Summary
Starvation blocks the actions of growth hormone (GH) and inhibits growth through mechanisms that are not well understood. In this report, we demonstrate that fibroblast growth factor 21 (FGF21), a hormone induced by fasting, causes GH resistance. In liver, FGF21 reduces concentrations of the active form of signal transducer and activator of transcription 5 (STAT5), a major mediator of GH actions, and causes corresponding decreases in the expression of its target genes including insulin-like growth factor 1 (IGF-1). FGF21 also induces hepatic expression of IGF-1 binding protein 1 and suppressor of cytokine signaling 2, which blunt GH signaling. Chronic exposure to FGF21 markedly inhibits growth in mice. These data suggest a central role for FGF21 in inhibiting growth as part of its broader role in inducing the adaptive response to starvation.
Introduction
Growth hormone (GH) is synthesized and secreted by somatotrophs in the anterior pituitary to regulate diverse physiological processes including growth and metabolism (Herrington et al., 2000; LeRoith and Yakar, 2007). Many of the anabolic actions of GH are mediated by insulin-like growth factor 1 (IGF-1). GH induces IGF-1 transcription through a complex regulatory cascade that is initiated when GH binds to the GH receptor (GHR) on the cell surface. This interaction causes the GHR to activate janus kinase 2 (JAK2), which in turn phosphorylates members of the signal transducers and activators of transcription (STAT) family. Once phosphorylated, STAT proteins translocate to the nucleus, where they bind to response elements in the regulatory regions of target genes including IGF-1. STAT5A and STAT5B have prominent roles in mediating the downstream effects of GH (Herrington et al., 2000). Mice lacking both STAT5A and STAT5B are 20–40% smaller than their wild type littermates and are resistant to the anabolic actions of exogenous GH (Teglund et al., 1998).
Starvation and malnutrition lower circulating levels of IGF-1 (Thissen et al., 1994). This reduction is caused in part by a decrease in IGF-1 mRNA levels in liver, where most IGF-1 is made (Bornfeldt et al., 1989; Emler and Schalch, 1987; Lowe et al., 1989; Maes et al., 1983). Fasting also decreases hepatic mRNA levels and circulating concentrations of acid labile subunit (ALS), which complexes with IGF-1 to stabilize it (Kong et al., 2002). While the mechanism underlying these fasting-induced effects is not known, a recent study showed that fasted rats have reduced hepatic phosphorylation of STAT5 in response to injected GH, suggesting that fasting might inhibit transcription of the IGF-1 and ALS genes by reducing STAT5 activity (Beauloye et al., 2002).
Fibroblast growth factor 21 (FGF21) is an atypical member of the FGF family that functions as an endocrine hormone (Kharitonenkov et al., 2005). In mice, FGF21 is induced in liver by fasting through a mechanism that requires the nuclear fatty acid receptor, peroxisome proliferator-activated receptor α(PPARα) (Badman et al., 2007; Inagaki et al., 2007). FGF21 induces the synthesis of ketone bodies, which are the principal source of energy during prolonged fasting and starvation. FGF21 also sensitizes mice to the energy-conserving state of torpor, which is characterized by decreased body temperature and physical activity (Inagaki et al., 2007). In this report, we show that FGF21 inhibits STAT5 signaling and blunts growth, revealing a broader role for FGF21 in promoting energy conservation during starvation.
Results
FGF21-transgenic mice have reduced growth
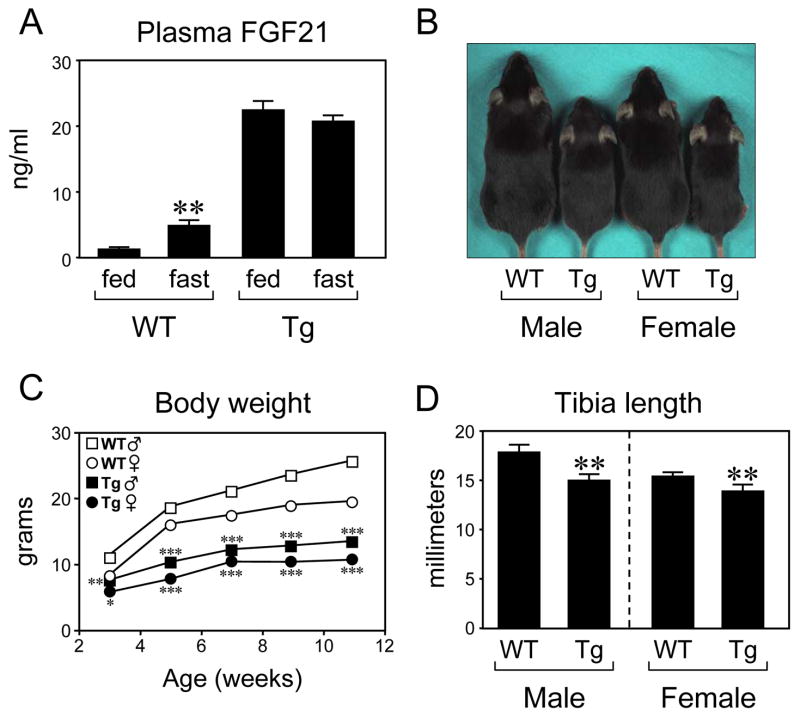
We previously generated transgenic mice that express FGF21 under the control of the apolipoprotein E promoter (Inagaki et al., 2007). Plasma FGF21 concentrations in these transgenic mice are ~5-fold higher than those in fasted wild type mice (Figure 1A). Notably, both male and female FGF21-transgenic mice were markedly smaller than their wild type counterparts (Figure 1B). Although wild type and FGF21-transgenic mice weighed the same at birth (data not shown), FGF21-transgenic mice gained less weight than wild type mice (Figure 1C). There was a significant reduction in tibia length in both male and female FGF21-transgenic mice (Figure 1D). Food consumption normalized to body weight was significantly increased in male FGF21-transgenic mice and trended higher in female FGF21-transgenic mice, and body composition studies showed increased adiposity in both male and female FGF21-transgenic mice (Supplementary Figure 1). These data demonstrate that chronic exposure to FGF21 inhibits growth without causing hypophagia.
Figure 1.
FGF21 inhibits growth. (A) Plasma FGF21 concentrations were measured in wild type (WT) and FGF21-transgenic (Tg) mice in either the fed state or after a 24 hour fast. n = 5 male mice/group. **, P<0.01 compared to WT, fed group. (B) Male and female WT and Tg mice are shown. (C) Body weights are shown for male (squares) and female (circles) WT (open symbols) and Tg mice (closed symbols). n = 5 mice/group. *, P<0.05; **, P<0.01; ***, P<0.001 compared to WT mice of same sex. (D) Tibia length was measured in male and female WT and Tg mice. n = 4 mice/group. **, P<0.01. In this and all other figures, error bars represent the mean ± SEM.
FGF21 reduces serum IGF-1
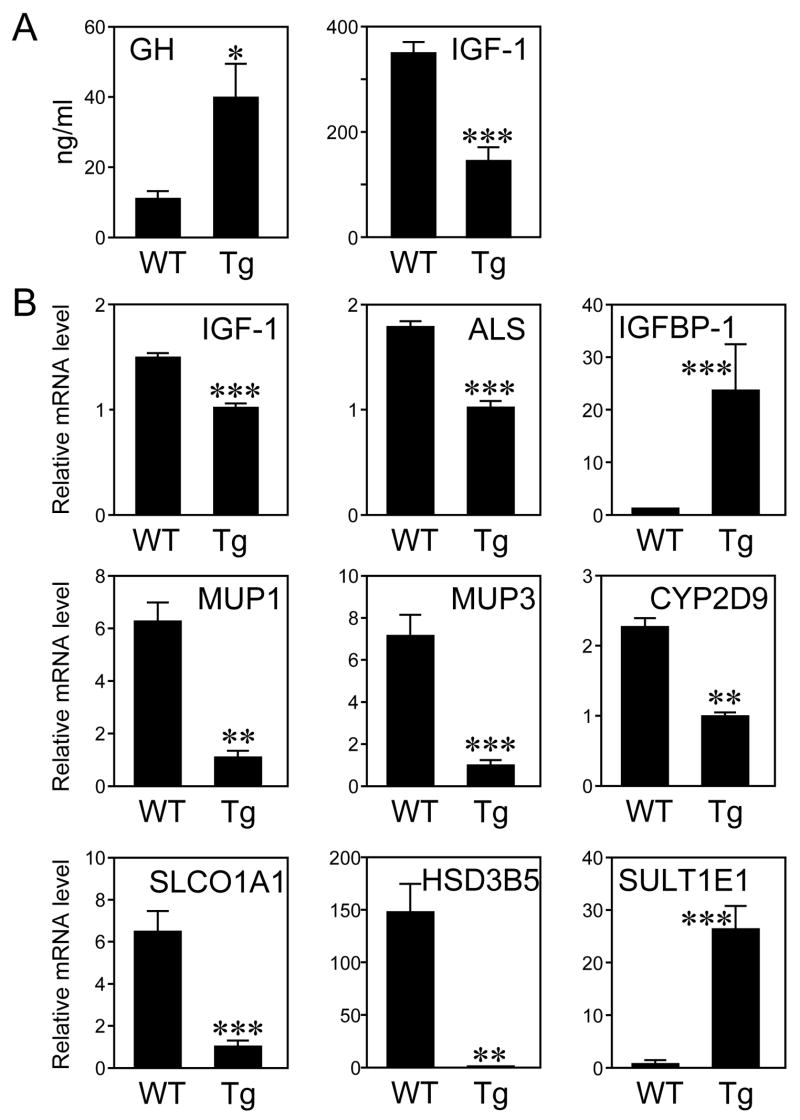
Plasma GH and IGF-1 concentrations were measured in wild type and FGF21-transgenic mice. Interestingly, GH concentrations were significantly increased in FGF21-transgenic mice (Figure 2A). Despite this increase in circulating GH, there was a >50% decrease in serum IGF-1 concentrations (Figure 2A). Thus, FGF21-transgenic mice are GH resistant.
Figure 2.
FGF21 inhibits IGF-1. (A) GH and IGF-1 concentrations in plasma from wild type (WT) and FGF21-transgenic (Tg) mice. n = 8 male mice/WT group, 11 male mice/Tg group. (B) mRNA levels of the indicated genes were measured by RT-qPCR in livers from WT and Tg mice. *, P<0.05; **, P<0.01; ***, P<0.001.
Approximately 75% of circulating IGF-1 is synthesized in the liver (Yakar et al., 1999). IGF-1 mRNA levels were reduced by ~30% in livers of FGF21-transgenic mice but were not changed in other tissues including skeletal muscle, kidney, and ovary (Figure 2B and data not shown). FGF21-transgenic mice also had a ~40% decrease in hepatic ALS mRNA (Figure 2B). Analysis of IGF-1 binding protein expression revealed a dramatic increase in hepatic IGFBP-1 mRNA (Figure 2B), which is induced by fasting (Murphy et al., 1991). IGFBP-1-transgenic mice have both intrauterine and postnatal growth retardation, suggesting that IGFBP-1 is involved in sequestering IGF-1 during periods of nutritional deprivation (Baxter and Martin, 1989; Silha and Murphy, 2002; Underwood et al., 1994). The coordinate changes in hepatic IGF-1, ALS, and IGFBP-1 expression likely account for the marked decrease in circulating IGF-1 in FGF21-transgenic mice.
FGF21 inhibits STAT5
Since both IGF-1 and ALS are induced and IGFBP-1 is repressed by STAT5 in liver (Davey et al., 2001; Ono et al., 2007; Seneviratne et al., 1990; Woelfle and Rotwein, 2004), we examined whether other STAT5-regulated genes are changed in the FGF21-transgenic mice. Significant decreases were seen in FGF21-transgenic mice in the hepatic mRNA levels of major urinary proteins (MUP) 1 and 3, CYP2D9, solute carrier organic anion transporter family member 1a1 (SLCO1A1) and hydroxysteroid dehydrogenase 3β5 (HSD3B5) (Figure 2B). By contrast, sulfotransferase family 1E member 1 (SULT1E1) mRNA levels were dramatically increased in FGF21-transgenic mice (Figure 2B). These changes in gene expression mirror those seen in STAT5A/B-knockout mice (Holloway et al., 2007). To determine directly whether STAT5 is altered in FGF21-transgenic mice, total and phosphorylated STAT5 levels were measured in whole-cell and nuclear extracts prepared from livers of wild type and FGF21-transgenic mice. Although there was no change in total STAT5 protein levels, there was a striking decrease in phosphorylated STAT5 levels and corresponding decreases in the concentrations of nuclear STAT5A and STAT5B in FGF21-transgenic mice (Figure 3A). Thus, both the STAT5A and STAT5B isoforms are affected. Injection of pharmacologic concentrations of recombinant GH into FGF21-transgenic mice acutely restored STAT5 phosphorylation (Supplementary Figure 2). Taken together, these data demonstrate that STAT5 activity is strongly impaired in FGF21-transgenic mice. We note that IGFBP-1 and other fasting-induced genes are regulated by the transcription factor FOXO1 (Guo et al., 1999). However, nuclear FOXO1 protein levels were unchanged in livers of FGF21-transgenic mice (Supplementary Figure 3), suggesting that FGF21 does not affect FOXO1 signaling.
Figure 3.
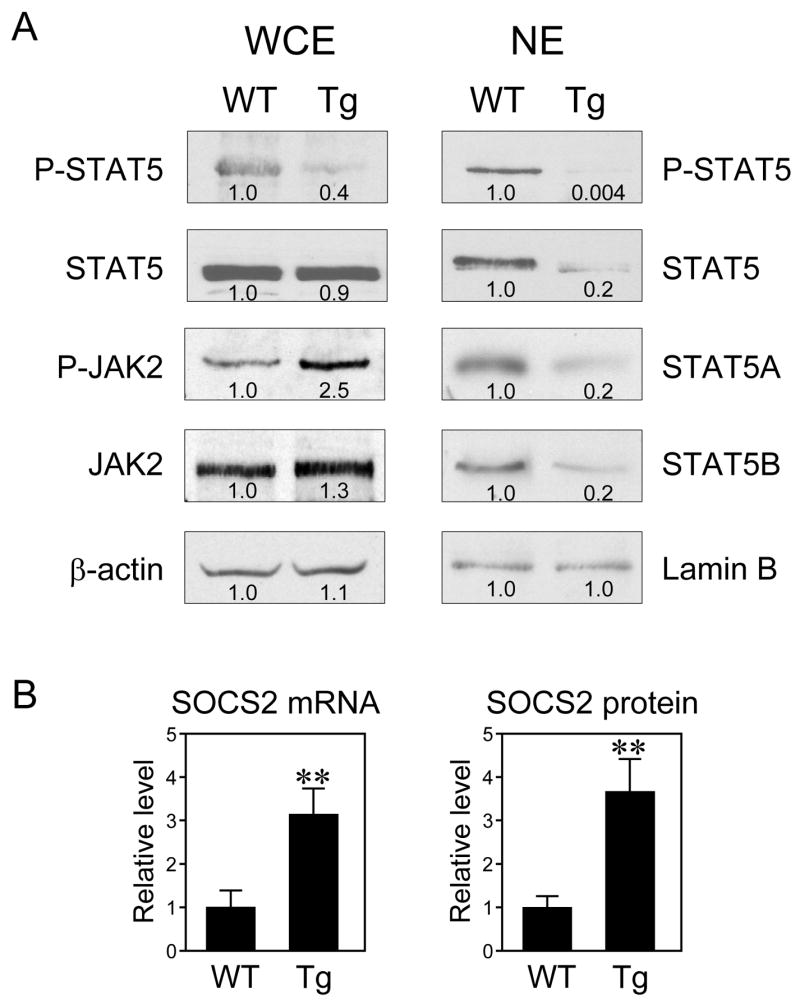
FGF21 reduces STAT5 phosphorylation. (A) Phosphorylated STAT5 (P-STAT5), total STAT5, STAT5A, STAT5B, phosphorylated JAK2 (P-JAK2), and total JAK2 protein levels were measured by immunoblotting in hepatic whole-cell extracts (WCE) or nuclear extracts (NE) from wild type (WT) or FGF21-transgenic (Tg) mice. β-Actin and Lamin B were used as loading controls. Immunoblotting was done with equal amounts of protein pooled from 4 male mice. Quantification by densitometry is indicted below each band. (B) SOCS2 mRNA and protein levels were measured by RT-qPCR and immunoblotting, respectively, in livers from WT and Tg mice. n = 7 male mice/group. **, P<0.01.
JAK2 plays an important role in the phosphorylation and activation of STAT5 (Herrington et al., 2000). While there was little or no change in total JAK2 protein levels in livers of FGF21-transgenic mice, phosphorylated JAK2 concentrations were increased in the FGF21-transgenic mice (Figure 3A). These data indicate that FGF21 interrupts the GH signaling cascade downstream of JAK2.
Suppressor of cytokine signaling 2 (SOCS2) inhibits the GH signaling cascade (Leroith and Nissley, 2005; Rico-Bautista et al., 2006). In liver of FGF21-transgenic mice, there was a significant increase in SOCS2 mRNA (Figure 3B) but no changes in the mRNAs encoding the SOCS2-related proteins cytokine-induced Src homology 2 protein and SOCS3 (data not shown). Western analysis revealed a corresponding increase in SOCS2 protein in livers of FGF21-transgenic mice (Figure 3B). These data suggest that increased SOCS2 activity may contribute to the decrease in phosphorylated STAT5 in FGF21-transgenic mice.
Shorter-term exposure to FGF21 inhibits STAT5 activity
Two strategies were used to test whether shorter-term exposure to FGF21 also inhibits STAT5 activity. Since FGF21 can be induced efficiently by PPARα, we first examined whether administration of the PPARα agonist, Wy14643, affects the GH-STAT5 pathway. Because Wy14643 causes a decrease in food consumption, pair-fed studies were performed. Wy14643 administration for 10 days caused a 5-fold increase in hepatic FGF21 mRNA (Supplementary Figure 4A) and decreased concentrations of phosphorylated STAT5 (Figure 4). Corresponding decreases occurred in hepatic IGF-1 and ALS mRNAs levels and serum IGF-1 concentrations. Wy14643 treatment also caused significant increases in hepatic IGFBP-1 mRNA and SOCS2 protein levels (Figure 4). Thus, administration of a PPARα agonist recapitulates the effects of long-term FGF21 exposure on the GH-STAT5 axis.
Figure 4.
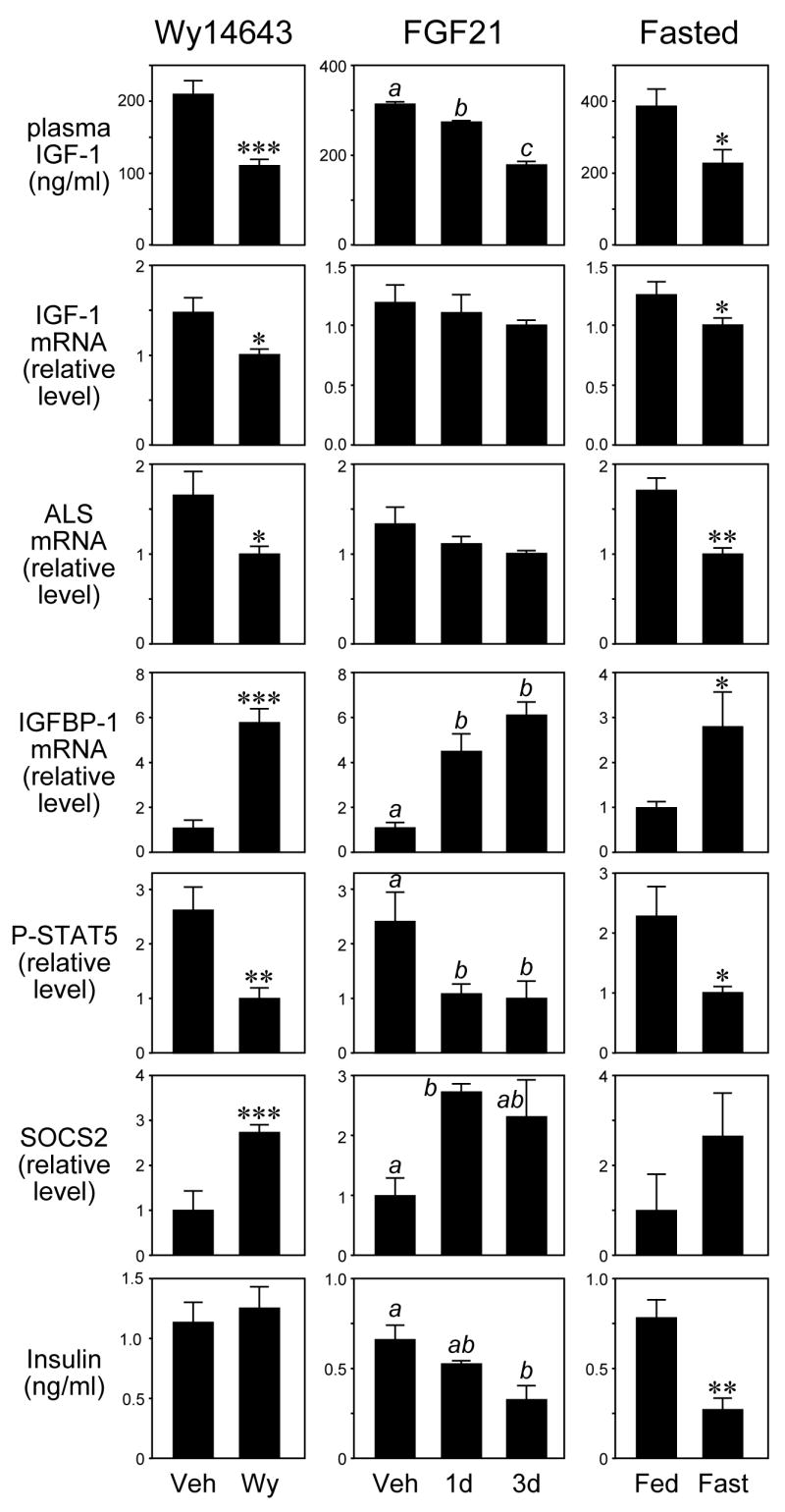
FGF21, PPARα activation, and fasting have similar effects on the IGF-1 pathway. Mice were administered vehicle (Veh) or Wy14643 (Wy) for 10 days (left panels); vehicle (Veh) or FGF21 for 1 or 3 days (middle panels); or were fed or fasted for 24 hours (right panels). Plasma IGF-1 concentrations, hepatic IGF-1, ALS, and IGFBP-1 mRNA levels, hepatic phosphorylated STAT5 (P-STAT5) and SOCS2 protein levels and plasma insulin levels were measured. n = 4-6 mice/group except for 1 day FGF21 treatment group, where n = 3. Male mice were used in the FGF21 and fasting experiments and female mice in the Wy14643 experiment. *, P<0.05; **, P<0.01; ***, P<0.001. For the middle panels, the presence of different lowercase letters indicates statistical significance (P<0.05) between groups.
While the Wy14,643 experiments were consistent with the hypothesis that induction of FGF21 interferes with the GH-STAT5 signaling pathway, they were not definitive. To determine directly whether short-term exposure to FGF21 blunts the GH-STAT5 axis, mice were injected with recombinant FGF21 for either 1 or 3 days. Notably, significant decreases in hepatic levels of phosphorylated STAT and circulating IGF-1 concentrations occurred as early as 1 day post-injection (Figure 4). The decrease in plasma IGF-1 concentrations was accompanied by increased IGFBP-1 mRNA levels and modest decreases in IGF-1 and ALS mRNA levels (Figure 4). Short-term FGF21 administration also increased SOCS2 protein concentrations (Figure 4). Importantly, the changes in hepatic levels of phosphorylated STAT5 and SOCS2 protein and IGFBP-1 mRNA and plasma IGF-1 caused by short-term FGF21 administration were comparable in magnitude to those caused by a 24 hour fast (Figure 4), which induced hepatic FGF21 mRNA levels ~10 fold (Supplementary Figure 4B). FGF21 administration also caused a decrease in plasma insulin concentration that was similar in magnitude to that caused by fasting (Figure 4). Interestingly, this decrease in insulin was not seen in mice administered Wy14643, revealing differences in the actions of Wy14643 and FGF21. Overall, these data provide strong evidence that FGF21 plays an important role in down-regulating the GH-STAT5 signaling pathway during fasting.
Discussion
Although it is well established that fasting blunts GH actions and reduces circulating IGF-1 concentrations in a variety of different mammalian species including man (Thissen et al., 1994), the molecular underpinnings of this phenomenon have remained obscure. In this report, we show that the fasting-induced hormone, FGF21, elicits the same spectrum of effects as fasting on the GH signaling cascade, including elevated plasma GH concentrations and decreased circulating IGF-1 levels. Moreover, chronic exposure of mice to FGF21 strongly inhibits growth. Taken together, these findings suggest that FGF21 plays a central role in causing growth hormone resistance in response to nutrient deprivation. Since GH exerts powerful effects on carbohydrate and lipid metabolism (Clemmons, 2004; Davidson, 1987; LeRoith and Yakar, 2007), these findings also raise the possibility that inhibition of the GH axis contributes to the diverse metabolic actions of FGF21.
FGF21 caused a marked reduction in hepatic concentrations of phosphorylated STAT5 and corresponding decreases in STAT5-regulated genes including IGF-1 and ALS. These changes along with the up-regulation of IGFBP-1, which is repressed by GH through a STAT5B-dependent mechanism (Ono et al., 2007; Seneviratne et al., 1990), are likely to account for the changes that are seen in IGF-1 levels in FGF21-transgenic mice. Notably, the FGF21-transgenic mice closely resemble STAT5A/B-knockout mice in several key respects. First, FGF21-transgenic mice and STAT5A/B-knockout mice have very similar changes in hepatic gene expression including reduced IGF-1, ALS, MUP1, MUP3, SLCO1A1 and HSD3B5 mRNAs and increased SULT1E1 mRNA (Holloway et al., 2007). Second, the ~50% decreases in serum IGF-1 concentrations and body weight in FGF21-transgenic mice are comparable to the changes reported for STAT5A/B-knockout mice (Teglund et al., 1998). Finally, female FGF21-transgenic mice are infertile and have few or no corpora lutea as reported for the STAT5A/B-knockout mice (V.Y.L., D.J.M. and S.A.K, unpublished) (Teglund et al., 1998). Taken together, the striking similarities between the FGF21-Tg and STAT5A/B-knockout mice strongly suggest that many of the effects of FGF21 are mediated through inhibition of STAT5.
How does FGF21 reduce phosphorylated STAT5 levels? We show that FGF21 inhibits the GH signaling pathway downstream of JAK2. FGF21 increased hepatic levels of SOCS2, which functions as a potent negative regulator of GH signaling in vivo (Leroith and Nissley, 2005; Rico-Bautista et al., 2006). SOCS2-knockout mice are significantly larger than wild type mice, and this increased growth requires both GH and STAT5B (Greenhalgh et al., 2002; Greenhalgh et al., 2005; Metcalf et al., 2000). Since SOCS2 binds to the tyrosine-phosphorylated GHR, it might blunt GH signaling by competing with STAT5 for access to the GHR. The induction of SOCS2 by FGF21 is surprising in that SOCS2 is also induced by GH and STAT5B (Davey et al., 1999; Woelfle and Rotwein, 2004), presumably as part of a feedback loop that attenuates GH signaling. Our results suggest that there is an additional, STAT5-independent mechanism through which SOCS2 can be induced by FGF21. We note that we have been unable to recapitulate the effects of FGF21 on STAT5 activity or SOCS2 expression in primary cultures of mouse or rat hepatocytes treated with FGF21 for periods of 1 to 3 days. One explanation for these data is that FGF21 does not act directly on the liver but instead through an indirect mechanism. In this regard, FGF21 does not efficiently activate FGF receptor 4, the predominant FGF receptor in liver (Ogawa et al., 2007; Suzuki et al., 2008). An alternate explanation is that primary hepatocytes have lost their ability to respond to FGF21 during the culturing process.
While FGF21 causes many of the same effects as fasting on the GH axis, there were two notable differences. First, whereas fasting in rats caused resistance to GH-mediated phosphorylation of JAK2 (Beauloye et al., 2002), hepatic levels of phosphorylated JAK2 were increased in FGF21-transgenic mice. Second, whereas SOCS3 mRNA is increased in livers of fasting rats (Beauloye et al., 2002), we did not detect any changes in hepatic SOCS3 mRNA levels in FGF21-transgenic mice (data not shown). These differences may be a consequence of either species-specific differences or the contributions of other signaling pathways to the fasting response.
Transcription of the FGF21 gene is stimulated directly by PPARα (Badman et al., 2007; Inagaki et al., 2007; Lundasen et al., 2007). As predicted, treatment of mice with a synthetic PPARα agonist elicited the same spectrum of effects as FGF21 on the GH/IGF-1 pathway, including reduced hepatic levels of phosphorylated STAT5, decreased IGF-1 and ALS mRNA levels, and increased SOCS2 concentrations. Cross-talk between PPARα and STAT5B has been previously described in cell-based assays, with GH-activated STAT5B inhibiting PPARα-regulated gene transcription, and conversely, ligand-activated PPARα inhibiting STAT5B-regulated transcription (Shipley and Waxman, 2004). Thus, cross-talk between PPARα and STAT5 may occur at multiple levels. Notably, in clinical studies the PPARα agonist bezafibrate significantly lowers IGF-1 levels in patients (Ruotolo et al., 2000). This finding together with data showing that FGF21 expression is induced by PPARα agonists in primary human hepatocytes (Inagaki et al., 2007; Lundasen et al., 2007) suggest that the PPARα/FGF21 pathway may be operative and affect IGF-1 signaling in humans.
Methods
Animal experiments
All animal experiments were approved by the Institutional Animal Care and Research Advisory Committee at the University of Texas Southwestern Medical Center. Mice were housed in a temperature-controlled environment with 12 hour light/dark cycles and fed standard rodent chow ad libitum. For fasting-refeeding experiments, the nonfasted group was fed ad libitum and the fasted group was fasted for 24 hr and killed at the end of dark cycle. For the FGF21 administration experiments, mice were injected subcutaneously with recombinant FGF21 protein (0.75mg/kg) or vehicle. Mice were injected at the beginning of the dark cycle and 2 hours prior to sacrifice at 10 a.m. The one-day-treatment group was injected with vehicle for two days prior to FGF21 administration so that all mice underwent the same number of injections during the three day period. For experiments with Wy14643 (Chemsyn Science Laboratories), mice were treated daily with Wy14643 (50 mg/kg/day in 1% Tween-80, 1% methylcellulose) or vehicle by gavage for 10 days. Since PPARα agonists can cause hypophagia, pair-fed studies were performed by restricting food intake in vehicle-treated mice to the amount ingested by Wy14643-treated animals given unlimited access to food. To measure tibia length, isolated tibia were incubated in protein lysis buffer containing 50 mM Tris, 100 mM EDTA, 100 mM NaCl, 1% SDS and 0.2 mg/ml proteinase K at 55°C overnight and the length measured using digital calipers (Marathon Watch Company Ltd., Richmond Hill, ON, Canada).
Plasma measurements
Plasma FGF21 concentrations were measured using a radioimmunoassay kit (Phoenix Pharmaceuticals, Inc. Belmont, CA). Plasma IGF-1 and GH concentrations were measured using ELISA kits (Diagnostic Systems Laboratories Inc., Webster, TX).
Immunoblot analyses
To prepare whole-cell extracts, 100 mg of liver was homogenized in 1 ml buffer (10 mM Tris-HCl, 5 mM EDTA, 150 mM NaCl, 30 mM sodium phosphate, 50 mM Sodium Fluoride, 1 mM Sodium Vanadate, 10% glycerol, and 0.5% NP40, pH at 7.4, containing Complete protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN)). After centrifugation at 16,000 g for 5 minutes at 4°C, the supernatant was collected. To prepare nuclear extracts, 100 mg of liver was homogenized in 1.2 ml buffer (20 mM Tris-HCl, 2 mM MgCl2, 0.25 M Sucrose, 10 mM EDTA, 10 mM EGTA, 5 mM DTT, 50 mM Sodium Fluoride and 1 mM Sodium Vanadate, pH at 7.4, containing Complete protease inhibitor cocktail). After centrifugation at 1,500 g for 5 minutes at 4°C, the pellet was resuspended in 1.8 ml of the same buffer and centrifuged at 1,500 g for 8 minutes at 4°C. The pellet was resuspended in 0.3 ml of extraction buffer (20 mM HEPES, 2.5% Glycerol, 0.42 M NaCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 50 mM Sodium Fluoride and 1 mM Sodium Vanadate containing Complete protease inhibitor cocktail), rotated at 4°C for 45 minutes and centrifuged at 355,000 g for 20 minutes. The supernatant was collected. Immunoblotting was performed using a phospho STAT-5 antibody (Cell Signaling Technology, Danvers MA), a phospho-JAK2 antibody (BioSource, Camarillo, CA), a JAK2 antibody (Upstate, Lake Placid, NY), a SOCS-2 antibody (Novus Biologicals, Littleton, CO), a β-actin antibody (Sigma-Aldrich, St Louis, MO), total STAT5, STAT5A, STAT5B, Lamin B and TBP antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). Immunoblots were quantified by digital densitometry using Scion Image software (Scion Corporation, Frederick, MD).
RT-qPCR analyses
RNA was prepared and RT-qPCR performed as previously described (Inagaki et al., 2007).
Recombinant proteins
Recombinant human FGF21 (residues 33–209) with a H-tag on the amino-terminus was expressed in E. coli and purified by sequential Ni-chelating and size exclusion chromatography. Recombinant mouse GH was obtained from Dr. A.F. Parlow and the National Hormone and Peptide Program.
Statistical Analyses
Statistical analyses were performed using Minitab Release 14 software (Minitab Inc.). Comparisons of two groups were performed using two-tailed Student’s t tests. Multiple groups were tested by one-way ANOVA followed by Fisher’s least significant difference test for unpaired data followed by Mann Whitney U test where appropriate. P<0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Norma Anderson for assistance with the metabolic cages, Dr. James Richardson and John Shelton for histology and photography, members of the Kliewer/Mangelsdorf laboratory for technical assistance, and Dr. Peter Rotwein for comments on the manuscript. This work was supported by National Institutes of Health grants P20RR20691 and 1RL1GM084436-01 (DJM and SAK), the Welch Foundation (DJM and SAK) and the Howard Hughes Medical Institute (VYL and DJM). DJM is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic Fibroblast Growth Factor 21 Is Regulated by PPARalpha and Is a Key Mediator of Hepatic Lipid Metabolism in Ketotic States. Cell Metab. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Baxter RC, Martin JL. Binding proteins for the insulin-like growth factors: structure, regulation and function. Prog Growth Factor Res. 1989;1:49–68. doi: 10.1016/0955-2235(89)90041-0. [DOI] [PubMed] [Google Scholar]
- Beauloye V, Willems B, de Coninck V, Frank SJ, Edery M, Thissen JP. Impairment of liver GH receptor signaling by fasting. Endocrinology. 2002;143:792–800. doi: 10.1210/endo.143.3.8692. [DOI] [PubMed] [Google Scholar]
- Bornfeldt KE, Arnqvist HJ, Enberg B, Mathews LS, Norstedt G. Regulation of insulin-like growth factor-I and growth hormone receptor gene expression by diabetes and nutritional state in rat tissues. J Endocrinol. 1989;122:651–656. doi: 10.1677/joe.0.1220651. [DOI] [PubMed] [Google Scholar]
- Clemmons DR. Role of insulin-like growth factor in maintaining normal glucose homeostasis. Horm Res. 2004;62 Suppl 1:77–82. doi: 10.1159/000080763. [DOI] [PubMed] [Google Scholar]
- Davey HW, McLachlan MJ, Wilkins RJ, Hilton DJ, Adams TE. STAT5b mediates the GH-induced expression of SOCS-2 and SOCS-3 mRNA in the liver. Mol Cell Endocrinol. 1999;158:111–116. doi: 10.1016/s0303-7207(99)00175-6. [DOI] [PubMed] [Google Scholar]
- Davey HW, Xie T, McLachlan MJ, Wilkins RJ, Waxman DJ, Grattan DR. STAT5b is required for GH-induced liver IGF-I gene expression. Endocrinology. 2001;142:3836–3841. doi: 10.1210/endo.142.9.8400. [DOI] [PubMed] [Google Scholar]
- Davidson MB. Effect of growth hormone on carbohydrate and lipid metabolism. Endocr Rev. 1987;8:115–131. doi: 10.1210/edrv-8-2-115. [DOI] [PubMed] [Google Scholar]
- Emler CA, Schalch DS. Nutritionally-induced changes in hepatic insulin-like growth factor I (IGF-I) gene expression in rats. Endocrinology. 1987;120:832–834. doi: 10.1210/endo-120-2-832. [DOI] [PubMed] [Google Scholar]
- Greenhalgh CJ, Bertolino P, Asa SL, Metcalf D, Corbin JE, Adams TE, Davey HW, Nicola NA, Hilton DJ, Alexander WS. Growth enhancement in suppressor of cytokine signaling 2 (SOCS-2)-deficient mice is dependent on signal transducer and activator of transcription 5b (STAT5b) Mol Endocrinol. 2002;16:1394–1406. doi: 10.1210/mend.16.6.0845. [DOI] [PubMed] [Google Scholar]
- Greenhalgh CJ, Rico-Bautista E, Lorentzon M, Thaus AL, Morgan PO, Willson TA, Zervoudakis P, Metcalf D, Street I, Nicola NA, et al. SOCS2 negatively regulates growth hormone action in vitro and in vivo. J Clin Invest. 2005;115:397–406. doi: 10.1172/JCI22710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Rena G, Cichy S, He X, Cohen P, Unterman T. Phosphorylation of serine 256 by protein kinase B disrupts transactivation by FKHR and mediates effects of insulin on insulin-like growth factor-binding protein-1 promoter activity through a conserved insulin response sequence. J Biol Chem. 1999;274:17184–17192. doi: 10.1074/jbc.274.24.17184. [DOI] [PubMed] [Google Scholar]
- Herrington J, Smit LS, Schwartz J, Carter-Su C. The role of STAT proteins in growth hormone signaling. Oncogene. 2000;19:2585–2597. doi: 10.1038/sj.onc.1203526. [DOI] [PubMed] [Google Scholar]
- Holloway MG, Cui Y, Laz EV, Hosui A, Hennighausen L, Waxman DJ. Loss of sexually dimorphic liver gene expression upon hepatocyte-specific deletion of Stat5a-Stat5b locus. Endocrinology. 2007;148:1977–1986. doi: 10.1210/en.2006-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, et al. Endocrine Regulation of the Fasting Response by PPARalpha-Mediated Induction of Fibroblast Growth Factor 21. Cell Metab. 2007;5:415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong SE, Baxter RC, Delhanty PJ. Age-dependent regulation of the acid-labile subunit in response to fasting-refeeding in rats. Endocrinology. 2002;143:4505–4512. doi: 10.1210/en.2002-220527. [DOI] [PubMed] [Google Scholar]
- Leroith D, Nissley P. Knock your SOCS off! J Clin Invest. 2005;115:233–236. doi: 10.1172/JCI24228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeRoith D, Yakar S. Mechanisms of disease: metabolic effects of growth hormone and insulin-like growth factor 1. Nat Clin Pract Endocrinol Metab. 2007;3:302–310. doi: 10.1038/ncpendmet0427. [DOI] [PubMed] [Google Scholar]
- Lowe WL, Jr, Adamo M, Werner H, Roberts CT, Jr, LeRoith D. Regulation by fasting of rat insulin-like growth factor I and its receptor. Effects on gene expression and binding. J Clin Invest. 1989;84:619–626. doi: 10.1172/JCI114207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundasen T, Hunt MC, Nilsson LM, Sanyal S, Angelin B, Alexson SE, Rudling M. PPARalpha is a key regulator of hepatic FGF21. Biochem Biophys Res Commun. 2007;360:437–440. doi: 10.1016/j.bbrc.2007.06.068. [DOI] [PubMed] [Google Scholar]
- Maes M, Underwood LE, Ketelslegers JM. Plasma somatomedin-C in fasted and refed rats: close relationship with changes in liver somatogenic but not lactogenic binding sites. J Endocrinol. 1983;97:243–252. doi: 10.1677/joe.0.0970243. [DOI] [PubMed] [Google Scholar]
- Metcalf D, Greenhalgh CJ, Viney E, Willson TA, Starr R, Nicola NA, Hilton DJ, Alexander WS. Gigantism in mice lacking suppressor of cytokine signalling-2. Nature. 2000;405:1069–1073. doi: 10.1038/35016611. [DOI] [PubMed] [Google Scholar]
- Murphy LJ, Seneviratne C, Moreira P, Reid RE. Enhanced expression of insulin-like growth factor-binding protein-I in the fasted rat: the effects of insulin and growth hormone administration. Endocrinology. 1991;128:689–696. doi: 10.1210/endo-128-2-689. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Kurosu H, Yamamoto M, Nandi A, Rosenblatt KP, Goetz R, Eliseenkova AV, Mohammadi M, Kuro-o M. BetaKlotho is required for metabolic activity of fibroblast growth factor 21. Proc Natl Acad Sci U S A. 2007;104:7432–7437. doi: 10.1073/pnas.0701600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M, Chia DJ, Merino-Martinez R, Flores-Morales A, Unterman TG, Rotwein P. Signal transducer and activator of transcription (Stat) 5b-mediated inhibition of insulin-like growth factor binding protein-1 gene transcription: a mechanism for repression of gene expression by growth hormone. Mol Endocrinol. 2007;21:1443–1457. doi: 10.1210/me.2006-0543. [DOI] [PubMed] [Google Scholar]
- Rico-Bautista E, Flores-Morales A, Fernandez-Perez L. Suppressor of cytokine signaling (SOCS) 2, a protein with multiple functions. Cytokine Growth Factor Rev. 2006;17:431–439. doi: 10.1016/j.cytogfr.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Ruotolo G, Bavenholm P, Brismar K, Efendic S, Ericsson CG, de Faire U, Nilsson J, Hamsten A. Serum insulin-like growth factor-I level is independently associated with coronary artery disease progression in young male survivors of myocardial infarction: beneficial effects of bezafibrate treatment. J Am Coll Cardiol. 2000;35:647–654. doi: 10.1016/s0735-1097(99)00591-4. [DOI] [PubMed] [Google Scholar]
- Seneviratne C, Luo JM, Murphy LJ. Transcriptional regulation of rat insulin-like growth factor-binding protein-1 expression by growth hormone. Mol Endocrinol. 1990;4:1199–1204. doi: 10.1210/mend-4-8-1199. [DOI] [PubMed] [Google Scholar]
- Shipley JM, Waxman DJ. Simultaneous, bidirectional inhibitory crosstalk between PPAR and STAT5b. Toxicol Appl Pharmacol. 2004;199:275–284. doi: 10.1016/j.taap.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Silha JV, Murphy LJ. Insights from insulin-like growth factor binding protein transgenic mice. Endocrinology. 2002;143:3711–3714. doi: 10.1210/en.2002-220116. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Uehara Y, Motomura-Matsuzaka K, Oki J, Koyama Y, Kimura M, Asada M, Komi-Kuramochi A, Oka S, Imamura T. {beta}Klotho Is Required for Fibroblast Growth Factor (FGF) 21 Signaling through FGF Receptor (FGFR) 1c and FGFR3c. Mol Endocrinol. 2008;22:1006–1014. doi: 10.1210/me.2007-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teglund S, McKay C, Schuetz E, van Deursen JM, Stravopodis D, Wang D, Brown M, Bodner S, Grosveld G, Ihle JN. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998;93:841–850. doi: 10.1016/s0092-8674(00)81444-0. [DOI] [PubMed] [Google Scholar]
- Thissen JP, Ketelslegers JM, Underwood LE. Nutritional regulation of the insulin-like growth factors. Endocr Rev. 1994;15:80–101. doi: 10.1210/edrv-15-1-80. [DOI] [PubMed] [Google Scholar]
- Underwood LE, Thissen JP, Lemozy S, Ketelslegers JM, Clemmons DR. Hormonal and nutritional regulation of IGF-I and its binding proteins. Horm Res. 1994;42:145–151. doi: 10.1159/000184187. [DOI] [PubMed] [Google Scholar]
- Woelfle J, Rotwein P. In vivo regulation of growth hormone-stimulated gene transcription by STAT5b. Am J Physiol Endocrinol Metab. 2004;286:E393–401. doi: 10.1152/ajpendo.00389.2003. [DOI] [PubMed] [Google Scholar]
- Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, LeRoith D. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci U S A. 1999;96:7324–7329. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.