Abstract
Purpose
Although prostate specific antigen velocity was proposed to increase the specificity of prostate specific antigen-based screening, there are little published data on the effect of differential prostate growth on prostate specific antigen velocity. If a patient presents with rising prostate specific antigen over a year or more, it would be useful to know whether such a change in prostate specific antigen could be explained by prostate growth. Thus, we investigated the relationship between changes in prostate size and prostate specific antigen changes in a large cohort of men without prostate cancer.
Materials and Methods
We identified 242 men without prostate cancer from the Baltimore Longitudinal Study of Aging who had 2 or greater serial pelvic magnetic resonance imaging studies and contemporaneous prostate specific antigen measurements. In this population we used the t test, correlation coefficients, and regression analysis to examine the relationship between prostate specific antigen changes and prostate volume changes, as assessed by magnetic resonance imaging.
Results
The mean age was 55 years. During 4.2 years of median followup, the median rate of volume change was 0.6 cc per year (range −9.9 to 11.8), and the median prostate specific antigen change was 0.03 ng/ml per year. There was no correlation between prostate specific antigen changes and prostate growth, as measured in cc per year (r = −0.01, p = 0.9) or the percent change per year (r = 0.07, p = 0.3). On multivariate analysis, there was no significant relationship between changes in prostate volume and prostate specific antigen changes.
Conclusions
Our data suggest that volume increases alone do not cause a high prostate specific antigen velocity. Despite growth rates as high as 10 cc per year, prostate specific antigen velocity was less than 0.1 ng/ml per year in most men without prostate cancer. Thus, differential rates of prostatic growth should not confound the use of prostate specific antigen velocity for prostate cancer detection and prognostication.
Keywords: prostate, organ size, prostatic neoplasms, prostate-specific antigen, mass screening
The majority of prostate cancer is currently diagnosed through PSA-based screening. Despite its utility in prostate cancer detection, the serum PSA level can also be affected by noncancerous conditions, including BPH. For example, Pinsky et al examined the relationship between DRE estimated prostate size and the PSA level in 35,323 men from the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial.1 After adjusting for measurement error, they reported a 3% increase in log PSA for every 1 cm3 increase in prostate volume. Moreover, for every 10 cm3 increase in prostate size, there was a 1.9-fold increased odds of having a PSA level of greater than 4 ng/ml. This could have vast implications, considering that 50% of men 51 to 60 years old have histological evidence of BPH.2
Thus, there has been considerable research into variations of PSA testing to reduce such confounding. For example, PSA density controls for the effects of prostate enlargement by dividing the serum PSA level by the prostate volume.3 More recently, free PSA isoforms have been identified that appear to be differentially expressed in BPH and prostate cancer.4,5
Finally, longitudinal changes in PSA with time have also been utilized to help distinguish prostate cancer from benign prostate conditions. In the initial report, our group showed that PSAV was significantly higher in patients with prostate cancer than in men with BPH or healthy controls.6 Nevertheless, there are little data on the precise relationship between the rate of prostate growth and serial changes in PSA with time.
Williams et al previously reported on MRI-determined prostate volume changes in a subset of 64 men from the BLSA.7 However, their study did not evaluate the correlation between changes in volume and longitudinal changes in PSA. Thus, we further investigated the relationship between changes in prostate size and PSA changes in a larger cohort of men without prostate cancer from the BLSA. Our hypothesis was that prostate volume changes may influence the use of PSAV in clinical practice.
Methods
Study Population
The study population consisted of participants in the BLSA. This prospective cohort study was initiated in 1958 by the National Institute on Aging, Bethesda, Maryland. To date, there are approximately 1,806 male participants in the open enrollment study, primarily residents of Maryland or Washington, D. C. Approximately every 2 years, participants undergo a comprehensive medical, physical, and neuropsychological evaluation, of which the details have been described previously.6,7 The study protocol was approved by the institutional review boards of Med Star and The Johns Hopkins Medical Institutions, Baltimore, Maryland, and written informed consent was obtained from all BLSA participants.
For participant visits after September 1991, PSA measurements and digital rectal examination were performed at each evaluation. A standard monoclonal immunoradiometric assay (Hybritech® Tandem-R) was used for all PSA measurements. Transrectal ultrasound guided prostate biopsy was recommended for a PSA level of greater than 4.0 ng/ml or suspicious digital rectal examination.
Beginning in February 1993, pelvic MRI was performed every 2 years as part of the urological examination for men without prostate cancer. Prostate volume was calculated from the T2 axial images using a semi-automated image analysis system, as previously described.7 MRI interpretation was blinded to the chronology of individual scans.
The inclusion criteria for this study were men with no evidence of prostate cancer, who underwent at least 2 pelvic MRI scans with concurrent PSA measurements. Of the 1,806 male subjects who participated in the BLSA between February 1958 and 2007, 242 had at least 2 prostate volume measurements on MRI with at least 2 concurrent PSA measurements. Men were excluded if they had a history of prostate cancer or finasteride use, or had MRI measurements without a concurrent PSA. Of the 242 men, 119 (49%) had 2 serial MRIs, 67 (28%) had 3, 43 (18%) had 4, and 13 (5%) had 5. Volume changes were calculated by a 2-point method using the first and last measurement for all participants. This approach was validated by comparing the results using the 2-point method to a linear regression using all values for men with more than 2 MRIs, and the Pearson correlation coefficient was 0.98. Changes in PSA were calculated as the simple rate of change between PSA at the first and last MRI, ie the difference between first and last PSA, divided by time between first and last PSA measurements.
Statistical Analysis
Descriptive statistics were used to examine the demographics, prostate volume distribution and longitudinal PSA changes in the overall study population. We then calculated the rate of change in prostate size, in both cc per year and percent change per year. Changes in prostate size were also calculated separately for men with an initial prostate volume of less than 40 and 40 cc or greater to examine the influence of baseline prostate size on the results.
The t test was used to compare clinical characteristics between participants with prostate growth and those with a stable/decreasing prostate size. Subgroup analysis was also performed, in which we excluded men with a negative PSA slope, to address the clinical question of whether changes in size are associated with a rising PSA level. In addition, we used Pearson correlation coefficients to evaluate the relationship between prostate volume changes and PSAV. Finally, multivariate regression analysis was performed to further examine the relationship between size changes and longitudinal PSA changes, in which both age and PSA changes were considered continuous variables.
Results
Characteristics of the Study Population
Serial PSA measurements and MRI measured prostate volume were available for 242 men. The majority of men were white (75.6%), with 18.6% black and 5.8% of other ethnic backgrounds. The mean age was 55 years at study entry and the mean ± SD American Urological Association symptom score was 5.8 ± 5.1. The table shows the baseline characteristics of the study population. At the initial visit, the median PSA level was 0.9 ng/ml. All participants underwent 2 to 5 serial MRIs. After a median followup of 4.2 years (range 1.8 to 9.8), the median PSA level was 1.0 ng/ml.
Study population baseline characteristics.
| Median (range) | Mean ± SD | |
|---|---|---|
| Age at first MRI | 56.7 (30.0–73.5) | 55.4 (11.7) |
| Initial prostate vol (cc) | 27.5 (4.4–135.0) | 30.4 (15.1) |
| PSA (ng/ml) | 0.9 (0.1–10.9) | 1.3 (1.4) |
| Followup (yrs) | 4.2 (1.8–9.8) | 4.8 (2.3) |
On the first MRI, the median prostate volume was 27 cc (range 4 to 135). The median prostate volume at the end of the study period was 30 cc (range 8.7 to 138). The median rate of change in volume was 0.6 cc per year (range −9.9 to 11.8), corresponding to a median 2.2% increase in prostate volume annually.
The median PSA change in the overall cohort was 0.03 ng/ml per year (range −1.7 to 3.5). This included 11 men (4.6%) with a change in PSA of greater than 0.5 ng/ml per year and 2 (0.83%) with a change in PSA of greater than 0.75 ng/ml per year.
Stratified by prostate volume, the median change in PSA was 0.03 ng/ml per year for men with an initial size of less than 40 cc, and 0.02 ng/ml per year for men with a prostate of 40 cc or greater. After excluding men with a negative PSA slope, the median value was 0.09 ng/ml per year (range 0.01 to 3.5).
Relationship Between Prostate Volume Changes and PSA Changes
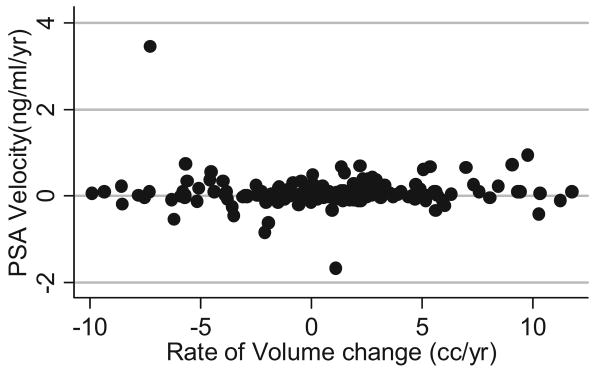
The figure shows the relationship between changes in PSA and the rate of change in prostate volume. Note that although there were some men with a growth rate as high as 10 cc per year, and others with significant shrinkage, the distribution of PSA changes had no apparent relationship with volume changes. Furthermore, there was no apparent trend in the coefficient of variation after stratification by volume quintile, suggesting that volume had no relationship with PSA variability.
Scatterplot shows relationship between changes in prostate volume and longitudinal PSA in overall study population.
Using Pearson correlation coefficients, changes in PSA were not significantly correlated with the rate of change in volume in cc per year (r = −0.01, p = 0.9) or with the percent change in prostate volume per year (r = 0.07, p = 0.3). Excluding participants with a negative PSA slope, there was also no significant correlation between a rising PSA and the rate of change in volume in cc per year (r = −0.08, p = 0.4) or the percent change in prostate volume per year (r = 0.02, p = 0.8). Finally, in the overall study population there was a small correlation between the rate of volume change and increasing age, but this did not attain statistical significance (r = 0.12, p = 0.06).
Using the t test, there was no significant difference between the absolute change in PSA (p = 0.1) or rate of change in PSA (p = 0.8) between men with an increasing prostate size, and those with a stable or decreasing size. Conversely, the rate of change in size was similar between men with a rising PSA, and those with a stable or decreasing PSA (p = 0.1).
Finally, we performed multivariate linear regression to further examine the relationship between changes in PSA and changes in prostate volume, controlling for patient age. Neither age at the first MRI (p = 0.8), age at the last MRI (p = 0.98), nor the rate of change in volume (p = 0.8) was associated with the rate of change in PSA.
Discussion
There is a growing body of evidence to support the utility of PSAV for prostate cancer risk assessment. Traditionally, a PSAV threshold of 0.75 ng/ml per year was used to help distinguish between prostate cancer and benign conditions.6 More recently, it has been shown that a PSAV in the range of 0.3 to 0.5 ng/ml per year may be associated with better performance characteristics for prostate cancer detection, particularly in men younger than 60 years.8,9 It has been suggested that PSAV may perform better in younger men due to the absence of BPH. However, there are limited data on the true relationship between PSAV and serial changes in prostate volume.
These issues are important since clinicians are beginning to place a greater emphasis on PSA kinetics to help guide treatment decisions. Indeed, there is evidence that PSAV not only helps in risk assessment, but also correlates strongly with prostate cancer aggressiveness and long-term treatment outcomes. Men with a PSAV of greater than 2 ng/ml per year in the year prior to diagnosis are at almost 10-fold increased risk for cancer specific mortality after radical prostatectomy, and similar results have been reported after radiation therapy.10,11 Even the PSAV more than 10 years prior to diagnosis, calculated as the running average of the rate of change for 3 consecutive visits, predicts the likelihood of later prostate cancer death.12
That notwithstanding, the potentially confounding effects of prostatic growth on PSAV have not been fully elucidated. In 1 study Bent et al randomized 225 men with moderately to severely symptomatic BPH to placebo or saw palmetto treatment.13 Although it was not one of the primary study end points, they reported that the median PSAV was 0.15 ng/ml during the 1-year study period. Interestingly, the sonographic prostate volume increased by a mean of 4.98 ml during the same interval.
In the current study, we found that prostate volume was not independently associated with longitudinal changes in PSA in the overall population after controlling for age. This argues against the traditional notion that PSAV may be significantly confounded by men with an enlarging prostate from BPH. Similar to Bent et al, who found only a slight PSA rise in men with highly symptomatic BPH,13 we did not find a strong relationship between the rate of change in size and PSAV. These findings suggest that PSAV provides a useful way to increase the specificity of screening for prostate cancer.
The National Comprehensive Cancer Network now recommends that a prostate biopsy should be considered for men with PSA levels 2.5 ng/ml or less and with a PSAV of greater than 0.35 ng/ml per year.14 Certainly, we believe that a long-term evaluation of PSAV using at least 3 serial measurements is most valuable, as was used in the study upon which these recommendations were based.12 In addition, despite concern that using a lower PSAV threshold would considerably increase the number of unnecessary biopsies, the data in the current study suggest that prostate volume changes are unlikely to result in a PSA increase of greater than 0.35 ng/ml per year. Indeed, in our cohort of men without prostate cancer, PSA increased by only 0.1 ng/ml during the entire 4.2-year study interval, and the median rate of change in PSA was 0.03 ng/ml per year.
Several limitations of our study deserve mention. First, prostate volume measurements in this study were based upon MRI. Although transrectal ultrasound is typically used to estimate prostate volume in daily clinical practice, there is a strong correlation between the 2 imaging modalities using the classic ellipsoid formula (r >0.8).15 Moreover, Rahmouni et al reported that MRI volume estimates are actually more accurate than transrectal ultrasound compared to the specimen weight at radical prostatectomy.16
Another limitation is that concurrent data on prostate volume and PSA measurements were not available for all 1,806 male participants in the BLSA. Rather, these data were available for only the 242 men in our study population, representing a possible selection bias. To evaluate for this, we compared the study population to 845 age matched BLSA participants with PSA measurements during the same interval but no MRI, and did not find any significant difference in the baseline characteristics (data not shown).
An additional limitation is that PSA testing was not performed on a yearly basis. Also, information on the estimated prostate size on digital rectal examination was not available for this population. Finally, the majority of men in the BLSA were white, potentially limiting the generalizability of this study to other ethnic groups.
Conclusions
Although changes in prostate size vary considerably in the aging male population, there was no significant association with longitudinal PSA changes. Moreover, the rate of change in PSA was less than 0.1 ng/ml per year in the majority of this population without prostate cancer, despite growth rates as high as 10 cc per year in some men. These results suggest that differential changes in prostate size should not confound the clinical use of PSAV for prostate cancer detection and prognostication.
Acknowledgments
Supported by the National Institutes of Health, National Institute on Aging Intramural Research Program.
Abbreviations and Acronyms
- BLSA
Baltimore Longitudinal Study of Aging
- BPH
benign prostatic hyperplasia
- MRI
magnetic resonance imaging
- PSA
prostate specific antigen
- PSAV
PSA velocity
Footnotes
Study received approval from the institutional review boards of Med Star and The Johns Hopkins Medical Institutions, Baltimore, Maryland.
References
- 1.Pinsky PF, Kramer BS, Crawford ED, Grubb RL, Urban DA, Andriole GL, et al. Prostate volume and prostate-specific antigen levels in men enrolled in a large screening trial. Urology. 2006;68:352. doi: 10.1016/j.urology.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 2.Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol. 1984;132:474. doi: 10.1016/s0022-5347(17)49698-4. [DOI] [PubMed] [Google Scholar]
- 3.Benson MC, Whang IS, Pantuck A, Ring K, Kaplan SA, Olsson CA, et al. Prostate specific antigen density: a means of distinguishing benign prostatic hypertrophy and prostate cancer. J Urol. 1992;147:815. doi: 10.1016/s0022-5347(17)37393-7. [DOI] [PubMed] [Google Scholar]
- 4.Slawin KM, Shariat S, Canto E. BPSA: A novel serum marker for benign prostatic hyperplasia. Rev Urol. 2005;7(suppl):S52. [PMC free article] [PubMed] [Google Scholar]
- 5.Mikolajczyk SD, Catalona WJ, Evans CL, Linton HJ, Millar LS, Marker KM, et al. Proenzyme forms of prostate-specific antigen in serum improve the detection of prostate cancer. Clin Chem. 2004;50:1017. doi: 10.1373/clinchem.2003.026823. [DOI] [PubMed] [Google Scholar]
- 6.Carter HB, Pearson JD, Metter EJ, Brant LJ, Chan DW, Andres R, et al. Longitudinal evaluation of prostate-specific antigen levels in men with and without prostate disease. JAMA. 1992;267:2215. [PMC free article] [PubMed] [Google Scholar]
- 7.Williams AM, Simon I, Landis PK, Moser C, Christens-Barry W, Carter HB, et al. Prostatic growth rate determined from MRI data: age-related longitudinal changes. J Androl. 1999;20:474. [PubMed] [Google Scholar]
- 8.Moul JW, Sun L, Hotaling JM, Fitzsimons NJ, Polascik TJ, Robertson CN, et al. Age adjusted prostate specific antigen and prostate specific antigen velocity cut points in prostate cancer screening. J Urol. 2007;177:499. doi: 10.1016/j.juro.2006.09.063. [DOI] [PubMed] [Google Scholar]
- 9.Loeb S, Roehl KA, Catalona WJ, Nadler RB. Prostate specific antigen velocity threshold for predicting prostate cancer in young men. J Urol. 2007;177:899. doi: 10.1016/j.juro.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 10.D'Amico AV, Chen MH, Roehl KA, Catalona WJ. Preoperative PSA velocity and the risk of death from prostate cancer after radical prostatectomy. N Engl J Med. 2004;351:125. doi: 10.1056/NEJMoa032975. [DOI] [PubMed] [Google Scholar]
- 11.D'Amico AV, Renshaw AA, Sussman B, Chen MH. Pretreatment PSA velocity and risk of death from prostate cancer following external beam radiation therapy. JAMA. 2005;294:440. doi: 10.1001/jama.294.4.440. [DOI] [PubMed] [Google Scholar]
- 12.Carter HB, Ferrucci L, Kettermann A, Landis P, Wright EJ, Epstein JI, et al. Detection of life-threatening prostate cancer with prostate-specific antigen velocity during a window of curability. J Natl Cancer Inst. 2006;98:1521. doi: 10.1093/jnci/djj410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bent S, Kane C, Shinohara K, Neuhaus J, Hudes ES, Goldberg H, et al. Saw palmetto for benign prostatic hyperplasia. N Engl J Med. 2006;354:557. doi: 10.1056/NEJMoa053085. [DOI] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology: Prostate Cancer Early Detection. [July 21, 2007]; Available at http://www.nccn.org/professionals/physician_gls/PDF/prostate_detection.pdf.
- 15.al-Rimawi M, Griffiths DJ, Boake RC, Mador DR, Johnson MA. Transrectal ultrasound versus magnetic resonance imaging in the estimation of prostatic volume. Br J Urol. 1994;74:596. doi: 10.1111/j.1464-410x.1994.tb09190.x. [DOI] [PubMed] [Google Scholar]
- 16.Rahmouni A, Yang A, Tempany CM, Frenkel T, Epstein J, Walsh P, et al. Accuracy of in-vivo assessment of prostatic volume by MRI and transrectal ultrasonography. J Comput Assist Tomogr. 1992;16:935. doi: 10.1097/00004728-199211000-00020. [DOI] [PubMed] [Google Scholar]