Abstract
Efficient entry of synthetic polymers inside cells is a central issue in polymeric drug delivery. Though polymers are widely believed to interact non-specifically with plasma membrane we present unexpected evidence that amphiphilic block copolymers depending on their aggregation state can distinguish between caveolae- or clathrin-mediated endocytosis. A block copolymer of poly(ethylene oxide) (PEO) and poly(propylene oxide) (PPO), Pluronic® P85 (P85), below critical micelle concentration (CMC) exists as single molecule coils (unimers) and above CMC forms 14.6 nm aggregated micelles with hydrophobic PPO core and hydrophilic PEO shell. The internalization pathways of P85 in mammalian cells were elucidated using endocytosis inhibitors and co-localization with endocytosis markers (clathrin-specific antibodies and transferrin for clathrin and caveolin-1-specific antibodies and cholera toxin B for caveolae). All together, our results indicate that P85 unimers internalize through caveolae-mediated endocytosis, while P85 micelles internalize through clathrin-mediated endocytosis. Furthermore, at concentrations above 0.01% P85 inhibits caveolae-mediated endocytosis (cholera toxin B), while having little or no effect on the clathrin-mediated endocytosis (transferrin). Selective interaction of Pluronic® with caveolae may explain its striking pharmacological activities including inhibition of drug efflux transport, activation of gene expression and dose-dependent hyperlipidemia.
Introduction
Amphiphilic triblock copolymers of poly(ethylene oxide)-b-poly(propylene oxide)-b-poly(ethylene oxide) (PEO-PPO-PEO), also known as Pluronic® or poloxamers, have been evaluated extensively as carriers for delivery of drugs and diagnostic imaging agents (1, 2). In aqueous solutions above the critical micelle concentration (CMC) the PPO chains of these block copolymers spontaneously segregate into a hydrophobic core of a micelle with the PEO chains forming a hydrophilic shell (1). The diameters of Pluronic® micelles usually vary from ca. 10 nm to 100 nm. The PPO core can incorporate considerable amounts (up to 20-30 % wt.) of many water-insoluble drugs (1). The micelles can be targeted to a specific body compartment, such as brain, by conjugating with neurospecific antibodies or insulin as targeting moieties (3-5). In addition, below the CMC Pluronic® exists in the form of “unimers”, which, in case of relatively hydrophobic copolymers, can bind with cell membranes and transport into cells (3, 6-9). Such block copolymers were also shown to inhibit a drug efflux pump, P-glycoprotein, Pgp, expressed in the membranes of multidrug resistant cells (7, 10, 11) and induce transcriptional activation of gene expression in cells through activation of certain cell signaling pathways (12-14). Finally, Pluronic® block copolymers also can serve as synthetic polymer transduction domains and carry proteins as large as horseradish peroxidase (HRP) into cells and across the blood brain barrier (BBB) (15).
Pluronic® unimers and micelles have been known to undergo internalization in cells (7), which can be abolished by metabolic inhibitors (7, 9). Based on this a general endocytosis mechanism was suggested for cellular entry of Pluronic®, although the specific pathways remained unknown. Endocytosis can be divided into two broad categories, phagocytosis (the uptake of large particles) and pinocytosis (the uptake of fluids and solutes) (16). Pinocytosis can be further classified either on the basis of membrane interaction or the type of membrane structures involved in the initial stages of internalization. The first classification includes (i) fluid phase endocytosis (e.g. HRP uptake), (ii) receptor mediated endocytosis (e.g. transferrin (Tf) uptake via Tf receptor (TfR)); or (iii) adsorptive endocytosis (e.g. albumin or wheat germ agglutinin uptake) (17). The second classification includes (i) clathrin-mediated endocytosis, and (ii) caveolae-mediated endocytosis (16).
Clathrin-mediated endocytosis involves polymerization of a cytosolic protein, clathrin, which binds to the cell plasma membrane and induces formation of a coated pit (18). After budding the vesicle sheds its clathrin coat, and then delivers its protein cargo to endosomes. The cargo can be then recycled or transported to lysosomes via late endosomes. Tf is commonly used as a marker for this pathway. Caveolae are cholesterol-rich “flask shaped invaginations” in the plasma membrane formed due to the presence of a dimeric protein, caveolin (19). These vesicles travel to caveosomes, which prevents lysosomal degradation of the cargo. Cholera toxin B (CTB) or Simian Virus 40 (SV40) typically use the caveolae-mediated mechanism for the cellular entry (18, 20, 21). Recent studies have also indicated the existence of clathrin- and caveolae-independent endocytosis, which still remains relatively poorly explored (22).
In this study we hypothesize that Pluronic® block copolymers can undergo endocytosis via clathrin- or caveolae-mediated mechanism. We further posit that Pluronic® can interfere with selected membrane trafficking mechanisms. The latter is based on the knowledge that Pluronic® molecules (i) incorporate into membranes by inserting their hydrophobic PPO chains into lipid bilayers (23, 24), (ii) decrease membrane micro-viscosity (7) and (iii) inhibit selected membranes enzymes, such as Pgp ATPase, which may be associated with cholesterol-rich membrane microdomains (25). To test this hypothesis we use Pluronic® P85 (P85) as a representative copolymer of the Pluronic® series, which exhibits all of the above listed activities (1, 3, 7, 26). Our approach involves 1) evaluation of P85 internalization in the presence of chemical inhibitors of selected endocytosis pathways; 2) co-localization of P85 unimers (below CMC) and micelles (above CMC) with clathrin- and caveolin-specific antibodies and various endocytosis markers (Tf and CTB); and 3) evaluation of the effect of P85 on the internalization of the clathrin- and caveolae-mediated and fluid phase endocytosis markers (Tf, CTB and HRP). The MDCK cells are used as an in vitro model. All together, our results indicate that P85 unimers at concentrations below the CMC (0.03% wt.) internalize through caveolae-mediated endocytosis, while P85 micelles at higher concentrations internalize mainly-through the clathrin-mediated endocytosis. Furthermore, at high concentration above 0.1% wt., P85 inhibits caveolae-mediated endocytosis, while having little or no effect on the clathrin-mediated endocytosis. For the first time we demonstrate that binding of the amphiphilic block copolymer with the cells may be constrained to selected membrane domains. The results are significant for understanding the mechanisms of transport of the block copolymers into cells as well as for relating the block copolymer-membrane interactions to the biological activities of the block copolymers, which may be mediated by the specific membrane micro-domains affected by these interactions.
Materials and Methods
Materials
P85, EO26-PO40-EO26 (lot # WPAU-549B) was kindly provided by BASF Corp. Synthesis of FITC labeled Pluronic® block co-polymers was performed by Dr. Srikanth Sriadibhatala at the University of Nebraska Medical Center (UNMC) as previously reported (9). Alexa 594 labeled CTB (Alexa 594-CTB), unlabeled CTB antibody and Alexa 647 labeled Tf (Alexa 647-Tf) were purchased from Invitrogen Inc (Carlsbad, CA). FITC labeled CTB (FITC-CTB), methyl-β-cyclodextrin (MBCD), sucrose, sodium azide, cytochalasin D, 2-deoxyglucose (2DG) were purchased from Sigma-Aldrich, (St. Louis, MO). Bovine serum albumin (BSA) (Fischer Scientific, Waltham, MA) RITC-HRP was prepared by labeling HRP (2mg/ml) Sigma-Aldrich (St. Louis, MO) in 0.1M bicarbonate buffer with RITC. RITC-HRP was separated from the mixture using Sephadex G-25 column Sigma-Aldrich (St. Louis, MO).
Cell Lines
Madin-Darby Canine Kidney (MDCK) cells were maintained in Dulbecco's Modified Eagle's Medium (DMEM), containing 10% heat inactivated fetal bovine serum (FBS), penicillin/streptomycin as described elsewhere (27). All tissue material media was obtained from Gibco Life Technologies, Inc. (Grand Island, NY). MDCK cells were used after 48 hrs of plating.
FACS analysis of FITC-P85 uptake
MDCK cells (5×104 cell/well in 24 well plates) were exposed to 0.001% FITC-P85 alone or to 0.001% FITC-P85 mixed with different concentrations of unlabelled P85 for 1 h at 37°C. Cells were then washed by phosphate-buffered saline (PBS), trypsinized, and resuspended in PBS. In select experiments cells were pre-treated for 30 min with inhibitors of endocytosis (5 mM MBCD, or 0.45 M sucrose, or 0.25 mM cytochalasin D), and then the same inhibitors were also present during subsequent incubation with the copolymer. The mean fluorescence intensity was analyzed using Becton Dickinson FACStarPlus flow cytometer operating under Lysis II (San Jose, CA) equipped with an argon ion laser. Data were acquired in linear mode and visualized in logarithmic mode. Data from 10,000 events were gated using forward and side scatter parameters to exclude debris and dead cells.
FACS analysis
MDCK cells (5×104 cell/well in 24 well plates) were exposed to 5 μg/ml Alexa 647-Tf or 5 μg/ml FITC-CTB with/without P85 for 1 h at 37°C. Cells were washed, trypsinized, resuspended and analyzed by FACS as described above. In select experiments cells were pre-treated with inhibitors of endocytosis (5 mM MBCD, or 0.45 M sucrose, or 0.25 mM cytochalasin D), and then the same inhibitors were also present during incubation with the endocytosis markers. All experiments were conducted thrice and measurements were conducted in triplicates and data presented as means +/− SEM. Statistical comparisons between groups were made using Student's t-test.
Confocal Analysis on Live Cells
Pulse-Chase experiments were conducted for FITC-P85 uptake using live cell confocal microscope (Carl Zeiss LSM 510 Meta, Peabody, MA). MDCK cells (1×106) were plated in Bioptech dishes (Fischer Scientific, Waltham, MA) and after two days (37°C, 5% CO2) were exposed for 30 min. to 0.001% FITC-P85 mixed with 0.01 % unlabelled P85 followed by another 30 min exposure 0.01 % unlabelled P85. In select experiments 5 μg/ml Alexa 594-CTB for 30 minutes, cells were washed, followed by concurrent addition of unlabeled CTB antibody with the co-polymer for 30 minutes. The exposures to the copolymer were carried out either at 4°C or 37°C. In other experiments, 5 μg/ml Alexa 647-Tf were added to cells for 30 min and concurrently incubated with unlabelled copolymer for additional 30 min. Cells were finally washed and kept in complete media for imaging using the confocal microscope. The live cell microscopy was also used to assess effects of P85 on uptake of RITC-HRP. In this case cells were incubated with 0.01 mg/ml RITC-HRP with/without P85 for 60 min. and then analyzed by confocal microscopy.
Immunocytochemistry on Fixed Cells
Cells were pretreated with DMEM without FBS for 30 min, and then treated with 0.001% FITC-P85 in the presence of 0.01 % or 0.1% unlabelled P85 for 1 h, washed and fixed with 4% paraformaldehyde. Rabbit anti-caveolin-1–Cy3 antibody (Sigma Aldrich, St Louis, MO) (1:100) and mouse anti-clathrin antibody (Affinity Bio-reagents, Golden, CO) (1:10) were incubated in blocking buffer overnight at 4°C. For detection of anti-clathrin antibody specific IgG antibody (1:100) conjugated to Alexa 568 (Invitrogen Inc, Carlsbad, CA.) was added to cells for 1 h at 37°C. Cells were examined under confocal microscope.
HRP assay
The enzymatic activity of HRP was assessed with o-phenylenediamine as described earlier (15). Briefly, cells were treated with HRP solution (0.1 mg/mL) with/without P85. In select experiments before adding the copolymer the cells were preincubated with 25 mM 2DG and 5mM sodium azide, which was then added also during the exposure to the copolymer. Cells were then lysed with 0.1% Triton X-100 (Sigma-Aldrich, St.Louis, MO) and the lysate was added to a 96-well plate with the solution of o-phenylenediamine (5 mg/mL) in 0.1 M citrate buffer (pH 5.0) containing 1 mg/mL BSA, 0.1% Triton X-100, and 0.02% hydrogen peroxide solution. After incubating the mixture for 5 min at 37°C the reaction was stopped by adding 0.5% sodium sulfite in 2 M sulfuric acid (Acros Organic, Waltham, MA), and absorbance was measured at 550 nm using the microplate reader Multiskan MCC/340. The amounts of HRP in the samples were determined using a standard calibration curve. All measurements were conducted in triplicates and data presented as means +/− SEM. Statistical comparisons between groups were made using Student's t-test.
Results
Internalization pathways of P85
The confocal analysis was indicative of the vesicular uptake of FITC-P85 at 37°C (Fig. 1A). The FACS analysis further demonstrated that the uptake of the block copolymer was temperature-dependent and was considerably inhibited at 4°C compared to 37°C (Fig 1B). Based on this we posit that endocytosis is a prominent pathway for internalization of P85.
Fig. 1.
Uptake of FITC-P85 in MDCK cells: (A) confocal microscopy on live cells at 37°C; (B) temperature dependence by FACS analysis. Cells were exposed to the copolymer at the corresponding temperatures for 60 min. The exposure solution contained 0.001 % FITC-P85 mixed with 0.01% P85. (B) Data are mean +/− S.E.M. (n = 3). Difference between 4°C and 37°C groups has a significance level of *** p = 0.001
To further elucidate the specific internalization pathways of P85 we examined the effects of the endocytosis inhibitors on the uptake of this copolymer into the cells. This study used three different types of inhibitors: 1) MBCD, a cholesterol-depleting agent, for caveolae-mediated endocytosis (28), 2) hypertonic sucrose for clathrin-mediated endocytosis (29, 30), and 3) cytochalasin D, a potent inhibitor of actin polymerization, for both pathways as well as macropinocytosis (31, 32). To differentiate the transport of P85 unimers and micelles the two concentrations of the copolymer were used. The first, 0.001% wt FITC-P85, was below the CMC (≈ 0.03% wt.) to examine the entry of the P85 unimers. The second, 0.001% wt FITC-P85 (as a tracer) mixed with 0.1% wt P85, was above the CMC, to characterize the entry of the micelles as well as unimers. At this high concentration, at least 70 % of FITC-P85 was incorporated into the micelles, while the rest was in equilibrium in the unimer form (33). In each case, the FACS analysis was performed 1 hr after exposure of the MDCK cells to the copolymer with or without the corresponding inhibitors. Since there is literature suggesting that various inhibitors may interfere with multiple endocytic pathways (34-36) we first demonstrated that MBCD inhibits CTB but not Tf uptake (i.e. is likely to be selective for caveolae) and sucrose inhibits Tf but not CTB uptake (i.e. is selective for clathrin) (data presented below in Fig. 6).
Fig. 6.
Effect of P85 (conc. reported as % wt) and various endocytosis inhibitors on the uptake of (A) FITC-CTB (B) Alexa 647-Tf in MDCK cells. The experiments were grouped for (A) as A1=0.0001 % P85, A2=0.001 % P85, A3=0.1 % P85, A4=1 % P85 A5=5 % P85, A6= FITC-CTB, A7=MBCD and A8=Sucrose and (B) as B1=0.0001 % P85, B2=0.1 % P85, B3=1 % P85 B4=5 % P85, B5=Sucrose, B6= Alexa 647-Tf and B7 = MBCD (A, B) Data are mean +/− S.E.M. (n = 3). Statistical comparisons between the control group (no inhibitor) and the corresponding inhibitor group are shown as follows: n.s. - not significant, ** p =0.05, *** p = 0.001.
As shown in Fig. 2A at the unimer concentration the FITC-P85 entry was blocked in the presence of MBCD but was not affected by the sucrose, suggesting that the unimers were entering cells via caveolae-mediated endocytosis. The situation was drastically changed at the higher P85 concentration (Fig. 2B). In this case, the greatest inhibition of FITC-P85 entry was observed with sucrose, suggesting that the most prominent pathway for the micelles uptake was the clathrin-mediated endocytosis. Although MBCD had some effect on FITC-P85 uptake at this concentration, its effect was much less pronounced than that of the sucrose. It could be due to the presence of a smaller portion of FITC-P85 in the form of unimers, which were taken into cells by caveolae. Additionally, a minor portion of the micelles could be internalized through the caveolae-mediated pathway. Interestingly, cytochalasin D inhibited FITC-P85 uptake at both concentrations although it was more pronounced at the unimers concentration.
Fig. 2.
Effect of endocytosis inhibitors on the uptake of FITC-P85 in MDCK cells: (A) 0.001 % FITC-P85 and (B) 0.001 % FITC-P85 mixed with 0.1% P85. Cells were exposed to the copolymer that corresponding temperatures for 60 min at 37°C. (A,B) Data are mean +/− S.E.M. (n = 3). Statistical comparisons between the control group (no inhibitor) and the corresponding inhibitor group are shown as follows: n.s. - not significant, ** p =0.01 ***p = 0.001.
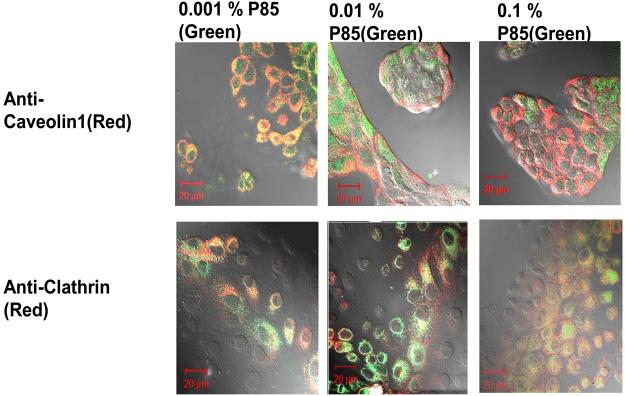
To validate the conclusions of the inhibitor analysis the cellular localization of FITC-P85 at different copolymer concentrations was examined by immunocytochemistry. In these studies following exposure to the copolymer for 60 min. the cells were fixed and stained with antibodies against clathrin or caveolin-1 and examined by confocal microscope (Fig. 3). Consistent with the inhibitor analysis at the lowest concentration of the copolymer (0.001%) there was a significant colocalization of the unimers with caveolin-1, which considerably diminished as the concentration of the copolymer increased. In contrast, co-localization with clathrin was minimal if any at low concentration of the copolymer, but it greatly increased as the copolymer concentration increased. To further corroborate this result we performed live cell microscopy on MDCK cells incubated with low concentration of labeled copolymer and either Alexa 594-CTB or Alexa 647-Tf. As shown in Fig. 4 there was strong co-localization of the FITC-P85 and CTB. When the cells were exposed to the copolymer and CTB at 4°C the localization of both labels appeared to be mainly constrained to the plasma membrane areas. In contrast, at 37°C the internalization and the co-localization of the labels were clearly evident. This suggests that FITC-P85 and Alexa 594-CTB are transported into cells through same pathways. Conversely, there was little if any co-localization of FITC-P85 and Alexa 647-Tf (data not shown).
Fig. 3.
Co-localization of FITC-P85 with caveolin-1 and clathrin in fixed MDCK cells. Live cells were exposed for 60 min. at 37°C to 0.001 % FITC-P85 alone, 0.001 % FITC-P85 mixed with 0.01% P85 or 0.001 % FITC-P85 mixed with 0.1% P85.
Fig. 4.
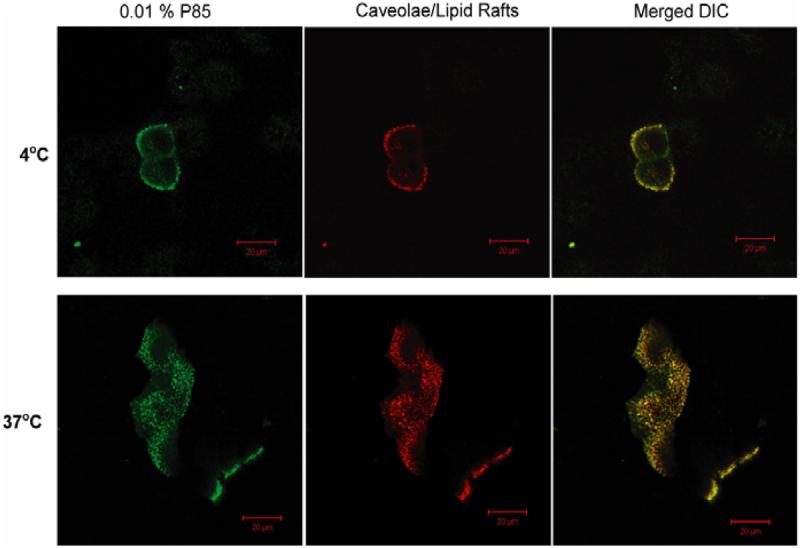
Co-localization of FITC-P85 and Alexa 594-CTB in live MDCK cells at 4°C and 37°C. Cells were exposed for 30 min. to 0.001% FITC-P85 mixed with 0.01 % unlabelled P85 followed by another 30 min exposure 0.01 % unlabelled P85 (see Materials and Methods). Some intracellular localization of the labels at 4°C may be due to the increase of the temperature to r.t., during fluorescence observation, which could initiate endocytosis in live cells.
Effect of P85 on endocytosis
To elucidate possible effects of P85 on different vesicular transport processes we used HRP, CTB, and Tf as the markers of fluid-phase, caveolae-mediated and clathrin-mediated endocytosis respectively. The MDCK cells were exposed to the corresponding markers with/without P85 at different concentrations. As seen in Fig. 5A the uptake of HRP was practically unchanged at 0.001% P85, significantly decreased at 0.01% and 0.1% P85 and practically abolished at 1% P85. In the latter case the inhibitory effect of the copolymer was practically the same as the effect of the metabolic inhibitor, 25 mM 2DG and 5mM sodium azide. The inhibition of internalization of RITC-HRP by high concentrations of P85 was also evident in the live cell confocal microscopy experiment (Fig. 5B). All together, these experiments demonstrate that at higher concentrations P85 inhibit non-specific fluid phase endocytosis of HRP.
Fig. 5.
Effect of P85 (conc. reported as % wt) on uptake of HRP in MDCK cells: (A) cellular levels of HRP as determined by enzymatic activity in cell lyzates, cells were treated in the absence or presence of P85 and are grouped as 1-HRP alone, 2-0.001% wt P85, 3-0.01% P85, 4-0.1% P85, 5-1% P85, 6- 2DG and sodium azide; (B) live cell confocal microscopy using RITC-HRP. (A) Data are mean +/− S.E.M. (n = 3).
In a separate experiment the effects of various concentrations of P85 on the uptake of FITC-CTB and Alexa 647-Tf were evaluated using FACS analysis. At low concentration 0.0001% and 0.001% the copolymer had no effect on the FITC-CTB uptake (Fig. 6A). However, significant inhibition of the uptake of this marker was observed already at 0.1% P85, while at 1% and 5% P85 the uptake was completely abolished. Notably, the cytotoxicity analysis using MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay (14) as well FACS analysis of the cells showed that 60 min exposure of the MDCK cells to these concentrations of P85 did not produce cytotoxicity (data not shown). Therefore, the plausible explanation of the data is that at higher concentrations P85 inhibits FITC-CTB uptake via caveolae-mediated endocytosis. This was reinforced by the effects of the endocytosis inhibitors on the FITC-CTB uptake, suggesting that cholesterol depletion by MBCD practically abolished the internalization of this marker (Fig. 6A). At the same time, inhibition of clathrin-mediated endocytosis by hypertonic sucrose had no effect of the FITC-CTB uptake. Finally, using Alexa 647-Tf as a clathrin-mediated endocytosis marker we demonstrated that P85 at any concentration did not inhibit this endocytosis pathway (Fig. 6B). Notably, this may explain the lack of overall toxicity of the copolymer under the conditions studied. The Alexa 647-Tf was inhibited by the hypertonic sucrose but not by the MBCD.
Discussion
Nanoscale size drug delivery systems based on synthetic polymers have attracted significant attention in pharmaceutics and medicine (37-39). An important and promising example of such systems is Pluronic® block copolymers, which have been evaluated as drug and gene delivery agents in numerous studies (3, 4, 26, 40-42). A characteristic of Pluronic® molecules is the ability to self-assemble into micelles in aqueous solutions (40). The non-covalent incorporation of drugs into the hydrophobic PPO core of the Pluronic® micelle increases 1) drug solubility, 2) drug metabolic stability, and 3) drug circulation time in the body. Furthermore, Pluronic® unimers can also act as biological response modifiers: they can 1) bind with the cell membranes, 2) alter membrane microviscosity, and 3) inhibit selected membrane proteins, notably Pgp (1, 7, 43) . This results in increased delivery of Pgp-dependent drugs in MDR tumors, as well as transport of such drugs across the BBB and intestinal epithelium, which are known to overexpress Pgp (3, 7, 9, 10, 44). Most recently, Pluronic® block copolymers were shown to increase gene expression in stably and transiently transfected cells by activating pro-inflammatory (NFκB) cell signaling (12, 14) and to enhance drug-induced apoptosis in MDR cells (45). These multiple effects may result from interaction of block copolymers with the cellular membranes. Furthermore, the profound inhibition of Pgp in MDR cells (10) and, possibly, enhanced pro-apoptotic signaling in MDR cells (paper in preparation) appear to be related to the ability of Pluronic® to transport inside the cells and interact with the mitochondrial membranes. Therefore, understanding of interaction of Pluronic® block copolymer with cell membranes and the transport of these copolymers inside the cells is critically important for understanding their diverse biological response modifying activities. Furthermore, since Pluronic® can exist in the form of 1) single chains (unimers), which exhibit the biological responses in cells, and 2) micelles, which can carry solubilized drugs inside the cells, it is essential to examine trafficking of these different aggregate forms of the copolymer. This study addresses such need by evaluating the early stages of entry of Pluronic® into cells.
Given a relative simplicity of the Pluronic® block copolymer structure and apparent lack of sites for binding with specific cellular trafficking molecules one could expect that the membrane interaction and cellular entry of this copolymer is quite unspecific. Surprisingly, to the contrary we demonstrate that Pluronic® unimers appear to co-localize with markers of caveolae and enter cells rather selectively via caveolae-mediated endocytosis. Caveolae are subsets of specific cellular domains, often known as “lipid rafts”, with which both the CTB and caveolin-1 are associated (46). Although, this result appears to be paradoxical at first glance its origin may be characteristic of interactions and self-assembly in polymeric systems. Such systems are known to exhibit highly cooperative behavior. For example, polyelectrolytes interacting with oppositely charged macromolecular species (other polyions, particles, etc.) can precisely “recognize” and “select” between different types of such species in the mixture (47). A recent example of this phenomenon is DNA topology recognition by synthetic polycations (48) . This selectivity rises from small differences in the binding energy between polymers counted on a per-unit base, which is amplified on a scale of the entire polymer molecule. It is therefore, possible, that PPO blocks in Pluronic® molecules may similarly recognize the cholesterol-rich micro-domains in the cell membranes. To the best of our knowledge this study is the first demonstration that amphiphilic block copolymers can interact selectively with membrane domains. One necessary factor for molecular recognition in polymeric systems is the reversibility of the binding and/or possibility of exchange between the bound polymeric species, so that the polymer can find the optimal partner by multiple trials (13). Such reversibility of binding with cellular membranes was previously shown for Pluronic® molecules (7).
As the copolymer concentration in the media increases the copolymer unimers self assemble into polymeric micelles. In particular P85 micelles are spherical core-shell structures of approximately 14.6 nm in diameter (33). They are composed of approximately 60 P85 molecules and have a PPO core of 5.4 nm in diameter surrounded by 3.7 nm PEO shell. The exterior surface of such micelles is hydrophilic and is unlikely to bind with the lipid portions of the cell membranes. For example, lack of PEO interactions with lipid domains in cell membranes was apparent in studies using hydrophobic fluorescent probes embedded in the membranes (9). However, the same study also suggested that copolymers with high PEO content may adhere to the cellular surface and limit the lateral mobility of membranes lipids. It is not surprising, therefore, that micelles have different pathway of cellular uptake compared to unimers. Indeed it was previously shown that drug solubilized in the micelles is transported into cells through a vesicular pathway rather than through trans-membrane diffusion (49). Furthermore, micelles can be conjugated with ligands, such as insulin and thus directed into cells via receptor mediated endocytosis (40, 50). In this study, however, for the first time we can suggest with high likelihood that P85 micelles enter into cells via clathrin-mediated endocytosis.
The reasons for the apparent selectivity of the micelles with respect to the clathrin-mediated pathway as opposed to the caveolae-mediated pathway may lie in the ability of the block copolymer at relatively high concentration to inhibit caveolae-mediated endocytosis, which was also demonstrated here for the first time. The inhibition of the uptake of the fluid-phase endocytosis marker, HRP and caveolae-mediated endocytosis marker, CTB, was observed already at 0.01% P85, which is below the CMC. (Although the mechanism of the fluid-phase endocytosis is still unclear it was shown to be non-clathrin mediated (51), and could be mediated through caveolae (manuscript in preparation)). All together, this indicates that the Pluronic® unimers can somehow perturb the caveolae structure, with which they bind, possibly, by changing membrane microviscosity (7) or membrane curvature due to incorporation of bulky hydrophobic PPO chains in the membranes. Notably, the inhibition of caveolae cannot be linked to depletion of cellular ATP because at these concentrations no ATP depletion in MDCK cells is observed (44). (It is unlikely that P85 interacts directly with endocytic markers (HRP, CTB and Tf) since first, P85 copolymer has neutral charge and second, all markers are hydrophilic in nature.) At higher concentrations of the copolymer, above the CMC, the unimers co-exist with the micelles, and thus can inhibit the micelle transport through the caveolae pathway. Furthermore, as the concentration of P85 increases above the CMC even more copolymer can bind with membranes and further contribute to the disruption of caveolae. Under these conditions the entry of the copolymer micelles can still proceed through the clathrin-mediated pathway, which is not inhibited. In addition, although there is no direct evidence of a specific receptor for micelles at clathrin microdomains one cannot exclude such possibility. Moreover, non-specific interaction of the micelle PEO corona with these domains is also possible. As already mentioned, despite of the fact that PEO is often considered inert some surface interactions with cell membranes have been suggested (9). This may include one or combination of hydrophobic and hydrogen binding interactions, for example, with membrane proteins. Interestingly, several studies suggest that either PEO alone or PEO-covered nanoparticles may induce complement activation (52). The structural similarity between the terminal region of the PEO chain and carbohydrates has also been noted, which may result in PEO binding with lectins (53) as well as carbohydrate receptors, which are endocytosed through clathrin-mediated route (54).
Finally, selective interactions of P85 unimers via PPO blocks with caveolae may be also related to the copolymer's functional activities in cells. The ability of Pluronic® to decrease micro-viscosity of some rigid membrane structures (membrane fluidization) was previously shown (7). It is possible, that these structures are the same as or similar to the cholesterol-rich lipid rafts/caveolae. Furthermore, there are indications in literature that Pgp, also affected by Pluronic® block copolymer, can co-localize with the lipid rafts/caveolae (55). Thus, the effects of Pluronic® block copolymers on Pgp and caveolae-mediated endocytosis may be related to each other. We plan to determine this in our subsequent studies. In addition, one needs to take into account that caveolae regulate cellular signaling of cholesterol, as well as activity of various ion channels. It is known that one side effect of overexposure to Pluronic® block copolymers is dose-dependent hyperlipidemia, in particular, rise in the cholesterol and triglyceride levels in the plasma (56). This can be due to impairment of cholesterol trafficking and metabolism resulting from inhibition of the caveolae-mediated endocytosis. Notably, caveolae are known to cluster multiple receptors, such as EGF, CCK, Endothelin, Tyrosine Kinases etc., which enable signal integration and amplification (13, 57). They also host signal transducers, for example, PKC, MAPK, eNOS and calmodulin, transporters (e.g. IP3 receptor and Porin) as well as structural molecules (e.g. actin and myosin). Therefore, detailed characterization of interactions of Pluronic® block copolymers with cell membranes is likely to provide foundation for future understanding of the diverse biological response modifying activities of these copolymers, including alteration of cell signaling, activation of transcription and other processes.
Acknowledgements
This study was supported by National Institutes of Health grants RO1 NS36229, 2RO1 CA89225, RO1 NS051334 awarded to (AVK). We thank Dr. Srikanth Sriadibhatala for assistance in synthesizing FITC labeled P85. We would also like to thank the flow cytometery and confocal microscopy core facilities at UNMC.
References
- 1.Kabanov AV, Alakhov VY. Pluronic block copolymers in drug delivery: from micellar nanocontainers to biological response modifiers. Crit Rev Ther Drug Carrier Syst. 2002;19:1–72. doi: 10.1615/critrevtherdrugcarriersyst.v19.i1.10. [DOI] [PubMed] [Google Scholar]
- 2.Kabanov AV, Batrakova EV, Alakhov VY. Pluronic block copolymers as novel polymer therapeutics for drug and gene delivery. J Control Release. 2002;82:189–212. doi: 10.1016/s0168-3659(02)00009-3. [DOI] [PubMed] [Google Scholar]
- 3.Batrakova EV, Li S, Miller DW, Kabanov AV. Pluronic P85 increases permeability of a broad spectrum of drugs in polarized BBMEC and Caco-2 cell monolayers. Pharm Res. 1999;16:1366–72. doi: 10.1023/a:1018990706838. [DOI] [PubMed] [Google Scholar]
- 4.Kabanov AV, Slepnev VI, Kuznetsova LE, Batrakova EV, Alakhov V, Melik-Nubarov NS, Sveshnikov PG, Kabanov VA. Pluronic micelles as a tool for low-molecular compound vector delivery into a cell: effect of Staphylococcus aureus enterotoxin B on cell loading with micelle incorporated fluorescent dye. Biochem Int. 1992;26:1035–42. [PubMed] [Google Scholar]
- 5.Chekhonin VP, Kabanov AV, Zhirkov YA, Morozov GV. Fatty acid acylated Fab-fragments of antibodies to neurospecific proteins as carriers for neuroleptic targeted delivery in brain. FEBS Lett. 1991;287:149–52. doi: 10.1016/0014-5793(91)80037-4. [DOI] [PubMed] [Google Scholar]
- 6.Alakhov V, Klinski E, Lemieux P, Pietrzynski G, Kabanov A. Block copolymeric biotransport carriers as versatile vehicles for drug delivery. Expert Opin Biol Ther. 2001;1:583–602. doi: 10.1517/14712598.1.4.583. [DOI] [PubMed] [Google Scholar]
- 7.Batrakova EV, Li S, Vinogradov SV, Alakhov VY, Miller DW, Kabanov AV. Mechanism of pluronic effect on P-glycoprotein efflux system in blood-brain barrier: contributions of energy depletion and membrane fluidization. J Pharmacol Exp Ther. 2001;299:483–93. [PubMed] [Google Scholar]
- 8.Kabanov AV, Lemieux P, Vinogradov S, Alakhov V. Pluronic block copolymers: novel functional molecules for gene therapy. Adv Drug Deliv Rev. 2002;54:223–33. doi: 10.1016/s0169-409x(02)00018-2. [DOI] [PubMed] [Google Scholar]
- 9.Batrakova EV, Li S, Alakhov VY, Miller DW, Kabanov AV. Optimal structure requirements for pluronic block copolymers in modifying Pglycoprotein drug efflux transporter activity in bovine brain microvessel endothelial cells. J Pharmacol Exp Ther. 2003;304:845–54. doi: 10.1124/jpet.102.043307. [DOI] [PubMed] [Google Scholar]
- 10.Batrakova EV, Li S, Elmquist WF, Miller DW, Alakhov VY, Kabanov AV. Mechanism of sensitization of MDR cancer cells by Pluronic block copolymers: Selective energy depletion. Br J Cancer. 2001;85:1987–97. doi: 10.1054/bjoc.2001.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Batrakova EV, Li S, Li Y, Alakhov VY, Kabanov AV. Effect of pluronic P85 on ATPase activity of drug efflux transporters. Pharm Res. 2004;21:2226–33. doi: 10.1007/s11095-004-7675-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Z, Zhu J, Sriadibhatla S, Gebhart C, Alakhov V, Kabanov A. Promoter- and strain-selective enhancement of gene expression in a mouse skeletal muscle by a polymer excipient Pluronic P85. J Control Release. 2005;108:496–512. doi: 10.1016/j.jconrel.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 13.Kabanov AV. Polymer genomics: an insight into pharmacology and toxicology of nanomedicines. Adv Drug Deliv Rev. 2006;58:1597–621. doi: 10.1016/j.addr.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sriadibhatla S, Yang Z, Gebhart C, Alakhov VY, Kabanov A. Transcriptional activation of gene expression by pluronic block copolymers in stably and transiently transfected cells. Mol Ther. 2006;13:804–13. doi: 10.1016/j.ymthe.2005.07.701. [DOI] [PubMed] [Google Scholar]
- 15.Batrakova EV, Vinogradov SV, Robinson SM, Niehoff ML, Banks WA, Kabanov AV. Polypeptide point modifications with fatty acid and amphiphilic block copolymers for enhanced brain delivery. Bioconjug Chem. 2005;16:793–802. doi: 10.1021/bc049730c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 17.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 18.Carver LA, Schnitzer JE. Caveolae: mining little caves for new cancer targets. Nat Rev Cancer. 2003;3:571–81. doi: 10.1038/nrc1146. [DOI] [PubMed] [Google Scholar]
- 19.Rothberg KG, Heuser JE, Donzell WC, Ying Y-S, Glenney JR, Anderson RGW. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- 20.Nichols B. Caveosomes and endocytosis of lipid rafts. J Cell Sci. 2003;116:4707–4714. doi: 10.1242/jcs.00840. [DOI] [PubMed] [Google Scholar]
- 21.Nabi IR, Le PU. Caveolae/raft-dependent endocytosis. J. Cell Biol. 2003;161:673–677. doi: 10.1083/jcb.200302028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8:603–612. doi: 10.1038/nrm2216. [DOI] [PubMed] [Google Scholar]
- 23.Kabanov AV, Levashov AV, Alakhov V. Lipid modification of proteins and their membrane transport. Protein Eng. 1989;3:39–42. doi: 10.1093/protein/3.1.39. [DOI] [PubMed] [Google Scholar]
- 24.Kabanov AV, Levashov AV, Alakhov V, Martinek K, Severin ES. Fatty acylation of proteins for translocation across cell membranes. Biomed Sci. 1990;1:33–6. [PubMed] [Google Scholar]
- 25.Barakat S, Demeule M, Pilorget A, Regina A, Gingras D, Baggetto LG, Beliveau R. Modulation of p-glycoprotein function by caveolin-1 phosphorylation. Journal of Neurochemistry. 2007;101:1–8. doi: 10.1111/j.1471-4159.2006.04410.x. [DOI] [PubMed] [Google Scholar]
- 26.Batrakova EV, Miller DW, Li S, Alakhov VY, Kabanov AV, Elmquist WF. Pluronic P85 enhances the delivery of digoxin to the brain: in vitro and in vivo studies. J Pharmacol Exp Ther. 2001;296:551–7. [PubMed] [Google Scholar]
- 27.Herzlinger DA, Easton TG, Ojakian GK. The MDCK epithelial cell line expresses a cell surface antigen of the kidney distal tubule. J. Cell Biol. 1982;93:269–277. doi: 10.1083/jcb.93.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hailstones D, Sleer LS, Parton RG, Stanley KK. Regulation of caveolin and caveolae by cholesterol in MDCK cells. J. Lipid Res. 1998;39:369–379. [PubMed] [Google Scholar]
- 29.Self TJ, Oakley SM, Hill SJ. Clathrin-independent internalization of the human histamine H1-receptor in CHO-K1 cells. Br J Pharmacol. 2005;146:612–624. doi: 10.1038/sj.bjp.0706337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heuser JE, Anderson RG. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J. Cell Biol. 1989;108:389–400. doi: 10.1083/jcb.108.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakase I, Niwa M, Takeuchi T, Sonomura K, Kawabata N, Koike Y, Takehashi M, Tanaka S, Ueda K, Simpson JC, Jones AT, Sugiura Y, Futaki S. Cellular Uptake of Arginine-Rich Peptides: Roles for Macropinocytosis and Actin Rearrangement. Mol Ther. 2004;10:1011–1022. doi: 10.1016/j.ymthe.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 32.Jones AT. Macropinocytosis: searching for an endocytic identity and role in the uptake of cell penetrating peptides. Journal of Cellular and Molecular Medicine. 2007;11:670–684. doi: 10.1111/j.1582-4934.2007.00062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kabanov AV, Nazarova IR, Astafieva IV, Batrakova EV, Alakhov VY, Yaroslavov AA, Kabanov VA. Micelle formation and solubilization of fluorescent probes in poly(oxyethylene-b-oxypropylene-b-oxyethylene) solutions. Macromolecules. 1995:2303–2314. [Google Scholar]
- 34.Ivanov AI. Pharmacological Inhibition of Endocytic Pathways: Is It Specific Enough to Be Useful? Exocytosis and Endocytosis. 2008:15–33. doi: 10.1007/978-1-59745-178-9_2. [DOI] [PubMed] [Google Scholar]
- 35.Rodal SK, Skretting G, Garred O, Vilhardt F, van Deurs B, Sandvig K. Extraction of cholesterol with methyl-beta-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol Biol Cell. 1999;10:961–74. doi: 10.1091/mbc.10.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Subtil A, Gaidarov I, Kobylarz K, Lampson MA, Keen JH, McGraw TE. Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc Natl Acad Sci U S A. 1999;96:6775–80. doi: 10.1073/pnas.96.12.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duncan R. Polymer conjugates as anticancer nanomedicines. Nat Rev Cancer. 2006;6:688–701. doi: 10.1038/nrc1958. [DOI] [PubMed] [Google Scholar]
- 38.Torchilin VP. Polymeric contrast agents for medical imaging. Curr Pharm Biotechnol. 2000;1:183–215. doi: 10.2174/1389201003378960. [DOI] [PubMed] [Google Scholar]
- 39.Kopecek J. Polymer chemistry: swell gels. Nature. 2002;417:388–9. doi: 10.1038/417388a. [DOI] [PubMed] [Google Scholar]
- 40.Batrakova EV, Han HY, Miller DW, Kabanov AV. Effects of pluronic P85 unimers and micelles on drug permeability in polarized BBMEC and Caco-2 cells. Pharm Res. 1998;15:1525–32. doi: 10.1023/a:1011942814300. [DOI] [PubMed] [Google Scholar]
- 41.Lemieux P, Guerin N, Paradis G, Proulx R, Chistyakova L, Kabanov A, Alakhov V. A combination of poloxamers increases gene expression of plasmid DNA in skeletal muscle. Gene Ther. 2000;7:986–91. doi: 10.1038/sj.gt.3301189. [DOI] [PubMed] [Google Scholar]
- 42.Oh KT, Bronich TK, Kabanov AV. Micellar formulations for drug delivery based on mixtures of hydrophobic and hydrophilic Pluronic block copolymers. J Control Release. 2004;94:411–22. doi: 10.1016/j.jconrel.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 43.Kabanov AV, Batrakova EV, Miller DW. Pluronic block copolymers as modulators of drug efflux transporter activity in the blood-brain barrier. Adv Drug Deliv Rev. 2003;55:151–64. doi: 10.1016/s0169-409x(02)00176-x. [DOI] [PubMed] [Google Scholar]
- 44.Batrakova EV, Li S, Alakhov VY, Elmquist WF, Miller DW, Kabanov AV. Sensitization of cells overexpressing multidrug-resistant proteins by pluronic P85. Pharm Res. 2003;20:1581–90. doi: 10.1023/a:1026179132599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Minko T, Batrakova EV, Li S, Li Y, Pakunlu RI, Alakhov VY, Kabanov AV. Pluronic block copolymers alter apoptotic signal transduction of doxorubicin in drug-resistant cancer cells. J Control Release. 2005;105:269–78. doi: 10.1016/j.jconrel.2005.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 47.Kabanov AV, Kabanov VA. Interpolyelectrolyte and block ionomer complexes for gene delivery: physico-chemical aspects. Advanced Drug Delivery Reviews. 1998;30:49–60. doi: 10.1016/s0169-409x(97)00106-3. [DOI] [PubMed] [Google Scholar]
- 48.Bronich TK, Nguyen HK, Eisenberg A, Kabanov AV. Recognition of DNA Topology in Reactions between Plasmid DNA and Cationic Copolymers. J. Am. Chem. Soc. 2000;122:8339–8343. [Google Scholar]
- 49.Miller DW, Batrakova EV, Waltner TO, Alakhov V, Kabanov AV. Interactions of pluronic block copolymers with brain microvessel endothelial cells: evidence of two potential pathways for drug absorption. Bioconjug Chem. 1997;8:649–57. doi: 10.1021/bc970118d. [DOI] [PubMed] [Google Scholar]
- 50.Batrakova EV, Han HY, Alakhov V, Miller DW, Kabanov AV. Effects of pluronic block copolymers on drug absorption in Caco-2 cell monolayers. Pharm Res. 1998;15:850–5. doi: 10.1023/a:1011964213024. [DOI] [PubMed] [Google Scholar]
- 51.Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J. Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O'Neil CP, Lee LK, Swartz MA, Hubbell JA. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotech. 2007;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 53.Moghimi SM, Hunter AC, Dadswell CM, Savay S, Alving CR, Szebeni J. Causative factors behind poloxamer 188 (Pluronic F68, Flocor(TM))-induced complement activation in human sera: A protective role against poloxamer-mediated complement activation by elevated serum lipoprotein levels. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2004;1689:103–113. doi: 10.1016/j.bbadis.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 54.Klink D, Yu Q-C, Glick MC, Scanlin T. Lactosylated Poly-L-Lysine Targets a Potential Lactose Receptor in Cystic Fibrosis and Non-Cystic Fibrosis Airway Epithelial Cells. Mol Ther. 2003;7:73–80. doi: 10.1016/s1525-0016(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 55.Jodoin J, Demeule M, Fenart L, Cecchelli R. o., Farmer S, Linton KJ, Higgins CF, Béliveau R,R. P-glycoprotein in blood brain barrier endothelial cells: interaction and oligomerization with caveolins. Journal of Neurochemistry. 2003;87:1010. doi: 10.1046/j.1471-4159.2003.02081.x. [DOI] [PubMed] [Google Scholar]
- 56.Palmer WK, Emeson EE, Johnston TP. Poloxamer 407-induced atherogenesis in the C57BL/6 mouse. Atherosclerosis. 1998;136:115–123. doi: 10.1016/s0021-9150(97)00193-7. [DOI] [PubMed] [Google Scholar]
- 57.Anderson RGW. THE CAVEOLAE MEMBRANE SYSTEM. Annual Review of Biochemistry. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]