Abstract
The von Hippel-Lindau tumor suppressor pVHL regulates the stability of Hypoxia-Inducible Factors (HIF) -1 and –2, oxygen-sensitive basic helix-loop-helix transcription factors, which mediate the hypoxic induction of angiogenic growth factors such as vascular endothelial growth factor (VEGF). Loss of VHL function results in constitutive activation of HIF-1 and HIF-2 and is associated with the development of highly vascularized tumors in multiple organs. We have used a conditional gene targeting approach to investigate the relative contributions of HIF-1 and HIF-2 to VHL-associated vascular tumorigenesis in a mouse model of liver hemangiomas. Here we demonstrate genetically that conditional inactivation of HIF-2α suppressed the development of VHL-associated liver hemangiomas and that angiogenic gene expression in hepatocytes is predominantly regulated by HIF-2 and not by HIF-1. These findings suggest that HIF-2 is the dominant HIF in the pathogenesis of VHL-associated vascular tumors and that pharmacologic targeting of HIF-2 may be an effective strategy for their treatment.
Patients with germ-line mutations in the von Hippel-Lindau tumor suppressor, pVHL, develop a familial tumor syndrome characterized by the development of highly vascularized tumors. The most common clinical manifestation of VHL disease are hemangioblastomas, which typically develop in the CNS and retina, but can also manifest in other organs such as the liver (Maher & Kaelin, 1997; McGrath et al., 1992). Although not malignant, hemangioblastomas are clinically devastating vascular tumors and consist of pericytes, endothelial, stromal, and mast cells. They are thought to originate from pVHL-deficient stromal cells, which express high levels of vascular endothelial growth factor (VEGF) and the transcription factor HIF-2α (Flamme et al., 1998; Vortmeyer et al., 1997) resulting in the proliferation of neighboring endothelial cells. pVHL is the substrate recognition component of an E3-ubiquitin ligase, which targets the oxygen-sensitive α-subunit of Hypoxia-Inducible Factors (HIF) for ubiquitination and proteasomal degradation under normoxia and thus plays a critical role in the regulation of molecular oxygen sensing. Loss of pVHL function results in constitutive activation of HIF-1 and HIF-2, whose individual contributions to VHL-associated tumorigenesis are currently under intense investigation (Kapitsinou & Haase, 2008).
VHL-associated vascular tumorigenesis can be modeled in mice by conditional inactivation of Vhlh (murine VHL) in hepatocytes, resulting in constitutive activation of Hif-1 and Hif-2, and the development of cavernous liver hemangiomas (Haase et al., 2001; Rankin et al., 2005). Although VHL-associated liver hemangiomas and CNS hemangioblastomas are distinct with regard to their cellular origin, they share common histological features, such as endothelial cell proliferation, angiectasis and lipid droplet accumulation in neoplastic cells (Haase et al., 2001). We have used this mouse model as a genetic tool to dissect the relative contributions of individual HIF transcription factors to VHL-associated vascular tumor development. We have previously shown that liver hemangioma formation does not require HIF-1α, but is dependent on the HIF-β subunit, also known as the Arylhydrocarbon receptor nuclear translocator (ARNT), suggesting that HIF-2α may play an essential role in VHL-associated vascular tumorigenesis (Rankin et al., 2005).
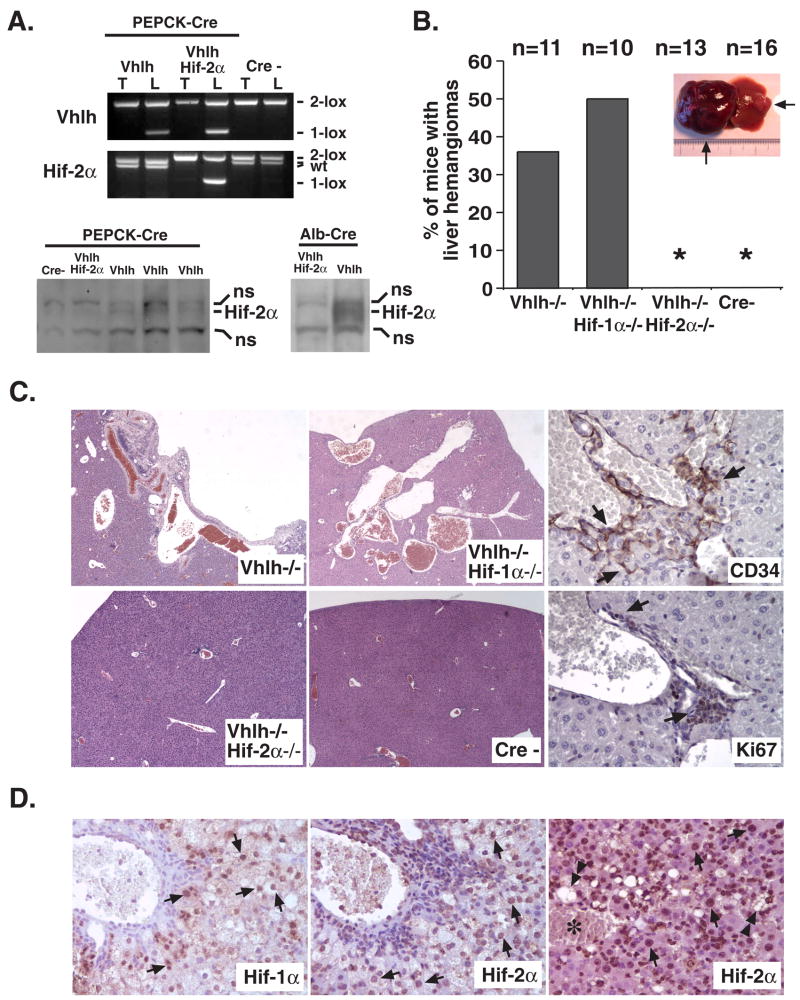
To investigate the role of HIF-2 in this process, we have used Cre-loxP recombination based gene targeting to inactivate Hif-2α in Vhlh-deficient mice that are prone to the development of cavernous liver hemangiomas (Rankin et al., 2005). In this model, Vhlh is targeted in approximately 20 to 30% of hepatocytes by Cre-recombinase under control of the phosphoenolpyruvate carboxykinase (PEPCK) promoter (Rankin et al., 2005). Recombination of Vhlh and Hif-2α conditional alleles occurred with similar efficiency in PEPCK-Cre expressing mice that were homozygous for both alleles, which from hereon are referred to as PEPCK-Vhlh/Hif-2α mutants (Figure 1A).
Figure 1. Inactivation of Hif-2α suppresses vascular tumor development in Vhlh mutant livers.
(A) Upper panel: Genotype analysis of PEPCK-Cre mutant mice by PCR. Mutant mice were generated in a mixed genetic background (Balb/C, 129Sv/J, C57BL/6) and littermates not carrying the Cre-transgene were used as controls. Due to mixed genetic backgrounds, two different Hif-2α wild type alleles were detected in Vhlh mutants, representing a polymorphism in the Hif-2α allele. Note that PEPCK-Cre efficiently recombines both the Vhlh and Hif-2α conditional alleles in mice homozygous for the Vhlh and Hif-2α conditional alleles (lanes 3 and 4). Abb.: 2-lox, non-recombined allele; 1-lox, recombined allele, wt, wild type allele; T, tail; L, liver. Generation and analysis of the Vhlh, Hif-1α, Hif-2α, and Arnt conditional alleles, Albumin-Cre and PEPCK-Cre transgenes was performed as described (Gruber et al., 2007; Rankin et al., 2007; Rankin et al., 2005). Lower panel: Hif-2α protein levels in Vhlh mutant mice. Western blot analysis of nuclear protein extracts isolated from the livers of PEPCK-Vhlh, -Vhlh/Hif-2α, and Cre-negative mice. Albumin-Vhlh and -Vhlh/Hif-2α protein extracts are shown as positive control for Hif-2α expression and to demonstrate that Hif-2α is efficiently deleted in hepatocytes (absence of Hif-2α in Vhlh mutants). Please note that only up to 20–30% of hepatocytes undergo recombination in PEPCK-Cre mutants compared to >90% of hepatocytes in Albumin-Cre mutants. This results in only a minor increase in Hif-2α protein levels in PEPCK-Vhlh mutants compared to Cre-negative controls. DNA and protein extracts were prepared and analyzed as previously described (Rankin et al., 2005). Abb.: ns, non-specific band. (B) Incidence of macroscopic liver hemangiomas detected in PEPCK-Vhlh, -Vhlh/Hif-1α, -Vhlh/Hif-2α and Cre-negative (Cre-) mice (>6 months of age). Picture shows examples of a large and small hemangioma located in the left and right lobes of a PEPCK-Vhlh mutant liver. (C) Microscopic appearance of VHL-associated hepatic vascular lesions. Shown are H&E stains of PEPCK-Cre mutant livers with the indicated genotypes (magnification ×100). Right panel shows immunohistochemical staining of a PEPCK-Vhlh hepatic vascular lesion for the endothelial marker CD34 and proliferation marker Ki67 (magnification ×400). Arrows indicate positive cells. (D) Immunohistochemical analysis of Hif-1α and Hif-2α expression in VHL-associated vascular lesions. Arrows point to hepatocytes with positive nuclear staining for Hif-α subunits. Note that the pattern of Hif-1α and Hif-2α staining is similar in Vhlh-deficient livers. Nuclear Hif-2α staining was also detected in clusters of non-hepatocyte cells that are associated with vascular lesions and contain Ki67- and CD34-positive cells (Figure 1C). Double-headed arrows depict Hif-2α positive cells, which contain macrovesicular lipid droplets; asterisk indicates a blood filled vascular cavity (magnification ×400). Anti-Hif-1α and anti-Hif-2α rabbit polyclonal antiserum (Novus) was used for the immunohistochemical detection of Hif-α subunits in paraffin-embedded liver sections employing a horseradish peroxidase-based high-amplification signal detection system (CSA, DAKO) with 3,3′-diaminobenzidine as enzymatic substrate.
Cavernous hemangiomas were observed in ~35% of PEPCK-Vhlh mutants 6 months of age or older (4/11), while ~80% of mutants developed microscopic vascular lesions (9/11), which were characterized by angiectasis, proliferating endothelial cells and the presence of lipid droplet containing hepatocytes (Figure 1B and C, and Rankin et al., 2005). Similarly, 5 of 10 PEPCK-Vhlh/Hif-1α mutant mice developed cavernous hemangiomas and 7 of 10 developed microscopic vascular lesions that were histologically identical to those in PEPCK-Vhlh mutant mice (Figure 1B and C). In contrast, PEPCK-Vhlh/Hif-2α double mutant livers were microscopically and macroscopically similar to control mice with the exception of one case (1/13) in which a small area of angiectasis was detected by macroscopic inspection (Figure 1B and C). Strikingly, however, cavernous hemangiomas were not observed in PEPCK-Vhlh/Hif-2α mutants at the ages of 8 months or older (Figure 1B). These results indicate that Hif-2 is required for the development of VHL-associated vascular tumors in the liver. Immunohistochemical analysis of Hif-α expression revealed that both Hif-1α and Hif-2α were detectable in hepatocytes that neighbored vascular lesions, suggesting a paracrine mechanism by which a HIF-induced excessive production of hepatocyte-derived angiogenic growth factors results in endothelial cell proliferation and vascular tumor development (PEPCK-Cre targets hepatocytes and not endothelial cells, Figure 1D). This is mechanistically consistent with hemangioblastoma development in the CNS, where pVHL-deficient stromal cells are the source of HIF-2 induced vascular growth factor production. Interestingly, the absence of Hif-1α did not change the histological appearance of vascular networks and lesions, nor did it affect lipid droplet accumulation in hepatocytes (Figure 1B and C), suggesting that Hif-1 is dispensable for hemangioma development in this model. Aside from its role in the pathogenesis of VHL-associated hemangiomas and hemangioblastomas, HIF-2 expression has been associated with poor clinical outcome in a variety of other tumor types, which include renal cell and hepatocellular cancer, melanoma and neuroblastoma (Holmquist-Mengelbier et al., 2006; Rankin & Giaccia, 2008). These observations, together with our findings suggest that pharmacological targeting of HIF-2 rather than HIF-1 may be a more effective strategy in the treatment of certain tumor types.
Since the development of VHL tumors is associated with increased angiogenic growth factor production, in particular VEGF (Flamme et al., 1998), we examined the role of Hif-2 in the regulation of Vegf in Vhlh knockout livers. Real time PCR analysis demonstrated that inactivation of Hif-2, but not Hif-1, was sufficient to suppress Vegf despite increased Hif-1 transcriptional activity, suggesting that Hif-2 is the dominant HIF for the regulation of hepatic Vegf (Figure 2A). We next sought to identify and characterize additional changes in angiogenic gene expression by cDNA microarray analysis. Due to limited Cre-recombinase expression in PEPCK-Cre livers, we utilized Albumin-Cre transgenic mice to inactivate Vhlh, Hif-1α, and Hif-2α in a larger percentage (>90%) of hepatocytes. Genes that were differentially expressed (> 1.5 fold) between Vhlh/Hif-1α/Hif-2α (mice that lack both Hif-1 and Hif-2) and Vhlh/Hif-1α or Vhlh/Hif-2α mutant livers were sorted according to functional categories using the NIH David gene ontology program. Using this approach, we identified 17 additional HIF-regulated genes that are involved in blood vessel morphogenesis and development, angiogenesis, and vasculature development, 12 of which were up-regulated in Vhlh or Vhlh/Hif-1α mutant livers (Figure 2B). Using real time PCR analysis, we determined that inactivation of Hif-2α suppressed the majority of up-regulated genes (12 of 13) more efficiently (> 75% reduction) than Hif-1α inactivation, suggesting Hif-2 dominance in the transcriptional regulation of angiogenic genes (Figure 2B). Among the genes identified was Hey1, a transcriptional target of Notch, which is involved in cardiovascular development (Figure 2C, (Fischer et al., 2004)). Recent reports demonstrated that Notch signaling is enhanced during hypoxia through functional interaction of HIF-1α with the Notch intracellular domain, providing a possible explanation for increased Notch target gene expression under conditions of HIF activation (Gustafsson et al., 2005). Other genes induced in a Hif-2 dependent manner included Bmp4, a TGF-β super family member previously shown to regulate tumor angiogenesis (Figure 2C, (Rothhammer et al., 2007)), Klf5, Nr2f2, Angpt13, Anxa2, and Cdh5 (Figure 2C). Whether increased expression of these genes resulted from direct transcriptional regulation by Hif-2 or was a consequence of increased angiogenesis is unclear and requires further analysis.
Figure 2. Hif-2α preferentially regulates angiogenic gene expression in Vhlh-deficient hepatocytes.
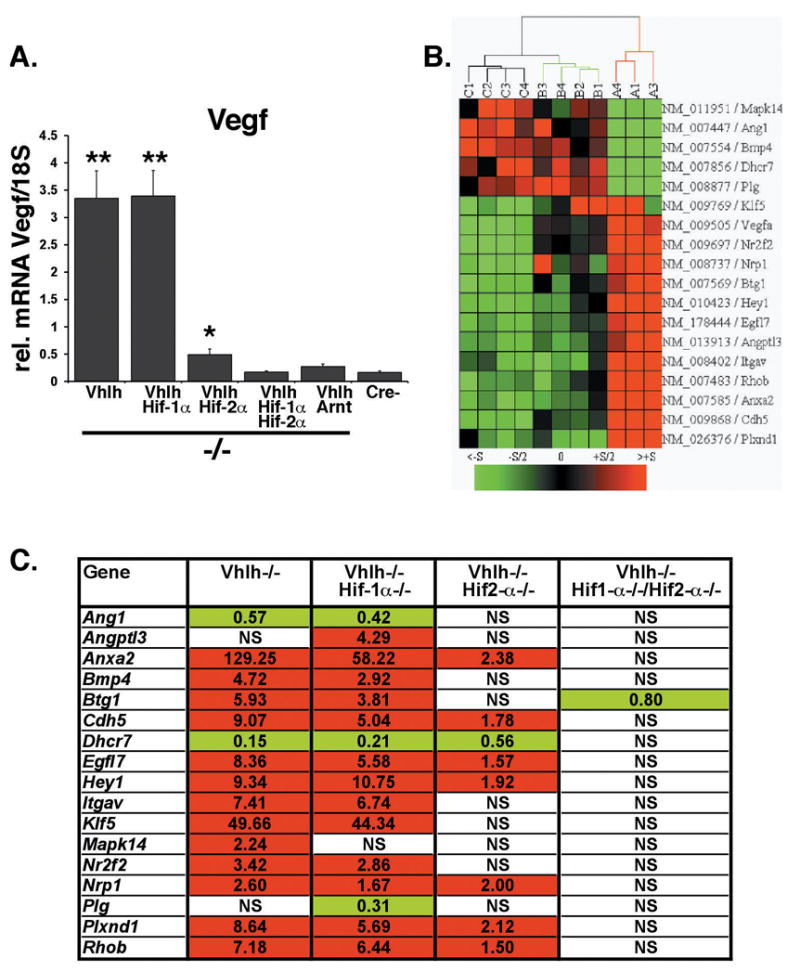
(A) Real time PCR analysis of Vegf mRNA levels in Albumin-Cre mutant livers. Columns represent the average mRNA transcript level of three mice for the Albumin-Vhlh/Hif-1α/Hif-2α group and four mice for all other groups. Error bars represent S.E.M. Asterisks represent a significant increase in gene expression compared to Cre-negative mice as determined by Student’s t-test (*, p<0.05; **, p<0.001). Primers used for real-time analysis are provided in supplementary Figure. (B) Hierarchical cluster analysis of angiogenic gene expression profiles in Albumin-Cre mutant livers. Microarray expression profiling was performed on PancChip version 6.0, a two color microarray developed by the Penn Diabetes and Endocrinology Research Center Functional Genomics Core (White et al., 2005). When compared to Vhlh/Hif-1α/Hif-2α mutant livers 1123 and 197 cDNAs were significantly changed in Vhlh/Hif-1α and Vhlh/Hif-2α livers respectively. Genes were grouped into functional gene ontology categories using NIH DAVID. Genes@Work (IBM) was used to generate a heat map depicting gene expression levels in Albumin-Vhlh/Hif-1α/Hif-2α (Group C, n=4), Albumin-Vhlh/Hif-1α (Group A, n=3), and Albumin-Vhlh/Hif-2α (Group B, n=4) mutant livers compared to a common reference RNA sample. Red and green boxes represent an increase and decrease in fold expression respectively. Each column represents an individual liver sample. Genes were clustered using Euclidian average linkage clustering. (C) Validation of microarray gene expression by real time PCR analysis. Shown are fold-changes in gene expression for the indicated genotype (n=4) compared to Cre-negative littermate livers (n=4); red and green boxes indicate an increase and decrease in mRNA levels respectively. mRNA levels were normalized to 18S. Abb.: NS, absence of statistically significant differences between mutant and control livers.
The expression of other HIF-2 targets that are commonly associated with pVHL-defective tumors, such as cyclin D1 (CCND1) in renal cancer cells (Bindra et al., 2002; Raval et al., 2005; Zatyka et al., 2002), did not change in the liver (data not shown), indicating that HIF-2 mediated induction of gene expression is tissue- and context-dependent. Although a specific polymorphism in the CCND1 gene was found to be associated with increased susceptibility to retinal and CNS hemangioblastomas in one study (Zatyka et al., 2002), no clear correlation between CCND1 genotype and expression was found in another clinical report (Gijtenbeek et al., 2005).
Collectively, our findings demonstrate that HIF-2 is the dominant HIF for the regulation of VEGF and other angiogenic factors in hepatocytes. A role for HIF-2 in the regulation of VEGF has recently been observed in human renal cell cancer and neuroblastoma cell lines, while in other cell types such as thymocytes and astrocytes Vegf expression was found to be Hif-1 dependent (Biju et al., 2004; Chavez et al., 2006; Holmquist-Mengelbier et al., 2006). Taken together these data suggest that both HIF-1 and HIF-2 have the ability to activate VEGF, however, they appear to preferentially regulate its expression within individual cell types. This may be attributable to different HIF-1 and HIF-2 expression levels in individual cell types or may be due to the presence of cell-specific transcriptional co-factors that modulate HIF activity. In the context of human VHL-associated hemangioblastomas, high levels of HIF-2 are associated with increased VEGF mRNA levels, while correlation of HIF-1 expression with tumor VEGF levels was poor (Flamme et al., 1998), which is consistent with our data in mice.
In summary, our studies provide genetic evidence that HIF-2 is the critical regulator of angiogenic gene expression and vascular tumorigenesis in pVHL-deficient livers and may therefore represent a therapeutic target for the treatment of VHL-associated vascular tumors.
Supplementary Material
Primer sequences used for quantitative real time PCR analysis of angiogenic gene expression in Vhlh mutant livers. Shown are the sequences of forward and reverse primers used to detect changes in gene expression with SYBR green on an Applied Biosystems platform. All genes were normalised to 18S levels using the 18S Taqman set from Applied Biosystems.
Acknowledgments
This work was supported by NIH grant DK073467 and CA100787 (both to VHH), the Center for Molecular Studies in Digestive and Liver Disease (P30-DK50306) and the Penn Diabetes and Endocrinology Research Center Functional Genomics Core (P30-DK19525). E.B.R. was supported by a fellowship grant from the American Heart Association.
References
- Biju MP, Neumann AK, Bensinger SJ, Johnson RS, Turka LA, Haase VH. Vhlh gene deletion induces Hif-1-mediated cell death in thymocytes. Mol Cell Biol. 2004;24:9038–9047. doi: 10.1128/MCB.24.20.9038-9047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindra RS, Vasselli JR, Stearman R, Linehan WM, Klausner RD. VHL-mediated hypoxia regulation of cyclin D1 in renal carcinoma cells. Cancer Res. 2002;62:3014–3019. [PubMed] [Google Scholar]
- Chavez JC, Baranova O, Lin J, Pichiule P. The transcriptional activator hypoxia inducible factor 2 (HIF-2/EPAS-1) regulates the oxygen-dependent expression of erythropoietin in cortical astrocytes. J Neurosci. 2006;26:9471–9481. doi: 10.1523/JNEUROSCI.2838-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Schumacher N, Maier M, Sendtner M, Gessler M. The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev. 2004;18:901–911. doi: 10.1101/gad.291004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamme I, Krieg M, Plate KH. Up-regulation of vascular endothelial growth factor in stromal cells of hemangioblastomas is correlated with up-regulation of the transcription factor HRF/HIF-2alpha. Am J Pathol. 1998;153:25–29. doi: 10.1016/s0002-9440(10)65541-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gijtenbeek JM, Boots-Sprenger SH, Franke B, Wesseling P, Jeuken JW. Cyclin D1 genotype and expression in sporadic hemangioblastomas. J Neurooncol. 2005;74:261–266. doi: 10.1007/s11060-004-7326-z. [DOI] [PubMed] [Google Scholar]
- Gruber M, Hu CJ, Johnson RS, Brown EJ, Keith B, Simon MC. Acute postnatal ablation of Hif-2alpha results in anemia. Proc Natl Acad Sci U S A. 2007;104:2301–2306. doi: 10.1073/pnas.0608382104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, et al. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Haase VH, Glickman JN, Socolovsky M, Jaenisch R. Vascular tumors in livers with targeted inactivation of the von Hippel- Lindau tumor suppressor. Proc Natl Acad Sci U S A. 2001;98:1583–1588. doi: 10.1073/pnas.98.4.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmquist-Mengelbier L, Fredlund E, Lofstedt T, Noguera R, Navarro S, Nilsson H, et al. Recruitment of HIF-1alpha and HIF-2alpha to common target genes is differentially regulated in neuroblastoma: HIF-2alpha promotes an aggressive phenotype. Cancer Cell. 2006;10:413–423. doi: 10.1016/j.ccr.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Kapitsinou PP, Haase VH. The VHL tumor suppressor and HIF: insights from genetic studies in mice. Cell Death Differ. 2008 doi: 10.1038/sj.cdd.4402313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher ER, Kaelin WG., Jr von Hippel-Lindau disease. Medicine (Baltimore) 1997;76:381–391. doi: 10.1097/00005792-199711000-00001. [DOI] [PubMed] [Google Scholar]
- McGrath FP, Gibney RG, Morris DC, Owen DA, Erb SR. Case report: multiple hepatic and pulmonary haemangioblastomas--a new manifestation of von Hippel-Lindau disease. Clin Radiol. 1992;45:37–39. doi: 10.1016/s0009-9260(05)81467-9. [DOI] [PubMed] [Google Scholar]
- Rankin EB, Biju MP, Liu Q, Unger TL, Rha J, Johnson RS, et al. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest. 2007;117:1068–1077. doi: 10.1172/JCI30117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin EB, Giaccia AJ. The role of hypoxia-inducible factors in tumorigenesis. Cell Death Differ. 2008 doi: 10.1038/cdd.2008.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin EB, Higgins DF, Walisser JA, Johnson RS, Bradfield CA, Haase VH. Inactivation of the arylhydrocarbon receptor nuclear translocator (Arnt) suppresses von Hippel-Lindau disease-associated vascular tumors in mice. Mol Cell Biol. 2005;25:3163–3172. doi: 10.1128/MCB.25.8.3163-3172.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raval RR, Lau KW, Tran MG, Sowter HM, Mandriota SJ, Li JL, et al. Contrasting Properties of Hypoxia-Inducible Factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-Associated Renal Cell Carcinoma. Mol Cell Biol. 2005;25:5675–5686. doi: 10.1128/MCB.25.13.5675-5686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothhammer T, Bataille F, Spruss T, Eissner G, Bosserhoff AK. Functional implication of BMP4 expression on angiogenesis in malignant melanoma. Oncogene. 2007;26:4158–4170. doi: 10.1038/sj.onc.1210182. [DOI] [PubMed] [Google Scholar]
- Vortmeyer AO, Gnarra JR, Emmert-Buck MR, Katz D, Linehan WM, Oldfield EH, et al. von Hippel-Lindau gene deletion detected in the stromal cell component of a cerebellar hemangioblastoma associated with von Hippel-Lindau disease. Hum Pathol. 1997;28:540–543. doi: 10.1016/s0046-8177(97)90075-7. [DOI] [PubMed] [Google Scholar]
- White P, Brestelli JE, Kaestner KH, Greenbaum LE. Identification of transcriptional networks during liver regeneration. J Biol Chem. 2005;280:3715–3722. doi: 10.1074/jbc.M410844200. [DOI] [PubMed] [Google Scholar]
- Zatyka M, Da Silva NF, Clifford SC, Morris MR, Wiesener MS, Eckardt KU, et al. Identification of cyclin D1 and other novel targets for the von Hippel-Lindau tumor suppressor gene by expression array analysis and investigation of cyclin D1 genotype as a modifier in von Hippel-Lindau disease. Cancer Res. 2002;62:3803–3811. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primer sequences used for quantitative real time PCR analysis of angiogenic gene expression in Vhlh mutant livers. Shown are the sequences of forward and reverse primers used to detect changes in gene expression with SYBR green on an Applied Biosystems platform. All genes were normalised to 18S levels using the 18S Taqman set from Applied Biosystems.