Abstract
Objective
Atherosclerotic plaques that are prone to disruption and acute thrombotic vascular events are characterized by large necrotic cores. Necrotic cores result from the combination of macrophage apoptosis and defective phagocytic clearance (efferocytosis) of these apoptotic cells. We previously showed that macrophages with tyrosine kinase-defective Mertk receptor (MertkKD) have a defect in phagocytic clearance of apoptotic macrophages in vitro. Herein we test the hypothesis that the MertkKD mutation would result in increased accumulation of apoptotic cells and promote necrotic core expansion in a mouse model of advanced atherosclerosis.
Methods and Results
MertkKD;Apoe−/− mice and control Apoe−/− mice were fed a Western-type diet for 10 or 16 wks, and aortic root lesions were analyzed for apoptosis and plaque necrosis. We found that the plaques of the MertkKD;Apoe−/− mice had a significant increase in TUNEL-positive apoptotic cells. Most importantly, there were more non-macrophage-associated apoptotic cells in the MertkKD lesions, consistent with defective efferocytosis. The more advanced (16-wk) MertkKD;Apoe−/− plaques were more necrotic, consistent with a progression from apoptotic cell accumulation to plaque necrosis in the setting of a defective efferocytosis receptor.
Conclusion
In a mouse model of advanced atherosclerosis, mutation of the phagocytic Mertk receptor promotes the accumulation of apoptotic cells and the formation of necrotic plaques. These data are consistent with the notion that a defect in an efferocytosis receptor can accelerate the progression of atherosclerosis and suggest a novel therapeutic target to prevent advanced plaque progression and its clinical consequences.
Keywords: Atherosclerosis-Pathophysiology, Apoptosis, Phagocytosis, Animal models of human disease
Prevention and regression of human atherothrombotic vascular disease requires an understanding of the underlying mechanisms that promote advanced plaque progression. Acute coronary artery syndromes are often associated with the presence of so-called vulnerable plaques. These plaques are characterized by focal thinning of the fibrous cap, a high level of inflammatory cytokines and matrix proteases, apoptosis of intimal cells, and expansion of a lipid-laden necrotic core.1 Expansion of the latter is likely the consequence of accelerated macrophage apoptosis coupled with defective phagocytic clearance (efferocytosis).2,3 Defective phagocytic clearance leads to post-apoptotic cellular necrosis and release of proinflammatory and pro-thrombotic intracellular debris.4
Previous reports have provided in-vivo evidence that efferocytosis is impaired in advanced atherosclerosis.2,3,5 For example, Schrijvers et al.6 showed that advanced human coronary artery lesions had more apoptotic cells outside of lesional phagocytes than a control tissue, human tonsil, where most of the apoptotic cells were inside phagocytes. Moreover, gene targeting of extracellular molecules that have been shown to bridge phagocytes to apoptotic cells in vitro, namely, complement C1q and lactadherin, promotes apoptotic cell accumulation7 and a vulnerable plaque phenotype in mouse atherosclerotic lesions.8 Walsh and colleagues9 showed a link between defective efferocytosis and accelerated atherosclerosis in hyperlipidemic mice lacking Fas ligand. Boisvert et al.10 found that Ldlr−/− mice lacking transglutaminase-2 (TG2), which has been implicated in efferocytosis, had increased atherosclerosis. However, the human study was correlative, not causative, and C1q, lactadherin, Fas ligand, and TG-2 have other important roles besides those in efferocytosis.
Mertk (also known as Eyk, Nyk, and Tyro-12) is a tyrosine kinase receptor for the phosphatidylserine-binding protein Gas6, which bridges apoptotic cells to phagocytes.11,12 Mer tyrosine kinase appears to signal through a pathway involving αvβ5 integrin, culminating in polymerization of the phagocyte cytoskeleton during apoptotic cell internalization.12 In vivo, apoptotic thymocyte removal is defective in mice carrying a kinase-defective Mertk (MertkKD), in turn promoting autoantibody production and stimulating lupus-like autoimmunity.12,13 In an in-vitro study, our group showed that macrophages from MertkKD mice have a defect in the ingestion of macrophages rendered apoptotic by cholesterol loading, which is a model of advanced lesional macrophage death.14
We hypothesized that if the Mertk receptor functions similarly in advanced atherosclerotic lesions as it does in vitro, then defective Mertk should promote advanced lesional macrophage apoptosis and plaque necrosis. To test this hypothesis, we crossed MertkKD mice onto the Apoe−/− background and then fed these mice and Apoe−/− control mice a Western-type high-cholesterol diet for 10 or 16 wks. We found that the plaques of the MertkKD;Apoe−/− mice had a significant increase in TUNEL-positive apoptotic cells and, at the 16-wk timepoint, more plaque necrosis. Furthermore, the ratio of free-to-macrophage-associated apoptotic cells was significantly increased in MertkKD lesions. These data are consistent with our hypothesis that a defect in an efferocytosis receptor can accelerate the progression of atherosclerosis. Future studies based on this principle may suggest novel therapeutic targets to prevent advanced plaque progression and its clinical consequences.
Methods
Animals and Diets
MertkKD mice on the C57BL6/J were bred onto the Apoe−/− C57BL6/J background to generate MertkKD;Apoe−/− breeding pairs. Starting at 8 wks of age, progeny from these breeding pairs were fed a high-fat (21.2%), high-cholesterol (0.2%) Western-type diet (Catalog # TD88137) from Harlan Teklad (Madison, WI) for 10 weeks or 16 weeks. All animals were housed and cared for according to NIH and IACUC guidelines in a barrier facility at Columbia University Medical Center, New York, NY.
Plasma Lipid and Cytokine Analysis
At the end of the study, the mice were fasted overnight and then weighed, anesthetized using isoflurane, and euthanized. Blood was collected from the left ventricle. Total plasma cholesterol was measured with a commercially available kit from Wako. Plasma lipoprotein profiles were determined by fast performance liquid chromatography (FPLC) gel filtration on a Superose 6 column at a flow rate of 0.2 ml per minute. Eluted fractions were assayed for cholesterol using the Wako kit. Plasma cytokines were performed at the Cytokine Core Laboratory in Baltimore MD.
Aortic Root Atherosclerotic Lesion Analysis
Mouse hearts and aortic arches were perfused in-situ with saline, removed, and aortic roots fixed in 10% formalin. Aortic roots were placed in biopsy cassettes, processed in a Leica tissue-processing machine, and embedded in paraffin blocks. Sections were cut serially at 6-μm intervals from the aortic sinus and mounted on slides. Prior to staining, sections were deparaffinized in xylene and rehydrated in graded series of ethanol. For area measurements and morphometric analysis, the sections were stained with Harris’ hematoxylin and eosin. Total intimal lesional area was quantified by averaging six sections that were spaced 30 μm apart, starting from the base of the aortic root. Images were viewed and captured with a Nikon Labophot 2 microscope and analyzed using Image Pro Plus software. Apoptotic cells in lesions were detected by TUNEL (Tdt-mediated dUTP nick end labeling) after proteinase K or Triton X-100 treatment, using the in-situ cell death detection kit, TMR red, from Roche. Nuclei were counterstained with Hoechst for 5 minutes, and the slides were viewed and imaged by fluorescent microscopy. Plaque necrosis was quantified by measuring the area of hematoxylin and eosin-negative acellular areas in the intima, as described previously 15.
Immunohistochemistry
Blocking was performed using immunoglobulin from the species of the secondary antibody. Macrophages were detected using a rabbit anti-macrophage antibody (AIA31240) from Accurate Chemical and Scientific Corporation. SMC actin was detected using a rabbit monoclonal (E184) to alpha smooth muscle actin (ab32575) from Abcam. Polyclonal Mertk antibody was from R&D Systems. Secondary antibodies were biotinylated conjugates that were subsequently detected using streptavidin-horseradish peroxidase (HRP) or alternatively Molecular Probes Alexa Fluor 488. The HRP substrate was diaminobenzidine. Images were viewed and captured as above.
Aortic Arch Gene Expression Analysis
Aortic arches were homogenized and RNA extracted. RNA was isolated using RNAeasy columns from QIAGEN. Total aortic RNA was subjected to RT-PCR and Real-time PCR. cDNA was synthesized from total RNA using Moloney Murine Leukemia Virus Reverse Transcriptase (M-MLV RT) from Invitrogen. The Mertk primers have previously been described.16 The forward Mertk primer was: GTG GCA GTG AAG ACC ATG AAG TTG. The reverse Mertk primer was: GAA CTC CGG GAT AGG GAG TCA T. The forward and reverse primers for TNF-α were CGG AGT CCG GGC AGG T and GCT GGG TAG AGA ATG GAT GAA CA, respectively. The forward GAPDH primer was: GGT GGC AGA GGC CTT TG. The reverse GAPDH primer was: TGC CCA TTT AGC ATC TCC TT. cDNA was subjected to either PCR or quantitative RT-PCR amplification using a SYBR Green PCR Master Mix (Applied Biosystems).
In Situ Efferocytosis Assay
Following established procedures described previously,6,9,17,18 efferocytosis was determined in situ by counting the number of free vs. macrophage-associated apoptotic cells in individual lesion sections. Apoptotic cells were considered “free” when they were not surrounded by or in contact with macrophages, as described in detail in the Results section. The analysis was performed in a blinded fashion by 2 independent observers.
Statistical Analysis
Data are displayed as mean ± S.E.M. For the 10-wk cohort, n = 5 per group, and for the 16-wk cohort, n = 10 per group. Statistically significant difference between values for the two groups of mice was determined using the Student’s paired t-test.
Results
MertkKD;Apoe−/− mice displayed no gross phenotypic differences compared with control Apoe−/− mice. After 10 or 16 wks on a Western-type diet, Mertk deficiency did not significantly affect overall body weight or plasma cholesterol parameters (Figure 1). Moreover, plasma levels of TNFα, an indicator of systemic inflammation,14,16 were below the detection limit of the assay (5 pg/ml) in both groups. We also assayed TNFα mRNA in aortae and no significant difference between the two groups of mice (P=0.84). To test whether Mertk was expressed in the aorta of Apoe−/− mice, we conducted immunohistochemistry using anti-Mertk antibody. As shown in Figure 2A, Mertk was expressed in macrophage-rich areas of the aortic root intimal of Apoe−/− mice. Because this polyclonal antibody recognizes the tyrosine kinase-truncated MertkKD mutant, we relied on RT-PCR of aortic RNA, using primers specific for the Mertk kinase region as previously described,16 to show evidence of mutated Mertk in MertkKD;Apoe−/− aorta. Using these primers, the Mertk PCR product was found in control Apoe−/− aorta and but not in MertkKD aorta (Figure 2B).
Figure 1. Total body weight and plasma lipoproteins of Apoe−/− and MertkKD;Apoe−/− mice.
Body weight, total plasma cholesterol, and fast performance liquid chromatography gel-filtration profiles (from pooled plasma samples) from male Apoe−/− and MertkKD;Apoe−/− mice fed a Western-type diet for either (A) 10 wks or (B) 16 wks. n.s., statistically non-significant difference between the two groups of mice.
Figure 2. Mertk expression and aortic root lesion area in Apoe−/− and MertkKD; Apoe−/− mice after 10 wks and 16 wks on a Western-type diet.
(A) Immunohistochemistry for Mertk and macrophages was performed as described in Materials and Methods. The isotype control IgG was matched for the anti-Mertk antibody. Sections were counterstained with hematoxylin. nec, necrotic area. (B) Aortic RNA from each genotype was subjected to RT-PCR for Mertk, as described in Materials and Methods. (C–D) Examples of hematoxylin and eosin-stained aortic root sections from each group of mice fed a Western-type diet for either (C) 10 wks or (D) 16 wks. Bar, 0.3 mm. Below each set of sections are graphs showing the quantified lesion area. n.s., statistically non-significant difference between the two groups of mice.
To determine the effect of Mertk deficiency on atherosclerosis progression, aortic roots from Apoe−/− and MertkKD;Apoe−/− mice were sectioned and total atherosclerotic lesional area was measured at the end of the 10 & 16 week Western diets. While apoptosis and efferocytosis can affect early lesion development,2 we hypothesized that Mertk would have a specific effect on advanced lesions.14 Consistent with this idea, lesion area was not significantly affected in MertkKD;Apoe−/− mice after either diet duration, as shown in Figure 2C–D. This finding enabled analysis of advanced lesions in a manner that is independent of early lesion development and size per se.
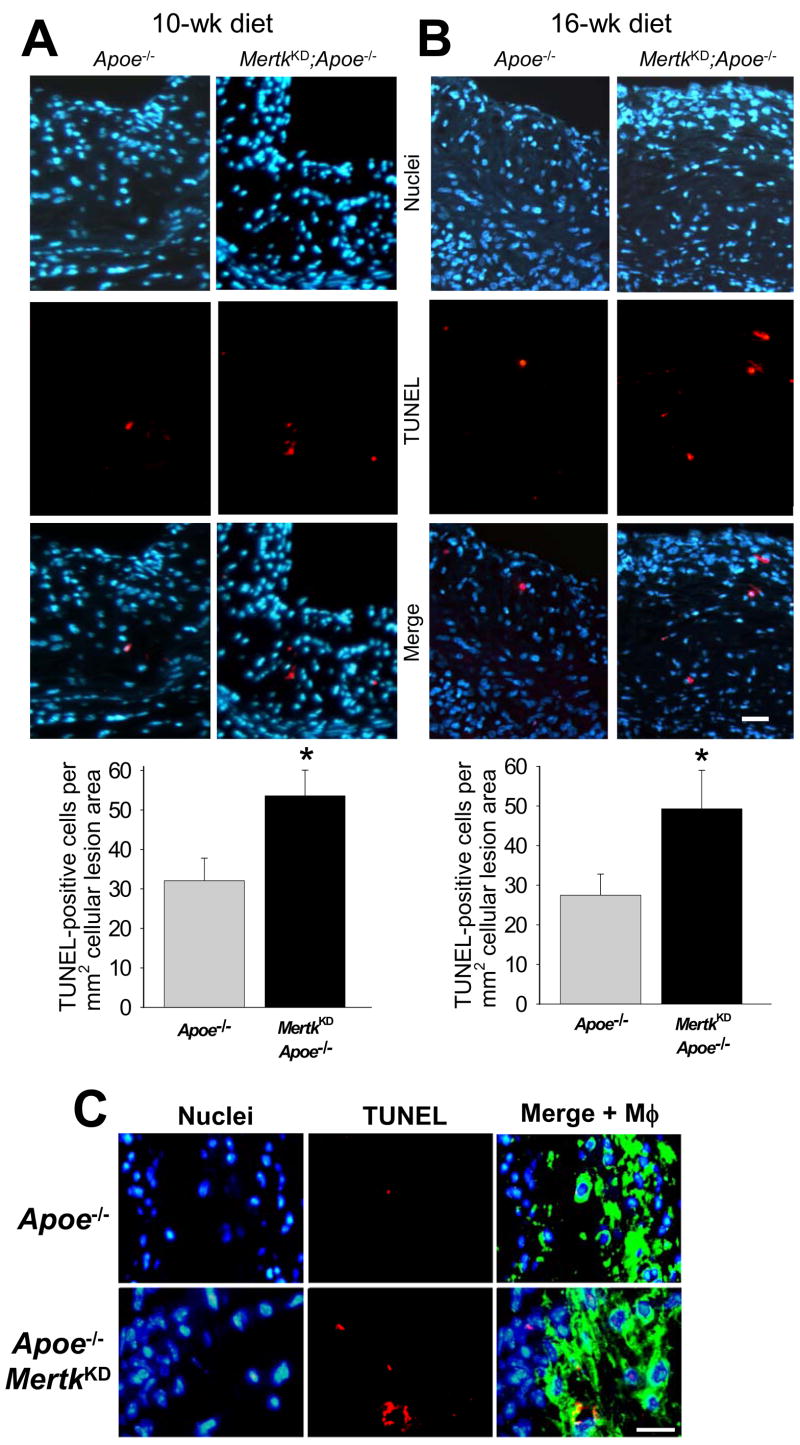
To detect apoptotic cells in the plaques, lesions from the two groups of mice were stained by the TUNEL method. In most cases, the TUNEL stain was punctate and co-localized with or very close to nuclei, but in some cases the TUNEL signal was more diffuse and appeared to be associated with disrupted cells, presumably representing relatively early- vs. late-stage apoptosis, respectively. As expected,19 TUNEL-positive cells were of low frequency in Apoe−/− lesions (Figure 3A–B; Supplemental Figure). Most importantly, lesions from both the 10- and 16-wk-fed MertkKD;Apoe−/− mice exhibited a significant increase in intimal TUNEL-positive cells relative to lesions from Apoe−/− mice (P<0.05) (graphs at bottom of images in Figure 3A–B). In the 16-wk group, additional sections were also stained for macrophages (examples in Figure 3C), and analysis showed that >90% of TUNEL-positive cells were located within macrophage-rich areas of the intima.
Figure 3. TUNEL-positive nuclei are increased in the aortic root lesions of MertkKD;Apoe−/− mice.
TUNEL analysis of aortic root sections from Mertk+/+;Apoe−/− and Mertk−/−;Apoe−/− mice fed a Western-type diet for either (A) 10 wks or (B) 16 wks. Micrographs display nuclei (blue; Hoechst), TUNEL-positive signal (red), and merged images. Bar, 10 μm. Quantified data are shown below the images (* indicates P<0.05). (C) Similar to the images in (B), but the right image in each group shows merged nuclear, TUNEL, and macrophage (green) staining. Bar, 10 μm.
We next determined whether the accumulation of apoptotic cells in the intima in the setting of the MertkKD mutation was associated with increased plaque necrosis. In previous studies, we found that intimal cell apoptosis precedes plaque necrosis,20 which is consistent with a cause-effect relationship.2,3 In the 10-wk-diet mice in the current study, plaque necrosis was not statistically different between the two groups of mice (data not shown). However, in the 16-wk-diet mice, plaque necrosis was substantially and significantly increased in the MertkKD;Apoe−/− lesions (Figure 4). Necrotic cores and surrounding cells stained positive with antibodies specific for macrophages and Mertk (see Figure 2B, above).
Figure 4. Plaque necrosis is increased in the aortic root lesions of MertkKD;Apoe−/− mice after 16 wks on a Western-type diet.

The images show examples of sections of hematoxylin and eosin-stained aortic roots from each group of mice fed a Western-type diet for 16 wks. nec, necrotic areas. Bar, 100 μm. The graph shows quantification of necrotic areas (n = 10 per group; * indicates P<0.05).
These data are consistent with the hypothesis that non-cleared apoptotic macrophages, conferred by defective Mertk, contributes at least in part to expansion of plaque necrosis in advanced atherosclerotic lesions. To determine whether lesional efferocytosis is compromised in MertkKD;Apoe−/−, lesions, we applied a previously established methodology that measures efferocytosis in situ.6,9,17,18 Specifically, we measured the ratio of free-to-macrophage-associated apoptotic cells in lesions by staining for nuclei, TUNEL, and macrophages. In this procedure, apoptotic bodies are detected as TUNEL-positive nuclei, whereas macrophage efferocytes are detected as cells having green cytoplasm. TUNEL-positive nuclei that do not overlap with the green stain are considered “free,” whereas TUNEL-positive nuclei that overlap with the green stain are considered “macrophage-associated.” The images in Figure 5A–B shows examples of this procedure. The upper and middle images on the left in Figure 5A show an apoptotic body (i.e., TUNEL-positive nucleus; arrows). The bottom left image also shows a green-stained cell, which is a lesional macrophage. In this case, the apoptotic body is in close association with the macrophage. In contrast, the apoptotic body shown in the right column of images in Figure 5A is not closely associated with a macrophage (“free”). Additional examples of macrophage-associated and free apoptotic cells are shown in the top and bottom images in Figure 5B, respectively. Two independent investigators, blinded to the identity of the samples, quantified free vs. macrophage-associated apoptotic cells in lesions from the two groups of mice. The final data, with concurrence between the observers of >90%, is shown in Figure 5C. There was a 2.4-fold increase in the ratio of free-to-macrophage-associated apoptotic cells in the lesions from MertkKD;Apoe−/− mice (P<0.05), consistent with a marked defect in efferocytosis.
Figure 5. Phagocytosis efficiency of apoptotic cells is decreased in MertkKD;Apoe−/− plaques.
(A) Examples of nuclear (blue; Hoechst), TUNEL (red), and macrophage (green) staining to differentiate macrophage-associated (left column of images) from free (right column of images) apoptotic cells in aortic root lesions of 16-wk diet-fed Apoe−/− and MertkKD;Apoe−/− mice, respectively. (B) Examples of merged nuclear, TUNEL, and macrophage-stained images differentiating macrophage-associated (top image) from free (bottom image) apoptotic cells in aortic root lesions of Apoe−/− and MertkKD;Apoe−/− mice, respectively. Arrows indicate apoptotic bodies. (C) Quantitation of the free-to-macrophage-associated ratio of apoptotic cells in the lesions of the two groups of mice (* indicates P<0.05).
Discussion
In view of the known role of Mertk in efferocytosis and our previous observation that MertkKD phagocytes have a defect in the ingestion of a cholesterol-induced apoptotic macrophages in vitro,11,12,14 the data in this study provide strong support for the hypothesis that Mertk plays an important role in efferocytosis in advanced atheromata. Moreover, the data are consistent with the idea that defective efferocytosis in advanced atheromata leads to the accumulation of apoptotic cells in these lesions and, eventually, to plaque necrosis.2,5,6 Importantly, this defect in Mertk does not affect plasma lipoproteins or overall lesion size, indicating a specific effect on advanced plaque morphology. This latter point is important, because it has been proposed that efficient efferocytosis of apoptotic macrophages in early lesions limits early lesion development.2 The explanation may lie in the possibility that the type of apoptotic macrophages in early lesions favors recognition by one or more of the many other efferocytosis receptors described in the literature.4 Indeed, it was remarkable in our previous study that Mertk had such an important, non-redundant role in the efferocytosis of macrophages that were rendered apoptotic by a process—accumulation of unesterified cholesterol—that appears to be limited to advanced atherosclerotic lesions.14,21
Although the Mertk mutation had a marked effect on efferocytosis, there were still macrophage-associated apoptotic cells in the lesions of these mice. Thus, it is almost certain that other efferocytosis receptors play a role in advanced atheromata. Moreover, in the context of the current study, other possible effects of the MertkKD mutation need to be considered. For example, cells with this mutation have a heightened inflammatory response,14,16 and if this effect occurs in atherosclerotic lesions, it may certainly contribute to advanced plaque progression.22 We found no evidence of systemic or increased aortic inflammation in these mice, but defective efferocytosis in general is associated with increased inflammation.4 Therefore, in the microenvironment of advanced atherosclerosis, post-apoptotic cellular necrosis and inflammation likely go hand-in-hand as consequences of decreased efferocytosis. In a similar manner, we recently showed that efferocytosis activates cell-survival pathways in macrophages,23 and so defective efferocytosis may also be intimately linked with accelerated macrophage apoptosis. Whatever the results of future studies related to the mechanisms of Mertk in plaque progression, the data reveal the importance of a single molecule in a critical stage in advanced atherosclerosis, namely, expansion of the necrotic core.
The central hypothesis of this study raises the question of mechanisms of defective efferocytosis in advanced lesions. Investigators have proposed a number of possibilities, including competitive inhibition of efferocytosis receptors by oxidized ligands, oxidant stress, and hypoxia.2,3 Although the goal of the current proof-of-concept study was to use genetic engineering to rid lesions of a known efferocytosis receptor, it is theoretically possible that Mertk could somehow be rendered dysfunctional in advanced atheromata. Interestingly, the metalloproteinase TACE/ADAM17, which is expressed in advanced lesions, has been shown to cleave the extracellular domain of Mertk into a soluble receptor that can competitively bind to the Mertk/phosphatidylserine ligand Gas6 and inhibit phagocytosis of apoptotic cells.24,25 Mertk-mediated phagocytic activity might also be limited by the availability of Gas6. In this regard, vascular smooth muscle cells appear to be a major Gas6 source in lesions,26,27 and vulnerable plaques have a deficiency of smooth muscles in areas of plaque disruption.28 Thus, it is possible that the genetic manipulation carried out in this study mimics an event that may naturally occur in very advanced lesions. Finally, polymorphisms in the Mertk gene are associated with abnormalities in systemic lupus erythrematosis, which is another disease associated with defective efferocytosis.29 It will be interesting to determine whether these polymorphisms are also associated with increased risk for atherothrombotic vascular disease.
Almost all humans in industrialized societies have evidence of atherosclerosis by the time they reach their teens or twenties.30 Although most of these lesions are of the so-called “benign” type, meaning they lack the features of vulnerable plaques,31 those lesions that become necrotic and acquire other vulnerable traits wreak havoc in terms of the toll of ischemic vascular disease.31,32 Moreover, there is an accelerating epidemic of diabetes-associated atherothrombotic vascular disease,33 and diabetic lesions are characterized by very large necrotic cores.34 For this reason, identifying specific molecular targets and processes that affect advanced plaque necrosis is critical. In the context of this report and others,2,5,6 measures to enhance efferocytosis in general,2 or Mertk function in particular, may lead to novel strategies to prevent acute atherothrombotic vascular disease.
Supplementary Material
Acknowledgments
We thank Dr. Matsushima for originally supplying the MertkKD mice for our studies.
Sources of Funding
This work was supported by an AHA-Heritage Affiliate Post-doctoral Fellowship grant (to E.T.); a Fonds Wetenschappelijk Onderzoek (FWO) grant and Fulbright Scholarship (to. D.M.S.); and NIH grants HL54591 and HL75662 and a Boehringer-Ingelheim research grant (to I.T.).
Disclosures
This study was supported partially by research grant from Boehringer-Ingelheim (to I.T.).
References
- 1.Virmani R, Burke AP, Kolodgie FD, Farb A. Vulnerable plaque: the pathology of unstable coronary lesions. J Interv Cardiol. 2002;15:439–446. doi: 10.1111/j.1540-8183.2002.tb01087.x. [DOI] [PubMed] [Google Scholar]
- 2.Tabas I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler Thromb Vasc Biol. 2005;25:2255–2264. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- 3.Schrijvers DM, De Meyer GR, Herman AG, Martinet W. Phagocytosis in atherosclerosis: Molecular mechanisms and implications for plaque progression and stability. Cardiovasc Res. 2007;73:470–480. doi: 10.1016/j.cardiores.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Vandivier RW, Henson PM, Douglas IS. Burying the dead: the impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease. Chest. 2006;129:1673–1682. doi: 10.1378/chest.129.6.1673. [DOI] [PubMed] [Google Scholar]
- 5.Tabas I. Mouse models of apoptosis and efferocytosis. Curr Drug Targets. 2008 doi: 10.2174/138945007783220623. [DOI] [PubMed] [Google Scholar]
- 6.Schrijvers DM, De Meyer GR, Kockx MM, Herman AG, Martinet W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25:1256–1261. doi: 10.1161/01.ATV.0000166517.18801.a7. [DOI] [PubMed] [Google Scholar]
- 7.Bhatia VK, Yun S, Leung V, Grimsditch DC, Benson GM, Botto MB, Boyle JJ, Haskard DO. Complement C1q reduces early atherosclerosis in low-density lipoprotein receptor-deficient mice. American Journal of Pathology. 2007;170:416–426. doi: 10.2353/ajpath.2007.060406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ait-Oufella H, Kinugawa K, Zoll J, Simon T, Boddaert J, Heeneman S, Blanc-Brude O, Barateau V, Potteaux S, Merval R, Esposito B, Teissier E, Daemen MJ, Leseche G, Boulanger C, Tedgui A, Mallat Z. Lactadherin deficiency leads to apoptotic cell accumulation and accelerated atherosclerosis in mice. Circulation. 2007;115:2168–2177. doi: 10.1161/CIRCULATIONAHA.106.662080. [DOI] [PubMed] [Google Scholar]
- 9.Aprahamian T, Rifkin I, Bonegio R, Hugel B, Freyssinet JM, Sato K, Castellot JJ, Jr, Walsh K. Impaired clearance of apoptotic cells promotes synergy between atherogenesis and autoimmune disease. Journal of Experimental Medicine. 2004;199:1121–1131. doi: 10.1084/jem.20031557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boisvert WA, Rose DM, Boullier A, Quehenberger O, Sydlaske A, Johnson KA, Curtiss LK, Terkeltaub R. Leukocyte transglutaminase 2 expression limits atherosclerotic lesion size. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006;26:563–569. doi: 10.1161/01.ATV.0000203503.82693.c1. [DOI] [PubMed] [Google Scholar]
- 11.Nagata K, Ohashi K, Nakano T, Arita H, Zong C, Hanafusa H, Mizuno K. Identification of the product of growth arrest-specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases. Journal of Biological Chemistry. 1996;271:30022–30027. doi: 10.1074/jbc.271.47.30022. [DOI] [PubMed] [Google Scholar]
- 12.Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, Matsushima GK. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 13.Cohen PL, Caricchio R, Abraham V, Camenisch TD, Jennette JC, Roubey RA, Earp HS, Matsushima G, Reap EA. Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. Journal of Experimental Medicine. 2002;196:135–140. doi: 10.1084/jem.20012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Gerbod-Giannone MC, Seitz H, Cui D, Thorp E, Tall AR, Matsushima GK, Tabas I. Cholesterol-induced apoptotic macrophages elicit an inflammatory response in phagocytes, which is partially attenuated by the Mer receptor. J Biol Chem. 2006;281:6707–6717. doi: 10.1074/jbc.M510579200. [DOI] [PubMed] [Google Scholar]
- 15.Feng B, Zhang D, Kuriakose G, Devlin CM, Kockx M, Tabas I. Niemann-Pick C heterozygosity confers resistance to lesional necrosis and macrophage apoptosis in murine atherosclerosis. Proc Natl Acad Sci U S A. 2003;100:10423–10428. doi: 10.1073/pnas.1732494100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camenisch TD, Koller BH, Earp HS, Matsushima GK. A novel receptor tyrosine kinase, Mer, inhibits TNF-alpha production and lipopolysaccharide-induced endotoxic shock. The Journal of Immunology. 1999;162:3498–3503. [PubMed] [Google Scholar]
- 17.Taylor PR, Carugati A, Fadok VA, Cook HT, Andrews M, Carroll MC, Savill JS, Henson PM, Botto M, Walport MJ. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. Journal of Experimental Medicine. 2000;192:359–366. doi: 10.1084/jem.192.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Potter PK, Cortes-Hernandez J, Quartier P, Botto M, Walport MJ. Lupus-prone mice have an abnormal response to thioglycolate and an impaired clearance of apoptotic cells. The Journal of Immunology. 2003;170:3223–3232. doi: 10.4049/jimmunol.170.6.3223. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez-Hernando C, Ackah E, Yu J, Suarez Y, Murata T, Iwakiri Y, Prendergast J, Miao RQ, Birnbaum MJ, Sessa WC. Loss of akt1 leads to severe atherosclerosis and occlusive coronary artery disease. Cell Metab. 2007;6:446–457. doi: 10.1016/j.cmet.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim WS, Timmins JM, Seimon TA, Sadler A, Kolodgie FD, Virmani R, Tabas I. STAT1 is critical for apoptosis in macrophages subjected to endoplasmic reticulum stress in vitro and in advanced atherosclerotic lesions in vivo. Circulation. 2008;117:940–951. doi: 10.1161/CIRCULATIONAHA.107.711275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tabas I. Consequences of cellular cholesterol accumulation: basic concepts and physiological implications. Journal of Clinical Investigation. 2002;110:905–911. doi: 10.1172/JCI16452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 23.Cui D, Thorp E, Li Y, Wang N, Yvan-Charvet L, Tall AR, Tabas I. Pivotal advance: macrophages become resistant to cholesterol-induced death after phagocytosis of apoptotic cells. J Leukoc Biol. 2007;82:1040–1050. doi: 10.1189/jlb.0307192. [DOI] [PubMed] [Google Scholar]
- 24.Sather S, Kenyon KD, Lefkowitz JB, Liang X, Varnum BC, Henson PM, Graham DK. A soluble form of the Mer receptor tyrosine kinase inhibits macrophage clearance of apoptotic cells and platelet aggregation. Blood. 2007;109:1026–1033. doi: 10.1182/blood-2006-05-021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Canault M, Peiretti F, Kopp F, Bonardo B, Bonzi MF, Coudeyre JC, Alessi MC, Juhan-Vague I, Nalbone G. The TNF alpha converting enzyme (TACE/ADAM17) is expressed in the atherosclerotic lesions of apolipoprotein E-deficient mice: possible contribution to elevated plasma levels of soluble TNF alpha receptors. Atherosclerosis. 2006;187:82–91. doi: 10.1016/j.atherosclerosis.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 26.Melaragno MG, Fridell YW, Berk BC. The Gas6/Axl system: a novel regulator of vascular cell function. Trends in Cardiovascular Medicine. 1999;9:250–253. doi: 10.1016/s1050-1738(00)00027-x. [DOI] [PubMed] [Google Scholar]
- 27.Yin J, McLachlan C, Chaufour X, McGuire MA, White G, Turner V, King NJ, Hambly BD. Growth arrest-specific gene 6 expression in proliferating rabbit vascular smooth muscle cells in vitro and in vivo. Electrophoresis. 2000;21:3851–3856. doi: 10.1002/1522-2683(200011)21:17<3851::AID-ELPS3851>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 28.Clarke MC, Figg N, Maguire JJ, Davenport AP, Goddard M, Littlewood TD, Bennett MR. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat Med. 2006;12:1075–1080. doi: 10.1038/nm1459. [DOI] [PubMed] [Google Scholar]
- 29.Cheong HS, Lee SO, Choi CB, Sung YK, Shin HD, Bae SC. MERTK polymorphisms associated with risk of haematological disorders among Korean SLE patients. Rheumatology (Oxford) 2007;46:209–214. doi: 10.1093/rheumatology/kel182. [DOI] [PubMed] [Google Scholar]
- 30.Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 31.Cheruvu PK, Finn AV, Gardner C, Caplan J, Goldstein J, Stone GW, Virmani R, Muller JE. Frequency and distribution of thin-cap fibroatheroma and ruptured plaques in human coronary arteries: a pathologic study. J Am Coll Cardiol. 2007;50:940–949. doi: 10.1016/j.jacc.2007.04.086. [DOI] [PubMed] [Google Scholar]
- 32.Braunwald E. Cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. New England Journal of Medicine. 1997;337:1360–1369. doi: 10.1056/NEJM199711063371906. [DOI] [PubMed] [Google Scholar]
- 33.Libby P. The forgotten majority: unfinished business in cardiovascular risk reduction. J Am Coll Cardiol. 2005;46:1225–1228. doi: 10.1016/j.jacc.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 34.Virmani R, Burke AP, Kolodgie F. Morphological characteristics of coronary atherosclerosis in diabetes mellitus. Can J Cardiol. 2006;22 Suppl B:81B–84B. doi: 10.1016/s0828-282x(06)70991-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.