Abstract
Purpose:
This study investigated the penetration of lidocaine around and through a sutured incision following the application of iontophoretic and passive patches in the CD Hairless rat.
Methods:
Concentrations in localized areas (suture, dermis, subcutaneous, and vascular) were determined using microdialysis sampling followed by analysis using liquid chromatography with UV detection.
Results:
Iontophoresis significantly enhanced the dermal penetration of lidocaine. In an intact skin model, dermal concentrations were 40 times greater following iontophoretic delivery compared to passive delivery. In a sutured incision model, iontophoresis enhanced localized concentrations in the dermis, suture, and subcutaneous regions by 6, 15, and 20 fold, respectively. Iontophoretic delivery to a region containing a sutured incision was focused to the incision resulting in a greater increase in the suture concentration and in the subcutaneous region directly below the incision.
Conclusions:
The four microdialysis probe design was successful in the determination of localized drug penetration in a sutured incision model. Iontophoresis enhanced skin penetration and allowed for site specific delivery when applied to a sutured incision.
Keywords: iontophoresis, microdialysis, liquid chromatography, sutured incision, skin, transdermal drug delivery, percutaneous, lidocaine
Introduction
Surgery is a stressful event with a major concern being postoperative pain. It has been reported that primary hyperalgesia, an extreme sensitivity to pain, can persist for at least 4 days following an abdominal surgery (1). The use of anesthetics is a general practice that helps reduce or eliminate some of the pain endured. Lidocaine, a common anesthetic, has been shown to have local and general anesthetic properties. Systemic treatment using lidocaine has been shown to reduce post operative pain (1, 2). However, several studies report systemic administration of lidocaine produced minimal effects on reducing primary hyperalgesia following thermal injury or capsaicin application (3-5). The half-life of lidocaine in humans has been reported to be 80-108 minutes (6). Therefore for prolonged therapeutic effects, a continual lidocaine dose would have to be administered. Dermal application of lidocaine is another form of administration that has been shown to have great promise. As dermal drug application directly targets the site of interest, it minimizes potential side effects and drug-drug interactions in comparison to systemic delivery routes (7).
One of the main limitations to dermal penetration is the restrictive nature of the stratum corneum, the major diffusional barrier. In order to enhance dermal penetration chemical or physical modifiers are often employed. Of particular interest for this project is the use of iontophoresis.
Iontophoresis is an electrically driven technique that pushes charged and neutral compounds through the skin by the application of a current. The flow of ions during iontophoresis has been shown to follow the path of least resistance (8). In a system with intact skin, the greatest amount of ion flow has been shown to be through the sweat glands and the hair follicles (9, 10). It is therefore believed that when the skin is damaged by an incision an increased amount of ions will flow through the incision.
In order to assess the use of iontophoresis in a region with a sutured incision the use of multi-probe microdialysis experiments was employed as it has been shown to be useful in gathering data about drug locations and drug flow throughout the body (11-13). Due to the relatively small size of microdialysis probes, implantation of multiple probes within one organ or region is generally possible. For instance, Davies and Lunte utilized multiple linear probes in order to determine regional differences of drug and metabolite concentrations within the liver (14). The use of multiple probe microdialysis is an ideal technique to study drug penetration around a sutured incision.
The goal of this project was to utilize multi-probe microdialysis in the determination of lidocaine transport through and around a sutured incision in the CD hairless rat under both passive and iontophoretic conditions. This was completed by utilizing a four probe design in the vicinity of the sutured wound. In addition, lidocaine penetration through intact skin was investigated for comparison against lidocaine penetration through sutured skin.
Materials and Methods
Chemicals
Lidocaine HCl solution (40 mg/mL) was provided by Hospira, Inc. (Lake Forest, IL). A 4% lidocaine HCl, 2% HPMC gel and 0.9% NaCl, 2% HPMC gel was provided by Travanti Pharma Inc. (Mendota Heights, MN). Ringer's solution consisted of 145 mM NaCl, 2.8 mM KCl, 1.2 mM CaCl2, and 1.2 mM MgCl2 all purchased from Fisher Scientific (Fair Lawn, NJ). HPLC grade acetonitrile was purchased from Fisher Scientific. Ketamine was manufactured by Fort Dodge Animal Health (Fort Dodge, IA), xylazine was made by Ben Venue Laboratories (Bedford, OH), acepromazine was manufactured by Boehringer Ingelheim (St. Joseph, MO), and AErrane (isoflurane) was purchased from Baxter (Deerfield, IL). Microdialysis probes and surgical instruments were sterilized by ethylene oxide using an Anprolene gas sterilizer (AN 74i, Anderson Products, Inc., Haw River, NC). Water for buffer preparation was filtered through a Labconco, Water Pro Plus.
Chromatographic System
The chromatographic system consisted of a Shimadzu LC-20AD pump, Shimadzu SCL-10AVP system controller, Shimadzu SIL-20AC auto sampler, and a Shimadzu SPD-10A UV-Vis spectrophotometric detector (Shimadzu Scientific instruments, Inc., Columbia, MD). Run conditions consisted of a 40:60 (v:v) mobile phase of acetonitrile in a 50 mM phosphate buffer (pH 6.6) at a flow rate of 0.20 mL/min and a wavelength of 210 nm. Chromatographic data were acquired using EZ Start Software (Shimadzu Scientific Instruments, Inc., Columbia, MD). A Zorbax Eclipse XDB-C18 column (150 × 2.1 mm, 3.5 um), purchased from Agilent (Palo Alto, CA), was used with a sample injection volume of 15 μL.
Animals
CD Hairless male rats (Charles River Laboratories, Raleigh, NC) 6-7 weeks of age were housed in cages that were maintained in temperature-controlled rooms with free access to food and water. All experiments were in accordance with the Principles of Laboratory Animal Care (NIH Publication no. 85-23, revised 1985).
Surgical Procedure
One day prior to patch/drug application, the rats were anesthetized by inhalation of isofluorane followed by subcutaneous injection of a ketamine (67.5 mg/kg), acepromazine (0.67 mg/kg), and xylazine (3.4 mg/kg) mixture. A subcutaneous injection of atropine (0.05 mg/kg) was given to reduce bronchial and salivary secretions. As necessary, additional ketamine (20 mg/kg, intramuscular) was given to maintain anesthesia. The animal's body temperature was maintained until it had recovered from surgery by placing the animal on a heating pad. Strict aseptic techniques were used during the surgical procedure.
The sparse amount of fur at the treatment site was shaved using an Oster® Vorteq clipper. After cleaning the skin by swabbing betadiene and 70% alcohol alternating three times each, a small incision was made to expose the jugular vein, which was separated from the artery and nerve. A vascular microdialysis probe was inserted in the vein and advanced distally and affixed by tying two sutures around the vein and probe. The end of the vascular probe was tunneled using a trocar to the nape of the neck. Finally, the incision was sutured shut. The rat was turned over and a 1-cm long full thickness skin incision was made on the left lumbar region of the rat. Two 5 mm linear microdialysis probes were implanted in-line with the surgical incision. One probe was sutured into the incision using a 4-0 Dexon II suture (Tyco Healthcare, King of Prussia, PA) and one probe was implanted in the subcutaneous layer. One additional probe was implanted in the dermis parallel to the incision but at a distance of 5-10 mm medially. The probes were implanted with a 23-gauge needle by threading one end of the probe's polyimide tubing through the needle. The needle was withdrawn leaving the dialysis membrane imbedded in the surrounding tissue. The polyimide inlet and outlet were protected using MRE-040 tubing. The tubing was then held secured to the polyimide by UV glue. This tubing kept the dermal probe fixed. In order to keep the subcutaneous probe stationary, a small suture was placed in the muscle and tied to the polyimide above and below the active membrane of the subcutaneous probe. The probes were then tunneled under the skin to the nape of the neck using a trocar. At the end of the surgical procedure, 10 mL sterile saline solution was given subcutaneously to replace fluids that may have been lost during surgery. The animal was placed in a harness for attachment to the Raturn awake animal containment system (Bioanalytical Systems, Inc., West Lafayette, IN). The probes were pulled through a small hole in the harness for attachment to the microdialysis system.
Dialysis System
Linear microdialysis probes with a 5 mm polyacrylonitrile (PAN) membrane and vascular microdialysis probes with a 10 mm PAN membrane were constructed in house. The inlet of the microdialysis probe was connected with fluorinated ethylenepropylene (FEP) tubing to a Hamilton syringe mounted on a CMA 400 syringe pump (North Chelmsford, MA). The microdialysis probe outlet was connected to a Honeycomb Refrigerated Fraction Collector (BAS, West Lafayette, IN) with FEP tubing. The infusion pump delivered the perfusion medium at a flow rate of 1 μL min−1.
Microdialysis Probe Construction
Linear microdialysis probes with a 5 mm dialysis membrane made of a polyacrylonitrile (PAN) membrane (Hospal Industrie, Meyzieu, France) were used. Probes were fabricated by placing two lengths of polyimide tubing (MicroLumen® Inc., Tampa, FL) (173 μm i.d., 216 μm o.d.) into the ends of a PAN membrane (240 μm i.d., 340 μm o.d., molecular weight cut off of 29 kDa). The connections were secured with UV glue (UVEXS, Sunnyville, CA).
Vascular probes (Figure 1) containing a 10 mm active membrane window were constructed by first inserting a piece of polyimide (o.d. 175 μm, i.d. 125 μm) into a 10 mm length of micro-renathane surgical tubing (MRE-033, Braintree Scientific, MA) so that 10 mm length of polyimide was exposed. The exposed polyimide was threaded into a piece of PAN membrane. The membrane was also threaded into the MRE-033 tubing. The end of the membrane was sealed with UV glue and glued to the MRE-033 tubing. A second piece of polyimide was inserted approximately one third into the top of the MRE-033 and secured with UV glue. The polyimide ends were protected by threading them into a piece of MRE-040 tubing. Two large beads of glue were placed at the junction of MRE-033 and MRE-040 tubing.
Figure 1.
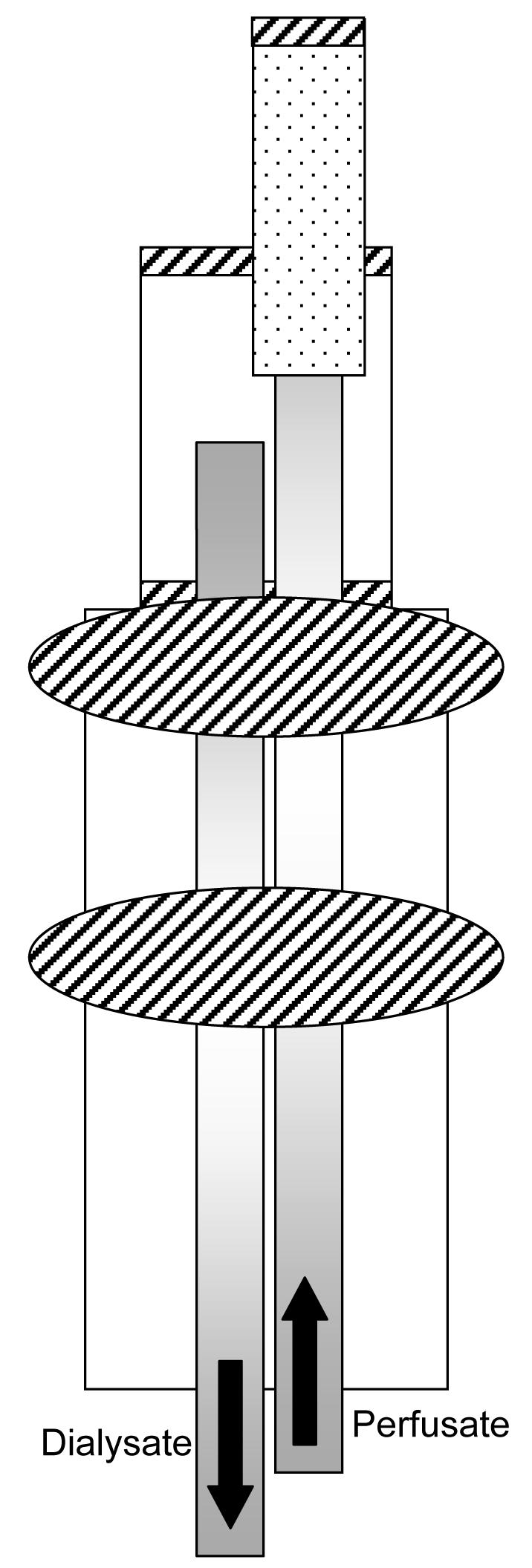
Vascular probe constructed in-house. Shaded rectangles represent polyimide tubing, dotted material is PAN membrane, diagonal stripes depict UV glue, and remaining tubing is MRE-033 or MRE-040. Picture not drawn to scale.
Iontophoretic Patch-Principle of Operation
The single-use, disposable iontophoretic patches (provided by Travanti Pharma, Inc., Mendota Heights, MN) consisted of medical adhesive tape, absorbent pads, an internal transistor, supplemental power source, and electrodes composed of silver and silver-silver chloride for the anode and cathode, respectively. Each patch had an active positive electrode delivery area of 2.25 cm2 which was positioned over the incision site. Iontophoretic patches operated at 0.15 mA/cm2 +/− 10%.
Dosing Protocol
Uniform layers of 4% Lidocaine HCL, 2% HPMC (0.4 mL) and 0.9% NaCl, 2% HPMC gel (0.4 mL) were applied to individual absorbent pads 1.5 hours prior to application. Once imbibed, the lidocaine pad was placed onto the positive electrode and the saline pad was placed onto the negative electrode. Prior to patch application, an additional 0.4 mL of lidocaine gel was applied over the suture region and 0.4 mL of saline gel was applied onto the contralateral (right) side of the rat back. This was completed by first dispensing the gel onto the dermis and then evenly spreading the gel over the patch site using a cotton swab.
Patch Application
Prior to patch application, the rat was lightly sedated using isoflurane (to effect) in order to limit movement that could hinder proper placement of the patch. The rat was removed from the rodent bowl and placed on a short table that allowed it to remain connected to the Raturn containment system. The suture region and site for saline application were covered using strips of adhesive while the back of the rat was sprayed with Mastisol®, a liquid adhesive. The patch was applied while the back of the rat was half arched and pressed securely into place. Three strips of adhesive were wrapped around the rat to ensure good contact between the patch and the application site. The rat was returned to the rodent bowl and allowed to recover.
Statistical Analysis
Statistical analysis was completed by first testing for homogeneity using Hartley's F-max test. The non-parametric test of Mann-Whitney U test was used for all data comparisons. Results were reported at the 95% confidence limit level. All results are reported as mean +/− standard deviation
Calibration of Microdialysis Probe
Determination of microdialysis probe extraction efficiency was completed by a delivery experiment of lidocaine. Following an overnight recovery, a 1 μg/mL solution of lidocaine was perfused through the microdialysis probes at a flow rate of 1 μL/min and sampled in thirty minute increments. After five steady state samples, probes were again perfused with Ringer's solution. The extraction efficiency was calculated using the equation below as previously described (15).
Results
Microdialysis Probe Calibration
Probe calibration was completed for each individual probe at the start of every experiment using the delivery method previously described. Mean percent delivery for each probe can be seen in Table I. In general, delivery to the subcutaneous, suture, and dermal probes were found to be approximately the same. Lidocaine delivery determined in the vascular probe was over two times greater than microdialysis probes in the other tissue regions. This is in part due to the larger membrane size (10 mm vs. 5 mm) and also related to the hydrodynamic environment of the blood vessel which allows for the removal of lidocaine near the probe membrane.
Table I.
Mean percent delivery of microdialysis probes
| Probe | Mean % Delivery |
|---|---|
| Vasculara | 70.5 ± 14.0 |
| Subcutaneousb | 29.7 ± 5.5 |
| Sutureb | 26.4 ± 7.5 |
| Dermalb | 22.9 ± 5.3 |
n∼15
n∼30
Several issues in microdialysis sampling have been shown to affect the extraction efficiency during the course of an experiment. These include immunological responses around the membrane and blood flow changes (15, 16). For example in vivo fouling, which is a result of protein or macromolecule absorption to the microdialysis membrane after extended implantation lengths, would result in a decrease in the extraction efficiency. The use of iontophoresis has the potential of multiple side effects that include change in blood flow, edema, erythema, changes in pH, and increase in temperature (17). Therefore to assess possible changes in extraction efficiencies that could result from the use of iontophoresis, control calibration studies were completed that included continuous calibration throughout the length of an experiment with and without the addition of an iontophoretic patch.
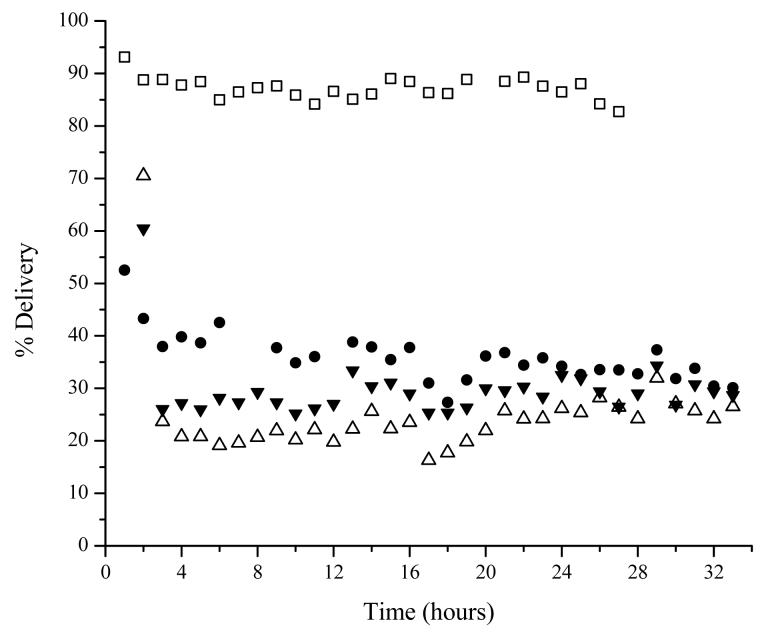
For the initial calibration study, a solution of 1 μg/mL lidocaine was perfused through the microdialysis probes for a total of 32 hours in order to allot time for the initial calibration completed at the start of every experiment (Figure 2). Steady state was found to occur following the first one hour collection period. All probes, except the subcutaneous probe, were found to have approximately the same percent delivery throughout the course of the 32 hours. The extraction efficiency of the subcutaneous probe was found to decrease to approximately 80% of its original value, when comparing the first four hours of collection to the end of the sampling period.
Figure 2.
Representative plot of a continuous calibration of 1 μg/mL lidocaine for 32 hours. Probe locations are: vascular □, subcutaneous ●, suture △, and dermis 5-10 mm medial to suture ▼.
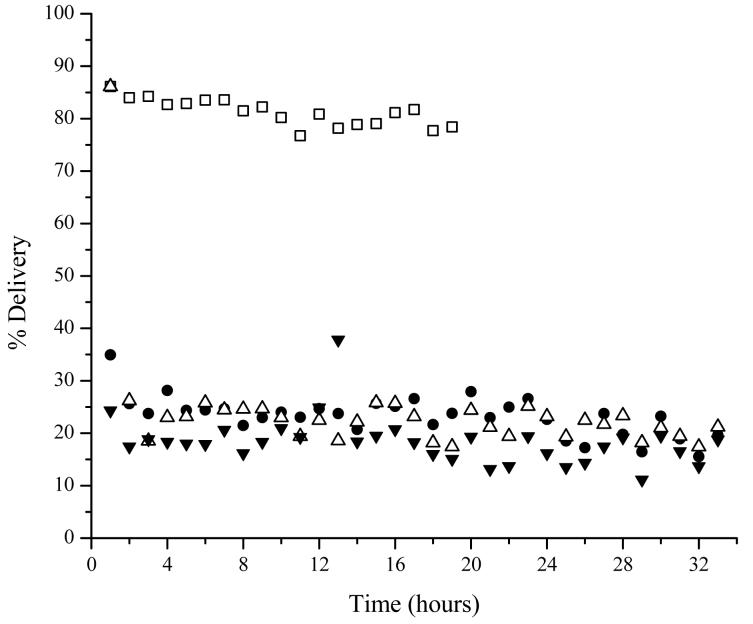
In order to assess the effects that iontophoresis has on probe calibration, the experiment was repeated with an iontophoresis patch, with saline in both drug reservoirs, applied after the first eight hours of calibration. A similar experiment was completed by Stagni et al. who monitored microdialysis recovery over a two hour sampling period. Their results indicated no change in microdialysis recovery (17). This experiment was repeated for this study since sampling times were much greater. Again, probe deliveries were found to be approximately the same throughout the course of the sampling period (Figure 3). In addition, lidocaine delivery in the subcutaneous probe was consistent the entire time and did not decrease as previously seen without the addition of an iontophoretic patch. This demonstrates that the recovery of lidocaine during the course of the 24 hour sampling period was constant and unaffected from any physiological responses caused from the iontophoretic patch.
Figure 3.
Representative plot of a continuous calibration of 1 μg/mL lidocaine for 32 hours. At the eight hour time point, a silver constructed iontophoretic patch with saline gel in both drug reservoirs was applied for the remainder of the calibration. Probe locations are: vascular □, subcutaneous ●, suture △, and dermis 5-10 mm medial to suture ▼.
Iontophoretic Patch Results
Intact Skin Model: Passive vs. Iontophoretic
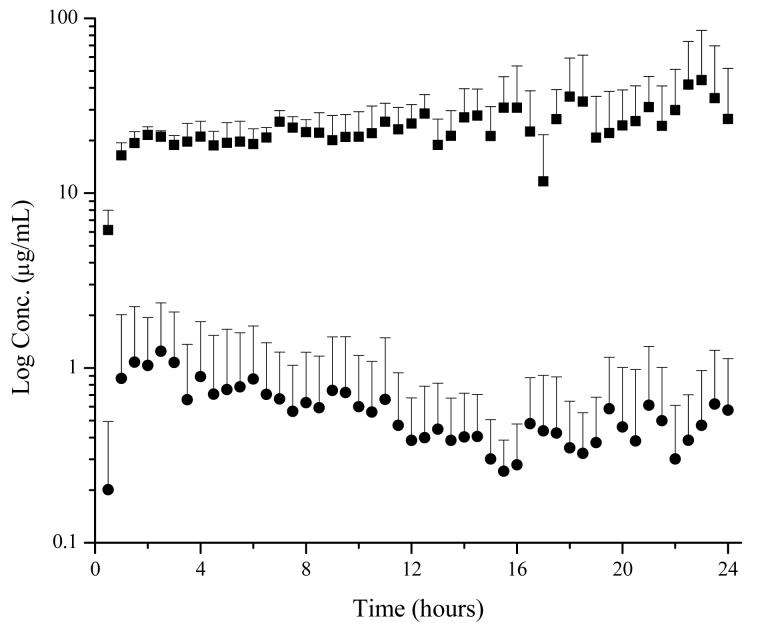
The primary focus of this study was to evaluate the effects of drug delivery by iontophoresis compared to passive delivery. Iontophoresis operating at 0.15 mA/cm2 significantly enhanced lidocaine penetration compared to passive diffusion when using an intact skin model (Figure 4). Localized skin concentrations after twelve hours of delivery using an iontophoretic patch was approximately 20 μg/mL, a forty fold increase over passive delivery. In addition, the total area under the curve (AUCtot) for dermal penetration was 55 times greater for iontophoretic delivery compared to passive delivery. The use of iontophoresis also resulted in an increased level of free lidocaine in the blood. On average, free lidocaine concentration in the blood after twelve hours of iontophoretic delivery was 0.015 μg/mL, a five fold increase to passive delivery. Similarly, the AUCtot in plasma was 15 times greater for iontophoretic delivery.
Figure 4.
Effects of iontophoresis on lidocaine penetration in a CD Hairless rat with intact skin. ■ 0.15 mA/cm2 iontophoresis, ● passive
Sutured Incision Model: Passive
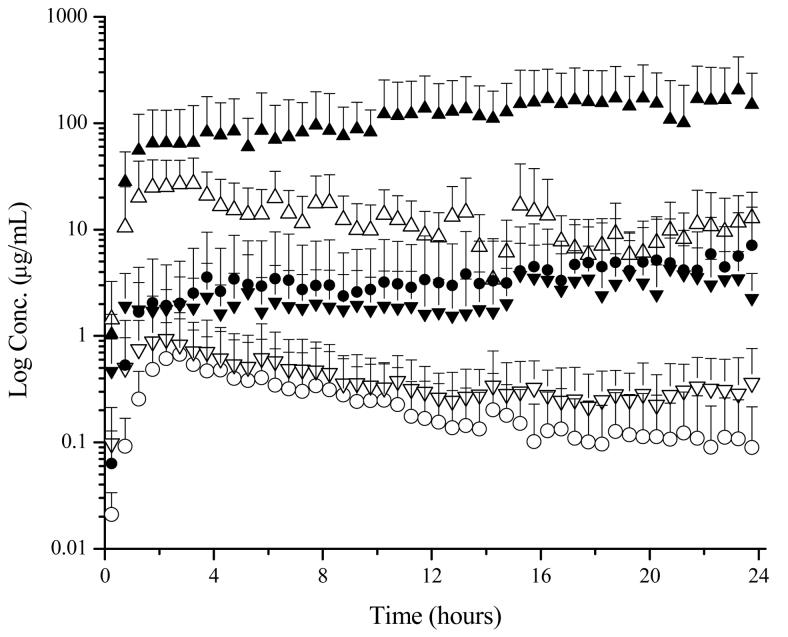
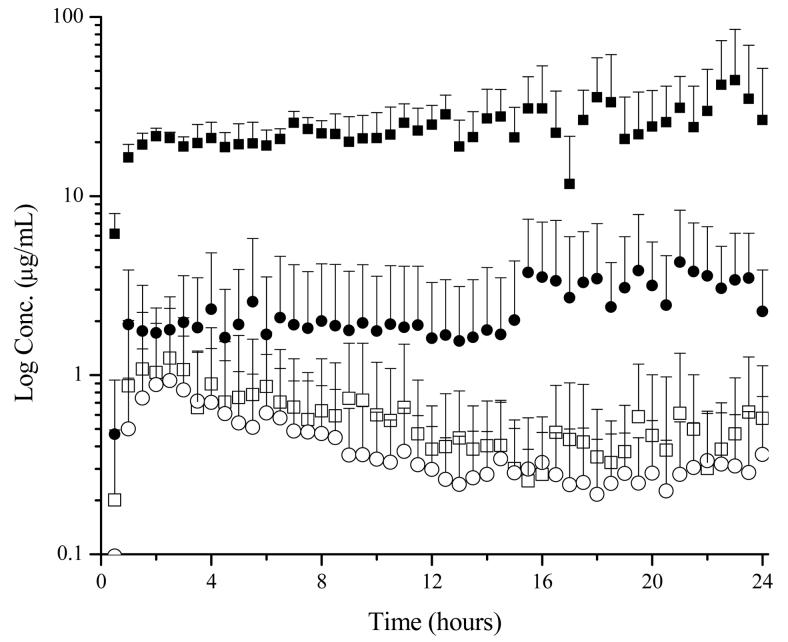
Next, the use of the four microdialysis probe design was tested in a system with a sutured incision following passive delivery of lidocaine. This design was successful in the determination of localized drug levels. As expected, an increased amount of drug was found to penetrate through the incision in comparison to the intact skin adjacent to the incision (Figure 5). Following twelve hours of delivery, the lidocaine concentration in the suture was approximately 8 μg/mL, 30 times greater than the dermal drug concentration. The concentration of lidocaine in the subcutaneous region directly below the incision was similar to the dermal concentration; however there was a slight delay in the initial absorption of lidocaine.
Figure 5.
Time course of lidocaine concentrations in selected regions following the application of a 4% lidocaine HCl, 2% HPMC (0.4 mL) gel. Probe locations are as follows: ● subcutaneous, ▲ suture, and ▼ dermis 5-10 mm medial to suture. Passive conditions are shown as open symbols and iontophoretic conditions (0.15 mA/cm2) are shown as shaded symbols.
Sutured Incision Model: Iontophoretic
Lastly, the four microdialysis probe design was utilized for the determination of localized concentrations in a sutured incision during iontophoretic delivery. As previously noted with intact skin, iontophoresis enhanced lidocaine delivery in all four regions tested (Figure 5). Lidocaine concentrations reached their plateau values approximately two hours after patch application. This demonstrated a faster delivery rate compared to passive diffusion where Cmax was reached approximately three hours after application. In addition lidocaine concentrations were found to slowly increase during the course of the experiment, a result that might be explained by skin hydration. Previous studies have demonstrated increases in water permeation for up to 30 hours of hydration which results in an increased permeation across the skin (8, 18).
A significant difference (p < 0.05) in the AUCtot was determined in both the vascular and suture microdialysis probes when using an iontophoretic patch operating at 0.15 mA/cm2 compared to the passive patch (Table II). This resulted in a nine fold increase in the AUCtot for the suture region and a three fold increase for the vascular AUCtot. The subcutaneous (p < 0.08) and dermal (p < 0.08) AUCtot were also found to be different compared to the passive patch values. A 16 fold increase in the AUCtot was observed for the subcutaneous region, while a four fold increase was noted for the dermal AUCtot.
Table II.
Mean data obtained for passive and iontophoretic (0.15 mA/cm2) patches in CD hairless rats with a sutured incision.
| Group | Units | Dermal | Suture | Subcutaneous | Vascular | |
|---|---|---|---|---|---|---|
| Passive n=4 |
AUCtot | hr·μg/mL | 9.6 ± 8.5 |
302 ± 208 | 5.4 ± 6.5 | 0.41 ± 0.19 |
| Iontophoresis n=4 |
AUCtot | hr·μg/mL | 44 ± 43 | 2720* ± 2560 |
80 ± 120 | 1.09* ± 0.16 |
p < 0.05
The enhancement of lidocaine delivery was also observed in the localized tissue concentrations. After 12 hours, lidocaine concentrations obtained from passive delivery achieved an approximate steady state and were therefore used for comparison purposes. At this time point, dermal lidocaine concentrations achieved using the iontophoretic patch were six times greater than those obtained by passive diffusion and the free blood lidocaine concentrations were approximately three and a half times the concentration obtained from passive diffusion. The greatest marked differences in local concentrations following iontophoretic delivery were lidocaine levels in the suture and subcutaneous regions. Lidocaine concentrations in the suture were 15 times greater while subcutaneous amounts were nearly 20 times greater using iontophoresis compared to delivery using the passive patch.
Discussion
Variability of Results
An in-depth analysis into the variation associated with the dermal, subcutaneous, and suture microdialysis probes was completed. Variations near 100 percent were determined for both the Cmax and AUCtot for these three tissue regions. However, the amount of variation in the free lidocaine concentration was more acceptable and ranged from 15 – 50% for the AUCtot.
As previously reported in the literature, high inter- and intra-individual variability of exogenous species for cutaneous microdialysis is generally noted (19-24). Several factors contribute to this, including differences in skin barrier function which could affect skin absorption rates, metabolism and diffusion rate to the systematic circulation, and differences in probe implantation, mainly probe depth. An analysis of the microdialysis probe depths revealed a weak correlation (R2 = 0.76) to the AUCtot but only when comparing probe depths greater than 0.4 mm. Furthermore, the amount of variation observed with this data set is comparable to previously reported data containing similar sample sizes (20-22, 25-27).
Several factors could have influenced the amount of variation noted in both the suture and subcutaneous microdialysis probes. First of all, the implantation of a microdialysis probe within an incision was a new surgical technique therefore probe placement was evaluated for each individual rat via histological examinations. The location of the microdialysis probe in the suture was consistently found to be at the deeper region of the dermis while the location of the subcutaneous probe was either directly below the suture microdialysis probe or was offset slightly, which could contribute to the variation seen in the subcutaneous measurements. Another consideration for the large variation was differences in the rate of healing between rats. It has been shown that incisional wounds often begin epithelialization within the first 24 – 48 hours following the injury (28). Since the patch is not applied until approximately 30 hours post-incision, differences in healing could lead to considerable changes in penetration. However, a study conducted by Spence and Pomeranz showed minimal increases in skin resistance up to five days after injury (29). In this study they made a one centimeter incision on the dorsal region and monitored wound healing by measuring the electrical resistance of the epidermis over the course of 20 days. Initial resistances were found to be low the first two days of healing with a marked increase in resistance beginning around day five (29). As the flow of ions under iontophoresis is through the region of least resistance, it would seem that a large increase in penetration should be detected in the suture region for the initial five days following the incision.
Other areas that could affect drug penetration through the suture include the angle in which the incision is made, the position of the incision on the rat, the lay of the skin following suturing, the tightness of the suture and the positioning of the patch over the incision. These particular details are defined by the surgeon and the particular procedure completed on the patient. The variation seen within this study was created by one surgeon and the deviations from multiple surgeons could be much greater.
As the amount of variation within the suture data was found to be large, it would be expected that the data obtained for a microdialysis probe directly below the suture would also contain a large amount of variation. For instance, rats that had greater amounts of lidocaine penetrating through the suture resulted in a greater concentration detected in the subcutaneous tissue directly below the incision. As previously mentioned, the position of the subcutaneous probe was found to be offset in some of the rats which would result in lower drug concentrations.
Iontophoretic Delivery in Skin Region Containing a Sutured Incision
The use of iontophoresis enhanced the amount of lidocaine delivered in both the intact skin model and in the sutured incision skin model. Using the intact skin model the localized dermal lidocaine concentration was 40 fold greater when using iontophoresis compared to passive delivery. The sutured skin model also saw localized concentration enhancement following iontophoretic treatment, however increased lidocaine concentrations in the dermal, suture, and subcutaneous regions were only 6.5, 14, and 20 fold, respectively.
A comparison of the localized lidocaine concentration between passive and iontophoretic delivery revealed differences in the magnitude each region was affected by iontophoresis. Lidocaine concentration in both the suture and the subcutaneous region directly below the suture had a greater percent increase compared to the dermis 5-10 mm medial to the incision. This suggests that drug delivery from the iontophoretic patch was not uniform throughout the patch surface area. Control experiments were completed where the rat skin remained intact and two dermal microdialysis probes were implanted 5-10 mm parallel to one another. Results from these studies demonstrated that drug delivery was uniform throughout the entire area of the iontophoretic patch. This demonstrates that when iontophoresis is applied to a region containing a sutured incision, drug delivery will be focused to the sutured skin region. As shown, this results in a greater increase in the suture concentration and in the subcutaneous region directly below the incision.
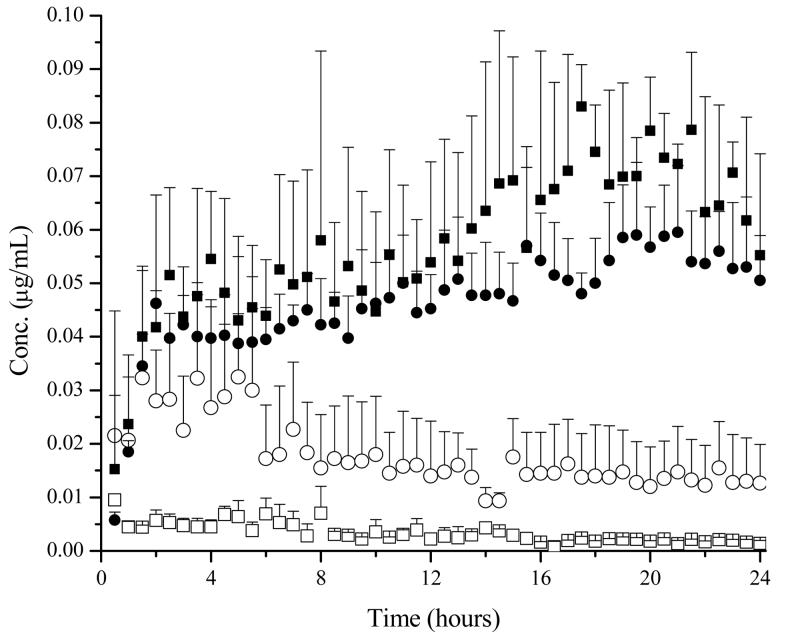
The use of iontophoresis as a site specific drug delivery device when applied to a region with a sutured incision was further verified when comparing the dermal concentrations in both intact skin and in the dermis 5-10 mm medial from an incision. When lidocaine was allowed to penetrate due to diffusion as with the passive patch, similar dermal concentrations were found in intact skin and sutured skin (Figure 6). When driven by current, dermal concentrations were found to be different based on the presence of a suture. With a suture present, dermal lidocaine levels approximately 5 – 10 mm medial from the suture were found to be roughly 2 – 3 μg/mL. However, when the entire skin was intact, dermal concentrations were approximately 20 μg/mL (Figure 6). The addition of an incision was found to significantly (p < 0.05) affect the dermal concentration when placed under iontophoresis (Table III). The AUCtot of lidocaine in the dermis was found to be approximately 12 times greater when the skin was intact compared to sutured skin under iontophoretic conditions. When a suture is present, it appears that a majority of ions will follow the path of least resistance and flow along the break in the dermis.
Figure 6.
Dermal concentrations of lidocaine when placed under passive and iontophoretic conditions (0.15 mA/cm2). Symbols: Intact skin ■; sutured skin ●. Passive conditions are shown as open symbols and iontophoretic conditions are shown as shaded symbols.
Table III.
Compiled dermal data for both skin models and treatment groups.
| Group | Units | Passive | Iontophoresis* | |
|---|---|---|---|---|
| With Incision | AUCtot | hr·μg/mL | 9.6 ± 8.5 | 44.3 ± 43.1 |
| Without Incision | AUCtot | hr·μg/mL | 10.3 ± 8.1 | 564 ± 187 |
p < 0.05
The addition of an incision also played an important role in the resulting free blood concentration of lidocaine. When lidocaine penetrated the skin under passive conditions, an increased concentration of free lidocaine was observed in the blood when a suture was present compared to intact skin (Figure 7). The AUCtot was also found to be five times greater when a suture was present compared to intact skin when tested using passive conditions (Table IV). However when a current was applied, the free lidocaine concentration between intact skin and sutured skin was found to be similar (Figure 7). The presence of an incision did not play a role in the total concentration of lidocaine to reach the vascular system when placed under iontophoresis. These results suggest that the free blood concentration is dictated by the overall current of the iontophoretic patch and is independent of the pathway the ions travel.
Figure 7.
Free lidocaine blood concentrations when placed under passive and iontophoretic conditions (0.15 mA/cm2). Symbols: Intact skin ■; sutured skin ●. Passive conditions are shown as open symbols and iontophoretic conditions are shown as shaded symbols.
Table IV.
Compiled vascular data for both skin models and treatment groups.
| Group | Units | Passive* | Iontophoresis | |
|---|---|---|---|---|
| With Incision | AUCtot | hr·μg/mL | 0.41 ± 0.19 | 1.09 ± 0.16 |
| Without Incision | AUCtot | hr·μg/mL | 0.080 ± 0.005 | 1.30 ± 0.36 |
p < 0.05
Overall, the presence of an incision was found to significantly affect drug flow as shown by both the dermal and suture concentrations. Under passive conditions, dermal concentrations were similar in both intact and sutured skin. However, free lidocaine concentrations in plasma were significantly greater when a suture was present. Under iontophoretic conditions free lidocaine concentrations in plasma were not affected by the presence of a suture, however dermal concentrations were significantly less when a suture was present. When sutured skin is placed under iontophoresis, the majority of drug flow is re-directed through the incision.
Conclusions
The use of iontophoresis for cutaneous drug delivery of lidocaine significantly enhanced dermal penetration. Using an intact skin model, an iontophoretic patch operating at 0.15 mA/cm2 resulted in a 40 fold increase in dermal lidocaine concentrations in comparison to passive drug delivery. When this system was applied to a sutured skin model, increased penetration values were determined in each of the suture, dermal, subcutaneous, and vascular microdialysis probe locations. Increases in localized regions of the vascular, dermal, suture, and subcutaneous were 3.5, 6, 15, and 20 fold, respectively.
The presence of an incision played a significant role in the path of drug flow. Overall, penetration of lidocaine was greater in the incision, when compared to adjacent intact skin, suggesting the dominant route of delivery is through the path of least resistance. This was noted under both passive and iontophoretic conditions. Under passive conditions, free lidocaine in plasma was found to be significantly greater with the existence of an incision. However, there was no change in the dermal lidocaine levels. When exposed to iontophoresis, the dermal concentration was found to be significantly less when a suture was present compared to intact skin. The total free lidocaine concentration however, was found to be unaffected by the presence of an incision. This demonstrates that total drug concentration in plasma is independent of the direction of drug flow.
Microdialysis was shown to be an important tool in the determination of lidocaine penetration in a region with a sutured incision for passive and iontophoretic delivery. Using traditional blood sampling methods, a difference in the total amount of drug to reach the systemic system would be determined for passive diffusion. Blood sampling would have shown that similar systemic levels were obtained following iontophoretic delivery but would provide no additional information on the route of drug penetration. In addition, dermal sampling by the standard tape stripping method would not allow for the appropriate site specific sampling needed for this study, nor would it provide continues analysis over time. Multi-probe microdialysis was successful in providing a detailed picture of drug flow in a sutured incision system.
Acknowledgements
The authors would like to thank the Lawrence Memorial Hospital, especially Dr. Mike Thompson, for their help with processing the histology slides. This work was funded by Travanti Pharma, Inc.
References
- 1.Kawamata M, Takahashi T, Kozuka Y, Nawa Y, Nashikawa K, Narimatsu E, Watanabe H, Namiki A. Experimental incision-induced pain in human skin: effects of systemic lidocaine on flare formation and hyperalgesia. Pain. 2002;100:77–89. doi: 10.1016/s0304-3959(02)00233-6. [DOI] [PubMed] [Google Scholar]
- 2.Wallace MS, Laitin S, Licht D, Yaksh TL. Concentration-effect relations for intravenous lidocaine infusions in human volunteers: effects on acute sensory thresholds and capsaicin-evoked hyperpathia. Anesth. 1997;86:1262–1272. doi: 10.1097/00000542-199706000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Dirks J, Fabricius P, Petersen KL, Rowbotham MC, Dahl JB. The effect of systemic lidocaine on pain and secondary hyperalgesia associated with the heat/capsaicin sensitization model in healthy volunteers. Anesth Analg. 2000;91:967–72. doi: 10.1097/00000539-200010000-00037. [DOI] [PubMed] [Google Scholar]
- 4.Gottrup H, Hansen PO, Arendt-Nielsen L, Jensen TS. Differential effects of systemically administered ketamine and lidocaine on dynamic and static hyperalgesia induced by intradermal capsaicin in humans. Br J Anesth. 2000;84:155–62. doi: 10.1093/oxfordjournals.bja.a013396. [DOI] [PubMed] [Google Scholar]
- 5.Holthusen H, Irsfeld S, Lipfert P. Effect of pre- or post-traumatically applied i.v. lidocaine on primary and secondary hyperalgesia after experimental heat trauma in humans. Pain. 2000;88:295–302. doi: 10.1016/S0304-3959(00)00338-9. [DOI] [PubMed] [Google Scholar]
- 6.Wilson DV, Barnes KS, Hauptman JG. Pharmacokinetics of combined intraperitoneal and incisional lidocaine in the dog following ovariohysterectomy. J Vet Pharmacol Ther. 2004;27:105–109. doi: 10.1111/j.1365-2885.2004.00552.x. [DOI] [PubMed] [Google Scholar]
- 7.Argoff CE, Galer BS, Jensen MP, Oleka N, Gammaitoni AR. Effectiveness of the lidocaine patch 5% on pain qualities in three chronic pain states: assessment with the neuropathic pain scale. Curr Med Res and Opin. 2004;20:S21–S28. doi: 10.1185/030079904X12960. [DOI] [PubMed] [Google Scholar]
- 8.Smith EW, Maibach HI. Percutaneous Penetration Enhancers. CRC; New York: 1995. [Google Scholar]
- 9.Banga AK. Electrically assisted transdermal and topical drug delivery. Taylor and Francis; 1998. [Google Scholar]
- 10.Turner NG, Ferry L, Price M, Cullander C, Guy RH. Iontophoresis of poly-L-lysines: the role of molecular weight? Pharm Res. 1997;14:1322–1331. doi: 10.1023/a:1012100100865. [DOI] [PubMed] [Google Scholar]
- 11.Huang Y-J, Liao J-F, Tung-Hu T. Concurrent determination of thalidomide in rat blood, brain, and bile using multiple microdialysis coupled to liquid chromatography. Biomed Chrom. 2005;19:488–493. doi: 10.1002/bmc.466. [DOI] [PubMed] [Google Scholar]
- 12.Tung-Hu T. Pharmacokinetics of pefloxacin and its interaction with cyclosporin A, a p-glycoprotein modulator in rat blood, brain, and bile, using simultaneous microdialysis. Br J Pharm. 2001;132:1310–1316. doi: 10.1038/sj.bjp.0703927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ting-Yu T, Tung-Hu T. Measurement of unbound geniposide in blood, liver, brain, and bile of anesthetized rats: an application of pharmacokinetic study and its influence on acupuncture. Anal Chem Acta. 517:47–52. 204. [Google Scholar]
- 14.Davies MI, Lunte CE. Simultaneous microdialysis sampling from multiple sites in the liver for the study of phenol metabolism. Life Sci. 1996;59:1003–1013. doi: 10.1016/0024-3205(96)00407-9. [DOI] [PubMed] [Google Scholar]
- 15.Song Y, Lunte CE. Calibration methods for microdialysis sampling in vivo: muscle and adipose tissue. Anal Chim Acta. 1999;400:143–152. [Google Scholar]
- 16.Stenken JA. Methods and issues in microdialysis calibration. Anal Chem Acta. 1999;379:337–358. [Google Scholar]
- 17.Stagni G, O'Donnell D, Liu YJ, Kellogg DL, Jr., Shepherd AMM. Iontophoretic current and intradermal microdialysis recovery in humans. J Pharm Tox. 1999;41:49–54. doi: 10.1016/s1056-8719(99)00027-1. [DOI] [PubMed] [Google Scholar]
- 18.Bronaugh RL, Maibach HI. Percutaneous Absorption. In: Calnan CD, Maibach HI, editors. Dermatology. Vol. 6. Marcel Dekker, Inc.; New York: 1985. [Google Scholar]
- 19.Schnetz E, Fartasch M. Microdialysis for the evaluation of penetration through the human skin barrier- a promising tool for future research? Eur J Pharm Sci. 2001;12:165–174. doi: 10.1016/s0928-0987(00)00155-x. [DOI] [PubMed] [Google Scholar]
- 20.Ault JM, Riley CM, Meltzer NM, Lunte CE. Dermal microdialysis sampling in vivo. Pharm Res. 1994;11:1631–1639. doi: 10.1023/a:1018922123774. [DOI] [PubMed] [Google Scholar]
- 21.Chaturvedula A, Joshi DP, Anderson C, Morris R, Sembrowich WL, Banga AK. Dermal, subdermal, and systematic concentrations of granisetron by iontophoretic delivery. Pharm Res. 2005;22:1313–1319. doi: 10.1007/s11095-005-5335-z. [DOI] [PubMed] [Google Scholar]
- 22.Kreilgaard M. Dermal pharmacokinetics of microemulsion formulations determined by in vivo microdialysis. Pharm Res. 2001;18:367–373. doi: 10.1023/a:1011067300397. [DOI] [PubMed] [Google Scholar]
- 23.Mathy F-X, Ntivunwa D, Verbeeck RK, Préat V. Fluconazole distribution in rat dermis following intravenous and topical application: a microdialysis study. J Pharm Sci. 2005;94:770–780. doi: 10.1002/jps.20290. [DOI] [PubMed] [Google Scholar]
- 24.Müller M, Schmid R, Wagner O, Osten B. v., Shayganfar H, Eichler HG. In vivo characterization of transdermal drug transport by microdialysis. J Controlled Release. 1995;37:49–57. [Google Scholar]
- 25.Kreilgaard M. Assessment of cutaneous drug delivery using microdialysis. Bulletin Technique Gattefosse. 2002;95:101–122. doi: 10.1016/s0169-409x(02)00117-5. [DOI] [PubMed] [Google Scholar]
- 26.Kreilgaard M. Dermal pharmacokinetics of microemulsion formulations determined by in vivo microdialysis. Pharm Res. 2001;18:367–373. doi: 10.1023/a:1011067300397. [DOI] [PubMed] [Google Scholar]
- 27.Kreilgaard M, Kemme MJB, Burggraaf J, Schoemaker RC, Cohen AF. Influence of a microemulsion vehicle on cutaneous bioequivalence of a lipophilic model drug assessed by microdialysis and pharmacodynamics. Pharm Res. 2001;18:593–599. doi: 10.1023/a:1011068907416. [DOI] [PubMed] [Google Scholar]
- 28.Coulombe PA. Wound epithelialization: accelerating the pace of discovery. Dermatology Foundation. 2003;37:219–230. doi: 10.1046/j.1523-1747.2003.12387.x. [DOI] [PubMed] [Google Scholar]
- 29.Spence DW, Pomeranz B. Surgical wound healing monitored repeatedly in vivo using electrical resistance of the epidermis. Physiol Meas. 1996;17:57–69. doi: 10.1088/0967-3334/17/2/001. [DOI] [PubMed] [Google Scholar]