ, Graham MW. Quantitative evaluation of Escherichia coli host strains for tolerance to cytosine methylation in plasmid and phage recombinants. Nucleic Acids Res. 1989;17:3469–3478. doi: 10.1093/nar/17.9.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip ES, Geszvain K, DeLoney-Marino CR, Visick KL. The symbiosis regulator RscS controls the syp gene locus, biofilm formation and symbiotic aggregation by Vibrio fischeri. Mol Microbiol. 2006;62:1586–1600. doi: 10.1111/j.1365-2958.2006.05475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip ES, Grublesky BT, Hussa EA, Visick KL. A novel, conserved cluster of genes promotes symbiotic colonization and σ54-dependent biofilm formation by Vibrio fischeri. Mol Microbiol. 2005;57:1485–1498. doi: 10.1111/j.1365-2958.2005.04784.x. [DOI] [PubMed] [Google Scholar]