Abstract
Raf-1 is an important effector of Ras mediated signaling and is a critical regulator of the ERK/MAPK pathway. Raf-1 activation is controlled in part by phosphorylation on multiple residues, including an obligate phosphorylation site at serine 338. Previously PAK1 and casein kinase II have been implicated as serine 338 kinases. To identify novel kinases that phosphorylate this site, we tested the ability of group II PAKs (PAKs 4-6) to control serine 338 phosphorylation. We observed that all group II PAKs were efficient serine 338 kinases, although only PAK1 and PAK5 significantly stimulated Raf-1 kinase activity. We also showed that PAK5 forms a tight complex with Raf-1 in the cell, but not A-Raf or B-Raf. Importantly, we also demonstrated that the association of Raf-1 with PAK5 targets a subpopulation of Raf-1 to mitochondria. These data indicate that PAK5 is a potent regulator of Raf-1 activity and may control Raf-1 dependent signaling at the mitochondria.
Keywords: Raf-1, PAK5, ERK, mitochondria, kinase, phosphorylation
Introduction
Raf family protein kinases are key effectors of Ras mediated signaling. Three Raf isoforms exist in mammals that are differentially expressed, Raf-1, A-Raf and B-Raf (O'Neill et al., 2004;Baccarini, 2005). The activation mechanism of Raf enzymes is complex and differs between isoforms. Growth factor mediated activation of Raf-1 requires binding to active Ras, release of the adaptor protein 14-3-3 from an amino-terminal binding site, and an increase in the phosphorylation of at least four distinct residues (Chong et al., 2003b). Two of these residues, serine 338 and tyrosine 341, are located adjacent to the catalytic domain and contribute to Raf-1 activation by blocking the function of an amino-terminal autoinhibitory domain (Tran et al., 2003;Chong et al., 2003a). The other two residues, threonine 491 and serine 494, are contained within the activation loop of the catalytic domain and contribute directly to Raf-1 kinase activation (Chong et al., 2001). Although not sufficient for Raf-1 activation, phosphorylation of serine 338 is critically important for Raf-1 activation since mutation of this site to a non-phosphorylatable residue blocks activation by growth factors and constitutively active Ras (Barnard et al., 1998;Chaudhary et al., 2000;Xiang et al., 2002).
Because of the importance of serine 338 phosphorylation to Raf-1 activation, the identification of kinases responsible for its phosphorylation is an area of considerable interest. Recent work indicates that p21 activated kinases (PAK) 1-3 can phosphorylate this site in vitro and in cells (King et al., 1998;Zang et al., 2002;Tran and Frost, 2003;Jin et al., 2005;Beeser et al., 2005). PAKs are serine/threonine kinases that are classified into two subfamilies, known as group I and group II (Jaffer et al., 2002;Bokoch, 2003). Group I PAKs (PAKs 1-3) are highly related and are activated by binding to active Rac1 or Cdc42. Group II PAKs (PAKs 4-6) are less homologous, bind to active Cdc42 but not active Rac1, and unlike group I PAKs, their kinase activities are not stimulated by binding to Cdc42. Group II PAKs also play distinct roles within the cell. For example, PAK4 and PAK5 regulate distinct aspects of cell survival while PAK6 regulates androgen receptor activity (Lu et al., 2003;Gnesutta et al., 2001;Cotteret et al., 2006;Yang et al., 2001).
Past work indicates that group I PAKs control Raf-1 activation in response to many but not all cellular stimuli. For example, expression of kinase-inactive forms of PAKs 1-3 blocks Raf-1 activation following exposure to nocodazole or by binding to integrins, but not after stimulation with EGF (Chaudhary, King, Mattaliano, Frost, Diaz, Morrison, Cobb, Marshall, and Brugge, 2000;Zang et al., 2001;Chiloeches et al., 2001). Similarly, inhibition of group I PAK activity by RNAi or by expression of the PAK1 autoinhibitory domain blocks the phosphorylation of Raf-1 on serine 338 in response to PDGF but not EGF (Beeser, Jaffer, Hofmann, and Chernoff, 2005). Casein kinase II has also been shown to phosphorylate serine 338 in response to EGF (Ritt et al., 2007). Thus, multiple kinases may contribute to the phosphorylation of Raf-1 on serine 338 in response to distinct stimuli. To identify novel serine 338 kinases we examined whether group II PAKs could phosphorylate this site on Raf-1. Our results indicate that PAK5 is a highly robust serine 338 kinase that potently stimulates Raf-1 activity and can target Raf-1 to mitochondria.
Materials and Methods
Cells, Plasmids and Antibodies
HEK293 cells were maintained and transfected as described (Tran and Frost, 2003). HA-tagged wt Raf-1, Flag-tagged wt Raf-1 and Raf-1 S338A, and Myc tagged constitutively active PAK1 L107F, were as described (Frost et al., 1997;Tran and Frost, 2003). Constitutively active PAK4, PAK5, and PAK6 (S445N, S573N, and S531N, respectively) were created by PCR using Pfu polymerase (Stratagene). Wild type and constitutively active PAK4 and PAK6 were in pCMV5M. HA-tagged wt PAK1 or PAK1 K299A were as described (Brown et al., 1996). Myc-tagged wt PAK5, kinase-inactive PAK5 K478M, and constitutively active PAK5 were in pcDNA3.1 (Pandey et al., 2002). The kinase-inactive PAK5 catalytic domain (K478M; residues 418-719) was amplified from the full length PAK5 K478M using Pfu polymerase and subcloned into pCMV5M. All cDNA constructs were sequenced to confirm correct amplification.
The following antibodies were used: mouse anti-pSer338Raf-1 (Upstate Biotechnology); mouse anti-Myc (National Cell Culture Center); mouse anti-Flag M2 (Sigma); mouse anti-Porin (Calbiochem); rabbit anti-MEK1 pSer218/222, mouse anti-pERK1/2; rabbit anti-ERK1/2 (Cell Signaling Technologies); mouse anti-Raf-1, mouse anti-A-Raf (BD Biosciences); rabbit anti-Raf-1, rabbit anti-A-Raf, mouse anti-B-Raf, rabbit anti-SOD-1, mouse anti-HA, mouse anti-GAPDH and mouse anti-MEK1 (Santa Cruz Biotechnology). Horseradish peroxidase-conjugated goat anti-rabbit and goat anti-mouse antibodies were from KPL. Western blots were developed using enhanced chemiluminescence and X-Ray film.
Raf-1 Kinase Assays and Co-immunoprecipitation
For Raf-1 kinase assays HEK293 cells were transiently transfected with Flag-tagged wt Raf-1 and different amounts of constitutively active PAKs. Cells were then starved in media without serum overnight, washed with PBS and lysed in Triton lysis buffer (Tran et al., 2005). Insoluble proteins were pelleted and Flag-tagged Raf-1 was immunoprecipitated as described (Tran and Frost, 2003). Half of the immune complex was tested for Raf-1 kinase activity towards recombinant, GST-MEK1 (2 μg per reaction) (Tran, Wu, and Frost, 2005). Kinase reactions were for 30 minutes at 30°C in kinase buffer (Tran and Frost, 2003). Reactions were stopped by adding 2× Laemmli sample buffer. Phosphorylation of MEK1 was detected by Western blotting using anti-MEK1 pSer218/222. Raf-1 in the immunoprecipitates was detected by Western blotting.
For co-immunoprecipitation assays cells were lysed in Triton lysis buffer and insoluble proteins were pelleted by centrifugation (Tran and Frost, 2003). The supernatants were incubated with the appropriate antibody plus Protein A-Sepharose for 2 hours at 4°C. Immunoprecipitates were washed with lysis buffer and proteins were identified by Western blotting. When necessary the Triton-insoluble fraction was extracted with SDS lysis buffer (50 mM Tris-HCl (pH 8.0), 100 mM NaCl, 1 mM EDTA, 2.0% SDS, 50 mM NaF, 80 mM β-glycerophosphate, 1 mM sodium orthovanadate, 10 μg/ml aprotinin, 10 μg/ml pepstatin A, 2 μg/ml aprotinin, 1 mM PMSF). When appropriate, protein concentrations were determined by bicinchoninic acid protein assay (BCA)(Pierce) and equal amounts of lysate were analyzed.
To test for co-immunoprecipitation of endogenous PAK5 with Raf-1 from adult rat brain, a single, freshly extracted brain was homogenized in hypotonic lysis buffer (50 mM Tris-HCl (pH 7.4), 50 mM NaF, 80 mM β-glycerophosphate, 1 mM sodium orthovanadate, 1 mM EDTA, 1 mM DTT, 10 μg/ml pepstatin A, 10 μg/ml leupeptin, 2 μg/ml aprotinin, 1 mM PMSF). Triton X-100 was then added to a final concentration of 1%, and after incubation on ice for 10 minutes insoluble proteins were pelleted by centrifugation (10,000 × g, 10 minutes, 4°C). Five mg of lysate were then immunoprecipitated with 2 μg of rabbit anti-Myc, rabbit anti-Raf-1 or rabbit anti-A-Raf antibodies plus 40 μl of Protein A-Sepharose. Precipitates were washed 3 times with wash buffer (1% Triton X-100, 20 mM Tris-HCl (pH 8.0), 100 mM NaCl), resuspended in 2× Laemmli sample buffer, resolved by SDS-PAGE and transferred to PVDF membrane. Co-precipitation of endogenous PAK5 was detected using a PAK5 antibody previously described (Wu et al., 2006).
Indirect Immunofluorescence
To test for co-localization of Raf-1 and PAK5, HEK293 cells were plated on glass cover slips pre-coated with 25 μg/ml fibronectin (Sigma), and then transfected with expression vectors for Flag-tagged wt Raf-1 and Myc-tagged wt PAK5, alone or together. After serum starvation for 24 h the cells were fixed with 3.7% paraformaldehyde in PBS for 10 minutes at 37°C, and then permeabilized with 0.2% Triton X-100 in PBS for 5 minutes at room temperature. The cells were then incubated at 37°C for 1 h with rabbit anti-Raf-1 and mouse anti-Myc antibodies diluted to 2 μg/ml in PBS + 0.05% Tween 20 (PBST) + 1% BSA. After washing with PBST the cells were incubated for 1 h at 37°C with Cy2-conjugated donkey anti-mouse, Cy3-conjugated donkey anti-rabbit (2.5 μg/ml each) and DAPI (0.5 μg/ml), each diluted in PBST + 1% BSA. After washing, the cells were mounted on glass slides using FluorSave reagent (Calbiochem).
To determine whether PAK5 co-expression caused Raf-1 relocalization to mitochondria, cells were transfected as described above and incubated with 1 μM MitoTracker Green (Invitrogen) for 1 hour prior to fixation. The cells were fixed and permeabilized as described, and then incubated with rabbit anti-Raf-1 diluted in PBST + 1% BSA for 1 h at 37°C. After washing the cells were incubated with Cy3-conjugated donkey anti-rabbit and DAPI, and mounted on glass slides, as described above. In all experiments cells were visualized using a Zeiss Axiophot epifluorescence microscope and images were captured using Axiovision software (Zeiss).
Subcellular Fractionation
Mitochondrial fractionation was performed using differential centrifugation (Graham J.M. et al., 1997). HEK293 cells were scraped into PBS containing protease inhibitors (5 μg/ml of pepstatin A, 5 μg/ml of leupeptin, 1 μg/ml of aprotinin, 0.5 mM of PMSF) and pelleted by centrifugation (400 × g, 5 min., 4°C). Cells were resuspended in four volumes of Buffer A (0.15 mM of MgCl2, 10 mM KCl, 10 mM Tris-HCl (pH 6.7), 25 mM NaF, 1 mM Na3VO4, 5 μg/ml of pepstatin A, 5 μg/ml of leupeptin, 1 μg/ml of aprotinin, 0.5 mM of PMSF) and lysed with a Dounce homogenizer. Sucrose was added to the homogenate (250 mM final concentration) and the homogenate was centrifuged (1,500 × g, 5 min., 4°C). The supernatant was re-centrifuged (4,000 × g, 10 min., 4°C) and the pellet, which corresponded to the mitochondria-enriched fraction, was washed with Buffer B (0.15 mM MgCl2, 10 mM Tris-HCl (pH 6.7), 250 mM sucrose) and resuspended in SDS lysis buffer. The supernatant containing the cytosolic and membrane fractions was collected and saved, or was centrifuged (100,000 × g, 45 min., 4°C) to isolate the cytosolic fraction. Protein concentrations in each fraction were determined by BCA assay.
Results
PAK5 phosphorylates Raf-1 on serine 338 and stimulates Raf-1 activity
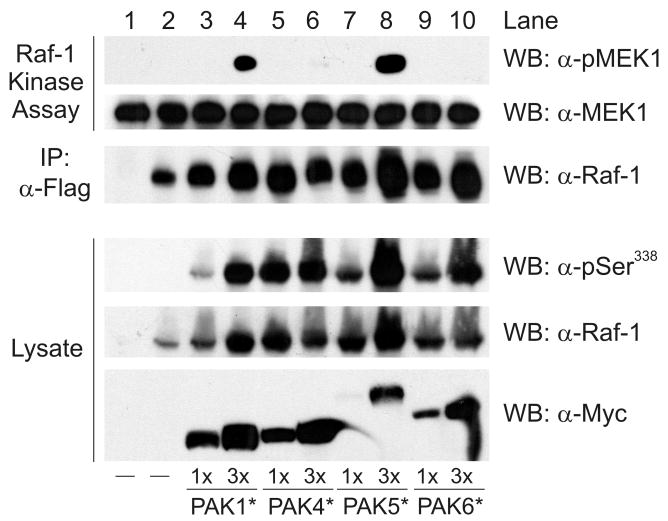
To determine whether group II PAKs (PAKs 4-6) are able to regulate Raf-1 activation, HEK293 cells were transfected with Flag-epitope tagged, wild type (wt) Raf-1 and different amounts of Myc-epitope tagged, constitutively active PAKs 4, 5 and 6. Constitutively active PAK1 was co-expressed with Raf-1 as a positive control for Raf-1 activation (Tran and Frost, 2003). After serum-starvation the Raf-1 was immunoprecipitated and tested for phosphorylation on serine 338 as well as for kinase activity towards recombinant GST-MEK1. In these assays MEK1 phosphorylation was measured by Western blotting using an antibody specific for the Raf-1 phosphorylation sites on MEK1. As expected, expression of constitutively active PAK1 strongly enhanced the phosphorylation of Raf-1 on serine 338 in cells and stimulated the kinase activity of Raf-1 towards MEK1 in vitro (Figure 1). Surprisingly, each of the group II PAKs were also efficient serine 338 kinases, exhibiting activities that were as good as or better than PAK1 (top panel, lysate, lanes 3-10). This was not due to an intrinsic difference in their degree of activation, since each PAK protein exhibited an equal activity towards the generic kinase substrate myelin basic protein in vitro (data not shown). Importantly, although each group II PAK phosphorylated serine 338 of Raf-1, only PAK5 effectively stimulated Raf-1 activation (top panel, compare lanes 5-10). The reason for this difference in ability to activate Raf-1 is not clear, but presumably indicates that PAK5, similar to PAKs 1-3, can regulate other aspects of the Raf-1 activation mechanism that are, as yet, unknown. Nevertheless, these data do indicate that PAK5 has the greatest potential among group II PAKs to regulate Raf-1 activation in cells, and also support the notion that phosphorylation of Raf-1 on serine 338 is necessary but not sufficient for Raf-1 activation (Beeser, Jaffer, Hofmann, and Chernoff, 2005).
Figure 1.
Activation of wt Raf-1 by constitutively active PAKs. HEK293 cells were co-transfected with Flag-tagged wt Raf-1 and different amounts of constitutively active, Myc-tagged PAKs 1, 4, 5 or 6. After serum starvation the Raf-1 was immunoprecipitated and tested for kinase activity using recombinant GST-MEK1 as a substrate. Phosphorylation of MEK1 was detected by Western blotting (α-pMEK1). The middle and lower panels are Western blots for GST-MEK1 and Raf-1 present in the kinase assays. The lower set of panels are Western blots showing expression of the transfected PAKs (α-Myc), Raf-1 phosphorylated on serine 338 (α-pSer338), and total Raf-1 in the cell lysate. Shown is a representative experiment from three independent experiments.
PAK5 binds tightly to Raf-1 and targets Raf-1 to mitochondria
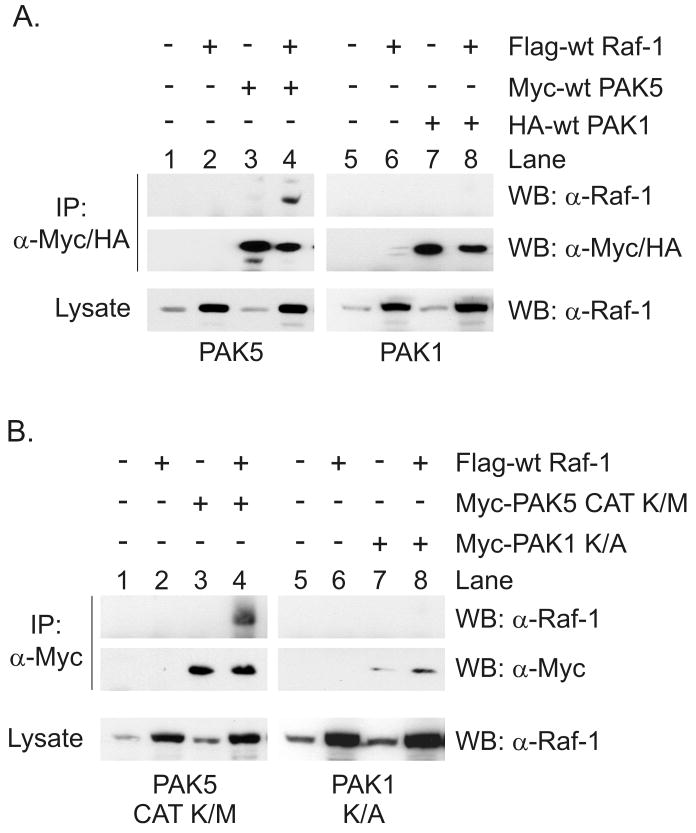
It has been shown that PAK1 binds to Raf-1 and that this interaction is required for PAK1-mediated Raf-1 activation (Zang, Hayne, and Luo, 2002). Since we observed that expression of constitutively active PAK5 activated Raf-1, we examined whether PAK5 also bound to Raf-1. HEK293 cells were transfected with wt Raf-1 and either Myc-tagged wt PAK5 or HA-tagged wt PAK1. After serum starvation the PAK proteins were immunoprecipitated with Myc- or HA-epitope specific antibodies and tested for the co-precipitation of Raf-1 by Western blotting. As shown in Figure 2A, Raf-1 efficiently co-precipitated with wt PAK5 from quiescent cells (top panel, lane 4). Under the same conditions we were unable to detect the co-precipitation of Raf-1 with wt PAK1 (top panel, lane 8), suggesting that PAK5 had a higher affinity for Raf-1 than PAK1. To determine whether the kinase activity of PAK5 was important for interaction with Raf-1, we also examined whether the kinase-inactive PAK5 catalytic domain (PAK5 CAT K/M) interacted with Raf-1. We observed that Raf-1 more readily co-precipitated with kinase-inactive PAK5 than kinase-inactive PAK1 (PAK1 K/A) (Figure 2B, compare lanes 4 and 8). These results indicate that the kinase activity of PAK5 is not required for interaction with Raf-1, and also suggest that PAK5 displays a higher affinity for Raf-1 than PAK1.
Figure 2.
Binding of PAK5 to Raf-1 in cells. (A) HEK293 cells were transfected with Flag-tagged wt Raf-1 and Myc-tagged wt PAK5 or HA-tagged wt PAK1. After serum starvation the cells were lysed and the PAKs were immunoprecipitated with Myc- or HA-epitope antibodies. Co-precipitation of Raf-1 was detected by Western blotting (upper panels). Immunoprecipitated PAK5 or PAK1 are shown in the middle panel, and Raf-1 in the cell lysate is shown in the bottom panels. (B) HEK293 cells were transfected with Flag-tagged wt Raf-1 and the Myc-tagged kinase-inactive catalytic domain of PAK5 (PAK5 CAT K/M) or full length, kinase-inactive PAK1 (PAK1 K/A). After serum starvation the cells were lysed and tested for the co-precipitation of Raf-1. Shown are representative experiments from four independent experiments.
To verify the interaction between PAK5 and Raf-1, HEK293 cells were transfected with expression vectors for each protein and then analyzed for their localization by indirect immunofluorescence. As shown in Figure 3A, Raf-1 was localized to the cytoplasm when expressed alone (top row). On the other hand, when expressed alone wt PAK5 tended to localize in a punctate pattern that was concentrated within the perinuclear space (middle row). This is not surprising given that PAK5 has been reported to localize to mitochondria in transfected cells (Cotteret et al., 2003;Cotteret and Chernoff, 2006;Wu and Frost, 2006). Importantly, when Raf-1 was co-expressed with PAK5 the two proteins co-localized in a punctate staining pattern within the cytoplasm of cells, with a concentration of staining within the perinuclear space (yellow staining, bottom row). Thus, these data confirm that PAK5 and Raf-1 interact in cells.
Figure 3.
Co-localization and mitochondrial targeting of Raf-1 and PAK5 in HEK293 cells. (A) HEK293 cells were transfected with Flag-tagged wt Raf-1 and Myc-tagged wt PAK5, alone or together. After serum starvation the cells were fixed and stained for the localization of each protein by indirect immunofluorescence. Shown are representative micrographs from three independent experiments. (B) Cells were transfected with Raf-1 alone or together with wt PAK5. One hour prior to fixation the cells were incubated with MitoTracker Green to visualize mitochondria. The cells were then fixed and stained for localization of Raf-1. Shown are representative micrographs from four independent experiments.
To determine whether co-expression of PAK5 was targeting Raf-1 to mitochondria, HEK293 cells were transfected with Raf-1 alone or together with wt PAK5. The cells were then stained for expression of Raf-1 and for mitochondria using the mitochondrial dye MitoTracker Green. As shown in Figure 3B, when expressed alone Raf-1 was localized throughout the cytoplasm with a minor degree of co-localization with mitochondria. On the other hand, co-transfection of PAK5 with Raf-1 clearly caused a relocalization of a portion of the expressed Raf-1 to mitochondria, with co-localization concentrated within a region adjacent to the nucleus. Interestingly, perinuclear concentration of mitochondria was not observed when Raf-1 or PAK5 were expressed alone, suggesting that PAK5 and Raf-1 may co-regulate the subcellular distribution of mitochondria. We also observed by subcellular fractionation that co-expression of PAK5 targeted a portion of the co-transfected Raf-1 to mitochondria (Figure 5). Taken together these data demonstrate that PAK5 co-expression targets a subpopulation of Raf-1 to the mitochondria. These data also suggest that the combined effect of Raf-1 and PAK5 expression may affect mitochondrial dynamics.
Figure 5.
PAK5 targets Raf-1 to mitochondria in a serine 338 independent manner. (A) HEK293 cells were transfected with Flag-tagged wt Raf-1 or Raf-1 S338A, alone or together with Myc-tagged wt PAK5. After serum starvation the mitochondrial and cytosolic fractions were isolated by differential centrifugation. Equal amounts of protein from each fraction were analyzed by Western blotting for the presence of PAK5 and Raf-1. Porin and SOD-1 are mitochondrial and cytosolic proteins, respectively. (B) HEK293 cells were transfected with Flag-tagged wt Raf-1 or Raf-1 S338A, alone or together with Myc-tagged wt PAK5. After serum starvation the cells were lysed, the PAK5 was immunoprecipitated, and the co-precipitation of Raf-1 with PAK5 was detected by Western blotting. Shown are representative experiments from four independent experiments. (C) HEK293 cells were transfected with HA-tagged wt Raf-1, alone or together with Myc-tagged wt PAK5, kinase-inactive full length PAK5 (PAK5 K/M), or the kinase-inactive PAK5 catalytic domain (PAK5 CAT K/M). The cells were then separated into mitochondrial and cytosolic/membrane fractions. Equal amounts of protein from each fraction were separated by SDS-PAGE, transferred to PVDF membrane, and tested for the presence of the antigens shown by Western blotting. Shown are the results from three independent experiments.
PAK5 is unique among group II PAKs for interaction with Raf-1 and does not bind to other Raf family members
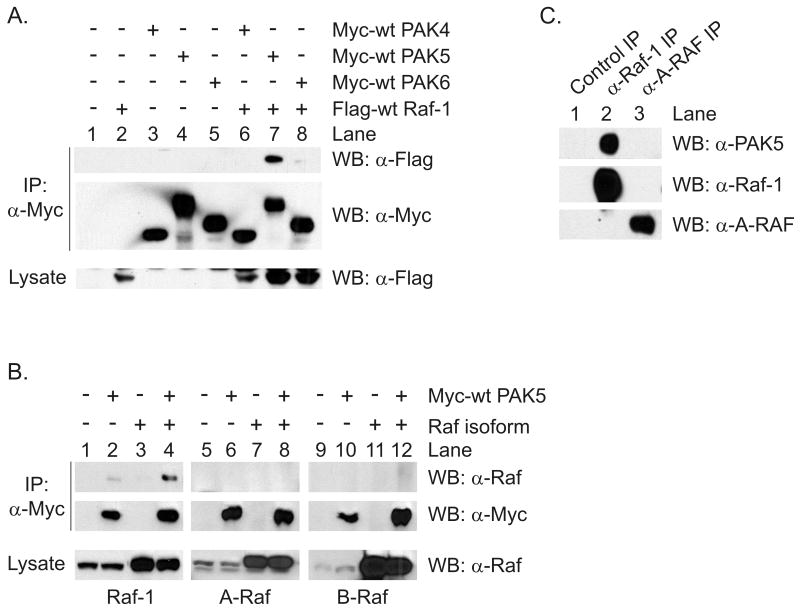
We then examined whether the interaction of PAK5 with Raf-1 was unique among group II PAKs, and whether other Raf family members interacted with PAK5. HEK293 cells were transfected with Myc-tagged, wild type forms of PAK4, PAK5 and PAK6, alone or together with Flag-tagged wt Raf-1. The PAK proteins were then immunoprecipitated and tested for co-precipitation of Raf-1. As shown in Figure 4A, Raf-1 efficiently co-precipitated with wt PAK5, but not with wt PAK4 or wt PAK6 (compare lanes 6-8). Thus, the interaction between PAK5 and Raf-1 is specific among group II PAKs. To determine whether PAK5 interacted with other Raf isoforms HEK293 cells were transfected with Flag-tagged wt Raf-1, A-Raf or B-Raf, alone or in combination with Myc-tagged wt PAK5. The PAK5 was then immunoprecipitated and tested for the co-precipitation of each Raf protein. As shown in Figure 4B, both endogenous and overexpressed Raf-1 co-precipitated with wt PAK5 (lanes 2 and 4, respectively). However, neither endogenous nor transfected A-Raf or B-Raf co-precipitated with PAK5. These data indicate that PAK5 uniquely interacts with Raf-1 in cells.
Figure 4.
PAK5 is unique among group II PAKs for interaction with Raf-1 and does not bind to other Raf proteins. (A) HEK293 cells were co-transfected with Flag-tagged wt Raf-1 and the Myc-tagged PAKs shown. After serum starvation the PAKs were immunoprecipitated and tested for co-precipitation of Raf-1 by Western blotting. (B) HEK293 cells were transfected with Myc-tagged wt PAK5 and Flag-tagged wt Raf-1, A-Raf, or B-Raf. The PAK5 was immunoprecipitated and the co-precipitation of Raf proteins was tested by Western blotting using Raf isoform specific antibodies. The upper panels are Western blots for co-precipitated Raf isoforms, the middle panels are Western blots for immunoprecipitated PAK5, and the bottom panels are Western blots for Raf isoforms in the cell lysates. (C) Endogenous Raf-1 or A-Raf were immunoprecipitated from a rat brain homogenate and tested for the co-precipitation of endogenous PAK5 by Western blotting. Shown are representative experiments from three independent experiments.
To determine whether endogenous PAK5 and Raf-1 interact in tissues, we tested whether these proteins co-immunoprecipitate from rat brain. This tissue was chosen because PAK5 has been shown to be mainly expressed in the brain and in neuroendocrine tissues (Pandey, Dan, Kristiansen, Watanabe, Voldby, Kajikawa, Khosravi-Far, Blagoev, and Mann, 2002;Dan et al., 2002). Endogenous Raf-1 or A-Raf were immunoprecipitated from a Triton X-100 soluble fraction of adult rat brain and then tested for the co-precipitation of endogenous PAK5. As shown in Figure 4C, endogenous PAK5 co-precipitated with Raf-1 (top panel, lane 2) but not with a control rabbit antibody or with an antibody specific for A-Raf (lanes 1 and 3, respectively). Thus, endogenous PAK5 interacts with Raf-1 but not A-Raf in the adult rat brain. Taken as a whole these experiments indicate that PAK5 selectively interacts with Raf-1 in cells and in native tissue, and that this interaction is unique among group II PAKs.
PAK5 targets Raf-1 to mitochondria independently of Raf-1 phosphorylation on serine 338
Since PAK1 has been shown previously to stimulate Raf-1 re-localization to mitochondria in a serine 338 phosphorylation dependent manner (Jin, Zhuo, Guo, and Field, 2005), we examined whether the ability of PAK5 to stimulate mitochondrial localization of Raf-1 was also serine 338-dependent. HEK293 cells were transfected with Flag-tagged wt Raf-1 or Raf-1 S338A, plus or minus Myc-tagged wt PAK5. After serum starvation the cells were separated into mitochondrial and cytoplasmic fractions and the presence of Raf-1 in each fraction was examined by Western blotting. As expected, very little wt Raf-1 was detected in the mitochondrial fraction when expressed alone (Figure 5A, top panel, lanes 1-3). However, co-expression of wt PAK5 significantly enhanced the mitochondrial localization of Raf-1 and dramatically increased its phosphorylation on serine 338 (lane 4, top and middle panels, respectively). Importantly, co-expression of wt PAK5 also stimulated a significant increase in the association of Raf-1 S338A with mitochondria. Thus, these data indicate that expression of wt PAK5 targets Raf-1 to mitochondria, and that this is largely independent of Raf-1 phosphorylation on serine 338. These data also indicate that the mechanism by which PAK5 targets Raf-1 to mitochondria is distinct from that of PAK1.
To confirm that the S338A substitution in Raf-1 did not affect the ability of PAK5 to bind to Raf-1, we examined the ability of this Raf-1 mutant to co-immunoprecipitate with PAK5. Wild type Raf-1 or Raf-1 S338A were transfected into HEK293 cells with or without wt PAK5. After serum starvation the PAK5 was immunoprecipitated and examined for the co-precipitation of Raf-1. As shown in Figure 5B, both wt Raf-1 and Raf-1 S338A co-precipitated with wt PAK5 (lanes 4 and 6, top panel). Thus, although the co-precipitation of Raf-1 S338A with PAK5 was slightly less efficient than for wt Raf-1, these data indicate that mutation of serine 338 to alanine did not significantly impair the interaction between PAK5 and Raf-1.
We also examined whether the kinase activity of PAK5 was required for mitochondrial targeting of Raf-1. HEK293 cells were transfected with HA-epitope tagged Raf-1 plus Myc tagged wt PAK5, kinase-inactive full length PAK5 (PAK5 K/M), or the kinase-inactive catalytic domain of PAK5 (PAK5 CAT K/M). The cells were then fractionated into mitochondrial and cytosolic/membrane fractions, and tested for the presence of Raf-1 and PAK5 proteins. In these assays we observed that each PAK5 protein was preferentially expressed in the mitochondrial fraction, and that the targeting of full length PAK5 proteins to mitochondria was more efficient than for the isolated PAK5 catalytic domain (Figure 5C). These data are consistent with there being multiple N- and C-terminal mitochondrial targeting sequences within PAK5 (Cotteret and Chernoff, 2006;Wu and Frost, 2006). Importantly, each PAK5 construct was able to target Raf-1 to mitochondria (lanes 3-5). Interestingly, wt PAK5 was more effective at targeting of Raf-1 than either kinase-inactive version of PAK5, presumably because wt PAK5 interacted more tightly with Raf-1 than kinase-inactive PAK5 (Figure 5B). Nevertheless, these data indicate that the kinase activity of PAK5 is not required for Raf-1 relocalization to mitochondria, and support our observation that phosphorylation of Raf-1 on serine 338 is not required for PAK5-dependent mitochondrial targeting of Raf-1.
PAK5 expression does not potentiate EGF-stimulated ERK/MAPK activation
We then examined whether expression of PAK5 affected the ability EGF to stimulate Raf-1 phosphorylation on serine 338, and if this affected ERK/MAPK activation in the cytosol or at the mitochondria. EGF is a strong activator of the ERK/MAPK pathway in HEK293 cells (Tran, Wu, and Frost, 2005). Since these cells do not normally express PAK5, we tested the ability of overexpressed PAK5 to potentiate EGF-mediated Raf-1 activation. HEK293 cells were transfected with Flag-tagged wt Raf-1 along with empty vector, Myc-tagged wt PAK5 or Myc-tagged constitutively active PAK5. After serum-starvation the cells were stimulated with EGF for 5 minutes, lysed and then separated into mitochondrial and cytoplasmic fractions. The phosphorylation of Raf-1 on serine 338 and the activation of ERK1/2 were then monitored by Western blotting. As shown in Figure 6, stimulation of cells with EGF caused an increase in the phosphorylation of Raf-1 on serine 338 in the cytoplasmic fraction that was accompanied by a strong activation of both ERK1 and ERK2 (lane 8). Furthermore, co-expression of wt PAK5 did not significantly alter Raf-1 phosphorylation or ERK activation in the cytosolic fraction (compare lanes 9 and 10). On the other hand, co-expression of constitutively active PAK5 caused a very strong phosphorylation of cytoplasmic Raf-1 on serine 338 that was not further stimulated by addition of EGF (compare lanes 11 and 12), indicating that PAK5 could access Raf-1 in this fraction. This suggests that PAK5 cannot contribute to EGF-dependent Raf-1 or ERK activation in the cytoplasm. Importantly, when we assayed for Raf-1 serine 338 phosphorylation in the mitochondrial fraction we observed that EGF was a very poor activator of Raf-1 in this fraction, and that expression of constitutively active PAK5 caused a robust, EGF-independent phosphorylation of Raf-1 on serine 338 (lanes 1-6). Taken as a whole, these results indicate that PAK5 is an efficient Raf-1 kinase, but that its expression is not limiting for EGF-stimulated Raf-1 and ERK/MAPK pathway activation in these cells.
Figure 6.
Expression of PAK5 does not potentiate EGF-stimulated ERK/MAPK pathway activation in HEK293 cells. HEK293 cells were transfected with Flag tagged wt Raf-1, minus or plus Myc-tagged wt PAK5 or Myc tagged constitutively active PAK5. After serum starvation the cells were stimulated with EGF for 5 minutes (50 ng/ml). The cells were then lysed and separated into cytosolic and mitochondrial fractions. Equal amounts of protein from each fraction were separated by SDS-PAGE, transferred to PVDF membrane. The localization and phosphorylation of Raf-1 and ERK1/2 and PAK5 proteins were determined by Western blotting. Shown is a representative experiment from three independent experiments.
Discussion
In the present work we have shown that PAK5 is unique among group II PAKs in that it efficiently phosphorylates Raf-1 on a key activating site, serine 338, and also stimulates Raf-1 activity. We have also shown that PAK5 forms a tight complex with Raf-1, but not A-Raf or B-Raf, and that this interaction occurs between the endogenous proteins in the adult rat brain, where both PAK5 and Raf-1 are expressed. We have also shown that the targeting of Raf-1 to mitochondria by PAK5 is independent of phosphorylation on serine 338, and does not require PAK5 kinase activity. Thus, these data identify PAK5 as a novel Raf-1 kinase that may control Raf-1 activation, primarily at mitochondria.
The ability of PAK5 to phosphorylate Raf-1 on serine 338 was not unique among group II PAKs, and in fact all of the PAKs we examined stimulated Raf-1 phosphorylation on this site. However, among the group II PAKs, only PAK5 efficiently stimulated Raf-1 activation in our hands. The explanation for why PAK4 and PAK6 were robust serine 338 kinases and yet failed to significantly stimulate Raf-1 activity is not clear. Phosphorylation of Raf-1 on serine 338 has previously been suggested to be necessary but insufficient for Raf-1 activation, and our data would support that conclusion (Beeser, Jaffer, Hofmann, and Chernoff, 2005). The Raf-1 activation mechanism is complex and is minimally thought to involve the binding of active Ras, the dephosphorylation of serine 259 by PP2A and subsequent release of 14-3-3 from this site, and the phosphorylation of Raf-1 on serine 338, tyrosine 341, threonine 491 and serine 494. Thus, it is possible that PAK5 uniquely contributes to Raf-1 activation by influencing Raf-1 phosphorylation at sites other than serine 338. It is also possible that PAK5 alters the interaction of Raf-1 with one or more phosphatases that may downregulate its activity. The distinctive capacity of PAK5 to form a tight complex with Raf-1 in the cell may also contribute to its ability to stimulate Raf-1 activity.
An important observation of the present work is that PAK5 targets a subpopulation of Raf-1 to mitochondria. Since this activity did not require the presence of serine 338 in Raf-1 this mechanism of targeting appears to be dissimilar from that of PAK1, which re-localizes Raf-1 to the mitochondria by stimulating its association with Bcl2 in a serine 338/serine 339 dependent manner (Jin, Zhuo, Guo, and Field, 2005). In this regard we have been unable to show that PAK5 expression causes Raf-1 to interact with transfected or endogenous Bcl2 in cells, thus further differentiating PAK5-dependent mitochondrial targeting from PAK1 (data not shown). PAK5 localizes to mitochondria by virtue of two separate mitochondrial targeting signals in the N- and C-terminus of the protein, and when overexpressed in cells at least of portion of the PAK5 localizes to mitochondria. PAK5 is also targeted to mitochondria irrespective of its kinase activity (Cotteret, Jaffer, Beeser, and Chernoff, 2003;Cotteret and Chernoff, 2006;Wu and Frost, 2006). Thus, our observation that expression of catalytically inactive PAK5 causes relocalization of Raf-1 to mitochondria supports the notion that the association of PAK5 with Raf-1 is sufficient for mitochondrial targeting.
PAK5 expression is mainly restricted to neuronal tissues (Pandey, Dan, Kristiansen, Watanabe, Voldby, Kajikawa, Khosravi-Far, Blagoev, and Mann, 2002;Dan, Nath, Liberto, and Minden, 2002), and in IMR32 neuroblastoma cells and C17.2 neural stem cells endogenous PAK5 has been reported to localize to both the mitochondria and the cytosol, indicating that the localization of overexpressed PAK5 in our experiments accurately recapitulates endogenous PAK5 targeting (Cotteret and Chernoff, 2006). These observations connected with our present data suggest that endogenous PAK5 may be expected to influence mitochondrial and cytosolic Raf-1 activation within neuronal cells. Thus it is possible that PAK5 may contribute to classical ERK/MAPK activation in the cytosol in response to stimuli governing axonal polarity and neurite outgrowth in immature neurons, or in response to signals that control synaptic plasticity in mature neurons (Sweatt, 2004;Mattson, 2007). Because PAK5 may also regulate Raf-1 signaling at the mitochondria, its activity may be important for the response of neural cells to various stresses. For example, endogenous PAK5 has been shown to contribute to cell survival in response to an apoptotic stimulus in IMR32 cells, suggesting that PAK5 dependent signaling may contribute to anti-apoptotic activity in primary neurons (Cotteret and Chernoff, 2006). Finally, it is important to note that the protein kinase mbt, which is the closest homolog to PAK5 in Drosophila, controls the generation and survival of neuronal structures involved in learning and memory (Melzig et al., 1998). Thus, PAK5 may play multiple roles in neuronal signaling, and the interaction between PAK5 and Raf-1 demonstrated here may be an important part of PAK5 function.
Acknowledgments
We thank Audrey Minden and Zijie Sun for the PAK4 and PAK6 cDNAs, respectively. This work was supported by grants to JAF from the American Cancer Society (RSG-01-193-01-TBE) and NIH (5R01CA116356-02).
References
- 1.Baccarini M. Second nature: biological functions of the Raf-1 “kinase”. FEBS Lett. 2005;579:3271–3277. doi: 10.1016/j.febslet.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Barnard D, Diaz B, Clawson D, Marshall M. Oncogenes, growth factors and phorbol esters regulate Raf-1 through common mechanisms. Oncogene. 1998;17:1539–1547. doi: 10.1038/sj.onc.1202061. [DOI] [PubMed] [Google Scholar]
- 3.Beeser A, Jaffer ZM, Hofmann C, Chernoff J. Role of group A p21-activated kinases in activation of extracellular-regulated kinase by growth factors. J Biol Chem. 2005;280:36609–36615. doi: 10.1074/jbc.M502306200. [DOI] [PubMed] [Google Scholar]
- 4.Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- 5.Brown JL, Stowers L, Baer M, Trejo J, Coughlin S, Chant J. Human Ste20 homologue hPAK1 links GTPases to the JNK MAP kinase pathway. Curr Biol. 1996;6:598–605. doi: 10.1016/s0960-9822(02)00546-8. [DOI] [PubMed] [Google Scholar]
- 6.Chaudhary A, King WG, Mattaliano MD, Frost JA, Diaz B, Morrison DK, Cobb MH, Marshall MS, Brugge JS. Phosphatidylinositol 3-kinase regulates Raf1 through Pak phosphorylation of serine 338. Curr Biol. 2000;10(9):551–554. doi: 10.1016/s0960-9822(00)00475-9. [DOI] [PubMed] [Google Scholar]
- 7.Chiloeches A, Mason CS, Marais R. S338 phosphorylation of Raf-1 is independent of phosphatidylinositol 3-kinase and Pak3. Mol Cell Biol. 2001;21(7):2423–2434. doi: 10.1128/MCB.21.7.2423-2434.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chong H, Guan KL. Regulation of Raf through phosphorylation and N terminus-C terminus interaction. J Biol Chem. 2003a;278:36269–36276. doi: 10.1074/jbc.M212803200. [DOI] [PubMed] [Google Scholar]
- 9.Chong H, Lee J, Guan KL. Positive and negative regulation of Raf kinase activity and function by phosphorylation. EMBO J. 2001;20:3716–3727. doi: 10.1093/emboj/20.14.3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chong H, Vikis HG, Guan KL. Mechanisms of regulating the Raf kinase family. Cell Signal. 2003b;15:463–469. doi: 10.1016/s0898-6568(02)00139-0. [DOI] [PubMed] [Google Scholar]
- 11.Cotteret S, Chernoff J. Nucleocytoplasmic shuttling of Pak5 regulates its antiapoptotic properties. Mol Cell Biol. 2006;26:3215–3230. doi: 10.1128/MCB.26.8.3215-3230.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cotteret S, Jaffer ZM, Beeser A, Chernoff J. p21-Activated kinase 5 (Pak5) localizes to mitochondria and inhibits apoptosis by phosphorylating BAD. Mol Cell Biol. 2003;23:5526–5539. doi: 10.1128/MCB.23.16.5526-5539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dan C, Nath N, Liberto M, Minden A. PAK5, a new brain-specific kinase, promotes neurite outgrowth in N1E-115 cells. Mol Cell Biol. 2002;22:567–577. doi: 10.1128/MCB.22.2.567-577.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frost JA, Steen H, Shapiro P, Lewis T, Ahn N, Shaw PE, Cobb MH. Cross-cascade activation of ERKs and ternary complex factors by Rho family proteins. EMBO J. 1997;16:6426–6438. doi: 10.1093/emboj/16.21.6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gnesutta N, Qu J, Minden A. The serine/threonine kinase PAK4 prevents caspase activation and protects cells from apoptosis. J Biol Chem. 2001;276:14414–14419. doi: 10.1074/jbc.M011046200. [DOI] [PubMed] [Google Scholar]
- 16.Graham JM, Rickwood D. Subcellular Fractionation, A Practical Approach. Oxford University Press; New York: 1997. [Google Scholar]
- 17.Jaffer ZM, Chernoff J. p21-activated kinases: three more join the Pak. Int J Biochem Cell Biol. 2002;34:713–717. doi: 10.1016/s1357-2725(01)00158-3. [DOI] [PubMed] [Google Scholar]
- 18.Jin S, Zhuo Y, Guo W, Field J. p21-activated Kinase 1 (Pak1)-dependent phosphorylation of Raf-1 regulates its mitochondrial localization, phosphorylation of BAD, and Bcl-2 association. J Biol Chem. 2005;280:24698–24705. doi: 10.1074/jbc.M413374200. [DOI] [PubMed] [Google Scholar]
- 19.King AJ, Sun H, Diaz B, Barnard D, Miao W, Bagrodia S, Marshall MS. The protein kinase Pak3 positively regulates Raf-1 activity through phosphorylation of serine 338. Nature. 1998;396:180–183. doi: 10.1038/24184. [DOI] [PubMed] [Google Scholar]
- 20.Lu Y, Pan ZZ, Devaux Y, Ray P. p21-activated protein kinase 4 (PAK4) interacts with the keratinocyte growth factor receptor and participates in keratinocyte growth factor-mediated inhibition of oxidant-induced cell death. J Biol Chem. 2003;278:10374–10380. doi: 10.1074/jbc.M205875200. [DOI] [PubMed] [Google Scholar]
- 21.Mattson MP. Mitochondrial regulation of neuronal plasticity. Neurochem Res. 2007;32:707–715. doi: 10.1007/s11064-006-9170-3. [DOI] [PubMed] [Google Scholar]
- 22.Melzig J, Rein KH, Schafer U, Pfister H, Jackle H, Heisenberg M, Raabe T. A protein related to p21-activated kinase (PAK) that is involved in neurogenesis in the Drosophila adult central nervous system. Curr Biol. 1998;8:1223–1226. doi: 10.1016/s0960-9822(07)00514-3. [DOI] [PubMed] [Google Scholar]
- 23.O'Neill E, Kolch W. Conferring specificity on the ubiquitous Raf/MEK signalling pathway. Br J Cancer. 2004;90:283–288. doi: 10.1038/sj.bjc.6601488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pandey A, Dan I, Kristiansen TZ, Watanabe NM, Voldby J, Kajikawa E, Khosravi-Far R, Blagoev B, Mann M. Cloning and characterization of PAK5, a novel member of mammalian p21-activated kinase-II subfamily that is predominantly expressed in brain. Oncogene. 2002;21:3939–3948. doi: 10.1038/sj.onc.1205478. [DOI] [PubMed] [Google Scholar]
- 25.Ritt DA, Zhou M, Conrads TP, Veenstra TD, Copeland TD, Morrison DK. CK2 is a component of the KSR1 scaffold complex that contributes to Raf kinase activation. Curr Biol. 2007;17:179–184. doi: 10.1016/j.cub.2006.11.061. [DOI] [PubMed] [Google Scholar]
- 26.Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Tran NH, Frost JA. Phosphorylation of Raf-1 by p21-activated kinase 1 and Src regulates Raf-1 autoinhibition. J Biol Chem. 2003;278:11221–11226. doi: 10.1074/jbc.M210318200. [DOI] [PubMed] [Google Scholar]
- 28.Tran NH, Wu X, Frost JA. B-Raf and Raf-1 are regulated by distinct autoregulatory mechanisms. J Biol Chem. 2005;280:16244–16253. doi: 10.1074/jbc.M501185200. [DOI] [PubMed] [Google Scholar]
- 29.Wu X, Frost JA. Multiple Rho proteins regulate the subcellular targeting of PAK5. Biochem Biophys Res Commun. 2006;351:328–335. doi: 10.1016/j.bbrc.2006.09.172. [DOI] [PubMed] [Google Scholar]
- 30.Xiang X, Zang M, Waelde CA, Wen R, Luo Z. Phosphorylation of 338SSYY341 regulates specific interaction between Raf-1 and MEK1. J Biol Chem. 2002;277:44996–45003. doi: 10.1074/jbc.M203953200. [DOI] [PubMed] [Google Scholar]
- 31.Yang F, Li X, Sharma M, Zarnegar M, Lim B, Sun Z. Androgen receptor specifically interacts with a novel p21-activated kinase, PAK6. J Biol Chem. 2001;276:15345–15353. doi: 10.1074/jbc.M010311200. [DOI] [PubMed] [Google Scholar]
- 32.Zang M, Hayne C, Luo Z. Interaction between active Pak1 and Raf-1 is necessary for phosphorylation and activation of Raf-1. J Biol Chem. 2002;277:4395–4405. doi: 10.1074/jbc.M110000200. [DOI] [PubMed] [Google Scholar]
- 33.Zang M, Waelde CA, Xiang X, Rana A, Wen R, Luo Z. Microtubule integrity regulates Pak leading to Ras-independent activation of Raf-1. Insights into mechanisms of Raf-1 activation. J Biol Chem. 2001;276:25157–25165. doi: 10.1074/jbc.M100152200. [DOI] [PubMed] [Google Scholar]