Abstract
Heparanase is an endoglycosidase that specifically cleaves heparan sulfate side chains, a class of glycosaminoglycans abundantly present in the extracellular matrix and on the cell surface. Heparanase activity is strongly implicated in tumor angiogenesis and metastasis attributed to remodeling of the subepithelial and subendothelial basement membranes. We hypothesized that similar to its proangiogenic capacity, heparanase is also engaged in lymphangiogenesis and utilized the D2-40 monoclonal antibody to study lymphangiogenesis in tumor specimens obtained from 65 head and neck carcinoma patients. Lymphatic density was analyzed for association with clinical parameters and heparanase staining. We provide evidence that lymphatic vessel density (LVD) correlates with head and neck lymph node metastasis (N-stage, p=0.007), and inversely correlates with tumor cell differentiation (p=0.007). Notably, heparanase staining correlated with LVD (p=0.04) and, moreover, with VEGF C levels (p=0.01). We further demonstrate that heparanase over expression by epidermoid, breast, melanoma, and prostate carcinoma cells induces a 3-5 fold elevation in VEGF C expression in vitro, and facilitates tumor xenograft lymphangiogenesis in vivo, while heparanase gene silencing was associated with decreased VEGF C levels. These findings suggest that heparanase plays a unique dual role in tumor metastasis, facilitating tumor cell invasiveness and inducing VEGF C expression, thereby increasing the density of lymphatic vessels that mobilize metastatic cells.
Keywords: Heparanase, head and neck carcinoma, lymphatic vessels, VEGF C, D2-40
Introduction
Heparanase is an endo-β-glucuronidase that cleaves heparan sulfate (HS) side chains of HS proteoglycans (HSPG). Heparanase activity has long been correlated with cell invasion associated with cancer metastasis, a consequence of structural modification that loosens the extracellular matrix (ECM) barrier 1, 2. This notion gained further support by employing siRNA and ribozyme technologies 3, 4, clearly depicting heparanase-mediated HS cleavage and ECM remodeling as critical requisites for inflammation, tumor angiogenesis, and tumor metastasis. More recently, heparanase up-regulation was documented in an increasing number of human carcinomas and hematological malignancies 5-7. In many cases, heparanase induction correlated with increased tumor metastasis, vascular density, and shorter post operative survival rate, thus providing a strong clinical support for the pro-metastatic function of the enzyme 5, 6. These studies depict compelling evidence for the clinical relevance of the enzyme, making it an attractive target for the development of anti-cancer drugs 5, 8-12. The role that heparanase plays in the primary tumor is less well understood, but likely involves angiogenic aspects. Elevation of microvessel density correlated with heparanase induction in solid 13-17 and hematological 18 malignancies, and was also evident in tumor xenografts produced by cells over expressing heparanase 3, 19, 20 and in heparanase treated wounds 21, 22. The angiogenic capacity of heparanase has been traditionally attributed to its enzymatic activity, facilitating the sprouting of endothelial cells through the underlying basement membrane to form new capillaries, and releasing HS-bound angiogenic growth factors such as bFGF and VEGF 23-25. More recently, heparanase was noted to induce the expression of angiogenic mediators such as tissue factor (TF) and, moreover, VEGF in a manner that involves no enzymatic activity and is mediated by p38 and Src activation 26, 27, thus expanding the scope of heparanase function. We hypothesized that similar to its pro-angiogenic capacity, heparanase also facilitates the formation of lymphatic vessels. According to this notion, heparanase enhances tumor metastasis by facilitating the dissemination of metastatic tumor cells, and by augmenting the formation of lymphatic vessels that mobilize these cells. Here, we utilized the D2-40 antibody which specifically decorates lymphatic endothelial cells to study lymphangiogenesis in head and neck carcinoma, and correlated lymphatic density with clinical parameters and heparanase staining. We provide evidence that lymphatic vessel density (LVD) correlates with head and neck lymph node metastasis (N-stage, p=0.007), and inversely correlates with tumor cell differentiation (p=0.007). Notably, heparanase staining correlated with LVD (p=0.04) and, moreover, with VEGF C expression (p=0.01). We further demonstrate that heparanase over expression by epidermoid (A431), breast (T47 D), melanoma (MDA-MB-435), and prostate (LNCaP) carcinoma cells induced the expression of VEGF C 3-5 fold in vitro, and aggravated tumor xenograft lymphangiogenesis in vivo. Likewise, heparanase gene silencing by means of siRNA was associated with decreased VEGF C levels, suggesting that VEGF C induction by heparanase is not restricted to head and neck carcinoma, but rather is a more general phenomena, leading to enhanced tumor lymphangiogenesis and tumor metastasis.
Material and methods
Experimental design
The study included 65 patients with head and neck cancer that were diagnosed in the Department of Otolaryngology, Head and Neck Surgery, Carmel Medical Center, Haifa, Israel, whose archival paraffin-embedded pathological material was available for immunohistochmical analysis 28. The study protocol was approved by the Institutional Review Board. The clinical data of all patients was reviewed and patients were re-staged according to the AJCC 2003 staging system. Clinical data included demographics, site of tumor, tumor-node-metastasis (TNM) staging, treatment modality, status at the end of the study (dead or alive), failure (local, regional or distant), time to failure, follow-up and survival. Pathological data included histology, tumor grade, local and regional spread of the disease (pT and pN), and extracapsular extension 28.
Immunohistochemistry
Staining of formalin-fixed, paraffin-embedded 5 micron sections was performed essentially as described 28. Briefly, slides were deparaffinized, rehydrated and endogenous peroxidase activity was quenched (30 min) by 3% hydrogen peroxide in methanol. Slides were then subjected to antigen retrieval by boiling (20 min) in 10 mM citrate buffer, pH 6 (for D2-40) or 2 mM EDTA, pH 9 (for VEGF C). Following washes with phosphate buffered saline (PBS), slides were incubated with 10% normal goat serum (NGS) in PBS for 60 min to block non specific binding and incubated (20 h, 4°C) with D2-40 monoclonal antibody (Dako, Glostrup, Denmark) or anti-VEGF C polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) diluted 1:100 in blocking solution. Slides were extensively washed with PBS containing 0.01% Triton X-100 and incubated with a secondary reagent (Envision kit) according to the manufacturer's (Dako) instructions. Following additional washes, color was developed with the AEC reagent (Dako), sections were counterstained with hematoxylin and mounted, as described 28. Immunostained specimens were examined by senior pathologist (IN) who was blind to clinical data of the patients. LVD was evaluated by counting D2-40-positive lymphatic vessels (0: < 1 lymph vessel per section; +1: 2-5 lymph vessels per section; +2: >5 lymph vessels per section). Since the D2-40 antibody often reacted with some tumor cells, high magnification was employed for LVD evaluation to ensure vessel morphology and accurate detection. VEGF C staining was scored according to the intensity of staining (0: none, +1: weak- moderate; +2: strong) and the percentage (extent staining) of tumor cells that were stained (0: <10%; +1: 10-50%; +2>50%). Specimens that were similarly stained with pre-immune serum, or applying the above procedure but lacking the primary antibody, yielded no detectable staining.
For double immunofluorescent staining, slides were similarly processed and incubated with anti-heparanase polyclonal (733) 29 and D2-40 monoclonal antibodies for 2 h at room temperature. Slides were then extensively washed with PBS and incubated with the relevant (Cy2/Cy3-conjugated) secondary antibody (Jackson ImmunoResearch, West Grove, PA) for 1 h, washed and mounted (Vectashield, Vector, Burlingame, CA), as described 29.
Statistical analysis
Univariate association between D2-40 (LVD) and VEGF C (intensity and extent of staining) parameters and clinical and pathological findings as well as patients' outcome, were analyzed using Chi Square tests (Pearson, Fisher exact test). A multivariable Cox's proportional hazard model was performed with stepwise selection, to identify independent predictors of survival (P for enter and p to stay were set as 0.1) and to estimate relevant Odds ratio (OR) with 95% confidence interval (CI). The model included all parameters with p<0.2 by the univariate analysis.
Cell culture, transfection and immunoblotting
Human LNCaP prostate, T47 D breast, and A431 epidermoid carcinoma cells were purchased from the American Type Culture Collection (ATCC). MDA-MB-435, previously considered as breast carcinoma-derived cells, have recently been characterized to be of melanoma origin 30 and considered here as such. Cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with glutamine, pyruvate, antibiotics and 10% fetal calf serum in a humidified atmosphere containing 8% CO2 at 37°C. For stable transfection, cells were transfected with the pSecTag 2 vector containing the full-length heparanase cDNA 31, using the FuGene reagent according to the manufacturer's (Roche, Mannheim, Germany) instructions, selected with Zeocin (100-200 μg/ml; Invitrogen, Carlsbad, CA) for 2 weeks, expanded and pooled. Cell transfection with control and anti-heparanase siRNA gene constructs was carried out essentially as described 3. Heparanase expression was evaluated by immunoblotting, essentially as described 20, 27. Briefly, cell cultures were washed twice with ice cold PBS containing 1 mM orththovanadate and scraped into lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Triton-X100, 1 mM orththovanadate, 1 mM PMSF) containing a cocktail of proteinase inhibitors (Roche). Total cellular protein concentration was determined by the BCA assay, according to the manufacturer's instructions (Pierce, Rockford, IL). Thirty μg of cellular protein were resolved on SDS polyacrylamid gel (SDS-PAGE) and immunoblotting was performed applying anti-heparanase, anti-VEGF C (Zymed, South San Francisco, CA) and anti-actin (Sigma, St. Louis, MI) antibodies, as described 20, 27. Experiments were repeated at least three times with similar results.
PCR analysis
Total RNA was extracted with TRIzol (Gibco BRL Life Technologies, Rockville, MD) and RNA (1 μg) was amplified using one step PCR amplification kit, according to the manufacturer's instructions (ABgene, Epsom, UK) essentially as described 27 . The PCR primer sets included F 5′-CAGTTACGGTCTGTGTCCAGTGTAG-3′, and R 5′-GGACACACATGGAGGTTTAAAGAAG-3′ for VEGF C; F 5′-AGCTCTGCATATGGAGGCGG-3′, and R 5′-TGAACTTCCTGGCCGGAGAG-3′ for heparanase; and F 5′-CCAGCCGAGCCACATCGCTC-3′ and R 5′-ATGAGCCCCAGCCTTCTCCAT-3′ for GAPDH.
Tumorigenicity
Cells from exponential cultures of control (Vo) or heparanase transfected MDA-MB-435 cells 27 were dissociated with trypsin/EDTA, washed with PBS and brought to a concentration of 5×107cells/ml. Cell suspension (5×106/ 0.1 ml) was inoculated subcutaneously at the right flank of 5-weeks old female Balb/C nude mice (n=7). At the end of the experiment, mice were sacrificed and xenografts were resected, weighted and fixed in formalin. Paraffin-embedded 5 micron sections were stained with anti LYVE polyclonal antibody (Abcam Inc, Cambridge, MA), using the Envision kit essentially as described above. All animal experiments were approved by the Animal Care Committee of the Technion, Haifa, Israel.
Results
Clinical significance of LVD in head and neck carcinoma
Sixty-five patients (60 males and 5 females) were included in this study; median age at diagnosis was 66.7±12 years. Mean follow-up was 42.1±6.3 months for the entire group and 64.8±11.05 months for surviving patients. Clinical description of the patients is presented in table 1. Larynx was the predominant site (52/65). Most patients had advanced disease as indicated by the T (primary tumor) and N (nodal metastasis) staging criteria (Table 1). Neck dissection was carried out in 32 patients. Among the 65 tumor biopsies available for staining, 18 were found negative for tumor lymphatic vessels; 33 exhibited low number of lymphatic vessels (+1; 1-5 lymphatic vessels per tumor section; Fig. 1A), and 14 bear high number of lymphatics (+2; >5 lymphatic vessels per tumor section; Fig. 1B). Although the D2-40 antigen, podoplanin, is considered specific for lymphatic endothelium, reactivity was often observed also in tumor cells, mainly residing at areas of cell-cell borders (Fig. 1C), in agreement with a similar staining pattern observed in squamous cell carcinoma of the skin and uterine 32, 33. Interestingly, staining of tumor cells with the D2-40 antibody inversely correlated with the follow-up time of patients: the mean follow-up time of patients with D2-40-positive staining was 33 ±10 months, whereas patients negative for D2-40 had a mean follow-up time of 77.8 ±13.4 months (p=0.003). Statistically significant correlation was found between lymphatic vessel density (LVD) and the tumor N-stage. Thus, while 11% of the patients scored negative for lymphatic vessels were diagnosed to bear advanced lymph node metastasis (N2/N3), 64% of the patients with high LVD were diagnosed as such (Table 2; p=0.007). Neck dissection was performed in 32 patients; significantly, none of the patients scored negative for lymphatic vessels had multiple metastatic lymph nodes (>1 node) compared with 44% and 67% of the patients that exhibited low and high LVD, respectively (Table 2), differences that are statistically highly significant (p=0.005). Furthermore, inverse correlation was found between tumor differentiation and LVD (Table 2). Notably, 93% of the tumors with high LVD (+2) were diagnosed as moderate-poorly differentiated compared with only 65 % of the tumors scored negative for lymphatic vessels (0), differences that are statistically highly significant (P=0.006). These findings indicate that high LVD is associated with poor prognosis of head and neck cancer patients.
Table 1.
Clinical description of patients.
| Parameter | Number of patients (%) |
||||
|---|---|---|---|---|---|
| Site of tumor | |||||
| Larynx | 52 (80) | ||||
| Pharynx | 3 (4.5) | ||||
| Oral cavity | 3 (4.5) | ||||
| Other | 7 (11) | ||||
| T-stage | |||||
| T 0-2 | 17 (26) | ||||
| T 3 | 26 (40) | ||||
| T 4 | 22 (34) | ||||
|
Lymphatic vessels density (LVD) Parameter |
0 (%) |
+1 (%) |
+2 (%) |
Total |
p |
| N-stage | |||||
| N0 | 34 (52) | ||||
| N1 | 7 (11) | ||||
| N2-3 | 24 (37) | ||||
| Grade | |||||
| Well diff. | 15 (24) | ||||
| Mod. Diff. | 40 (63) | ||||
| Poorly diff. | 8 (13) | ||||
Figure 1.

Immunohistochemical staining of lymphatic vessels and VEGF C in human head and neck tumor specimens. Formalin-fixed, paraffin-embedded 5 micron sections of 65 head and neck tumors were subjected to immunostaining, applying D2-40 monoclonal (A-C) and anti-VEGF C (G-I) polyclonal antibodies, as described under ‘Materials and Methods’. Shown are representative photomicrographs of specimens depicting low (A; +1) and high (B; +2) number of lymphatic vessels. Lymphatic vessel (arrow) adjacent to D2-40-positive tumor cells is shown in C. Shown are also photomicrographs of VEGF C negative (G), and positively stained specimens scored as weak (H; +1) and strong (I; +2) intensity. D-F. Double immunofluorescent staining. Head and neck tumor specimen was stained with anti heparanase polyclonal (green, D) and D2-40 monoclonal (red, E) antibodies, illustrating heparanase-positive tumor cells inside a lymphatic vessel lumen (merge, F), penetrating the lymphatic endothelium (arrow). Original magnifications: A,B × 20; D-F × 100; C, G-I × 40
Table 2.
LVD correlates with clinical staging and pathological findings.
| N-stage | 0.007 | ||||
| N0-1 | 16 (89%) | 20 (61%) | 5 (36%) | 41 | |
| N2-3 | 2 (11%) | 13 (39%) | 9 (64%) | 24 | |
| Total | 18 | 33 | 14 | 65 | |
|
Number of metastatic nodes in neck dissection |
0.005 | ||||
| None (0) | 3 (75%) | 1 (6%) | 1 (8%) | 5 | |
| Single node (1) | 1 (25%) | 8 (50%) | 3 (25%) | 12 | |
| Multiple nodes (>1) | 0 | 7 (44%) | 8 (67%) | 15 | |
| Total | 4 | 16 | 12 | 32 | |
| Tumor Grade | 0.006 | ||||
| Well differentiated | 6 (35%) | 8 (33%) | 1 (7%) | 15 | |
| Moderate-Poorly differentiated |
11(65%) | 24 (67%) | 13 (93%) | 48 | |
| Total | 17 | 32 | 14 | 63* |
Pathological data of two patients were missing
Heparanase expression correlates with LVD
We have recently reported that heparanase expression by head and neck carcinoma correlates with tumor progression and inversely correlates with patients' status 28. In order to investigate the possible contribution of heparanase to tumor lymphangiogenesis, LVD was correlated with heparanase staining 28. Heparanase staining extent 28 correlated with LVD. Here, 93% of the patients that exhibit high LVD were noted to bear high heparanase staining extent (i.e., more than 50% of the tumor cells stained positively for heparanase), compared with 65% of the patients scored negative for lymphatic vessels (Table 3, p=0.04). Moreover, all 11 cases in which tumor cells were clearly evident in lymph vessels (Fig. 1A, C) are also scored as high heparanase staining extent (Table 3, p=0.02). This is further demonstrated by subjecting tumor biopsies to double staining applying rabbit anti-heparanase polyclonal (green, Fig. 1D) and D2-40 monoclonal (red, Fig. 1E) antibodies, the composite of which clearly shows heparanase expressing tumor cells inside a lymphatic vessel lumen (Fig. 1F). In fact, heparanase expressing tumor cells appear to penetrate and disrupt the lymphatic vessel endothelium (Fig. 1F, arrow). These findings suggest that heparanase plays a unique, dual role in tumor metastasis, facilitating tumor cell invasiveness and increasing the density of lymphatic vessels that mobilize metastatic cells.
Table 3.
Heparanase staining extent correlates with LVD and VEGF C levels.
| Heparanase extent | 0-1 | 2 | Total | p |
|---|---|---|---|---|
| Parameter | ||||
| Lymphatic vessels density | 0.04 | |||
| 0-1 | 18 (35%) | 33 (65%) | 51 | |
| 2 | 1 (7%) | 13 (93%) | 14 | |
| 19 | 46 | 65 | ||
|
Tumor cells in lymphatic vessels |
0.02 | |||
| No | 18 (35%) | 34 (65%) | 52 | |
| Yes | 0 | 11 (100%) | 11 | |
| 18 | 45 | 63* | ||
| VEGF C Intensity | 0.01 | |||
| 0 | 6 (67%) | 3 (33%) | 9 | |
| 1 (low) | 7 (33%) | 14 (67%) | 21 | |
| 2 (high) | 2 (11%) | 16 (89%) | 18 | |
| 15 | 33 | 48 | ||
| Heparanase localization | Nuclear | Cytoplasmic | 0.04 | |
| VEGF C intensity | ||||
| 0 | 7 (78%) | 2 (22%) | 9 | |
| 1 (low) | 17 (81%) | 4 (19%) | 21 | |
| 2 (high) | 8 (44%) | 10 (56%) | 18 | |
| 32 | 16 | 48 |
Data of two patients were missing
Heparanase staining correlates with VEGF C expression
We hypothesize that VEGF C is responsible for the lymphangiogenesis promoting effect of heparanase and examined this possibility by staining the cohort of tumor biopsies with anti-VEGF C antibody. Forty-eight tumor biopsies, for which heparanase and lymphatic (D2-40) staining were performed, were included. Positive VEGF C staining was found in 81% (39/48) of the tumor specimens, whereas 19% (9/48) of the specimens were negative (Fig. 1G). The VEGF C-positive group was further categorized according to the intensity of staining. Thus, weak staining (+1; Fig. 1H) was found in 53% (21/39) of the positive specimens, while 47% (18/39) were stained strongly (+2) for VEGF C (Fig. 1I; Table 3). VEGF C staining correlated with the number of metastatic nodes in patients who underwent neck dissection: positive VEGF C staining was observed in 90% of the patients with multiple metastatic nodes, compared to 50% of the patients with a single metastatic node or without metastatic nodes who were stained positively for VEGF C (p=0.04), in close association with the LVD score (Table 2). Similarly, VEGF C staining was correlated with the outcome of patients: 78% of the patients (7/9) stained negatively for VEGF C were alive at the end of the study, compared to 44% (17/39) of the patients stained positively for VEGF C. Although the statistical significance of this observation was borderline (p=0.06), the results suggest that VEGF C levels associate with lymph nodes metastasis and poor prognosis of head and neck cancer patients. No correlation was found between VEGF C staining and the T-stage, N-stage, tumor grade, disease recurrent or extra-capsular extension. Notably, heparanase expression significantly correlated with VEGF C staining intensity. Thus, 89% (16/18) of the patients exhibiting strong VEGF C staining (+2) were also stained positively for heparanase, whereas 67% of the patients scored negative for heparanase were also found negative for VEGF C (p=0.01) (Table 3). Moreover, VEGF C staining intensity correlated with the cellular localization of heparanase. Thus, cytoplasmic localization of heparanase correlated with intense staining of VEGF C in 56% of the patients, whereas most patients (78%) with nuclear localization of heparanase were stained negatively for VEGF C (Table 4; p=0.04), in agreement with the favorable outcome associated with nuclear localization of heparanase in head and neck 28, gastric and esophageal 34, 35 carcinomas. Cox's proportional hazard model was subsequently performed for overall survival, including age, T-stage, N-stage, and VEGF C intensity. The most significant parameters are VEGF C (p=0.05), followed by N-stage (p=0.07) and age (p=0.07) (Table 4, Ist stage). However, if heparanase localization parameter is included, significant and independent parameters are age (p=0.02), heparanase localization (p=0.02), and N-stage (p=0.09), whereas VEGF C intensity becomes borderline (p=0.1) (Table 4, IInd stage), suggesting that VEGF C intensity depends on heparanase expression and localization.
Table 4.
Cox's Proportional Hazard Model for overall survival. First stage analysis (Ist) included the N-stage, age, and VEGF C intensity. Heparanase localization (cytoplasmic vs. nuclear) was added in a second stage (IInd) analysis.
| Ist Stage | OR | 95% CL | P |
|---|---|---|---|
| N Stage (0-1 vs. 2-3) | 2.33 | 0.93 -5.61 | 0.07 |
| Age | 1.036 | 0.99-1.08 | 0.07 |
| VEGF C intensity (0 vs. 1-2) | 3.39 | 0.96-21.5 | 0.05 |
|
IInd Stage (including heparanase localization) |
|||
| N-Stage (0-1 vs. 2-3) | 2.17 | 0.86-5.25 | 0.09 |
| Age | 1.043 | 1.00-1.08 | 0.02 |
| VEGF C intensity (0 vs. 1-2) | 2.83 | 0.79-18.1 | 0.11 |
| Heparanase localization (Cytoplasmic vs. nuclear) |
1.63 | 1.07-2.5 | 0.02 |
OR-Odds ratio
Heparanase induces VEGF C expression and facilitates tumor xenograft lymphangiogenesis
The clinical analyses suggest that heparanase facilitates tumor lymphangiogenesis which is mediated, possibly, by VEGF C gene induction. In order to investigate this possibility we examined VEGF C levels in tumor-derived cell lines engineered to over express heparanase. Heparanase activity was markedly elevated in transfected cells (Fig. 2A, upper panel, Hepa), and elevated heparanase levels were further confirmed by subjecting cell lysates (Fig. 2A, second panel; Ly.) and conditioned medium (Fig. 2A, second panel, Med.) to immunoblotting applying anti-heparanase antibody (Fig. 2A, second panel), and by immunofluorescent staining (Fig. 2A, lower panels). Notably, we found a consistent, 3-5 fold increase in VEGF C levels upon heparanase over expression in prostate carcinoma LNCaP (Fig. 2B, upper panel), epidermoid A431 (Fig. 2B, third panel), breast carcinoma T47 D (Fig. 2B, fifth panel), and melanoma MDA-MB-435 (Fig. 2B, seventh panel) cells, and VEGF C induction was further confirmed by immunofluorescent staining (Fig. 2C). Furthermore, VEGF C induction by heparanase is due, at least in part, to enhanced VEGF C gene transcription as revealed by RT-PCR analysis (Fig. 2B, ninth panel). Similarly, transfection of LNCaP cells with anti-heparanase siRNA construct resulted in a profound decrease in VEGF C protein (Fig. 2D, upper panel) and mRNA (Fig. 2D, third panel) levels, suggesting that endogenous heparanase is actively involved in VEGF C gene regulation. Over expression of VEGF C by MDA-MB-435 cells has been shown by Skobe et al to elicit tumor xenograft lymphangiogenesis and lymph node metastasis 36. We, therefore, utilized MDA-MB-435 cells and examined lymphangiogenesis in tumor xenograft produced by cells transfected with heparanase or control, empty vector (Vo). We have previously utilized this cell system and demonstrated that heparanase over expression by MDA-MB-435 cells results in tumors bigger in size, exhibiting elevated VEGF levels and higher vascular density 27. Here, tumor xenograft sections were stained with anti LYVE antibody which specifically labels lymphatic endothelial cells in the mouse 36. Staining revealed the presence of lymphatic vessels in the tissue surrounding the tumor xenograft produced by control Vo cells, whereas the tumor mass was devoid of intratumoral lymphatics (Fig. 3, upper panel). In striking contrast, tumor xenografts produced by heparanase transfected MDA-MB-435 cells were infiltrated with numerous lymphatic vessels (Fig. 3, middle panel). These include small capillaries as well as larger vessels with open lumen that often contained tumor cells (Fig. 3, lower panel). These results clearly support the notion that heparanase facilitates tumor lymphangiogenesis by inducing VEGF C expression levels. The observed modulation of VEGF C expression in tumor-derived cell lines suggests that this function of heparanase is not restricted to head and neck carcinoma, but rather is a general phenomenon, leading to enhanced tumor lymphangiogenesis and tumor metastasis.
Figure 2.

Heparanase induces VEGF C expression. A. Tumor-derived cell lines were stably transfected with control empty vector (Vo, ◆) or heparanase (Hepa, ■) plasmid and total cell lysates were subjected to heparanase activity assay (upper panel) and immunoblotting applying anti-heparanase (second panel) and anti-actin (third panel) antibodies, as describe in “Materials and methods”. Immunofluorescent staining of control (Vo) and heparanase (Hepa) transfected cells is shown in the lower panels applying anti-heparanase monoclonal antibody (green). Nuclei counter staining is shown in red. Shown are representative results obtained by prostate carcinoma LNCaP cells. B. Control (Vo) and heparanase transfected (Hepa) prostate carcinoma LNCaP (I panels), epidermoid A431 (II panels), breast carcinoma T47 D (III panels), and melanoma MDA-MB-435 (IV panels) cell lysates were subjected to immunoblotting applying anti VEGF C (upper panels) and actin (lower panels) antibodies. VEGF C induction by heparanase in MDA-MB-435 cells is further demonstrated by RT-PCR (V panels) and immunofluorescent staining applying anti-VEGF C antibody (green). Nuclei counter staining is shown in red (C). D. siRNA experiments. LNCaP cells were left untreated as control (Con) or transfected with control (si-Con) or anti-heparanase (si-Hepa) siRNA constructs and VEGF C levels were evaluated by immunoblotting (upper panels) and RT-PCR (lower panels) analyses. Actin (second panel) and GAPDH (lower panel) were used as internal controls.
Figure 3.
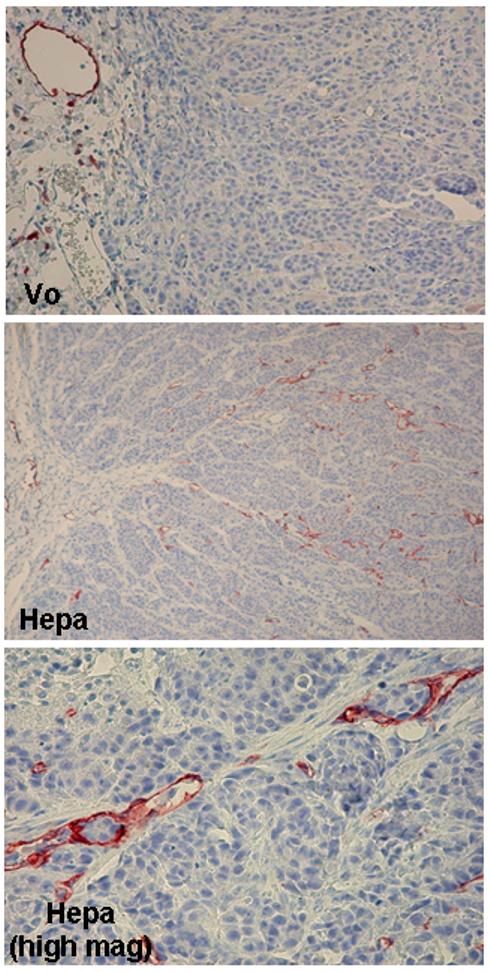
Heparanase facilitates tumor xenograft lymphangiogenesis. Control (Vo) and heparanase (Hepa) transfected MDA-435 cells (5×106) were inoculated at the flank of Balb C/nude mice. Tumor xenografts were harvested after 6 weeks and 5 micron sections were immunostained with anti-LYVE antibody. Note numerous intratumoral lymphatic vessels in tumor xenograft produced by cells over expressing heparanase (middle panel) compared with peritumor lymphatics in the Vo control specimen (upper panel). Lymphatic vessels infiltrated with tumor cells are evident at higher magnification in tumor xenograft produced by heparanase transfected cells (lower panel). Original magnifications: A, B × 20; C × 100.
Discussion
By providing a pathway for cell dissemination, tumor lymphatic vessels are considered as a primary route for metastatic spread of one half of all malignancies, including those of the head and neck 37. Indeed, regional lymph node involvement is a main prognostic factor in a variety of malignancies including head and neck carcinomas 38, and its assessment is critically important in clinical decision and treatment modality. Actively proliferating lymphatic endothelial cells observed within the tumor mass indicate that in head and neck carcinomas, lymphatic formation is dynamically occurring 39, 40 and is associated with increased lymph node metastasis, more advanced TNM stage, a higher risk for local relapse, and poor disease-specific prognosis 37, 39-41. Thus, head and neck carcinoma apparently utilizes de novo lymphatic formation (lymphangiogenesis) for its dissemination, yet mechanisms that regulate lymphangiogenesis are poorly defined. We have recently reported that heparanase expression is elevated in the majority of head and neck cancer patients, elevation that was associated with tumor progression and inversely correlated with patients' status 28. Heparanase activity has long been associated with the metastatic capacity of tumor cells as a consequence of HS cleavage and remodeling of the subepithelial and subendothelial basement membranes 5-7. The results presented here suggest that heparanase plays a unique dual role in tumor metastasis, facilitating the invasiveness and motility of metastatic tumor cells, and enhancing the formation of lymphatic vessels that mobilize these cells. This conclusion emerges from the close association between heparanase staining and LVD (Table 3). The functionality of the lymphatic system in head and neck carcinoma is clearly evident by the appearance of metastatic tumor cells, often in the form of tumor emboli, inside lymphatic vessels (Fig. 1A, C) 40. Significantly, all cases exhibiting lymphatic invasion by tumor cells are also marked by high levels of heparanase (Table 3), a situation best exemplified by double staining employing anti-heparanase and lymphatic specific (D2-40) antibodies (Fig. 1D-F). This finding is in accordance with the inverse correlation found between heparanase staining and the status of head and neck cancer patients 28. Elevation of tumor LVD is likely due to induction of VEGF C, a potent pro-lymphatic mediator secreted by tumor cells 42-45. VEGF C appears to be a relevant mediator since the expression of its close homolog, VEGF D, was noted to be decreased in head and neck tumors compared with the adjacent normal tissue 46. Notably, VEGF C expression closely correlated with heparanase staining (Table 3). Moreover, not only the expression but also the cellular localization of heparanase was found critical for VEGF C induction. Significantly, cytoplasmic heparanase was related to VEGF C induction while most cases exhibiting nuclear localization of heparanase were scored as VEGF C-negative (Table 3), in agreement with favorable outcome of these patients 28 and a more differentiated state of cells and tumors exhibiting nuclear localization of heparanase 35, 47, 48. The association between heparanase and VEGF C is further supported by Cox's proportional hazard model for overall survival (Table 4). Interestingly, while VEGF C levels appear significant for patients' survival in the absence of the heparanase localization parameter (Table 4, Ist stage analysis), its relevance drops to borderline level once heparanase localization is included (Table 4, IInd stage analysis), clearly signifying that the two factors are interrelated. VEGF C induction was noticeably evident in cells over-expressing heparanase (Fig. 2B,C). Moreover, decreased VEGF C levels were observed following heparanase gene silencing by means of siRNA (Fig. 2D), suggesting that endogenous heparanase is intimately involved in VEGF C gene regulation. It worth mentioning that all the genes so far identified to be induced by heparanase [Cox 2 49, TF 26, VEGF A 27, VEGF C] are involved in the regulation of blood or lymphatic vessel formation. Whereas VEGF A is well known for its potent pro-angiogenic repertoire, it was also noted to stimulate lymphatic vessels formation 50. Thus, lymphangiogenesis stimulation by heparanase may also involve VEGF A. Interestingly, most of the pro-lymphatic growth factors identified (i.e., VEGF, FGF-2, angiopoietins, PDGF) 51 are also found to be associated with heparin 52 and can thus be released by active heparanase, suggesting that enzymatically active and inactive heparanase may cooperate to facilitate lymphangiogenesis. These aspects are currently under investigation.
Taken together, we show, for the first time that heparanase facilitates tumor lymphatic vessels formation, in addition to its well documented pro-angiogenic function. This, and the pro-invasive behavior acquired by heparanase-expressing tumor cells 53 position heparanase as a unique mediator of tumor metastasis. Here, we combined clinical, in vitro, and in vivo studies to show that enhanced lymphangiogenesis likely involves VEGF C induction, implying that heparanase modulates the expression levels of selected genes associated with blood and lymphatic vessels formation. Inhibitors of heparanase are thus expected to profoundly affect tumor growth, by cutting its blood supply and decreasing lymphatic formation thereby inhibiting tumor cell invasion and metastasis.
Acknowledgments
This work was supported by grants from the Israel Science Foundation (grant 549/06); National Cancer Institute, NIH (grant RO1-CA106456); the DKFZ-MOST cooperation program in cancer research; and the Rappaport Family Institute Fund. Israel Vlodavsky is a Research Professor of the Israel Cancer Research Fund.
Abbreviations
- ECM
extracellular matrix
- HS
heparan sulfate
- HSPG
heparan sulfate proteoglycan
- LVD
lymphatic vessel density
- VEGF C
vascular endothelial growth factor C
References
- 1.Nakajima M, Irimura T, DiFerrante D, DiFerrante N, Nicolson GL. Heparan sulfate degradation: relation to tumor invasion and metastatic properties of Mouse B 16 Melanoma sublines. Science (Wash. DC) 1983;220:611–3. doi: 10.1126/science.6220468. [DOI] [PubMed] [Google Scholar]
- 2.Vlodavsky I, Fuks Z, Bar-Ner M, Ariav Y, Schirrmacher V. Lymphoma cells mediated degradation of sulfated proteoglycans in the subendothelial extracellular matrix: relation to tumor cell metastasis. Cancer Res. 1983;43:2704–11. [PubMed] [Google Scholar]
- 3.Edovitsky E, Elkin M, Zcharia E, Peretz T, Vlodavsky I. Heparanase gene silencing, tumor invasiveness, angiogenesis, and metastasis. J Natl Cancer Inst. 2004;96:1219–30. doi: 10.1093/jnci/djh230. [DOI] [PubMed] [Google Scholar]
- 4.Edovitsky E, Lerner I, Zcharia E, Peretz T, Vlodavsky I, Elkin M. Role of endothelial heparanase in delayed-type hypersensitivity. Blood. 2005;107:3609–3616. doi: 10.1182/blood-2005-08-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ilan N, Elkin M, Vlodavsky I. Regulation, function and clinical significance of heparanase in cancer metastasis and angiogenesis. Int J Biochem Cell Biol. 2006;38:2018–39. doi: 10.1016/j.biocel.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Vlodavsky I, Abboud-Jarrous G, Elkin M, Naggi A, Casu B, Sasisekharan R, Ilan N. The impact of heparanese and heparin on cancer metastasis and angiogenesis. Pathophysiol Haemost Thromb. 2006;35:116–27. doi: 10.1159/000093553. [DOI] [PubMed] [Google Scholar]
- 7.Vreys V, David G. Mammalian heparanase: what is the message? J Cell Mol Med. 2007;11:427–52. doi: 10.1111/j.1582-4934.2007.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferro V, Dredge K, Liu L, Hammond E, Bytheway I, Li C, Johnstone K, Karoli T, Davis K, Copeman E, Gautam A. PI-88 and novel heparan sulfate mimetics inhibit angiogenesis. Sem Thromb and Hemost. 2007;33:557–68. doi: 10.1055/s-2007-982088. [DOI] [PubMed] [Google Scholar]
- 9.Ferro V, Hammond E, Fairweather JK. The development of inhibitors of heparanase, a key enzyme involved in tumour metastasis, angiogenesis and inflammation. Mini Rev Med Chem. 2004;4:693–702. doi: 10.2174/1389557043403729. [DOI] [PubMed] [Google Scholar]
- 10.McKenzie EA. Heparanase: a target for drug discovery in cancer and inflammation. Br J Pharmacol. 2007;151:1–14. doi: 10.1038/sj.bjp.0707182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miao HQ, Liu H, Navarro E, Kussie P, Zhu Z. Development of heparanase inhibitors for anti-cancer therapy. Curr Med Chem. 2006;13:2101–11. doi: 10.2174/092986706777935230. [DOI] [PubMed] [Google Scholar]
- 12.Vlodavsky I, Ilan N, Naggi A, Casu B. Heparanase: structure, biological functions, and inhibition by heparin-derived mimetics of heparan sulfate. Curr Pharm Des. 2007;13:2057–73. doi: 10.2174/138161207781039742. [DOI] [PubMed] [Google Scholar]
- 13.El-Assal ON, Yamanoi A, Ono T, Kohno H, Nagasue N. The Clinicopathological significance of heparanase and basic fibroblast growth factor expressions in hepatocellular carcinoma. Clin Cancer Res. 2001;7:1299–305. [PubMed] [Google Scholar]
- 14.Gohji K, Hirano H, Okamoto M, Kitazawa S, Toyoshima M, Dong J, Katsuoka Y, Nakajima M. Expression of three extracellular matrix degradative enzymes in bladder cancer. Int J Cancer. 2001;95:295–301. doi: 10.1002/1097-0215(20010920)95:5<295::aid-ijc1051>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 15.Sato T, Yamaguchi A, Goi T, Hirono Y, Takeuchi K, Katayama K, Matsukawa S. Heparanase expression in human colorectal cancer and its relationship to tumor angiogenesis, hematogenous metastasis, and prognosis. J Surg Oncol. 2004;87:174–81. doi: 10.1002/jso.20097. [DOI] [PubMed] [Google Scholar]
- 16.Shinyo Y, Kodama J, Hongo A, Yoshinouchi M, Hiramatsu Y. Heparanase expression is an independent prognostic factor in patients with invasive cervical cancer. Ann Oncol. 2003;14:1505–10. doi: 10.1093/annonc/mdg407. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe M, Aoki Y, Kase H, Tanaka K. Heparanase expression and angiogenesis in endometrial cancer. Gynecol Obstet Invest. 2003;56:77–82. doi: 10.1159/000072821. [DOI] [PubMed] [Google Scholar]
- 18.Kelly T, Miao H-Q, Yang Y, Navarro E, Kussie P, Huang Y, MacLeod V, Casciano J, Joseph L, Zhan F, Zangari M, Barlogie B, et al. High heparanase activity in multiple myeloma is associated with elevated microvessel density. Cancer Res. 2003;63:8749–56. [PubMed] [Google Scholar]
- 19.Cohen I, Pappo O, Elkin M, San T, Bar-Shavit R, Hazan R, Peretz T, Vlodavsky I, Abramovitch R. Heparanase promotes growth, angiogenesis and survival of primary breast tumors. Int J Cancer. 2006;118:1609–1617. doi: 10.1002/ijc.21552. [DOI] [PubMed] [Google Scholar]
- 20.Zetser A, Bashenko Y, Miao H-Q, Vlodavsky I, Ilan N. Heparanase affects adhesive and tumorigenic potential of human glioma cells. Cancer Res. 2003;63:7733–41. [PubMed] [Google Scholar]
- 21.Elkin M, Cohen I, Zcharia E, Orgel A, Guatta-Rangini Z, Peretz T, Vlodavsky I, Kleinman HK. Regulation of heparanase gene expression by estrogen in breast cancer. Cancer Res. 2003;63:8821–6. [PubMed] [Google Scholar]
- 22.Zcharia E, Zilka R, Yaar A, Yacoby-Zeevi O, Zetser A, Metzger S, Sarid R, Naggi A, Casu B, Ilan N, Vlodavsky I, Abramovitch R. Heparanase accelerates wound angiogenesis and wound healing in mouse and rat models. FASEB J. 2005;19:211–21. doi: 10.1096/fj.04-1970com. [DOI] [PubMed] [Google Scholar]
- 23.Bashkin P, Doctrow S, Klagsbrun M, Svahn CM, Folkman J, Vlodavsky I. Basic fibroblast growth factor binds to subendothelial extracellular matrix and is released by heparitinase and heparin-like molecules. Biochemistry. 1989;28:1737–43. doi: 10.1021/bi00430a047. [DOI] [PubMed] [Google Scholar]
- 24.Elkin M, Ilan N, Ishai-Michaeli R, Friedmann Y, Papo O, Pecker I, Vlodavsky I. Heparanase as mediator of angiogenesis: mode of action. FASEB J. 2001;15:1661–3. doi: 10.1096/fj.00-0895fje. [DOI] [PubMed] [Google Scholar]
- 25.Folkman J, Klagsbrun M, Sasse J, Wadzinski M, Ingber D, Vlodavsky I. A heparin-binding angiogenic protein--basic fibroblast growth factor--is stored within basement membrane. Am J Pathol. 1988;130:393–400. [PMC free article] [PubMed] [Google Scholar]
- 26.Nadir Y, Brenner B, Zetser A, Ilan N, Shafat I, Zcharia E, Goldshmidt O, Vlodavsky I. Heparanase induces tissue factor expression in vascular endothelial and cancer cells. J Thromb Haemost. 2006;4:2443–51. doi: 10.1111/j.1538-7836.2006.02212.x. [DOI] [PubMed] [Google Scholar]
- 27.Zetser A, Bashenko Y, Edovitsky E, Levy-Adam F, Vlodavsky I, Ilan N. Heparanase induces vascular endothelial growth factor expression: correlation with p38 phosphorylation levels and Src activation. Cancer Res. 2006;66:1455–63. doi: 10.1158/0008-5472.CAN-05-1811. [DOI] [PubMed] [Google Scholar]
- 28.Doweck I, Kaplan-Cohen V, Naroditsky I, Sabo E, Ilan N, Vlodavsky I. Heparanase localization and expression by head and neck cancer: correlation with tumor progression and patient survival. Neoplasia. 2006;8:1055–61. doi: 10.1593/neo.06577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zetser A, Levy-Adam F, Kaplan V, Gingis-Velitski S, Bashenko Y, Schubert S, Flugelman MY, Vlodavsky I, Ilan N. Processing and activation of latent heparanase occurs in lysosomes. J Cell Sci. 2004;117:2249–58. doi: 10.1242/jcs.01068. [DOI] [PubMed] [Google Scholar]
- 30.Rae JM, Creighton CJ, Meck JM, Haddad BR, Johnson MD. MDA-MB-435 cells are derived from M14 melanoma cells--a loss for breast cancer, but a boon for melanoma research. Breast Cancer Res Treat. 2007;104:13–9. doi: 10.1007/s10549-006-9392-8. [DOI] [PubMed] [Google Scholar]
- 31.Levy-Adam F, Miao HQ, Heinrikson RL, Vlodavsky I, Ilan N. Heterodimer formation is essential for heparanase enzymatic activity. Biochem Biophys Res Commun. 2003;308:885–91. doi: 10.1016/s0006-291x(03)01478-5. [DOI] [PubMed] [Google Scholar]
- 32.Longatto-Filho A, Pinheiro C, Pereira SM, Etlinger D, Moreira MA, Jube LF, Queiroz GS, Baltazar F, Schmitt FC. Lymphatic vessel density and epithelial D2-40 immunoreactivity in pre-invasive and invasive lesions of the uterine cervix. Gynecologic oncology. 2007;107:45–51. doi: 10.1016/j.ygyno.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 33.Schacht V, Dadras SS, Johnson LA, Jackson DG, Hong YK, Detmar M. Up-regulation of the lymphatic marker podoplanin, a mucin-type transmembrane glycoprotein, in human squamous cell carcinomas and germ cell tumors. Am J Pathol. 2005;166:913–21. doi: 10.1016/S0002-9440(10)62311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takaoka M, Naomoto Y, Ohkawa T, Uetsuka H, Shirakawa Y, Uno F, Fujiwara T, Gunduz M, Nagatsuka H, Nakajima M, Tanaka N, Haisa M. Heparanase expression correlates with invasion and poor prognosis in gastric cancers. Lab Invest. 2003;83:613–22. doi: 10.1097/01.lab.0000067482.84946.bd. [DOI] [PubMed] [Google Scholar]
- 35.Ohkawa T, Naomoto Y, Takaoka M, Nobuhisa T, Noma K, Motoki T, Murata T, Uetsuka H, Kobayashi M, Shirakawa Y, Yamatsuji T, Matsubara N, et al. Localization of heparanase in esophageal cancer cells: respective roles in prognosis and differentiation. Lab Invest. 2004;84:1289–304. doi: 10.1038/labinvest.3700159. [DOI] [PubMed] [Google Scholar]
- 36.Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7:192–8. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 37.Maula SM, Luukkaa M, Grenman R, Jackson D, Jalkanen S, Ristamaki R. Intratumoral lymphatics are essential for the metastatic spread and prognosis in squamous cell carcinomas of the head and neck region. Cancer Res. 2003;63:1920–6. [PubMed] [Google Scholar]
- 38.Quon H, Liu FF, Cummings BJ. Potential molecular prognostic markers in head and neck squamous cell carcinomas. Head Neck. 2001;23:147–59. doi: 10.1002/1097-0347(200102)23:2<147::aid-hed1010>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 39.Beasley NJ, Prevo R, Banerji S, Leek RD, Moore J, van Trappen P, Cox G, Harris AL, Jackson DG. Intratumoral lymphangiogenesis and lymph node metastasis in head and neck cancer. Cancer Res. 2002;62:1315–20. [PubMed] [Google Scholar]
- 40.Kyzas PA, Geleff S, Batistatou A, Agnantis NJ, Stefanou D. Evidence for lymphangiogenesis and its prognostic implications in head and neck squamous cell carcinoma. J Pathol. 2005;206:170–7. doi: 10.1002/path.1776. [DOI] [PubMed] [Google Scholar]
- 41.Franchi A, Gallo O, Massi D, Baroni G, Santucci M. Tumor lymphangiogenesis in head and neck squamous cell carcinoma: a morphometric study with clinical correlations. Cancer. 2004;101:973–8. doi: 10.1002/cncr.20454. [DOI] [PubMed] [Google Scholar]
- 42.Alitalo K, Carmeliet P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell. 2002;1:219–27. doi: 10.1016/s1535-6108(02)00051-x. [DOI] [PubMed] [Google Scholar]
- 43.Pepper MS. Lymphangiogenesis and tumor metastasis: myth or reality? Clin Cancer Res. 2001;7:462–8. [PubMed] [Google Scholar]
- 44.Pepper MS, Tille JC, Nisato R, Skobe M. Lymphangiogenesis and tumor metastasis. Cell and tissue research. 2003;314:167–77. doi: 10.1007/s00441-003-0748-7. [DOI] [PubMed] [Google Scholar]
- 45.Wissmann C, Detmar M. Pathways targeting tumor lymphangiogenesis. Clin Cancer Res. 2006;12:6865–8. doi: 10.1158/1078-0432.CCR-06-1800. [DOI] [PubMed] [Google Scholar]
- 46.O-charoenrat P, Rhys-Evans P, Eccles SA. Expression of vascular endothelial growth factor family members in head and neck squamous cell carcinoma correlates with lymph node metastasis. Cancer. 2001;92:556–68. doi: 10.1002/1097-0142(20010801)92:3<556::aid-cncr1355>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 47.Kobayashi M, Naomoto Y, Nobuhisa T, Okawa T, Takaoka M, Shirakawa Y, Yamatsuji T, Matsuoka J, Mizushima T, Matsuura H, Nakajima M, Nakagawa H, et al. Heparanase regulates esophageal keratinocyte differentiation through nuclear translocation and heparan sulfate cleavage. Differentiation. 2006;74:235–43. doi: 10.1111/j.1432-0436.2006.00072.x. [DOI] [PubMed] [Google Scholar]
- 48.Nobuhisa T, Naomoto Y, Takaoka M, Tabuchi Y, Ookawa K, Kitamoto D, Gunduz E, Gunduz M, Nagatsuka H, Haisa M, Matsuoka J, Nakajima M, et al. Emergence of nuclear heparanase induces differentiation of human mammary cancer cells. Biochem Biophys Res Commun. 2005;331:175–80. doi: 10.1016/j.bbrc.2005.03.129. [DOI] [PubMed] [Google Scholar]
- 49.Okawa T, Naomoto Y, Nobuhisa T, Takaoka M, Motoki T, Shirakawa Y, Yamatsuji T, Inoue H, Ouchida M, Gunduz M, Nakajima M, Tanaka N. Heparanase is involved in angiogenesis in esophageal cancer through induction of cyclooxygenase-2. Clin Cancer Res. 2005;11:7995–8005. doi: 10.1158/1078-0432.CCR-05-1103. [DOI] [PubMed] [Google Scholar]
- 50.Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C, D'Amore PA, Dana MR, Wiegand SJ, Streilein JW. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040–50. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Achen MG, Stacker SA. Tumor lymphangiogenesis and metastatic spread-new players begin to emerge. Int J Cancer. 2006;119:1755–60. doi: 10.1002/ijc.21899. [DOI] [PubMed] [Google Scholar]
- 52.Raman R, Sasisekharan V, Sasisekharan R. Structural insights into biological roles of protein-glycosaminoglycan interactions. Chemistry & biology. 2005;12:267–77. doi: 10.1016/j.chembiol.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 53.Vlodavsky I, Friedmann Y, Elkin M, Aingorn H, Atzmon R, Ishai-Michaeli R, Bitan M, Pappo O, Peretz T, Michal I, Spector L, Pecker I. Mammalian heparanase: gene cloning, expression and function in tumor progression and metastasis. Nat Med. 1999;5:793–802. doi: 10.1038/10518. [DOI] [PubMed] [Google Scholar]


