Abstract
Chiral phosphonyl imines attached by 1-naphthyl protection group were found to react with lithium ester enolates smoothly and give chiral β-amino esters in good yields (70−88%) and up to excellent diastereoselectivity (>99:1 dr). Triisopropoxytitanium (IV) chloride was found to enhance diastereoseletivity when used as the Lewis acid promoter. The chiral auxiliary can be readily removed by treating with HBr to give free amino esters. The absolute structure has been unambiguously determined by converting one of the products into an authentic sample. This reaction provides an easy access to β-amino acid derivatives.
Keywords: chiral phosphoramide, chiral N-phosphonyl imines, β-amino acid, lithium enolate, triisopropoxytitanium(IV) chloride
1. Introduction
There has been a continuing interest in the development of new methods for asymmetric synthesis β-amino acids and their derivatives in organic and medicinal chemistry, because β-amino acids belong to extremely important building blocks for studying natural products, pharmaceuticals and peptide and peptidomimetics (1-9). In addition, they are also precursors to chiral amino alcohols which are important chemical and biological molecules (10-11).
In recent years, many methods have been developed for the asymmetric synthesis of β-amino acids (12-27). Among these methods the reaction of chiral imines with ester enolates has become one of the most powerful and practical tool for this synthesis including the use of chiral N-sulfinyl imines (27-35).
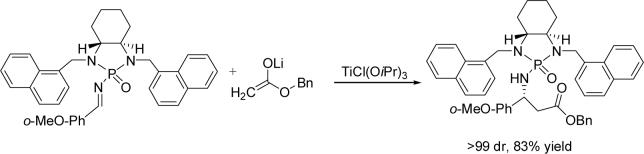
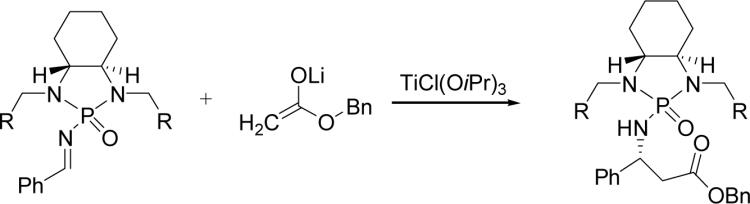
Very recently, we have established novel chiral N-phosphonyl imine chemistry, and successfully utilized this chemistry for asymmetric aza-Darzens reaction (36), asymmetric aza-Henry reaction (37) and asymmetric Mannich reaction (38). As a continuing effort on this important new chemistry, we would like to report the addition of lithium ester enolates onto chiral phosphonyl imines in the presence of triisopropoxytitanium (IV) chloride for the asymmetric synthesis of chiral β-amino esters (Scheme 1). Initially, we utilized N-phosphonyl imines for this reaction that were substituted with either benzyl or iso-propyl groups. Unfortunately, the reaction of lithium ester enolates with these imines afforded either low diastereoselectivity or modest chemical yields. Next, we modified the structure of phosphonyl imine by using different groups on the two nitrogen atoms of the auxiliary. Pleasantly, we found that the replacement of the original protection groups with a 1-nathphyl counterpart can enhance diastereoselectivity and provide a good chemical yield of 81% (entry 1, Table 1). Obviously, the bulky 1-naphthyl groups showed a larger steric effect on asymmetric induction during this addition process.
Scheme 1.
Addition of lithium ester enolates to N-phosphonyl imines.
Table 1.
Optimization of chiral phosphonyl auxiliary.a
 | ||||
|---|---|---|---|---|
| entry | imine | R | yield (%)b | drc |
| 1 | 1b | 1-nathphyl | 81 | 82:18 |
| 2 | 2 | Ph | 82 | 77:23 |
| 3 | 3 | 2-MeO-Ph | 80 | 72:28 |
| 4 | 4 | iPr | 60 | 76:24 |
Reaction condition: 0.1 mmol imine, 0.2 mmol ester, 0.22 mmol base, 2 equiv TiCl(OiPr)3, 2 mL solvent.
Isolated yield.
Determined by 31P NMR.
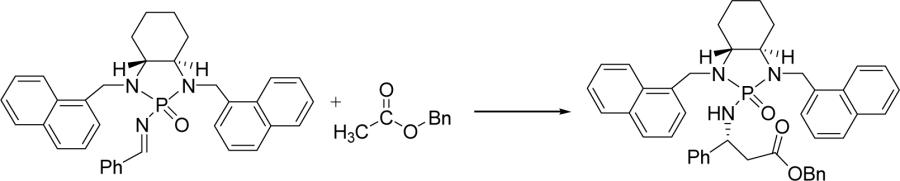
Imine 1b was then chosen as the model substrate for further optimizing reaction conditions by changing bases for enolate generation (Table 2). Among the various bases listed in Table 2, LDA was found to be the best candidate. Other bases such as n-BuLi, LiHMDS and (Me3Si)2NK gave either poor diastereoselectivity or low yields. Even though LiHMDS gave almost the same yield as LDA did under the same condition, diastereoselectivity was decreased to 79:21 (entry 3, Table 2). Interestingly, the potassium enolate generated by using (Me3Si)2NK as the base afforded a much lower yield of 45% (entry 4, Table 2), which could be attributed to the fact that potassium cation cannot act as a good Lewis acid for phosphonyl imine activation.
Table 2.
Solvent effect on the asymmetric reaction of phosphonyl imines with lithium ester enolatesa
 | |||||
|---|---|---|---|---|---|
| entry | base | solvent | temp (°C) | yield (%) | drb |
| 1 | LDA | THF | −78 | 81 | 82:18 |
| 2 | n-BuLi | THF | −78 | 50 | 70:30 |
| 3 | LiHMDS | THF | −78 | 83 | 79:21 |
| 4 | (Me3Si)2NK | THF | −78 | 45 | 72:28 |
| 5 | LDA | Et2O | −78 | 25 | 55:45 |
| 6 | LDA | CH2Cl2 | −78 | 35 | 70:30 |
| 7 | LDA | THF | −78 | 80 | 69:31c |
Reaction condition: 0.1 mmol imine, 0.2 mmol ester, 0.22 mmol base, 2 equiv TiCl(OiPr)3, 2 mL solvent.
Determined by 31P NMR.
No TiCl(OiPr)3 was added.
The solvent effect was also examined for this reaction. Among the three common solvents including THF, Et2O and CH2Cl2, THF was found to be the best choice regarding both chemical yield and diasereselectivity. For the cases of Et2O and CH2Cl2, very poor yields (25% and 35%, respectively) and diastereoselectivity (55:45 and 70:30, respectively) were obtained (entries 5 and 6, Table 2). Similar to the sulfinyl imine-based systems, triisopropoxytitanium(IV) chloride was proven to be beneficial to diastereoselectivity by acting as a Lewis acid promoter (entry 7, Table 2) (31-32, 36).
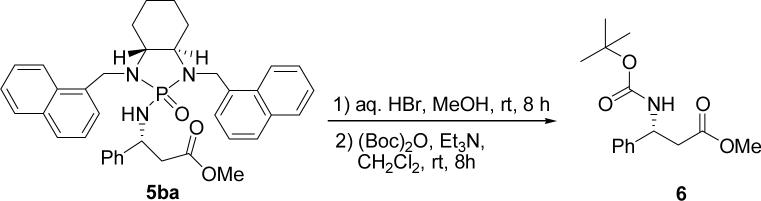
Based on the above optimal condition, we then examined the scope of substrates. As shown in Table 3, a variety of phosphoryl imines derived from benzaldehyde (entries 4 and 5, Table 3), 1-nathphaldehyde (entry 13 Table 3), aldehyde containing electron withdrawing groups on aromatic rings (entries 6−8, 11−12, Table 3) and electron donating groups on aromatic rings (entries 1−3, 9−10, Table 3) are all suitable for this reaction to give good chemical yields (70−88%). Good to excellent diastereoselectivities, ranging from 77:23 to >99:1, were obtained in all cases that were examined. Slightly better diastereoselectivities were obtained in cases of 2-substituted benzaldehyde-derived imines. Besides benzyl acetate, methyl acetate and t-butyl acetate were also proven to be suitable substrates for generating lithium enolates to give good to excellent yields and diastereoselectivities. To determine absolute structure, the product 5ba was subjected to deprotection reaction with HBr/MeOH at room temperature (37) followed by protection using (Boc)2O in the presence of triethylamine. The in situ procedure resulted in methyl (R)-N-t-BOC-3-phenyl-β-alanineate 6 which is a known compound. An overall yield of 82% was obtained under theses conditions, which enables the present method to be an efficient approach to 3-aryl-β-alanines and their ester derivatives (Scheme 2) (27, 39-40).
Table 3.
Examination of the scope of reaction substratesa
 | ||||||
|---|---|---|---|---|---|---|
| entry | imine | Ar | R | product | yield (%)b | drc |
| 1 | 1a | 2-MeO-Ph | Me | 5aa | 84 | 96:4 |
| 2 | 1a | 2-MeO-Ph | t-Bu | 5ab | 75 | 98:2 |
| 3 | 1a | 2-MeO-Ph | Bn | 5ac | 83 | >99:1 |
| 4 | 1b | Ph | Me | 5ba | 88 | 77:23 |
| 5 | 1b | Ph | Bn | 5bb | 81 | 82:18 |
| 6 | 1c | 2-F-Ph | Bn | 5c | 79 | 94:6 |
| 7 | 1d | 2-Cl-Ph | Bn | 5d | 73 | 93:7 |
| 8 | 1e | 2-Br-Ph | Bn | 5e | 83 | 93:7 |
| 9 | 1f | 2-Me-Ph | Bn | 5f | 80 | >99:1 |
| 10 | 1g | 2,6-di-Me-Ph | Bn | 5g | 70 | >99:1 |
| 11 | 1h | 4-Cl-Ph | t-Bu | 5h | 77 | 97:3d |
| 12 | 1i | 4-Br-Ph | t-Bu | 5i | 80 | 94:6d |
| 13 | 1j | 1-Naphthyl | Bn | 5j | 88 | 85:15 |
All the reactions were run in 0.05 M solution of THF.
Isolated yields after column.
Diastereoselectivities were determined by 31P NMR spectra of crude samples.
Ti(OiPr)4 was used.
Scheme 2.
Removal of chiral phosphonyl auxiliary of 5ba
In summary, a new method has been developed for the asymmetric synthesis of chiral β-amino esters in good to excellent diastereoselectivity (up to >99:1 dr) and good yields (up to 88%). Triisopropoxytitanium (IV) chloride was found to be an efficient Lewis acid promoter for the asymmetric addition of lithium ester enolates to chiral phosphonyl imines. Various aromatic phosphonyl imines with both electron withdrawing and electron donating groups on their rings are suitable substrates for this reaction.
Experimental Section
General Methods
All solvents used for the reactions were purified and dried by passing through an alumina column immediately prior to use. THF was distilled freshly from sodium/benzophenone ketyl. Chial phosphonyl imines were prepared according to the reported methods (36-38). The addition reactions were performed in oven-dried vials under protection of nitrogen gas. Flash column chromatography was performed using silica gel (Merck 60, 230−400 mesh). NMR spectra were recorded at 500/300, 125, 202 MHz for 1H, 13C and 31P respectively. Internal TMS (δ = 0.0 ppm) was used as the reference for 1H NMR and 13C NMR. 31P NMR specta were referenced to external H3PO4. Melting points were measured in open capillaries. 2 M LDA solution in THF, 1 M TiCl4 solution in dichloromethane and TiCl(OiPr)3 were obtained from Aldrich. These and other chemicals were used as obtained from commercial sources without further purifications.
Enolate addition to chiral N-phosphonyl imines
Into an oven dried reaction vial flushed with N2 were loaded ester (0.6 mmol) and 3.0 mL of dry THF. The reaction vial was cooled to −78 °C and 0.33 mL of 2 M LDA solution in THF was added dropwise with stirring over 10 minutes and the solution was stirred for another 30 min at −78 °C. To this solution, TiCl(OiPr)3 (0.72 mmol) in 0.5 mL THF was added dropwise. The solution was stirred for another 1 h. Into the resulting mixture was added dropwise 3.0 mL of THF off phosphonyl imine 1 (0.3 mmol). The reaction was stirred at −78 °C for 6 h and was quenched with 1.0 mL of saturated NH4Cl solution followed by 5.0 mL water at room temperature. The mixture was transferred into a separation funnel, and the aqueous layer was extracted with 2 × 20 mL of ethyl acetate. Combined organic layers were dried on anhydrous sodium sulfate. Sodium sulfate was filtered off and evaporated the organic solvent. Purification by column obtained adducts 5.
5aa
White solid. Mp 60−62 °C. = +2.2° (c = 0.46, CHCl3); 1H NMR (300 MHz, CDCl3): δ 8.13 (d, J = 8.4 Hz, 1H), 7.92 (d, J = 7.2 Hz, 1H), 7.85−7.88 (m, 1H), 7.76−7.80 (m, 3H), 7.64 (d, J = 8.1 Hz, 1H), 7.44−7.58 (m, 3H), 7.30−7.43 (m, 6H), 6.98−7.03 (m, 1H), 6.86 (d, J = 8.1 Hz, 1H), 4.87 (dd, J = 11.7, 16.2 Hz, 1H), 4.61−4.74 (m, 1H), 4.47 (dd, J = 12.0, 16.2 Hz, 1H), 4.10 (dd, J = 12.0, 17.4 Hz, 1H), 3.98 (t, J = 11.7 Hz, 1H), 3.80 (s, 3H), 3.51 (dd, J = 8.4, 17.7 Hz, 1H), 3.49 (s, 3H), 3.07−3.24 (m, 2H), 2.50 (dd, J = 9.3, 15.9 Hz, 1H), 1.99−2.08 (m, 1H), 1.93−1.97 (m, 1H), 1.73−1.77 (m, 1H), 1.49−1.58 (m, 2H), 1.41−1.49 (m, 1H), 1.08−1.35 (m, 3H); 13C NMR (125 MHz, CDCl3): δ 171.2 (d, J = 1.0 Hz), 156.9, 135.4, 135.4, 134.4, 134.3, 133.5, 133.2, 135.4, 135.4, 134.4, 134.3, 133.5, 133.2, 131.2, 130.4, 130.3, 130.3, 129.6, 128.7, 128.6, 128.5, 127.4, 126.8, 126.0, 125.6, 125.5, 125.4, 125.3, 125.1, 124.4, 123.0, 122.4, 120.5, 110.9, 65.3 (d, J = 10.1 Hz), 64.0 (d, J = 8.3 Hz), 55.1, 51.2, 50.4, 44.8, 44.8, 44.3, 44.3, 40.7, 40.6, 30.3, 30.2, 29.8, 29.7, 24.3, 24.2, 24.2; 31P NMR (202 MHz, CDCl3): δ 28.5.
5ab
Liquid. = +8.0° (c = 0.43, CHCl3); 1H NMR (300 MHz, CDCl3): δ 8.13 (d, J = 8.4 Hz, 1H), 7.97 (d, J = 6.9 Hz, 1H), 7.85−7.88 (m, 1H), 7.57−7.75 (m, 3H), 7.63 (d, J = 8.1 Hz, 1H), 7.46−7.58 (m, 3H), 7.32−7.43 (m, 6H), 6.99−7.04 (m, 1H), 6.88 (d, J = 8.4 Hz, 1H), 4.96 (dd, J = 11.7, 16.2 Hz, 1H), 4.64 (ddd, J = 4.5, 11.4, 21.6 Hz, 1H), 4.47 (dd, J = 9.9, 16.2 Hz, 1H), 4.11 (dd, J = 11.7, 17.4 Hz, 1H), 4.00 (t, J = 11.4 Hz, 1H), 3.76 (s, 3H), 3.49 (dd, J = 8.4, 17.4 Hz, 1H), 3.07−3.20 (m, 2H), 2.44 (dd, J = 9.9, 14.7 Hz, 1H), 2.05 (dd, J = 4.5, 14.7 Hz, 1H), 1.82−1.88 (m, 1H), 1.65−1.67 (m, 1H), 1.38−1.51 (m, 3H), 1.25 (s, 9H), 1.04−1.24 (m, 3H); 13C NMR (125 MHz, CDCl3): δ 169.9 (d, J = 1.4 Hz), 157.1, 135.4, 135.4, 134.5, 134.5, 133.4, 133.1, 131.1, 130.4, 130.2, 129.8, 128.6, 128.5, 128.4, 127.3, 126.7, 125.9, 125.4, 125.4, 125.3, 125.2, 125.1, 125.0, 124.2, 122.8, 122.3, 120.2, 110.9, 80.1, 65.3 (d, J = 9.8 Hz), 64.2 (d, J = 8.9 Hz), 55.1, 50.9, 44.8, 44.8, 44.3, 44.3, 42.3, 42.3, 30.2, 30.1, 29.9, 29.8, 27.8, 24.3, 24.2; 31P NMR (202 MHz, CDCl3): δ 28.7.
5ac
White solid. Mp 82−84 °C. = +3.4° (c = 0.32, CHCl3); 1H NMR (300 MHz, CDCl3): δ 8.11 (d, J = 8.1 Hz, 1H), 7.91 (d, J = 6.9 Hz, 1H), 7.64−7.85 (m, 5H), 7.28−7.55 (m, 12H), 7.16−7.20 (m, 2H), 6.90−7.01 (m, 1H), 6.86 (d, J = 8.4 Hz, 1H), 4.89 (d, J = 9.0 Hz, 2H), 4.85−4.99 (m, 1H), 4.67 (ddd, J = 4.2, 9.9, 21.6 Hz, 1H), 4.45 (dd, J = 11.1, 15.9 Hz, 1H), 4.08 (dd, J = 12.0, 17.7 Hz, 1H), 3.95 (t, J = 11.7 Hz, 1H), 3.74 (s, 3H), 3.46 (dd, J = 8.7, 17.7 Hz, 1H), 3.05−3.19 (m, 2H), 2.55 (dd, J = 11.1, 15.3 Hz, 1H), 2.01 (dd, J = 4.2, 15.6 Hz, 1H), 1.89−1.96 (m, 1H), 1.70−1.71 (m, 1H), 1.39−1.52 (m, 3H), 1.04−1.25 (m, 3H); 13C NMR (125 MHz, CDCl3): δ 170.6 (d, J = 1.0 Hz), 157.1, 135.7, 135.4, 135.4, 134.4, 134.4, 133.5, 133.2, 131.2, 130.4, 130.2, 129.8, 128.8, 128.6, 128.5, 128.4, 128.1, 127.4, 126.8, 126.0, 125.5, 125.5, 125.3, 125.3, 125.1, 124.4, 122.9, 122.4, 120.5, 111.0, 66.0, 65.3 (d, J = 10.3 Hz), 64.2 (d, J = 8.9 Hz), 55.2, 50.7, 44.9, 44.9, 44.4, 44.3, 41.0, 40.9, 30.3, 30.2, 29.9, 29.8, 24.4, 24.2; 31P NMR (202 MHz, CDCl3): δ 28.5.
5ba
White solid. Mp 174−176 °C. = −45.7° (c = 0.11, CHCl3); 1H NMR (300 MHz, CDCl3): δ 8.17 (d, J = 8.1 Hz, 1H), 7.82−7.98 (m, 3H), 7.71−7.80 (m, 3H), 7.65 (d, J = 8.4 Hz, 1H), 7.46−7.59 (m, 3H), 7.36−7.43 (m, 3H), 7.27−7.32 (m, 4H), 7.15−7.20 (m, 1H), 4.77 (dd, J = 10.8, 15.0 Hz, 1H), 4.49 (t, J = 14.7 Hz, 1H), 4.31−4.44 (m, 1H), 4.14 (dd, J = 11.7, 17.4 Hz, 1H), 3.41 (s, 3H), 3.31−3.46 (m, 1H), 2.90−3.17 (m, 2H), 2.05−2.09 (m, 1H), 1.95 (dd, J = 8.1, 15.6 Hz, 1H), 1.81 (dd, J = 4.5, 15.3 Hz, 1H), 1.71−1.76 (m, 1H), 1.46−1.57 (m, 2H), 0.90−1.38 (m, 5H); 13C NMR (125 MHz, CDCl3): δ 170.5, 143.5, 143.6, 135.3, 135.3, 134.0, 134.0, 133.6, 133.2, 131.5, 130.4, 128.7, 128.6, 128.5, 128.4, 127.7, 127.4, 126.9, 126.8, 126.6, 126.3, 126.1, 125.5, 125.5, 125.4, 125.4, 125.2, 124.4, 123.2, 122.4, 64.9 (d, J = 10.3 Hz), 64.0 (d, J = 8.9 Hz), 51.9, 51.3, 44.9, 44.1, 44.0, 42.6, 42.5, 30.1, 30.0, 29.6, 29.5, 24.3, 24.2; 31P NMR (202 MHz, CDCl3): δ 27.4.
5bb
White solid. Mp 74−76 °C. = −24.5° (c = 0.44, CHCl3); 1H NMR (300 MHz, CDCl3): δ 8.15 (d, J = 8.4 Hz, 1H), 7.89 (d, J = 6.9 Hz, 1H), 7.64−7.84 (m, 5H), 7.45−7.55 (m, 2H), 7.34−7.44 (m, 4H), 7.27−7.34 (m, 8H), 7.07−7.20 (m, 3H), 4.77−4.90 (m, 3H), 4.36−4.57 (m, 2H), 4.10 (dd, J = 10.8, 16.5 Hz, 1H), 3.48 (t, J = 9.9 Hz, 1H), 3.29 (dd, J = 8.4, 17.4 Hz, 1H), 2.90−3.17 (m, 2H), 2.02−2.13 (m, 2H), 1.86 (dd, J = 4.5, 15.3 Hz, 1H), 1.72−1.76 (m, 1H), 1.43−1.61 (m, 2H), 0.88−1.37 (m, 4H); 13C NMR (125 MHz, CDCl3): δ 170.8, 169.9, 169.9, 143.5, 143.4, 135.4, 135.3, 135.3, 134.0, 134.0, 133.5, 133.2, 131.4, 130.4, 128.6, 128.6, 128.5, 128.4, 128.1, 128.1, 127.7, 127.4, 126.9, 126.9, 126.5, 126.1, 125.5, 125.5, 125.4, 125.4, 125.1, 124.4, 123.1, 122.3, 66.0, 64.9 (d, J = 10.3 Hz), 64.0 (d, J = 8.4 Hz), 52.0, 45.0, 45.0, 44.0, 44.0, 42.8, 42.7, 30.1, 30.0, 29.6, 29.5, 24.3, 24.2; 31P NMR (202 MHz, CDCl3): δ 27.5.
5c
White solid. Mp 78−80 °C. = −19.0° (c = 0.43, CHCl3); 1H NMR (300 MHz, CDCl3): δ 8.12 (d, J = 8.4 Hz, 1H), 7.87 (d, J = 6.9 Hz, 1H), 7.78−7.82 (m, 2H), 7.65−7.76 (m, 3H), 7.50−7.55 (m, 1H), 7.42−7.46 (m, 2H), 7.35−7.40 (m, 4H), 7.27−7.30 (m, 3H), 7.26−7.29 (m, 2H), 7.13−7.20 (m, 2H), 6.98−7.09 (m, 2H), 4.73−4.97 (m, 4H), 3.98 (t, J = 15.3 Hz, 1H), 4.87 (dd, J = 11.7, 17.4 Hz, 1H), 3.91 (t, J = 10.8 Hz, 1H), 3.51 (dd, J = 8.4, 17.4 Hz, 1H), 3.02−3.23 (m, 2H), 2.27 (dd, J = 8.7, 15.9 Hz, 1H), 1.97−2.09 (m, 2H), 1.71−1.78 (m, 1H), 1.49−1.58 (m, 2H), 1.34−1.44 (m, 1H), 1.06−1.34 (m, 3H); 13C NMR (125 MHz, CDCl3): δ 169.9, 161.1, 159.1, 135.4, 135.1, 135.1, 134.0, 133.9, 133.5, 133.2, 131.1, 130.4, 129.4, 129.4, 129.1, 129.0, 128.6, 128.6, 128.4, 128.2, 127.7, 127.0, 126.3, 126.1, 125.5, 125.5, 125.4, 125.4, 125.2, 124.5, 124.3, 124.2, 123.0, 122.2, 115.8, 155.6, 66.1, 65.0 (d, J = 10.4 Hz), 64.1 (d, J = 8.5 Hz), 47.1, 44.9, 44.9, 44.0, 44.0, 41.0, 40.9, 30.1, 30.1, 29.6, 29.5, 24.3, 24.2; 31P NMR (202 MHz, CDCl3): δ 27.8.
5d
White solid. Mp 78−80 °C. = −14.2° (c = 0.35, CHCl3); 1H NMR (300 MHz, CDCl3): δ 8.17 (d, J = 8.4 Hz, 1H), 7.92 (d, J = 6.9 Hz, 1H), 7.78−7.82 (m, 2H), 7.65−7.75 (m, 3H), 7.53−7.58 (m, 1H), 7.39−7.49 (m, 5H), 7.35−7.37 (m, 3H), 7.24−7.33 (m, 4H), 7.11−7.18 (m, 3H), 4.83 (d, J = 2.1 Hz, 2H), 4.80−4.93 (m, 1H), 4.49 (t, J = 15.3 Hz, 1H), 4.18 (dd, J = 12.0, 17.4 Hz, 1H), 4.01 (t, J = 11.1 Hz, 1H), 3.33 (dd, J = 8.1, 17.4 Hz, 1H), 2.92−3.17 (m, 2H), 2.08 (dd, J = 4.2, 15.6 Hz, 1H), 2.04−2.06 (m, 1H), 1.72−1.84 (m, 2H), 1.51−1.61 (m, 2H), 0.94−1.39 (m, 5H); 13C NMR (125 MHz, CDCl3): δ 170.1, 140.1, 135.3, 135.2, 135.1, 133.9, 133.9, 133.6, 133.2, 131.8, 131.4, 130.4, 129.7, 129.7, 128.7, 128.6, 128.5, 128.4, 128.2, 128.2, 127.7, 127.0, 126.9, 126.6, 126.1, 125.6, 125.5, 125.5, 125.4, 125.1, 124.5, 123.2, 122.2, 66.1, 64.9 (d, J = 10.4 Hz), 64.0 (d, J = 8.9 Hz), 48.8, 44.9, 44.1, 44.1, 39.5, 39.5, 30.2, 30.1, 29.6, 29.5, 24.3, 24.2; 31P NMR (202 MHz, CDCl3): δ 27.9.
5e
White solid. Mp 64−66 °C. = −7.0° (c = 0.37, CHCl3); 1H NMR (300 MHz, CDCl3): δ 8.19 (d, J = 8.1 Hz, 1H), 7.93 (d, J = 6.9 Hz, 1H), 7.78−7.82 (m, 2H), 7.65−7.74 (m, 3H), 7.54−7.59 (m, 2H), 7.40−7.50 (m, 4H), 7.35−7.40 (m, 3H), 7.25−7.29 (m, 3H), 7.19−7.21 (m, 2H), 7.11−7.15 (m, 2H), 4.78−4.91 (m, 3H), 4.50 (t, J = 15.3 Hz, 1H), 4.15−4.27 (m, 2H), 3.27 (dd, J = 8.1, 17.4 Hz, 1H), 2.96−3.16 (m, 2H), 2.07−2.19 (m, 2H), 1.61−1.73 (m, 2H), 1.51−1.54 (m, 2H), 0.96−1.42 (m, 5H); 13C NMR (125 MHz, CDCl3): δ 170.1, 141.7, 135.3, 135.2, 135.2, 133.9, 133.8, 133.6, 133.2, 132.9, 131.4, 130.4, 129.9, 128.9, 128.7, 128.6, 128.4, 128.3, 128.2, 127.7, 127.7, 126.9, 126.6, 126.1, 125.6, 125.5, 125.5, 125.4, 125.1, 124.5, 123.2, 122.2, 122.1, 66.1, 64.9 (d, J = 10.3 Hz), 64.0 (d, J = 8.4 Hz), 50.9, 44.9, 44.9, 44.2, 44.2, 39.2, 39.2, 30.2, 30.1, 29.6, 29.5, 24.3, 24.2; 31P NMR (202 MHz, CDCl3): δ 27.4.
5f
White solid. Mp 66−68 °C. = −28.6° (c = 0.22, CHCl3); 1H NMR (300 MHz, CDCl3): δ 8.19 (d, J = 8.4 Hz, 1H), 7.83 (d, J = 7.2 Hz, 1H), 7.78−7.83 (m, 2H), 7.72−7.73 (m, 2H), 7.65 (d, J = 8.1 Hz, 1H), 7.52−7.57 (m, 1H), 7.36−7.50 (m, 7H), 7.25−7.28 (m, 3H), 7.13−7.21 (m, 2H), 7.06−7.10 (m, 3H), 4.81 (s, 2H), 4.61−4.78 (m, 2H), 4.48 (t, J = 15.3 Hz, 1H), 4.21 (dd, J = 11.7, 17.4 Hz, 1H), 3.55 (t, J = 9.9 Hz, 1H), 3.28 (dd, J = 8.1, 17.4 Hz, 1H), 2.77−3.16 (m, 2H), 2.38 (s, 3H), 2.04−2.09 (m, 1H), 1.80−1.97 (m, 1H), 1.70−1.78 (m, 1H), 1.39−1.54 (m, 2H), 0.96−1.31 (m, 5H); 13C NMR (125 MHz, CDCl3): δ 170.1, 141.1, 135.4, 135.3, 133.9, 133.9, 133.8, 133.6, 133.2, 131.5, 130.6, 130.5, 128.7, 128.5, 128.4, 128.1, 127.7, 127.2, 127.1, 126.9, 126.7, 126.3, 126.0, 125.6, 125.5, 125.5, 125.4, 125.2, 124.5, 123.2, 122.5, 66.0, 65.0 (d, J = 10.4 Hz), 63.7 (d, J = 8.5 Hz), 48.2, 44.8, 44.8, 44.2, 44.1, 41.1, 41.1, 30.1, 30.1, 29.6, 29.5, 24.3, 24.3, 19.3; 31P NMR (202 MHz, CDCl3): δ 27.8.
5g
White solid. Mp 76−78 °C. = −24.4° (c = 0.22, CHCl3); 1H NMR (300 MHz, CDCl3): δ 8.17 (d, J = 8.7 Hz, 1H), 7.91 (d, J = 6.9 Hz, 1H), 7.77−7.83 (m, 2H), 7.73−7.74 (m, 2H), 7.65 (d, J = 8.4 Hz, 1H), 7.51−7.57 (m, 3H), 7.36−7.50 (m, 4H), 7.26−7.29 (m, 3H), 7.05−7.14 (m, 3H), 6.96−7.03 (m, 2H), 4.87−5.01 (m, 1H), 4.76 (d, J = 2.1 Hz, 2H), 4.69−4.74 (m, 1H), 4.48 (t, J = 15.3 Hz, 1H), 4.12 (dd, J = 11.4, 17.4 Hz, 1H), 3.20 (dd, J = 8.7, 17.4 Hz, 1H), 3.08−3.16 (m, 1H), 2.79−2.87 (m, 2H), 2.52 (s, 3H), 2.42 (dd, J = 9.3, 14.4 Hz, 1H), 2.35 (s, 3H), 2.02−2.05 (m, 1H), 1.83 (dd, J = 6.6, 14.7 Hz, 1H), 1.69−1.73 (m, 1H), 1.37−1.53 (m, 2H), 0.85−1.27 (m, 4H); 13C NMR (125 MHz, CDCl3): δ 169.7 (d, J = 1 Hz), 138.1, 136.4, 135.9, 135.4, 135.4, 135.4, 133.7, 133.6, 133.6, 133.3, 131.5, 130.7, 130.5, 129.0, 128.6, 128.6, 128.4, 128.1, 128.0, 127.7, 127.3, 126.9, 126.9, 126.1, 125.6, 125.5, 125.5, 125.2, 124.7, 123.2, 122.5, 66.1, 64.4 (d, J = 10.9 Hz), 63.7 (d, J = 8.4 Hz), 48.0, 44.9, 44.9, 43.4, 43.4, 40.3, 40.2, 30.3, 30.2, 29.4, 29.3, 24.3, 24.3, 21.2, 21.1; 31P NMR (202 MHz, CDCl3): δ 27.7.
5h
White solid. Mp 178−180 °C. = −43.3° (c = 0.32, CHCl3); 1H NMR (300 MHz, CDCl3): δ 7.97 (d, J = 7.8 Hz, 1H), 7.89 (d, J = 7.8 Hz, 1H), 7.83−7.86 (m, 2H), 7.77 (d, J = 7.2 Hz, 1H), 7.71−7.74 (m, 2H), 7.62 (d, J = 6.6 Hz, 1H), 7.44−7.54 (m, 5H), 7.35−7.41 (m, 1H), 7.04−7.11 (m, 4H), 4.52−4.62 (m, 3H), 4.31 (dd, J = 11.7, 15.9 Hz, 1H), 4.21 (dd, J = 7.5, 17.1 Hz, 1H), 3.69 (t, J = 11.7 Hz, 1H), 3.09−3.12 (m, 2H), 2.32 (dd, J = 5.1, 15.3 Hz, 1H), 2.10 (dd, J = 6.6, 15.3 Hz, 1H), 1.70−1.72 (m, 1H), 1.55−1.63 (m, 3H), 1.23 (s, 9H), 1.14−1.30 (m, 4H); 13C NMR (125 MHz, CDCl3): δ 170.2, 141.9, 141.9, 135.1, 135.1, 133.6, 133.6, 133.5, 133.4, 132.7, 131.2, 130.7, 128.7, 128.6, 128.3, 127.9, 127.3, 127.2, 126.0, 125.9, 125.7, 125.4, 125.4, 125.3, 124.6, 122.8, 122.7, 81.0, 64.9 (d, J = 9.7 Hz), 63.6 (d, J = 9.4 Hz), 51.8, 44.5, 44.4, 44.2, 43.7, 43.6, 29.6, 29.5, 29.3, 29.2, 27.8, 24.2, 24.2; 31P NMR (202 MHz, CDCl3): δ 27.3.
5i
White solid. Mp 193−195 °C. = −31.5° (c = 0.39, CHCl3); 1H NMR (300 MHz, CDCl3): δ 7.97 (d, J = 8.1 Hz, 1H), 7.89 (d, J = 8.1 Hz, 1H), 7.84−7.86 (m, 2H), 7.77 (d, J = 6.9 Hz, 1H), 7.71−7.74 (m, 2H), 7.61 (d, J = 7.2 Hz, 1H), 7.44−7.55 (m, 5H), 7.35−7.42 (m, 2H), 7.20−7.25 (m, 1H), 7.02−7.05 (m, 2H), 4.49−4.64 (m, 3H), 4.30 (dd, J = 11.7, 15.9 Hz, 1H), 4.21 (dd, J = 7.8, 17.1 Hz, 1H), 3.69 (t, J = 10.8 Hz, 1H), 3.06−3.15 (m, 2H), 2.32 (dd, J = 5.1, 15.3 Hz, 1H), 2.10 (dd, J = 6.6, 15.3 Hz, 1H), 1.70−1.74 (m, 1H), 1.56−1.59 (m, 3H), 1.23 (s, 9H), 1.15−1.28 (m, 4H); 13C NMR (75 MHz, CDCl3): δ 170.4, 142.7, 135.4, 135.3, 133.8, 133.8, 133.7, 133.6, 131.5, 131.5, 130.9, 128.9, 128.8, 128.5, 127.6, 127.4, 126.2, 126.2, 126.0 , 125.7, 125.6, 124.9, 123.0, 122.9, 121.1, 81.3, 65.2 (d, J = 8.0 Hz), 63.7 (d, J = 9.2 Hz), 52.1, 44.7, 44.7, 44.4, 44.4, 43.9, 43.9, 29.9, 29.8, 29.6, 29.4, 28.1, 24.4; 31P NMR (202 MHz, CDCl3): δ 26.7.
5j
White solid. Mp 74−76 °C. = −7.9° (c = 0.29, CHCl3); 1H NMR (300 MHz, CDCl3): δ 8.22 (d, J = 8.4 Hz, 1H), 8.06 (d, J = 8.4 Hz, 1H), 7.95 (d, J = 7.2 Hz, 1H), 7.89−7.93 (m, 1H), 7.73−7.84 (m, 4H), 7.59−7.66 (m, 2H), 7.53−7.56 (m, 1H), 7.43−7.51 (m, 5H), 7.30−7.36 (m, 4H), 7.18−7.25 (m, 3H), 6.99−7.07 (m, 3H), 5.19−5.28 (m, 1H), 4.79−4.88 (m, 1H), 4.81 (s, 2H), 4.49 (t, J = 15.0 Hz, 1H), 4.04 (dd, J = 11.7, 17.4 Hz, 1H), 3.68 (t, J = 9.9 Hz, 1H), 2.97−3.13 (m, 2H), 2.65−2.72 (m, 1H), 2.15−2.16 (m, 1H), 2.06−2.09 (m, 1H), 1.38−1.71 (m, 4H), 0.84−1.36 (m, 4H); 13C NMR (125 MHz, CDCl3): δ 170.1, 139.0, 135.3, 135.2, 135.2, 133.9, 133.8, 133.6, 133.1, 131.5, 130.3, 130.0, 129.1, 128.7, 128.5, 128.3, 128.1, 128.1, 127.8, 126.8, 126.8, 126.3, 126.1, 125.7, 125.6, 125.5, 125.4, 125.4, 125.3, 125.1, 124.3, 123.2, 122.7, 122.2, 66.0, 64.3 (d, J = 10.4 Hz), 63.7 (d, J = 8.5 Hz), 44.8, 44.8, 43.8, 43.8, 41.8, 41.8, 29.9, 29.8, 29.5, 29.4, 24.3, 24.1; 31P NMR (202 MHz, CDCl3): δ 27.4.
Removal of N-phosphonyl group of 5ba and synthesis of N-Boc protected β-amino ester 6
Into a 50 mL round flask were loaded 309 mg of 5ba (0.5 mmol) and 15.0 mL of methanol. To this solution was added 48% aq. HBr (5 mL), and the reaction was stirred at r.t. for 8 h. The cleavage was monitored by TLC. Volatiles were removed to obtain yellow oil, to which was added 15.0 mL of methylenechloride and 0.70 mL of triethyl amine (5.0 mmol) at room temperature. The resulting mixture was added 0.654 g of di-tert-butyl dicarbonate (3.0 mmol) and stirred at room temperature overnight. The crude reaction mixture obtained after standard aqueous work-up was purified by using silica gel column chromatography (230−400 mesh) with 15% ethyl acetate in hexanes as the eluent. N-Boc β-amino ester 6 was obtained as a white solid in 82% yield (114 mg). = +14.7° (c = 1.02, CHCl3); 1H NMR (300 MHz, CDCl3): δ 7.22−7.36 (m, 5H), 5.44(s, br, 1H), 5.10 (s, br, 1H), 3.61 (s, 3H), 2.77−2.92 (m, 2H), 1.42 (s, 9H).
Supplementary Material
Acknowledgements
Financial assistance from Robert A. Welch Foundation (D-1361) and National Institutes of Health (DA018224 Amen 01) is gratefully acknowledged.
References
- 1.Lelais G, Seebach D. Beta (2)-amino acids - Syntheses, occurrence in natural products, and components of beta-peptides. Biopolymers. 2004;76:206–243. doi: 10.1002/bip.20088. [DOI] [PubMed] [Google Scholar]
- 2.Lee M-R, Raguse TL, Schinerl M, Pomerantz WC, Wang X, Wipf P, Gellman SH. Origins of the high 14-helix propensity of cyclohexyl-rigidified residues in peptides. Org Lett. 2007;9:1801–1804. doi: 10.1021/ol070511r. [DOI] [PubMed] [Google Scholar]
- 3.Liu M, Sibi MP. Recent advances in the stereoselective synthesis of beta-amino acids. Tetrahedron. 2002;58:7991–8035. [Google Scholar]
- 4.Hayashi Y, Katada J, Harada T, Tachiki A, Iijima K, Takiguchi Y, Muramatsu M, Miyazaki H, Asari T, Okazaki T, Sato Y, Yasuda E, Yano M, Uno I, Ojima I. GPIIb/IIIa integrin antagonists with the new conformational restriction unit, trisubstituted β-amino acid derivatives, and a substituted benzamidine structure. J Med Chem. 1998;41:2345–2360. doi: 10.1021/jm980126v. [DOI] [PubMed] [Google Scholar]
- 5.Iijima K, Katada J, Hayashi Y. Symmetrical anhydride-type serine protease inhibitors: Structure-activity relationship studies of human chymase inhibitors. Bioorg Med Chem Lett. 1999;9:413–418. doi: 10.1016/s0960-894x(99)00012-8. [DOI] [PubMed] [Google Scholar]
- 6.Bai RL, Verdier-Pinard P, Gangwar S, Stessman CC, McClure KJ, Sausville EA, Pettit GR, Bates RB, Hamel E. Dolastatin 11, a Marine Depsipeptide, Arrests Cells at Cytokinesis and Induces Hyperpolymerization of Purified Actin. Mol Pharmacol. 2001;59:462–469. doi: 10.1124/mol.59.3.462. [DOI] [PubMed] [Google Scholar]
- 7.Bates RB, Brusoe KG, Burns JJ, Caldera S, Cui W, Gangwar S, Gramme MR, McClure KJ, Rouen GP, Schadow H, Stessman CC, Taylor SR, Vu VH, Yarick GV, Zhang JX, Pettit GR, Bontems R. Dolastatins. 26. Synthesis and Stereochemistry of Dolastatin 11. J Am Chem Soc. 1997;119:2111–2113. [Google Scholar]
- 8.Jefferson EA, Swayze EE. β-Amino acid facilitates macrocyclic ring closure in a combinatorial library. Tetrahedron Lett. 1999;40:7757–7760. [Google Scholar]
- 9.Kaseda T, Kikuchi T, Kibayashi C. Enantioselective total synthesis of (+)-(S)-dihydroperiphylline. Tetrahedron Lett. 1989;30:4539–4542. [Google Scholar]
- 10.Enders D, Moser M, Geibel G, Laufer MC. Diastereo- and Enantioselective Synthesis of Differently N,O-Protected 1,3-Amino Alcohols with Three Neighbouring Stereogenic Centers. Synthesis. 2004;2004:2040–2046. and references cited therein. [Google Scholar]
- 11.Kochi T, Tang TP, Ellman JA. Development and Application of a New General Method for the Asymmetric Synthesis of syn- and anti-1,3-Amino Alcohols. J Am Chem Soc. 2003;125:11276–11282. doi: 10.1021/ja0363462. [DOI] [PubMed] [Google Scholar]
- 12.Wenzel AG, Jacobsen EN. Enantioselective Synthesis of β-Amino Acids. 2nd Ed. John Wiley & Sons; MA: 2005. [Google Scholar]
- 13.Juaristi E. Enantioselective Synthesis of β-Amino Acids. 1st ed. Wiley-VCH; New York: 1997. [Google Scholar]
- 14.Chi Y, English EP, Pomerantz WC, Horne W, Seth J, Leo A, Alexander LR, Fleming WS, Hopkins EA, Gellman SH. Practical synthesis of enatiomerically pure 2-amino acids via proline-catalyzed diastereoselective aminomethylation of aldehydes. J Am Chem Soc. 2007;129:6050–6055. doi: 10.1021/ja070063i. [DOI] [PubMed] [Google Scholar]
- 15.Juaristi E, Quintana D, Balderas M, Garcial PE. Enantioselective synthesis of beta-amino acids .7. Preparation of enantiopure alpha-substituted beta-amino acids from 1-benzoyl-2(S)-tert-butyl-3-methylperhydropyrimidin-4-one. Tetrahedron Asymmetry. 1996;7:2233–2246. [Google Scholar]
- 16.Chi Y, Gellman SH. Enantioselective Organocatalytic Aminomethylation of Aldehydes: A Role for Ionic Interactions and Efficient Access to β2-Amino Acids. J Am Chem Soc. 2006;128:6804–6805. doi: 10.1021/ja061731n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsiao Y, Rivera NR, Rosner T. Highly Efficient Synthesis of β-Amino Acid Derivatives via Asymmetric Hydrogenation of Unprotected Enamines. J Am Chem Soc. 2004;126:9918–9919. doi: 10.1021/ja047901i. [DOI] [PubMed] [Google Scholar]
- 18.Sibi MP, Itoh K. Organocatalysis in Conjugate Amine Additions. Synthesis of β-Amino Acid Derivatives. J Am Chem Soc. 2007;129:8064–8065. doi: 10.1021/ja071739c. [DOI] [PubMed] [Google Scholar]
- 19.Sibi MP, Prabagaran N, Ghorpade SG, Jasperse CP. Enantioselective Synthesis of α,β-Disubstituted-β-amino Acids. J Am Chem Soc. 2003;125:11796–11797. doi: 10.1021/ja0372309. [DOI] [PubMed] [Google Scholar]
- 20.Sibi MP, Shay JJ, Liu M, Jasperse CP. Chiral Lewis Acid Catalysis in Conjugate Additions of O-Benzylhydroxylamine to Unsaturated Amides. Enantioselective Synthesis of β-Amino Acid Precursors. J Am Chem Soc. 1998;120:6615–6616. [Google Scholar]
- 21.Myers JK, Jacobsen EN. Asymmetric Synthesis of β-Amino Acid Derivatives via Catalytic Conjugate Addition of Hydrazoic Acid to Unsaturated Imides. J Am Chem Soc. 1999;121:8959–8960. [Google Scholar]
- 22.Zhu G, Chen Z, Zhang X. Highly Efficient Asymmetric Synthesis of β-Amino Acid Derivatives via Rhodium-Catalyzed Hydrogenation of β-(Acylamino)acrylates. J Org Chem. 1999;64:6907–6910. doi: 10.1021/jo990565h. [DOI] [PubMed] [Google Scholar]
- 23.Elaridi J, Thaqi A, Prosser A, Jackson WR, Robinson AJ. An enantioselective synthesis of β2-amino acid derivatives. Tetrahedron: Asymmetry. 2005;16:1309–1319. [Google Scholar]
- 24.Miyabe H, Fujii K, Naito T. Radical addition to oxime ethers for asymmetric synthesis of β-amino acid derivatives. Org Biomol Chem. 2003;1:381–390. doi: 10.1039/b208823a. [DOI] [PubMed] [Google Scholar]
- 25.Beddow JE, Davies SG, Ling KB, Roberts PM, Russell AJ, Smith AD, Thomson JE. Asymmetric synthesis of β2-amino acids: 2-substituted-3-aminopropanoic acids from N-acryloyl SuperQuat derivatives. Org Biomol Chem. 2007;5:2812–2825. doi: 10.1039/b707689d. [DOI] [PubMed] [Google Scholar]
- 26.Allef P, Kunz H. Glycosylation-induced asymmetric synthesis: β-amino acid esters via Mannich reactions. Tetrahedron: Asymmetry. 2000;11:375–378. [Google Scholar]
- 27.Tillman AL, Ye J, Dixon DJ. Direct enantio- and diastereoselective Mannich reactions of malonate and β-keto esters with N-Boc and N-Cbz aldimines catalysed by a bifunctional cinchonine derivative. Chem Commun. 2006;2006:1191–1193. doi: 10.1039/b515725k. [DOI] [PubMed] [Google Scholar]
- 28.Kise N, Ueda N. A New Method for the Synthesis of β-Amino Acid Derivatives and β-Lactams. Reaction of N-Alkoxycarbonyl-1-methoxyamines with Esters. J Org Chem. 1999;64:7511–7514. [Google Scholar]
- 29.Cainelli G, Panunzio M, Bandini E, Martelli G, Spunta G. Diastereofacial selectivity in the reaction of chiral N-trimethylsilyl imines with ester enolates: Preparation of trans-azetidin-2-ones in high stereocontrolled fashion. Tetrahedron. 1996;52:1685–1698. [Google Scholar]
- 30.Cozzi PG, Simone BD, Umani-Ronchi A. Highly diastereoselective addition of silyl enolates to chiral imines derived from (S)-valine methyl ester using lanthanide triflate. Tetrahedron Lett. 1996;37:1691–1694. [Google Scholar]
- 31.Baldoli C, Buttero PD, Licandro E, Papagni A. Tricarbonyl(η6arene)Chromium(0) complexes as chiral auxiliaries: Asymmetric synthesis of β-aminoesters and β-lactams by Reformatsky condensation. Tetrahedron. 1996;52:4849–4856. [Google Scholar]
- 32.Tang TP, Ellman JA. Asymmetric Synthesis of β-Amino Acid Derivatives Incorporating a Broad Range of Substitution Patterns by Enolate Additions to tert-Butanesulfinyl Imines. J Org Chem. 2002;67:7819–7832. doi: 10.1021/jo025957u. [DOI] [PubMed] [Google Scholar]
- 33.Tang TP, Ellman JA. The tert-Butanesulfinyl Group: An Ideal Chiral Directing Group and Boc-Surrogate for the Asymmetric Synthesis and Applications of β-Amino Acids. J Org Chem. 1999;64:12–13. doi: 10.1021/jo9820824. [DOI] [PubMed] [Google Scholar]
- 34.Davis FA, Reddy RE, Szewczyk JM. Asymmetric Synthesis of (R)-(+)-beta.-Phenylalanine from (S)-(+)-Benzylidene-p-toluenesulfinamide. Regeneration of the Sulfinimine Precursor. J Org Chem. 1995;60:7037–7039. [Google Scholar]
- 35.Fujisawa T, Kooriyama Y, Shimizu M. Switchover of diastereofacial selectivity in the condensation reaction of optically active N-sulfinimine with ester enolate. Tetrahedron Lett. 1996;37:3881–3884. [Google Scholar]
- 36.Kattuboina A, Li G. Chiral N-phosphonyl imine chemistry: new reagents and their applications for asymmetric reactions. Tetrahedron Lett. 2008;49:1573–1577. [Google Scholar]
- 37.Kattuboina A, Kaur P, Ai T, Li G. Asymmetric Aza-Henry Reaction Using Chiral N-phosphonyl Imines. Chem. Biol. & Drug Design. 2008;71:216–223. doi: 10.1111/j.1747-0285.2008.00633.x. 2008. [DOI] [PubMed] [Google Scholar]
- 38.Han JL, Ai T, Olmos A, Li G. 2008. to be published.
- 39.Siegel C, Thornton ER. Asymmetric aldol reactions. A titanium enolate giving very high diastereofacial selectivities. J Am Chem Soc. 1989;111:5722–5728. [Google Scholar]
- 40.Crombie L, Haigh D, Jones RCF, Mat-Zin AR. Synthesis of the alkaloid homaline in (±) and natural (S,S)-(−) forms, using amination and transamidative ring expansion in liquid ammonia. J Chem Soc, Perkin Trans 1. 1993;1993:2047–2054. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.