Abstract
This experiment investigated whether the neural correlates of inter-item associative encoding vary according to study task. At study, pairs of unrelated words were subjected to either semantic or phonological relational judgments. Test items comprised studied word pairs (intact), pairs comprised of words belonging to different study pairs (rearranged), and novel pairs. The test requirement was to discriminate between these different classes of test item. fMRI was employed to contrast the neural activity elicited by studied pairs that were correctly endorsed as intact on the later associative recognition test, as opposed to pairs for which associative information was unavailable. In contrast to prior findings for the encoding of single items, there was no evidence that the loci of subsequent associative memory effects varied according to study task. Instead, in both tasks, pairs that were later correctly endorsed as intact elicited enhanced activity in mid- and ventral regions of the left ventrolateral prefrontal cortex (VLPFC). These findings were accompanied by extensive task-invariant reversed subsequent memory effects in medial and lateral parietal and frontal cortices. The findings suggest that the left VLPFC may play a domain-general role in the encoding of item-item associations, and in addition highlight the importance of elucidating the functional significance of reversed subsequent memory effects.
Keywords: associative memory, episodic memory, fMRI, relational memory, ventrolateral frontal cortex
Episodic memory – memory for unique events – depends upon the ability to encode and store new associations, both between the central elements of an event and their background context, and between the elements themselves. In the laboratory, these two kinds of associations are operationalized in tests of source memory (item-context associations) and associative memory (item-item associations). In the present study we investigate the neural correlates of the successful encoding of item-item associations using the fMRI ‘subsequent memory’ paradigm, focusing on whether these correlates differ according to the nature of the processing mediating the association.
Beginning with Brewer et al. (1998) and Wagner et al. (1998), the subsequent memory paradigm has been employed in numerous prior fMRI studies investigating the neural correlates of successful episodic encoding (for reviews see Paller and Wagner, 2002; Davachi, 2006; see Sanquist et al., 1980, for the first use of the subsequent memory procedure). In this paradigm, neural activity elicited at study by subsequently remembered items is contrasted with the activity elicited by subsequently forgotten items, allowing identification of regions where study activity is ‘predictive’ of later memory performance. The majority of studies employing this procedure have focused on regions where activity is greater for subsequently remembered items (henceforth, subsequent memory effects). A few studies, however, have also reported the outcomes of the reversed contrast, identifying regions where activity is greater for subsequently forgotten items (henceforth reversed subsequent memory effects; Otten and Rugg, 2001; Wagner and Davachi, 2001; Clark and Wagner, 2003; Daselaar et al., 2004; Reynolds et al., 2004).
The majority of studies employing the subsequent memory procedure have investigated the encoding of single test items or item-context associations. Relatively few studies have investigated the neural correlates of successful encoding of item-item associations, despite recent claims (Diana et al., 2007; Eichenbaum et al., 2007) that these associations are supported by neural mechanisms distinct from those that support either item memory or item-context associations. Of the relatively few studies that have investigated subsequent memory effects for item-item associations (Sperling et al., 2003; Jackson and Schacter, 2004; Kirwan and Stark, 2004; Prince et al., 2005; Summerfield et al., 2006; Chua et al., 2007), all employed either intentional or semantically-oriented study tasks, and none contrasted subsequent memory effects according to the nature of the study task1. Across these studies, the regions most consistently demonstrating associative subsequent memory effects are anterior medial temporal lobe, including the hippocampus, and left ventrolateral prefrontal cortex (VLPFC). In addition, two studies (Daselaar et al., 2004; Chua et al., 2007) reported that successful associative encoding was also associated with reversed subsequent memory effects (see above). These effects were found in regions – such as medial parietal cortex – where reversed effects have also been reported in studies investigating the encoding of single items rather than item pairs (e.g. Otten and Rugg, 2001; Wagner and Davachi, 2001).
Previous studies of subsequent memory effects for the encoding of both single items (Otten and Rugg, 2001; Otten et al., 2002; Mitchell et al., 2004) and item-context associations (Park et al., 2008) have reported that the cortical loci of these effects vary according to the nature of the study task. Notably, across the studies of Otten and colleagues, subsequent memory effects associated with study tasks that emphasize semantic processing were localized to left ventral inferior and medial prefrontal regions, while successful phonologically-based encoding was associated with effects in bilateral posterior cortex and left dorsal inferior prefrontal cortex. The sensitivity of subsequent memory effects to the nature of the study task has been interpreted as evidence that the effects reflect modulation of on-line processing engaged in service of the task. Consistent with this account, task-specific subsequent memory effects have been found to overlap with regions selectively activated by the respective study tasks (Otten and Rugg, 2001; see also Park and Rugg, 2008).
In the present study, we investigated whether the neural correlates of successful item-item encoding differ according to the nature of the study task. Thus, we addressed the question whether previous findings implicating left VLPFC and the anterior medial temporal lobe in successful associative encoding reflect a role for these regions in the encoding of inter-item associations in general as opposed to the encoding of semantically-mediated associations only. The study of Jackson and Schacter (2004) also investigated subsequent memory effects for study items comprised of word pairs. However, unlike in that study, in the present case participants were cued to relationally process either the semantic or the phonological features of the members of each study pair in the present study. Thus, we were able to search both for regions that exhibited subsequent associative memory effects common to the two study tasks, and regions where effects were associated selectively with one or other of the two tasks. In addition, we were able to assess the extent to which task-selective subsequent memory effects overlapped with regions selectively activated by engagement in one task rather than the other. This allowed us to assess the proposal that subsequent memory effects co-localize with regions selectively engaged by the study task (Rugg et al., 2002; Mitchell et al., 2004; Rugg et al., 2008).
Materials and Methods
Participants
Twenty-four participants (14 female; ages 18 - 28 years) were recruited from the University of California at Irvine (UCI) community. All were right-handed, native English speakers with no self-reported history of neurological or psychiatric illness. Four participants were excluded from all data analyses, two due to excessive head movement, and two to inadequate behavioral performance. Informed consent was obtained before participation in accordance with the requirements of the UCI Institutional Review Board, which approved the experimental protocol. Participants were remunerated for their time.
Stimulus Materials
The critical experimental stimulus pool consisted of 340 word pairs that were both semantically and phonologically unrelated. The words were selected from the word association norms compiled by Nelson et al. (1998; http://www.usf.edu/FreeAssociation), and ranged in length from 3 to 12 letters with a mean word frequency of 53.13 (Kučera and Francis, 1967). In addition, 90 word pairs were constructed in which each member belonged to the same semantic category (e.g. drawer–couch), and a further 90 pairs were constructed where the two words rhymed, but were relatively dissimilar orthographically (e.g. weight–mate).
A study list comprised a pseudorandom ordering of 240 unrelated pairs (drawn from the pool of 340 pairs described above), 80 semantically-related pairs, and 80 phonologically related pairs, along with six buffer pairs. The unrelated pairs were pseudorandomly assigned to the semantic and phonological judgment tasks such that, across subjects, each pair appeared equally often in each task context and each member of a pair was presented equally often above and below fixation. A test list comprised 320 critical unrelated word pairs along with two buffer pairs. One hundred and sixty of the test pairs were presented in the same pairing as at study, half from the semantic judgment task, the other half from the phonological judgment task (hereafter, intact pairs). Eighty test pairs (40 from the semantic task, and 40 from the phonological task) comprised studied items that, while from the same task, had been re-paired from study (henceforth rearranged pairs). Eighty entirely new unrelated pairs were also included in the test list. Both study and test lists were constrained such that no pair type occurred more than three times in succession. For the practice phase, an additional 20 unrelated word pairs and 10 related pairs were used.
Procedure
Participants were given instructions and practice for the study session prior to the experiment proper. The experiment consisted of a single study-test cycle. On each study trial, a red fixation cross appeared for 100 ms and was replaced by a task cue for 500 ms, indicating the task to be performed on the upcoming pair (an ‘o’ for the semantic task and an ‘x’ for the phonological task). Cues were randomly ordered for unrelated pairs, but were always matched for the corresponding related pairs. A word pair was then displayed for 2000 ms, with one word presented just above and the other just below fixation. The study pair was replaced by a white cross for 1900 ms that served as a prompt for a response. Stimulus onset asynchrony was 4500 ms. For the semantic judgment task, participants were instructed to rate the degree to which the two words shared a semantic theme, using a 1-3 scale, and to depress a corresponding button with the appropriate finger of the right hand. Assignment of fingers to responses was counterbalanced across subjects. For the phonological task, participants rated how similar the two words sounded, again on a 1-3 scale. Participants were not informed that their memory for the study pairs would be tested until the test phase. The study list was presented across three scanning sessions that were separated by approximately 2 min breaks.
The associative recognition test was administered outside of the scanner approximately 10 min after the end of the study phase. Before the test proper, participants were given instructions for test and practice using items that had been presented in the pre-scan practice study list. The memory test required participants to indicate whether each test pair was i) intact: two items studied in the same pairing as at study, ii) rearranged: two studied items that had been paired with different items at study, iii) single: one item studied but the other item new, or iv) new: two unstudied items. Although no ‘single’ pairs were actually presented, this option was included in order to discourage the use of the ‘rearranged’ option when only one member of a studied pair was recognized (cf. Jackson and Schacter, 2004). The test was self-paced.
fMRI Scanning
A Philips Achieva 3T MR scanner (Philips Medical Systems, Andover, MA) fitted with an 8 channel RF receiver head coil was used to acquire both T1–weighted anatomical volume images (256 × 238 matrix, 1mm3 voxels) and T2*–weighted echo-planar images (EPIs) (80 × 80 matrix, 3mm × 3mm in-plane resolution, transverse acquisition, flip angle 70°, TE 30ms) per volume. EPIs were acquired using a sensitivity encoding (SENSE) reduction factor of 2. Each EPI volume comprised 30 3mm-thick axial slices acquired in a descending sequential order and separated by 1mm, providing coverage of almost the entire brain. Data were acquired during the study phase in three scanning sessions comprising 323 volumes each, with a repetition time (TR) of 2s. Five additional volumes were collected at the beginning of each run but discarded to allow for T1 equilibration. The 4.5s SOA allowed an effective sampling rate of the hemodynamic response of 2Hz.
fMRI Data Analysis
Data preprocessing and statistical analyses were performed with Statistical Parametric Mapping (SPM 5, Wellcome Department of Cognitive Neurology, London, UK: http://www.fil.ion.ucl.ac.uk), implemented in MATLAB 7 (Mathworks, Natick, MA). For each subject, functional images were registered to the first image of each scan session and then spatially realigned to the mean functional image across sessions. The anatomical image was coregistered to the mean functional image. The unified segmentation procedure (Ashburner and Friston, 2005) was used to segment each subject's T1-image into grey matter, white matter and cerebrospinal fluid. The segmented images were also deformed to probabilistic maps of each tissue type in MNI space (International Consortium for Brain Mapping: www.loni.ucla.edu/ICBM). The resulting segmented deformation parameters were used to normalize the functional images which were also resampled into 3mm3 voxels using nonlinear basis functions (Asburner and Friston, 1999). The normalized images were smoothed with an isotropic 8 mm full-width half-maximum Gaussian kernel. The time series in each voxel were high-pass filtered to 1/128 Hz to remove low-frequency noise and scaled within-session to a grand mean of 100 across both voxels and scans.
Prior to model estimation, image time-series were concatenated across sessions. For each subject, neural activity was modeled by delta functions (impulse events) at stimulus onset. The event-related blood-oxygen-level dependent (BOLD) response was modeled by convolving these delta functions with a canonical hemodynamic response function (HRF) and its temporal and dispersion derivatives. In addition, six regressors were employed to model movement-related variance, and session-specific constant terms were employed to model the mean image intensity in each session.
In the first stage of data analysis, parameter estimates for events of interest were estimated for each subject using a General Linear Model. Non-sphericity of the error covariance was accommodated by an AR(1) model in which the temporal autocorrelation was estimated by pooling over suprathreshold voxels (Friston et al., 2002). The parameters for each covariate and the hyperparameters governing the error covariance were estimated using Restricted Maximum Likelihood (ReML). In a second stage, linear contrasts of these subject-specific parameter estimates were computed, treating subjects as a random effect.
For the principal analysis of subsequent associative memory effects, four events of interest were defined: ‘semantic–hit’ (semantically studied unrelated pairs that were correctly endorsed as intact on the later test); ‘semantic–miss’ (semantically studied unrelated pairs that were incorrectly judged as rearranged, single, or new), and the analogous ‘phonological–hit’ and ‘phonological–miss’ study pairs. All other study pairs including buffers, semantically- and phonologically-related pairs and pairs contributing to rearranged test pairs were modeled as events of no-interest. In a subsidiary analysis, semantically and phonologically studied intact pairs that were endorsed as intact (hits) were contrasted with the intact pairs that were later incorrectly judged as rearranged (associative misses). In this case all other study pairs, including those endorsed as single or new, were modeled as events of no-interest.
Unless otherwise specified, only effects surviving an uncorrected threshold of p < .001 with five or more contiguous voxels were interpreted. When exclusive masking was employed to identify voxels where effects were not shared between two contrasts, the mask threshold was set at a one-tailed threshold of p < .05. Note that the more liberal the threshold of an exclusive mask, the more conservative is the masking procedure. The thresholds employed for inclusive masking are described in the results section below. The peak voxels of clusters exhibiting reliable effects are reported in MNI coordinates. Separate contrasts were performed with parameter estimates derived from the canonical HRF and each of the two derivatives. The results from the temporal and dispersion derivatives did not add substantially to the results obtained from the canonical HRF, and are not reported.
Results
Behavioral Results
Study phase
The proportions of unrelated study pairs receiving ‘none’ or ‘somewhat’ relatedness judgments were .94 (SD = .10) for the semantic task and .97 (SD = .04) for the phonological task. The difference between these means, while small, was reliable [t(19) = 2.40, p < .05] . The mean reaction time (RT) for the semantic judgments to unrelated pairs was 1924 ms (SD = 423), against 1850 ms (SD = 426) for phonological judgments, and these means did not significantly differ [p > .06]. For both tasks, 92% of the related pairs were endorsed as highly similar.
Study RTs for the intact study pairs are shown in Table 1 segregated according to later memory performance. To parallel the approach taken in the principal fMRI analyses (see below), RTs were contrasted both according to task and to whether the pairs were later correctly endorsed as intact (hits) or received an incorrect judgment (misses). ANOVA [factors of task (semantic vs. phonological) and response (hit vs. miss)] revealed neither a significant subsequent memory effect nor an interaction between subsequent memory and study task (all Fs < 2.5, ps > .1).
Table 1.
RT (ms) to unrelated study pairs according to test response (SD in parenthesis)
| Semantic task | Phonological task | |||||||
|---|---|---|---|---|---|---|---|---|
| Test response |
intact | rearranged | single | novel | intact | rearranged | single | novel |
| 1932 | 1899 | 1940 | 1956 | 1888 | 1796 | 1875 | 1890 | |
| (430) | (463) | (517) | (546) | (436) | (397) | (457) | (538) | |
Test phase
Mean associative hit rates (correct intact judgments for intact test pairs) were .65 (SD = .19) and .36 (SD = .17) for the semantically and phonologically studied pairs respectively. Mean associative false alarm rates (incorrect intact judgments for rearranged test pairs) were .17 (SD = .10) and .16 (SD = .12) respectively. Associative recognition performance, measured as pHit – pFA, was greater in the semantic than the phonological task [F(1,19) = 39.54, p < .001], but was significantly above chance in both cases [t(19) = 15.17, p < .001 for the semantic task; t(19) = 6.21, p < .001 for the phonological task].
Post-test debriefing
On oral debriefing following the test phase, no subject reported suspecting that their memories would be tested for the word pairs presented in the scanner, or that they intentionally attempted to memorize the pairs.
fMRI Results
Analysis overview
The principal subsequent memory analyses were based on contrasts between encoding activity elicited at study by intact test pairs later correctly endorsed as intact (semantic and phonological hits) as opposed to those receiving an incorrect endorsement (i.e. rearranged, single, or new; i.e., semantic and phonological misses). These analyses were performed on the data from all 20 subjects. The employment of these contrasts was motivated by the fact there were 5 subjects who did not have sufficient (≥ 8) trials corresponding to one or more events of interest to allow estimates to be calculated for pairs later incorrectly judged as rearranged. Thus, we elected to maximize statistical power by pooling the three classes of study pairs for which associative information was unavailable. As is reported below, the outcomes of the contrasts between pairs later judged as intact and rearranged conducted on the data from the 15 eligible subjects were very similar to those of the principal analyses we first describe.
Task-invariant subsequent associative memory effects
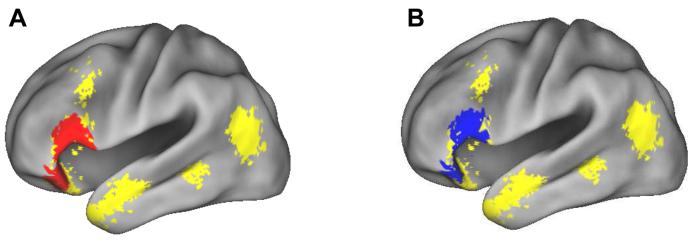
In this analysis we identified subsequent associative memory effects that were common to the two study tasks. We accomplished this by first computing the main effect of subsequent associative memory (all hits > all misses), peak thresholded at p < .001 with a cluster extent threshold k ≥ 5. To eliminate voxels where subsequent memory effects were not independently significant in each task, the main effect was then inclusively masked with both simple effects, each thresholded at p < .05. Finally, the contrast was exclusively masked by the interaction (F contrast, p < .1) between study task and subsequent memory. The resulting SPM thus identifies clusters of five or more voxels where, collapsed across study task, subsequent memory effects are statistically significant at p < .001, demonstrate task-specific subsequent memory effects at p < .05, and do not differ reliably in magnitude (p < .05) across the two study tasks. The outcome of this analysis is shown in Figure 1A. Voxels demonstrating a main effect of subsequent associative memory were localized exclusively to left VLPFC (−51, 30, 12, Z = 5.33, 185 voxels).
Figure 1.
Regions demonstrating task-invariant (A) and task-invariant reversed (B) subsequent associative memory effects (p < .001), rendered onto the PALS brain atlas (Van Essen, 2005) with Caret5 (Van Essen et al., 2001: http://brainmap.wustl.edu/caret).
We also searched for task-invariant reversed subsequent memory effects, using a procedure exactly analogous to that described above. As is evident from Figure 1B and Table 2, extensive reversed effects were evident in midline and bilateral parietal and frontal cortex.
Table 2.
Task-invariant reversed subsequent memory effects
| Coordinates (x y z) | Z | (# of voxels) | Region | BA | ||
|---|---|---|---|---|---|---|
| −30 | 33 | 21 | 3.92 | 37 | L middle frontal gyrus | 10 |
| −27 | 57 | 12 | 3.89 | 14 | L superior frontal gyrus | 10 |
| 9 | 66 | 6 | 4.01 | 6 | R medial frontal gyrus | 10 |
| 42 | 48 | 9 | 3.65 | 6 | R middle frontal gryus | 46 |
| 30 | 45 | 30 | 4.31 | 212 | R middle/superior frontal cortex | 10 |
| −57 | −12 | 0 | 3.60 | 7 | L superior temporal gryus | 22/21 |
| 57 | −42 | 12 | 3.94 | 15 | R superior temporal gyrus | 22 |
| 0 | 18 | 30 | 3.28 | 6 | Anterior cingulate gyrus | 24 |
| 6 | −45 | 36 | 4.89 | 685 | Posterior cingulate cortex | 29/31/7 |
| 57 | −30 | 42 | 4.11 | 12 | R postcentral gyrus | 2 |
| −57 | −36 | 36 | 4.54 | 175 | L inferior parietal cortex | 40 |
| 57 | −39 | 30 | 4.99 | 230 | R inferior parietal cortex | 40 |
Coordinates and Z-values refer to the peak voxels of each cluster. L, left; R, right; BA, Brodmann area (approximate).
Task-selective subsequent associative memory effects
In these analyses we searched for regions where subsequent associative memory effects dissociated according to study task. This was accomplished by inclusively masking the effects for each study task (thresholded at p < .001) by the appropriate interaction contrast (thresholded at p < .01). Thus, to identify task-selective semantic effects, we inclusively masked the subsequent memory effect for the semantic task (semantic hit > miss) with the interaction identifying regions where subsequent memory effects were larger for the semantic than the phonological task. Only one small left VLPFC cluster demonstrated effects that were of greater magnitude in the semantic than in the phonological task (−39, 30, −15, Z = 3.82, 6 voxels). No regions could be identified where effects were larger for the phonological than the semantic task.
Reversed task-selective subsequent memory effects were identified using an analogous procedure. A single cluster was identified where reversed effects were larger for the semantic task (57, −36, 36, Z = 4.05, 17 voxels), and two small clusters were also identified where phonological reversed effects exceeded those for the semantic task (−27, 30, 39, Z = 3.57, 8 voxels; 9, −21, 54, Z = 3.67, 6 voxels).
Medial temporal lobe subsequent memory effects
In light of prior findings of the involvement of the hippocampus and adjacent medial temporal lobe in the encoding of inter-item associations (e.g. Sperling et al., 2003; Jackson and Schacter, 2004; Kirwan and Stark, 2004; Prince et al., 2005; Summerfield et al., 2006; Chua et al., 2007), we searched for evidence of medial temporal lobe subsequent associative memory effects at a reduced statistical threshold (p < .05). We identified a small cluster exhibiting a task-invariant subsequent memory effect on the medial edge of the left anterior hippocampus (−18, −12, −24, Z = 2.42, 4 voxels). Using the same approach as described previously, we were unable to identify task-selective effects in the medial temporal lobe for either task. However, as is illustrated in Figure 2, the unmasked contrast of hit > miss for the semantic task (analogous to the contrast employed by Jackson and Schacter, 2004) identified a 105-voxel cluster in the left anterior medial temporal lobe (−15, −9, −12, Z = 2.77) that extended into anterior hippocampus.
Figure 2.
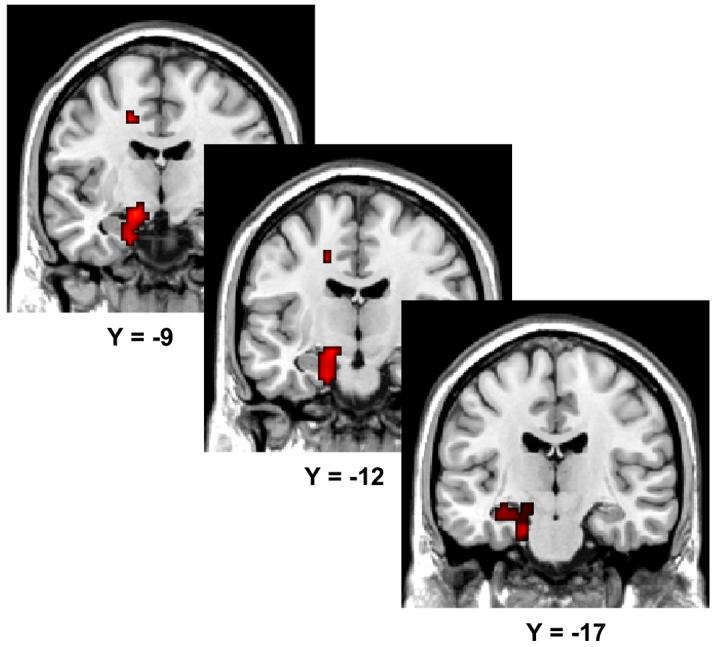
Medial temporal lobe semantic subsequent associative memory effects (p < .05) Effects are overlaid on sections of the MNI canonical brain.
Intact versus rearranged judgments
Task-invariant and task-selective subsequent associative memory effects were also sought with contrasts between intact study pairs later endorsed as intact (hits) versus rearranged (‘associative misses’), using the same procedures as were employed for the contrasts between hits (intact) and misses (rearranged, single, new). As noted previously, these analyses were performed on a subset (n = 15) of the subjects included in those prior analyses.
As in the previous analyses, a task-invariant subsequent memory effect was identified in left VLPFC (−39, 33, −18, Z = 3.85, 18 voxels), and a semantically-selective effect was identified in an adjacent region (−39, 30, −15, Z = 3.60), albeit only for two voxels. No regions selective for the phonological task were identified. Task-invariant reversed effects were again evident in midline and lateral parietal and frontal regions. No task-selective reversed effects were evident for the semantic task, and one small cluster demonstrating such effects was identified for the phonological task (−24, −18, 54, Z = 4.06, 8 voxels).
Overlap between subsequent memory and task processing effects
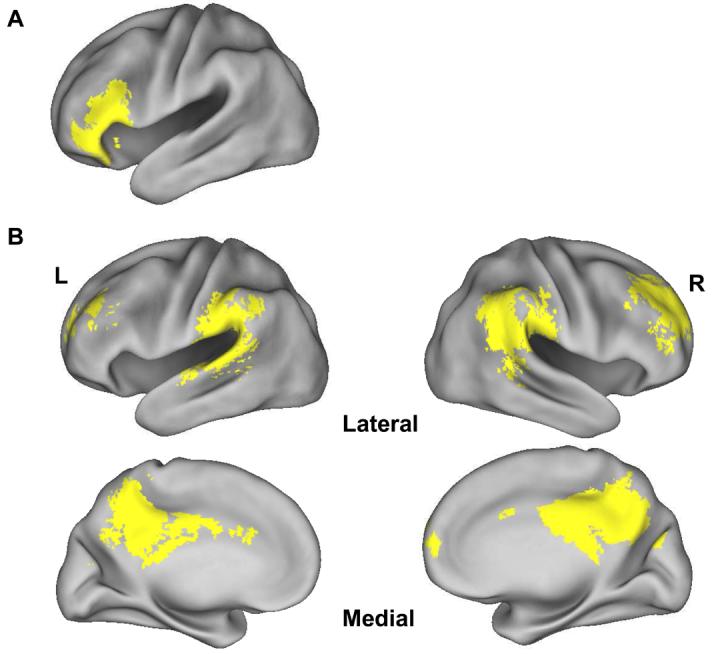
For the reasons noted in the Introduction, we searched for regions demonstrating overlap between subsequent associative memory effects and the main effect of task. This was accomplished by inclusively masking the subsequent memory effects for each task (operationalized by the hit > miss contrast for the semantic and phonological tasks respectively, each thresholded at p < .01) with the corresponding contrast between all unrelated study pairs subjected to each type of judgment (thresholded at p < .001 for each side of the contrast). The conjoint significance of these two contrasts is p < .0001 as estimated by Fisher's procedure (Lazar et al. 2002). As illustrated in Figure 3, an extensive (114 voxels) region of left VLPFC demonstrated overlap between semantic subsequent memory effects and the semantic task effect. By contrast, no voxels could be identified where there was overlap between phonological subsequent memory and phonological task effects. Strikingly, however, phonological subsequent memory effects demonstrated an overlap with semantic task effects that did not fall far short (76 voxels) of the overlap found for the semantic subsequent memory effects.
Figure 3.
Overlap between subsequent associative memory effects in each study task (A: semantic; B: phonological; p < .01) and regions where activity was greater for semantically studied than phonologically studied pairs (yellow, p < .001). Results are rendered onto the PALS atlas.
An analogous procedure was used to identify overlap between the contrasts of hits versus associative misses and task effects. Once again, a region of left VLPFC demonstrated overlap between semantically-selective subsequent associative effects and semantic task effects (28 voxels). No voxels were identified where there was overlap between phonological subsequent memory effects and phonological task effects. As in the prior analysis, however, phonological subsequent memory effects again overlapped with the semantic task effect (13 voxels).
Discussion
Behavioral findings
Response latency in the two study tasks was equivalent, as was the proportion of related pairs that attracted the highest similarity ratings. Importantly, in neither task were there detectable RT differences between unrelated study pairs according to whether the pairs were later accurately judged intact (hits) or incorrectly endorsed as rearranged, single or new (misses). Thus the fMRI subsequent memory effects discussed below are unlikely to reflect gross differences in the efficiency with which the unrelated study pairs were processed.
Associative recognition was markedly more accurate for semantically studied pairs than for phonologically studied pairs, an example of the well-known effects of ‘depth of processing’ (Craik and Lockhart, 1972). This disparity in performance implies that a higher proportion of correct associative judgments were the result of ‘lucky guesses’ following phonological than following semantic study (e.g. Rugg et al., 1998), leading to a relative dilution of phonological associative subsequent memory effects. This may have contributed to the finding that subsequent memory effects tended to be more robust in the semantic task, as is discussed below.
fMRI findings
The principal aim of the present study was to determine whether the neural correlates of successful associative encoding vary according to study task. Whereas robust subsequent memory effects common to the two tasks were evident in left VLPFC, there was little evidence of task-selective effects. These findings raise the possibility that a common set of cognitive processes underlie the formation of new inter-item memory representations regardless of the nature of the study processing. This possibility receives further support from the analyses of the reversed subsequent memory effects. Extensive task-invariant effects were found in medial parietal and lateral frontal and parietal cortex, in marked contrast to the few small clusters where the effects varied according to task.
Before discussing the implications of the fMRI findings further, it is important to note the similarities between the outcomes of our principal analyses, in which the contrast was between study pairs correctly endorsed as intact and pairs receiving any form of incorrect judgment, and our subsidiary analyses, where the contrasts were restricted to study pairs endorsed as intact versus rearranged. As was noted in the Results section, our motivation for the former analyses derived from the increased power accompanying the inclusion of an additional five subjects. This comes at the cost however of a potential confounding of associative and item memory, a confound that was absent in the second analysis, which specifically targeted the encoding of inter-item associations. The strong qualitative similarities between the outcomes of the two sets of analyses indicate that any confounding effect of item memory had little or no influence on the subsequent memory effects identified in each case. We therefore assume that the findings from our principal analyses largely reflect encoding processes supporting item-item associations, and draw no further distinctions between the two sets of analyses.
Task-invariant subsequent associative memory effects
The present finding of task-invariant effects suggests that successful encoding of item-item associations depended on processes that were shared between the two study tasks. Thus, on the assumption that the two tasks encouraged associations between distinct, domain-specific representations of the study items (semantic vs. phonological), it follows that left VLPFC plays a domain-general role in the formation of item-item associations.
As noted in the Introduction, subsequent memory effects in left VLPFC have been reported in several previous studies of inter-item associative encoding (Sperling et al., 2003; Jackson and Schacter, 2004; Prince et al., 2005; Summerfield et al., 2006; Chua et al., 2007). The locus of the peak effect in the present study (−51, 30, 12) is close to those reported in the prior studies (e.g. −45, 33, −6 in Jackson and Schacter, 2004; −51, 21, 6 in Sperling et al., 2003) and spans what, according to Badre and Wagner (2007), are two functionally distinct prefrontal regions, labeled by them as anterior and mid-VLPFC (pars orbitalis and pars triangularis, respectively). Badre and Wagner proposed that anterior VLPFC supports the domain-specific function of ‘controlled semantic retrieval’, whereas the mid-VLPFC supports the domain-general process of ‘controlled selection’. Thus, they argued for a distinction between these regions both in terms of process (retrieving representations versus selecting between the contents of retrieved representations), and representation (semantically-specific versus domain-general).
It is easy to imagine why the present study tasks would place demands on both controlled retrieval and controlled selection of item representations. In both tasks, assessment of the level of similarity of dissimilar word pairs likely depended upon the iterative retrieval and matching of candidate meanings or pronunciations of the two words. For reasons that remain to be elucidated, it appears that the more extensively these cognitive operations are engaged for a given word pair, the more likely it is that the words will be ‘bound’ into an episodic memory representation. In this context, the finding that the left VLPFC regions demonstrating task-invariant subsequent associative memory effects overlapped extensively with the prefrontal region that was preferentially activated by the semantic task (Figure 3) is intriguing. This finding suggests that the cognitive processes preferentially engaged in service of the semantic task also supported the encoding of inter-item associations in both the semantic and the phonological task.
As already noted, according to Badre and Wagner (2007), whereas mid-VLPFC is domain-general, anterior VLPFC is domain-specific, supporting the retrieval specifically of semantic representations. At first glance, our finding that semantically and phonologically-mediated subsequent memory effects co-localize to this region is inconsistent with this proposal, and supports the alternative view that, like mid-VLPFC, anterior VLPFC supports domain-general cognitive operations (Gold and Buckner, 2002). According to this view, the reason anterior VLPFC was engaged preferentially by the semantic task is not because of its specialization for semantic processing, but because this task placed greater demands on the domain-general processes supported by the region than the phonological task did.
An alternative possibility, however, is that anterior VLPFC does indeed selectively support processing in the semantic domain, as proposed by Badre and Wagner (2007; see also Poldrack et al., 1999). By this account, the co-localization of semantic and phonological subsequent memory effects in this region reflects the benefit to later memory of incidental semantic processing in the phonological task. According to this argument, study pairs in the phonological task that, for some reason, were also subjected to semantic processing were more likely to be later remembered than those where processing was exclusively phonological (see Otten et al., 2001 for a similar ‘semantic spillover’ account in respect of the overlap between subsequent memory effects elicited by ‘deeply’ and ‘shallowly’ studied words).
It is difficult to adjudicate between these two alternative accounts on the basis of the current data. That said, the finding that the area of the VLPFC that was preferentially activated in the semantic task extended into the putatively domain-general mid-VLPFC arguably favors the proposal that the anterior VLPFC also supports domain-general processing. In support of this point, it is also worth reiterating that the same left VLPFC region has been identified in a study of associative encoding that employed materials – unfamiliar face-name pairs – possessing minimal semantic content (Sperling et al., 2003; VLPFC peak effects at −51, 21, 6 in the Sperling et al.'s study and −51, 30, 12 in the present study). Regardless of how this issue is resolved, the present findings, together with the results of prior studies that between them employed a diverse range of stimulus materials and tasks, highlight the importance of left VLPFC for the encoding of inter-item associations.
Task-selective subsequent memory effects
The present study provided little or no evidence of task-selective associative subsequent memory effects. Although two small clusters were identified in left VLIFC where effects were present solely for semantically-studied items, we were unable to find regions demonstrating the reverse dissociation. In the absence of this complementary dissociation, the most plausible explanation for the finding of the aforementioned semantically-selective effects is that, for the reasons already discussed, they merely reflect the greater power of the semantic study task to detect subsequent memory effects. Whereas null findings such as these must be interpreted with caution, they stand in contrast to previous findings of task-dependent double-dissociations in subsequent memory effects for both item memory and item-context associations (Otten and Rugg, 2001; Otten et al., 2002; Park et al., 2008) and raise the possibility that the encoding of inter-item associations depends upon mechanisms distinct from those supporting other aspects of episodic memory (Diana et al., 2007; Eichenbaum et al., 2007; see Hockley and Cristi, 1996, for relevant behavioral evidence). This conclusion is of course subject to the caveat that successful associative encoding in the phonological task may have been mediated semantically.
Medial temporal lobe effects
Subsequent associative memory effects in left anterior hippocampus and adjacent medial temporal cortex have been reported in several prior studies investigating associative encoding (Sperling et al., 2003; Jackson and Schacter, 2004; Kirwan and Stark, 2004; Prince et al., 2005; Summerfield et al., 2006; Chua et al., 2007). In the present study, medial temporal lobe effects were weak and, indeed, were not detected at our pre-experimental statistical threshold. The effects identified at a lower threshold were most prominent for the semantic task, with a peak (−15, −9, −12) near to those reported in previous studies (e.g. −22, −4, −18 in Chua et al., 2007; −18, −6, −21 in Jackson and Schacter, 2004; −18, −11, −18 in Summerfield et al., 2006). For the reasons already noted, the finding that our medial temporal effects predominated in the semantic task should not be taken as evidence for task-selectivity in this region. All that can safely be concluded on the basis of the present findings is that the findings provide further support for the proposal that the anterior hippocampus and medial temporal cortex play a key role in the encoding of inter-item associations (Chua et al., 2007).
Reversed subsequent associative memory effects
Reversed subsequent memory effects were evident in widespread cortical regions and, for the most part, were task-invariant. The present findings thus provide a dramatic replication of prior reports, and highlight just how prominent these effects can be (whereas a total of 185 voxels met our statistical criteria for a task-invariant subsequent memory effect, more than 1400 voxels met the equivalent criteria for a reversed effect). As has been noted previously (Daselaar et al., 2004), the regions demonstrating reversed subsequent memory effects seemingly overlap with those comprising what has become known as the ‘default-mode network’–regions that consistently show relatively greater activity during rest than during task engagement (Gusnard and Raichle, 2001; Raichle et al., 2001). The functional role of this network is currently unclear. One hypothesis is that the regions comprising the network play a role in the monitoring and representation of the external and internal environments, and that their task-related disengagement reflects the reallocation of attentional resources away from these functions and toward goal-relevant cognitive processes (Raichle et al., 2001). From this perspective, the present and previous findings indicate that the extent to which a stimulus event elicits such attentional reallocation has a substantial impact on whether it will be successfully encoded into episodic memory. The present findings further suggest that this impact is largely uninfluenced by the nature of the cognitive operations engaged by the stimulus event (see also Daselaar et al., 2004). A large number of issues remain to be addressed, however, not least among which are whether reversed subsequent memory effects vary in their magnitude or loci according to the type of memory being encoded (e.g. item-context vs. item-item associations), and the need for a more precise specification of the cognitive operation(s) or resources reflected by these effects (cf. Otten and Rugg, 2001; Daselaar et al., 2004).
Conclusions
The present study diverges from prior experiments in its failure to find an influence of study task on subsequent memory effects. Instead, the findings suggest a domain-general role for left anterior and mid-VLPFC in the encoding of item-item associations. To the extent this possibility is borne out by future studies that explore the effects of a wider range of study tasks and materials, the proposal that no single cortical region plays a pre-eminent role in episodic encoding (e.g. Rugg et al., 2002) may require significant qualification.
Acknowledgements
This research was supported by the National Institute of Mental Health Grant MH074528. The authors thank the members of the University of California–Irvine Research Imaging Center for their assistance with fMRI data acquisition.
Footnotes
Prince et al. (2005) used both semantic and perceptual study tasks with intentional study instructions. The memory test following semantic study was similar to the one employed here. In their perceptual task, two words in a pair were presented in the same font during study. At test, some of these pairs were re-presented in the same font, whereas others were presented in an intact form but in a different font. Thus, discrimination between these two classes of items did not require memory for inter-item associations, but instead for associations between single study items and the font in which they had been presented.
Conflict of Interest: None declared.
References
- Ashburner J, Friston K. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston K. Non linear spatial normalization using basis functions. Hum. Brain Mapp. 1999;7:254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Brewer JB, Zhao Z, Desmond JE, Glover GH, Gabrieli JDE. Making memories: Brain activity that predicts how well visual experience will be remembered. Science. 1998;281:1185–1187. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- Chua E, Schacter DL, Rand-Giovannetti E, Sperling RA. Evidence for a specific role of the anterior hippocampal region in successful associative encoding. Hippocampus. 2007;17:1060–1070. doi: 10.1002/hipo.20340. [DOI] [PubMed] [Google Scholar]
- Clark D, Wagner AD. Assembling and encoding word presentations: fMRI subsequent memory effects implicate a role for phonological control. Neuropsychologia. 2003;41:304–317. doi: 10.1016/s0028-3932(02)00163-x. [DOI] [PubMed] [Google Scholar]
- Cocosco CA, Kollokian V, Kwan RS, Evans AC. Brainweb: Online interface to a 3D MRI simulated brain database. NeuroImage. 1997;5:S425. [Google Scholar]
- Craik FIM, Lockhart RS. Levels of processing: A framework for memory research. Journal of Verbal Learning and Verbal Behavior. 1972;11:671–684. [Google Scholar]
- Daselaar SM, Prince SE, Cabeza R. When less means more: Deactivations during encoding that predict subsequent memory. NeuroImage. 2004;23:921–927. doi: 10.1016/j.neuroimage.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Curr. Opin. Neurobiol. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Davachi L, Maril A, Wagner AD. When keeping in mind supports later bringing to mind: Neural markers of phonological rehearsal predict subsequent remembering. J. Cogn. Neurosci. 2001;13:1059–1070. doi: 10.1162/089892901753294356. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath D. Imaging recollection and familiarity in the medial temporal lobe: A three-component model. Trends Cogn. Sci. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AR, Ranganath C. The medial temporal lobe and recognition memory. Annu. Rev. Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Penny W, Phillips C, Kiebel S, Hinton G, Ashburner J. Classical and Bayesian inference in neuroimaging: Applications. NeuroImage. 2002;16:484–512. doi: 10.1006/nimg.2002.1091. [DOI] [PubMed] [Google Scholar]
- Gold BT, Buckner RL. Common prefrontal regions co-activate with dissociable regions during controlled semantic and phonological tasks. Neuron. 2002;35:803–812. doi: 10.1016/s0896-6273(02)00800-0. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: Functional imaging and the resting human brain. Nat. Rev. Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hockley WE, Cristi C. Tests of encoding trade-offs between item and associative information. Memory & Cognition. 1996;24:202–216. doi: 10.3758/bf03200881. [DOI] [PubMed] [Google Scholar]
- Jackson O, Schacter DL. Encoding activity in anterior medial temporal lobe supports associative recognition. NeuroImage. 2004;21:456–464. doi: 10.1016/j.neuroimage.2003.09.050. [DOI] [PubMed] [Google Scholar]
- Kirchhoff BA, Wagner AD, Maril A, Stern CE. Prefrontal-temporal circuitry for episodic encoding and subsequent memory. J. Neurosci. 2000;20:6173–6180. doi: 10.1523/JNEUROSCI.20-16-06173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan CB, Stark CEL. Medial temporal lobe activation during encoding and retrieval of novel face-name pairs. Hippocampus. 2004;14:919–930. doi: 10.1002/hipo.20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kučera H, Francis WN. Computational analysis of present-day American English. Brown University Press; Providence, RI: 1967. [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Encoding-specific effects of social cognition on the neural correlates of subsequent memory. J. Neurosci. 2004;24:4912–4917. doi: 10.1523/JNEUROSCI.0481-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar NA, Luna B, Sweeney JA, Eddy W. Combining brains: A survey of methods for statistical pooling of information. NeuroImage. 2002;16:538–550. doi: 10.1006/nimg.2002.1107. [DOI] [PubMed] [Google Scholar]
- Nelson DL, McEvoy CL, Schreiber TA. The University of South Florida word association, rhyme, and word fragment norms. 1998 doi: 10.3758/bf03195588. http://www.usf.edu/FreeAssociation. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Henson RNA, Rugg MD. Depth of processing effects on neural correlates of memory encoding-relationship between findings from across-and within-task comparisons. Brain. 2001;124:399–412. doi: 10.1093/brain/124.2.399. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Rugg MD. Task-dependency of the neural correlates of episodic encoding as measured by fMRI. Cereb. Cortex. 2001;11:1150–1160. doi: 10.1093/cercor/11.12.1150. [DOI] [PubMed] [Google Scholar]
- Paller KA, Wagner AD. Observing the transformation of experience into memory. Trends Cogn. Sci. 2002;6:93–102. doi: 10.1016/s1364-6613(00)01845-3. [DOI] [PubMed] [Google Scholar]
- Park H, Rugg MD. The relationship between study processing and the effects of cue congruency at retrieval: fMRI support for Transfer Appropriate Processing. Cereb. Cortex. 2008;18:868–875. doi: 10.1093/cercor/bhm130. [DOI] [PubMed] [Google Scholar]
- Park H, Uncapher MR, Rugg MD. Effects of study task on the neural correlates of source encoding. Learn. Memory. 2008;15:417–425. doi: 10.1101/lm.878908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. NeuroImage. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Prince SE, Daselaar SM, Cabeza R. Neural correlates of relational memory: Successful encoding and retrieval of semantic and perceptual associations. J. Neurosci. 2005;25:1203–1210. doi: 10.1523/JNEUROSCI.2540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc. Natl. Acad. Sci. USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JR, Donaldson DI, Wagner AD, Braver TS. Item- and task-level processes in the left inferior prefrontal cortex: positive and negative correlates of encoding. NeuroImage. 2004;21:1472–1483. doi: 10.1016/j.neuroimage.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Johnson JD, Park H, Uncapher MR. Encoding-retrieval overlap in human episodic memory: a functional neuroimaging perspective. In: Sossin W, Lacaille JC, Castellucci V, Belleville S, editors. Progress in brain research. Vol. 169. Elsevier; Amsterdam: 2008. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Otten LJ, Henson RN. The neural basis of episodic memory: Evidence from functional neuroimaging. Philos. T. Roy. Soc. B. 2002;357:1097–1110. doi: 10.1098/rstb.2002.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg MD, Schloerscheidt AM, Mark RE. An electrophysiological comparison of two indices of recollection. J. Mem. Lang. 1998;39:47–69. [Google Scholar]
- Sanquist TF, Rohrbaugh JW, Syndulko K, Lindsley DB. Electrophysiological signs of levels of processing: Perceptual analysis and recognition memory. Psychophysiology. 1980;17:568–576. doi: 10.1111/j.1469-8986.1980.tb02299.x. [DOI] [PubMed] [Google Scholar]
- Sperling R, Chua E, Cocchiarella A, Rand-Giovannetti E, Poldrack R, Schacter DL, Albert M. Putting names to faces: Successful encoding of associative mnemories activates the anterior hippocampal formation. NeuroImage. 2003;20:1400–1410. doi: 10.1016/S1053-8119(03)00391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield C, Greene M, Wager T, Egner T, Hirsch J, Mangels J. Neocortical connectivity during episodic memory formation. Plos. Biol. 2006;4:855–886. doi: 10.1371/journal.pbio.0040128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC. A population-average, landmark- and surface-based (PALS) atlas of human cerebral cortex. NeuroImage. 2005;28:635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Dickson J, Harwell J, Hanlon D, Anderson CH, Drury HA. An integrated software system for surface-based analyses of cerebral cortex. J. Am. Med. Inform. Assoc. 2001;8:443–459. doi: 10.1136/jamia.2001.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Davachi L. Cognitive Neuroscience: Forgetting of things past. Curr. Biol. 2001;11:R964–967. doi: 10.1016/s0960-9822(01)00575-9. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen B, Buckner RL. Building memories: Remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]