Abstract
Aim
To present a visual representation of changes in body composition, leptin, insulin, estradiol and follicular stimulating hormone (FSH) levels in relation to menarche in girls.
Methods
Participants were a subset of healthy girls (n = 108) enrolled in a longitudinal study of growth and development conducted at the General Clinical Research Center at the Massachusetts Institute of Technology (MIT). Participants were seen annually from before menarche until 4 years postmenarche for measures of body composition and serum levels of leptin, insulin, estradiol and FSH. Body composition was determined by bioelectrical impedance. Standardized body composition and hormone levels were smoothed and plotted relative to menarche to visualize patterns of change. Results: At menarche, the mean percentage body fat (%BF) of girls was 24.6% (SD = 4.1%) after menarche %BF was ~27%. Leptin levels averaged 8.4 ng/mL (SD = 4.6) at menarche and were ~12 ng/mL after menarche. Changes in leptin levels closely paralleled changes in %BF. Insulin, estradiol and FSH levels followed expected patterns relative to menarche. Leptin began rising closer to menarche than did insulin or the other sex hormones.
Conclusion
We provide a visual presentation of hormonal and body composition changes occurring throughout the pubertal period in girls which may be useful in generating new hypotheses related to the timing of menarche.
Keywords: Body fatness, Leptin, Menarche, Sexual maturation
The time between childhood and adolescence in girls is characterized by dynamic changes in body composition and the hormonal milieu. Although pubertal transitions begin years before the onset of menarche and continue for several years afterwards, menarche is a definable event that provides a subject-specific time point to which changes occurring over the pubertal period can be compared.
Many studies have examined inter-relationships between hormones and body composition during puberty in girls (1,2). Although the role of the adipocyte hormone, leptin, discovered in 1994 (3) has begun to be incorporated into our understanding of puberty (4–10), the inter-relationships of leptin, insulin, the sex hormones and body composition relative to age at menarche have not been thoroughly examined.
In this study we followed a cohort of healthy girls from several years before menarche until 4 years after menarche. This study builds on work by others (2,5,7,8) by including leptin, sex hormones, insulin and body composition in analyses and providing a visual representation that allows for comparison of patterns relative to menarche.
METHODS
Study participants
Participants in this study, initiated in 1993, were part of a larger cohort of girls enrolled in the Massachusetts Institute of Technology (MIT) Growth and Development study (11) conducted at the MIT Clinical Research Center. The criteria for enrollment were premenarcheal status and a triceps skinfold less than the 85th percentile for age and sex (12). Participants (n = 196) aged 8–12 years (mean = 10 years) were enrolled between 1990 and 1993, having been recruited from the Cambridge and Somerville public schools in Massachusetts, the MIT summer day camp and friends and siblings of enrolled subjects. All participants were healthy; and at study entry were not taking any medication that might affect body composition or energy expenditure. Participants included in this analysis (n = 108) were older at menarche than the girls in the larger cohort (13.2 years vs. 12.9 years) because the laboratory assays were added in year 3 of study entry and participants had to have at least one blood sample available for hormonal analysis. The study protocol was approved by both the Committee on the Use of Humans as Experimental Subjects at MIT and the Human Investigation Review Committee of the New England Medical Center.
Subjects enrolled in the MIT growth and development study were followed annually until 4 years after menarche. At each visit, they were examined by a physician to insure that they were in good health. Menarche was determined prospectively. Participants were asked to phone when they got their first period, and they also were asked at each annual visit if they had reached menarche. Body composition at baseline and at each follow-up visit was measured by bioelectrical impedance and percentage body fat (%BF) was estimated using cohort-specific equations validated within the cohort against measures of total body water, as previously described (13).
Serum hormone levels
Participants were asked to provide a blood sample for analysis of leptin, insulin, estradiol and follicular stimulating hormone (FSH) at each visit. Blood samples were drawn before 9 a.m. and after a 10- to 12-h fast. For subjects who had reached menarche, blood was drawn 5–10 days after the start of menses. When a subject’s visit did not fall within the day 5- to day 10-window relative to her menstrual period, she was invited to return during the appropriate time in her cycle in the subsequent months to provide an additional blood sample for determination of sex hormone levels. Our analyses included measurements of insulin and leptin, but not of FSH or estradiol for blood samples obtained outside of the 5–10 day window of the follicular phase. With the exception of insulin, we excluded from analyses hormone data for any participants taking oral contraceptives at the time of the visit.
Hormone assays
Serum samples were stored at −80°C before analyses. Serum samples were analyzed for leptin, insulin, estradiol and FSH by radioimmunoassay. The set of samples for each subject were run together when possible. Additional samples obtained in later years were run within a year of blood draw and were not run with the subject’s original set. Plasma insulin and leptin were analyzed by a double antibody radioimmunoassay (LINCO RIA Laboratories, St. Charles, MO). Insulin and leptin inter-assay coefficients of variation were 9.3% and 8.7% and the intra-assay coefficients of variation were 4.5% and 6.5%. Estradiol was measured using a radioimmunoassay from Diagnostic System Laboratory (DSL). The inter- and intra-assay coefficients of variation were respectively, 9.3% and 5.3%. FSH was measured by an immunoradiometric assay (IRMA). The intra-assay coefficient of variation for FSH was 6.2%.
Analysis
Means and standard deviations of hormone and body composition variables were calculated by maturational status categories that were defined as: between 3 years and 1 year before menarche, between 1 year before and 1 year after menarche (the menarcheal interval), between 1 year and 3 years after menarche, and 3 or more years after menarche. Generalized additive modeling (GAM) was used to visualize the smoothed relationship between leptin and percentage body fat, total body fat, and fat-free mass relative to menarche. We also used GAM to visualize the relationship of leptin, FSH, insulin and estradiol relative to menarche (14). We applied internally referenced z-score transformations to hormone and body composition values to allow for comparison among variables measured in different scales, to observe patterns relative to menarche. Finally, to establish the trend in these variables while accounting for repeated measurements on the same individuals, we used linear mixed effects models. In these models, the slope represents the annual rate of change in the z-score modeled over time, with time defined relative to each participant’s menarche. The intercept reflects the level of each variable, expressed as a z-score, at menarche.
RESULTS
Data from 108 participants were included in this analysis. Of these, 78% were white, 13% were African American and 9% were of other race/ethnicities. Average (SD) age at menarche of participants was 13.19 (1.58) years. These 108 participants provided 377 measurements of body composition and hormone levels across a period ranging from 4.5 years before menarche to 4.3 years after menarche as part of a longitudinal study of the relationship of energy expenditure to weight gain during adolescence (15). Subjects in this analysis provided a mean (SD) of 3.5 (1.9) measurements each, with 63% of subjects contributing three or more repeated measures. The maximum number of measurements provided by a participant was nine, 14% of participants provided one measurement and 17% provided six or more repeated measures.
Table 1 provides means and standard deviations for hormone and body composition measures, in the units in which they were measured, by maturational status category. At the menarcheal interval participants weighed on average 48 kg, of which 36 kg was fat-free mass (FFM) and 12 kg was fat mass (25% body fat). Leptin levels were 8.4 ng/mL.
Table 1.
Body composition and serum hormone levels relative to menarche
| Maturational stage
|
||||
|---|---|---|---|---|
| 3 years to 1 year before menarche | Within 1 year of menarche | 1 year to 3 years after menarche | >3 years after menarche | |
| n | 61 | 97 | 56 | 57 |
| Age (years) | 12.0 (1.0) | 13.6 (1.1) | 15.5 (1.3) | 17.2 (1.1) |
| Weight (kg) | 39.7 (6.3) | 48.0 (6.4) | 56.2 (6.2) | 59.2 (6.9) |
| Fat-free mass (kg) | 29.7 (3.5) | 36.1 (4.0) | 40.9 (3.6) | 42.9 (4.1) |
| Fat (kg) | 10.1 (3.9) | 12.0 (3.2) | 15.4 (3.5) | 16.3 (3.6) |
| Percentage body fat | 24.8 (5.8) | 24.6 (4.1) | 27.1 (3.9) | 27.3 (3.5) |
| Leptin (ng/mL) | 7.1 (5.5) | 8.4 (4.6) | 12.5 (6.7) | 12.2 (6.1) |
| Insulin (μU/mL) | 12.3 (5.6) | 14.4 (6.9) | 13.3 (7.7) | 11.4 (5.2) |
| FSH (mIU/mL) | 2.5 (1.4) | 3.9 (1.6) | 2.8 (1.0) | 2.7 (1.7) |
| Estradiol (pg/mL) | 20.3 (13.3) | 36.6 (19.6) | 46.5 (34.4) | 50.9 (47.5) |
Data represent means (standard deviations). If a subject had more than one observation within a maturational stage, only one observation was included in this table. Decisions were as follows: for all categories except >3 years after menarche, the assessment at which the subject’s age relative to menarche was closest to the middle of the maturational stage was chosen, and for the category of >3 years after menarche the latest assessment (when subject was oldest) was chosen to maximize range.
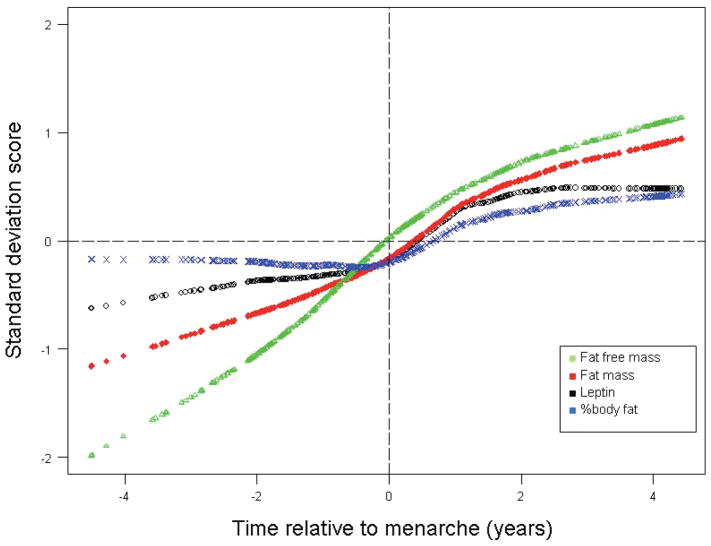
Figure 1 shows body composition and serum leptin levels relative to menarche. These points represent averaged and smoothed data from GAM models that included all available observations. As can be seen in Figure 1, the change in slope that occurs around menarche for leptin parallels changes that occur in percentage body fat. FFM and total body fat are higher at later maturational ages, reflecting growth and the accretion of both lean and fat tissue over the pubertal period.
Figure 1.
Body composition changes and serum leptin levels relative to menarche. Standard deviation score transformations were applied based on FFM (mean = 36.44, SD = 6.38 kg), Fat (mean = 12.95, SD = 4.20 kg), leptin (mean = 9.48, SD = 5.86 ng/mL) and %BF (mean = 25.75, SD = 4.46).
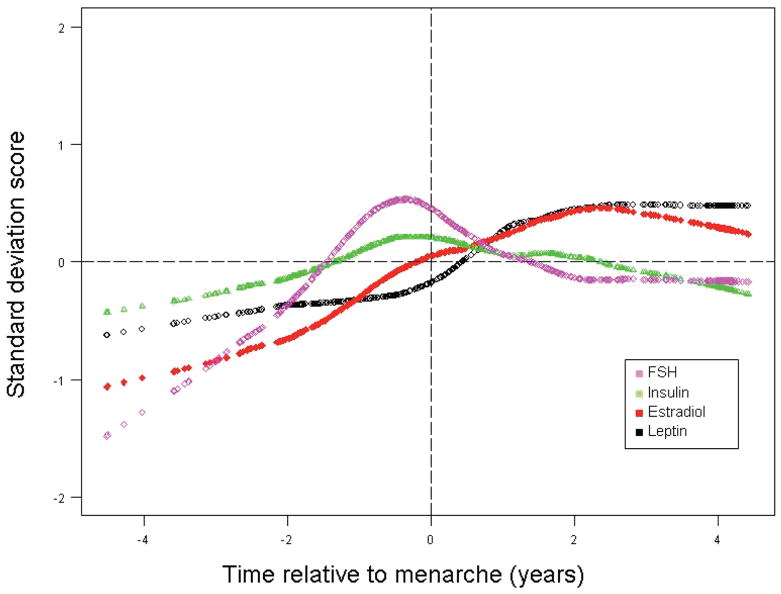
Figure 2 shows standardized levels of the hormones, FSH, insulin, estradiol and leptin plotted relative to menarche from GAM models. The relationship between leptin levels and FSH, insulin and estradiol in girls at menarche has not been well understood. This figure allowed us to examine relationships between changing levels of insulin, FSH and estradiol in association with leptin. To increase interpretability, we describe these patterns relative to maturation from 2 years before menarche to 4 years after menarche, acknowledging that these figures are a combination of cross-sectional information and prospective information. FSH rose in the premenarcheal period, peaked about 6 months before menarche, and then declined to a plateau approximately 2 years after menarche. Insulin also peaked about 6 months before menarche and then declined to prepubertal levels, consistent with the insulin resistance associated with puberty (16). Estradiol rose steadily before menarche and did not decline until 2–3 years after menarche. In contrast, leptin levels began to increase just prior to menarche, later than estradiol, FSH and insulin. Unlike insulin and FSH, leptin levels did not peak but rather rose until 2 years after menarche and then leveled off. All other hormone levels appeared to stabilize around 2 years after menarche with only insulin returning to prepubertal levels.
Figure 2.
Change in serum hormone levels relative to menarche in years. Standard deviation score transformations were applied based on FSH (mean = 3.11, SD = 1.64 mIU/mL), insulin (mean = 13.17, SD = 6.51 μU/mL), estradiol (mean = 46.12, SD = 36.41 pg/mL) and leptin (mean = 9.48, SD = 5.86 ng/mL).
Results of linear mixed effects modeling to assess the presence of a linear change in body composition and hormone levels expressed as z-scores over time are presented in Table 2. The intercept represents the mean value in z-score units at the time of menarche. The slope represents the mean annual change in z-score units. As expected based on the visual representation, significant increases with age relative to menarche are seen for percent body fat, fat-free mass, fat mass, leptin and estradiol, but not for insulin and FSH.
Table 2.
Results of linear mixed effects models predicting body composition and hormone levels (as z-scores) over time expressed as age relative to menarche
| Intercept | Slope (p-value) | |
|---|---|---|
| Fat mass | −0.138 | 0.254 (0.0001) |
| Fat-free mass | −0.199 | 0.363 (0.0001) |
| Percentage body fat | −0.055 | −0.090 (0.0001) |
| Leptin | −0.050 | 0.147 (0.0001) |
| Insulin | −0.005 | 0.003 (0.89) |
| FSH | 0.041 | 0.034 (0.22) |
| Estradiol | −0.058 | 0.146 (0.0001) |
DISCUSSION
Our analysis of data collected from a cohort of girls who were followed from before menarche until 4 years after menarche offers a visualization of the interrelationships between changes in body composition and changes in leptin, estradiol, FSH and insulin, during the pubertal transition. Several studies have examined the relationship between leptin and body composition over the pubertal period (4,6,8,17), and two have investigated the relationship between sex hormones and body composition during puberty (1,2). Only a few have examined both leptin and sex hormones in relation to body composition at this time (5,7). Adding to that knowledge, we have presented hormone levels and body composition in relation to menarche, and thus have standardized them to a biological time point. Hormonal and body composition changes during the pubertal period are best studied in relation to age at menarche rather than chronological age because of the large variability in maturational development among girls who are the same chronological age (18).
Our observations are consistent with those of others. As would be expected insulin, FSH and estradiol rose well before menarche (16,18). Our findings of an increase in FSH and estradiol 2 years before menarche are in agreement with those of Legro et al. (2). We observed a decrease in FSH levels around the time of menarche and a leveling off of estradiol 2 years after menarche. Body composition and serum leptin levels at menarche were similar to those reported by Matkovic (8).
As has been found in other studies (4,6,8,17), we observed a very close association between relative body fatness and serum leptin levels as girls matured. Until about 3–6 months before menarche, mean percentage body fat in these girls was unchanged. Likewise until shortly before menarche, serum leptin levels were stable or only slightly increasing. The increase in slope of percentage body fat that occurred in the 6 month period before menarche coincided with an increase in slope of serum leptin levels, and when percentage body fat began to plateau a year or so after menarche, serum leptin levels also stabilized. Our data provide further evidence that changes in leptin levels reflect changes in body fatness across puberty in girls. We acknowledge, however, that we used an indirect measure of body composition, bioelectrical impedance, to estimate body fatness. We were able to use a cohort-specific equation developed from multiple measures of total body water at different time points. In order to calculate body fat from this measure, an assumption is made that the hydration of FFM is constant. Individual variability in the hydration constant could introduce some error in the measurement of FFM and percent body fat.
Our ability to draw conclusions about the dynamics of leptin changes more than 2 years before menarche is limited by having fewer observations from this maturational period. This is to be expected because premenarcheal girls between ages 8 and 12 years (mean 10.1 years) were enrolled in the study and at enrollment their age at menarche was unknown. In addition blood collection did not begin until 1993. Therefore when our observations are arrayed relative to age at menarche, as in the figures and GAM analyses that generated them, fewer observations are available from the early pubertal period.
Frisch suggested years ago that menarche required sufficient body fat stores to support reproduction (19). Despite considerable research, this hypothesis remains controversial, in part, because of the lack of a molecular mechanism to signal that fat stores are adequate for reproduction. The discovery of leptin (3) led to speculation that it might represent that molecular signal. Matkovic et al. (8) studied pubertal girls for 4 years and investigated associations between leptin levels and age at menarche in relation to body composition. They concluded that for menarche to occur, leptin levels must reach a critical level. Although it is possible that a sufficient level of leptin is required to initiate menarche, our data do not support the notion that leptin rises sharply prior to menarche. Because changes in leptin parallel changes in body fatness, it remains uncertain whether leptin is the signal. The observation that leptin more closely tracks percentage body fat than fat mass beyond 2 years after menarche suggests that relative fatness may be more important than absolute body fat in body weight regulation and reproduction.
The strengths of our study include the prospective assessment of menarche, repeated measurements over the pubertal period, and concurrent measures of body composition and hormone levels. However, our study, like several others (7,8), is limited by the number and timing of samples. In theory, patterns of change in hormone levels and body composition relative to menarche in girls could be studied in cross-sectional or prospective study designs, with different strengths and limitations of each approach.
Advantages of cross-sectional studies, such as reported by Horlick (5), Ellis (17) and Arslanian (4) include ensuring consistency of methodology across measurements, speed and logistics. Disadvantages to this approach are that measurements cannot be ordered relative to age at menarche, because the time to menarche for premenarcheal girls is unknown, and for girls who are postmenarcheal their recalled age at menarche must be used and reliance on memory would be expected to introduce some misclassification error (20). In addition, an important assumption underlying interpretation of hormone levels and body composition patterns relative to pubertal development in a study with a single measurement occasion is that the main difference between subjects is in sexual development. However, race/ethnicity, body type, or athleticism might also be associated with hormone levels and body composition. Thus, it is difficult to know whether differences in hormone levels or body composition across maturational stage observed in a cross-sectional study are due to maturation or to who was measured.
Optimally, a prospective study design would include closely spaced measurements of hormone levels and body composition in a large group of young girls followed for many years. This design would provide individual trajectories of change in hormone levels and body composition for each girl, and one could average these trajectories to estimate the pattern of change in hormone levels and body composition relative to age at menarche. The advantage of this type of study design would be direct measurement of change within individuals; however the difficulties in conducting such a study would be great in terms of time, cost, subject burden and logistics. Ahmed et al. (6) and Matkovic et al. (8) studied girls measured repeatedly during puberty; however, their analytic approach resulted in linear models of association. For non-linear trajectories to be estimated, individual observations would need to be closely spaced in time.
The analyses presented in our report, and those by Demerath (7) represent a combination of cross-sectional and prospective designs. The participants in our study were followed over time, and age at menarche was determined prospectively, but participants varied in their number and spacing of measurements. The visualization of the interrelationships of hormone levels and body composition relative to age at menarche that we provide in the figures, using a GAM approach, allows for inclusion of all available measurements. Forty-five percent of subjects were measured on four or more occasions, 63% were measured on three or more occasions and 6% were measured on seven or more occasions. However, 14% of subjects were measured on only one occasion. With this number and variability in measurements, one can only fit a straight line. However, it is evident that much information is lost with this approach. For reasons of practicality, it was difficult to obtain repeated measures of sex hormone levels on the entire sample because the measurements need to be done during a small window of time relative to the menstrual cycle. Although only approximately half of the girls in the cohort elected to participate, reasons for non-participation are unlikely to bias our results.
In conclusion, this visual presentation of the hormonal and body composition changes occurring throughout the pubertal period in girls may be useful in generating new hypotheses related to the timing of menarche.
Acknowledgments
We gratefully acknowledge Katherine Getzewich, and Zoom Vu and the staff at the clinical research centre for their assistance with the study, and we thank the girls who enrolled for their commitment to the study. We also gratefully acknowledge Aida Grossman for her analysis of the hormones. The study was supported by NIH grants DK-HD50537, M01-RR-00088, M01-RR-01066 and 5-PD-DK46200.
Footnotes
DISCLAIMER
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC.
References
- 1.de Ridder CM, Bruning PF, Zonderland ML, Thijssen JHH, Bonfrer JMG, Blankenstein MA, et al. Body fat mass, body fat distribution, and plasma hormones in early puberty in females. J Clin Endocrinol Metab. 1990;70:888–93. doi: 10.1210/jcem-70-4-888. [DOI] [PubMed] [Google Scholar]
- 2.Legro RS, Lin HM, Demers LM, Lloyd T. Rapid maturation of the reproductive axis during perimenarche independent of body composition. J Clin Endocrinol Metab. 2000;85:1021–5. doi: 10.1210/jcem.85.3.6423. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Proenca R, Maffei M, Barone M, Leoplod L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 4.Arslanian S, Suprasongsin C, Kalhan SC, Drash AL, Brna R, Janosky JE. Plasma leptin in children: relationship to puberty, gender, body composition, insulin sensitivity, and energy expenditure. Metabolism. 1998;47:309–12. doi: 10.1016/s0026-0495(98)90262-1. [DOI] [PubMed] [Google Scholar]
- 5.Horlick MB, Rosenbaum M, Nicolson M, Levine LS, Fedun B, Wang J, et al. Effect of puberty on the relationship between circulating leptin and body composition. J Clin Endocrinol Metab. 2000;85:2509–18. doi: 10.1210/jcem.85.7.6689. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed ML, Ong KKL, Morrell DJ, Cox L, Drayer N, Perry L, et al. Longitudinal study of leptin concentrations during puberty: sex differences and relationship to changes in body composition. J Clin Endocrinol Metab. 1999;84:899–905. doi: 10.1210/jcem.84.3.5559. [DOI] [PubMed] [Google Scholar]
- 7.Demerath EW, Towne B, Wisemandle W, Blangero J, Chumlea WC, Siervogel RM. Serum leptin concentration, body composition, and gonadal hormones during puberty. Int J Obes. 1999;23:678–85. doi: 10.1038/sj.ijo.0800902. [DOI] [PubMed] [Google Scholar]
- 8.Matkovic V, Ilich JZ, Skugor M, Badenhop NE, Goel P, Clairmont A, et al. Leptin is inversely related to age at menarche in human females. J Clin Endocrinol Metab. 1997;82:3239–45. doi: 10.1210/jcem.82.10.4280. [DOI] [PubMed] [Google Scholar]
- 9.Li HJ, Ji CY, Wang W, Hu YH. A twin study for serum leptin, soluble leptin receptor and free insulin-like growth factor-I in pubertal females. J Clin Endocrinol Metab. 2005;90:3659–64. doi: 10.1210/jc.2004-2079. [DOI] [PubMed] [Google Scholar]
- 10.Tam CS, de Zegher F, Garnett SP, Baur LA, Cowell CT. Opposing influences of prenatal and postnatal growth on the timing of menarche. J Clin Endocrinol Metab. 2006;91:4369–73. doi: 10.1210/jc.2006-0953. [DOI] [PubMed] [Google Scholar]
- 11.Bandini LG, Must A, Spadano JL, Dietz WH. Relation of body composition, parental overweight, pubertal stage, and race-ethnicity to energy expenditure among premenarcheal girls. Am J Clin Nutr. 2002;76:1040–7. doi: 10.1093/ajcn/76.5.1040. [DOI] [PubMed] [Google Scholar]
- 12.Must A, Dallal GE, Dietz WH. Reference data for obesity: 85th and 95th percentiles of body mass index (w/ht2)-a correction. Am J Clin Nutr. 1991;54:773. doi: 10.1093/ajcn/53.4.839. [DOI] [PubMed] [Google Scholar]
- 13.Phillips SM, Bandini LG, Compton DV, Naumova EN, Must A. A longitudinal comparison of body composition by total body water and bioelectrical impedance in adolescent girls. J Nutr. 2003;133:1419–25. doi: 10.1093/jn/133.5.1419. [DOI] [PubMed] [Google Scholar]
- 14.Hastie TJ, Tibshirani RM, editors. Generalized additive models. Monographs on statistical and applied probablity 43. New York: Chapman and Hall; 1990. [Google Scholar]
- 15.Bandini LG, Must A, Phillips SM, Naumova EN, Dietz WH. Relation of body mass index and body fatness to energy expenditure: longitudinal changes from preadolescence through adolescence. Am J Clin Nutr. 2004;80:1262–9. doi: 10.1093/ajcn/80.5.1262. [DOI] [PubMed] [Google Scholar]
- 16.Amiel SA, Sherwin RS, Simonson DC, Lauritano AA, Tamborlane WV. Impaired insulin action in puberty: a contributing factor to poor glycemic control in adolescents with diabetes. New Engl J Med. 1986;315:215–9. doi: 10.1056/NEJM198607243150402. [DOI] [PubMed] [Google Scholar]
- 17.Ellis KJ, Nicolson M. Leptin levels and body fatness in children: effects of gender, ethnicity, and sexual development. Pediatr Res. 1997;42:484–8. doi: 10.1203/00006450-199710000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Tanner JM. Growth at adolescence. Oxford: Blackwell Scientific; 1962. [Google Scholar]
- 19.Frisch RE. Body fat, menarche, fitness and fertility. Hum Reprod. 1987;2:521–33. doi: 10.1093/oxfordjournals.humrep.a136582. [DOI] [PubMed] [Google Scholar]
- 20.Must A, Phillips SM, Naumova EN, Blum M, Harris S, Dawson-Hughes B, et al. Recall of early menstrual history and menarcheal body size: after 30 years, how well do women remember? Am J Epidemiol. 2002;155:672–9. doi: 10.1093/aje/155.7.672. [DOI] [PubMed] [Google Scholar]