Abstract
The sequence of 20 amino acids from the N terminus of Clostridium perfringens epsilon-toxin was determined. Some differences between this sequence and the previously published sequence (A. S. Bhown and A. F. S. A. Habeeb, Biochem. Biophys. Res. Commun. 78:889-896, 1977) were found. A degenerate 23-bp pair oligonucleotide probe was designed from the amino acid sequence data and used to isolate a DNA fragment containing the gene encoding epsilon-toxin (etx) from C. perfringens type B. The gene encoded a protein with a molecular weight of 32,981. Upstream of the gene, promoter sequences which resembled the Escherichia coli sigma 70 consensus sequences were identified. The gene was expressed in E. coli, and the cloned gene product reacted with epsilon-toxin-specific monoclonal antibodies and had a molecular weight and isoelectric point similar to those of the native protein. Downstream of etx, two overlapping open reading frames were identified. Each encoded part of a protein which was homologous to the transposase from Staphylococcus aureus transposon Tn4001. Southern hybridization experiments indicated that the etx gene was found only in C. perfringens types B and D, the types which produce epsilon-toxin.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BATTY I., BULLEN J. J. The effect of Clostridium welchii type D culture filtrates on the permeability of the mouse intestine. J Pathol Bacteriol. 1956 Apr;71(2):311–323. doi: 10.1002/path.1700710206. [DOI] [PubMed] [Google Scholar]
- Bhown A. S., Habeerb A. F. Structural studies on epsilon-prototoxin of Clostridium perfringens type D. Localization of the site of tryptic scission necessary for activation to epsilon-toxin. Biochem Biophys Res Commun. 1977 Oct 10;78(3):889–896. doi: 10.1016/0006-291x(77)90506-x. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Findlay P. R., Johnson M. W. The relationship between base composition and codon usage in bacterial genes and its use for the simple and reliable identification of protein-coding sequences. Gene. 1984 Oct;30(1-3):157–166. doi: 10.1016/0378-1119(84)90116-1. [DOI] [PubMed] [Google Scholar]
- Blaschek H. P., Solberg M. Isolation of a plasmid responsible for caseinase activity in Clostridium perfringens ATCC 3626B. J Bacteriol. 1981 Jul;147(1):262–266. doi: 10.1128/jb.147.1.262-266.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boarer C. D., Sojka M. G., White V. J., Roeder P. L. The production and evaluation of monoclonal antibodies to Clostridium perfringens type D epsilon toxin. J Biol Stand. 1988 Jul;16(3):207–218. doi: 10.1016/0092-1157(88)90008-x. [DOI] [PubMed] [Google Scholar]
- Byrne M. E., Rouch D. A., Skurray R. A. Nucleotide sequence analysis of IS256 from the Staphylococcus aureus gentamicin-tobramycin-kanamycin-resistance transposon Tn4001. Gene. 1989 Sep 30;81(2):361–367. doi: 10.1016/0378-1119(89)90197-2. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Doi R. H., Wang L. F. Multiple procaryotic ribonucleic acid polymerase sigma factors. Microbiol Rev. 1986 Sep;50(3):227–243. doi: 10.1128/mr.50.3.227-243.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairweather N. F., Lyness V. A., Pickard D. J., Allen G., Thomson R. O. Cloning, nucleotide sequencing, and expression of tetanus toxin fragment C in Escherichia coli. J Bacteriol. 1986 Jan;165(1):21–27. doi: 10.1128/jb.165.1.21-27.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Garnier T., Cole S. T. Characterization of a bacteriocinogenic plasmid from Clostridium perfringens and molecular genetic analysis of the bacteriocin-encoding gene. J Bacteriol. 1986 Dec;168(3):1189–1196. doi: 10.1128/jb.168.3.1189-1196.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAUSCHILD A. H. SEPARATION OF CLOSTRIDIUM PERFRINGENS TYPE D TOXINS. J Immunol. 1965 Apr;94:551–554. [PubMed] [Google Scholar]
- Habeeb A. F., Lee C. L., Atassi M. Z. Conformational studies on modified proteins and peptides. VII. Conformation of epsilon-prototoxin and epsilon-toxin from Clostridium perfringens. Conformational changes associated with toxicity. Biochim Biophys Acta. 1973 Oct 18;322(2):245–250. doi: 10.1016/0005-2795(73)90300-0. [DOI] [PubMed] [Google Scholar]
- Habeeb A. F. Studies on epsilon-prototoxin of Clostridium perfringens type D. Physicochemical and chemical properties of epsilon-prototoxin. Biochim Biophys Acta. 1975 Nov 18;412(1):62–69. doi: 10.1016/0005-2795(75)90339-6. [DOI] [PubMed] [Google Scholar]
- Hill L. R. An index to deoxyribonucleic acid base compositions of bacterial species. J Gen Microbiol. 1966 Sep;44(3):419–437. doi: 10.1099/00221287-44-3-419. [DOI] [PubMed] [Google Scholar]
- Minton N. P., Atkinson T., Sherwood R. F. Molecular cloning of the Pseudomonas carboxypeptidase G2 gene and its expression in Escherichia coli and Pseudomonas putida. J Bacteriol. 1983 Dec;156(3):1222–1227. doi: 10.1128/jb.156.3.1222-1227.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara N. Markedly unbiased codon usage in Bacillus subtilis. Gene. 1985;40(1):145–150. doi: 10.1016/0378-1119(85)90035-6. [DOI] [PubMed] [Google Scholar]
- Percival D. A., Shuttleworth A. D., Williamson E. D., Kelly D. C. Anti-idiotypic antibody-induced protection against Clostridium perfringens type D. Infect Immun. 1990 Aug;58(8):2487–2492. doi: 10.1128/iai.58.8.2487-2492.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P., Peterkofsky A., McKenney K. Translational efficiency of the Escherichia coli adenylate cyclase gene: mutating the UUG initiation codon to GUG or AUG results in increased gene expression. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5656–5660. doi: 10.1073/pnas.82.17.5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sakurai J., Nagahama M. Amino groups in Clostridium perfringens epsilon prototoxin and epsilon toxin. Microb Pathog. 1986 Oct;1(5):417–423. doi: 10.1016/0882-4010(86)90003-3. [DOI] [PubMed] [Google Scholar]
- Sakurai J., Nagahama M. Carboxyl groups in Clostridium perfringens epsilon toxin. Microb Pathog. 1987 Dec;3(6):469–474. doi: 10.1016/0882-4010(87)90017-9. [DOI] [PubMed] [Google Scholar]
- Sakurai J., Nagahama M. Role of one tryptophan residue in the lethal activity of Clostridium perfringens epsilon toxin. Biochem Biophys Res Commun. 1985 Apr 30;128(2):760–766. doi: 10.1016/0006-291x(85)90112-3. [DOI] [PubMed] [Google Scholar]
- Sakurai J., Nagahama M. The inactivation of Clostridium perfringens epsilon toxin by treatment with tetranitromethane and N-acetylimidazole. Toxicon. 1987;25(3):279–284. doi: 10.1016/0041-0101(87)90256-x. [DOI] [PubMed] [Google Scholar]
- Sakurai J., Nagahama M. Tryptophan content of Clostridium perfringens epsilon toxin. Infect Immun. 1985 Jan;47(1):260–263. doi: 10.1128/iai.47.1.260-263.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinedling S., Gayle M., Pribnow D., Gold L. Mutations affecting translation of the bacteriophage T4 rIIB gene cloned in Escherichia coli. Mol Gen Genet. 1987 May;207(2-3):224–232. doi: 10.1007/BF00331582. [DOI] [PubMed] [Google Scholar]
- Worthington R. W., Mülders M. S. Physical changes in the epsilon prototoxin molecule of Clostridium perfringens during enzymatic activation. Infect Immun. 1977 Nov;18(2):549–551. doi: 10.1128/iai.18.2.549-551.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
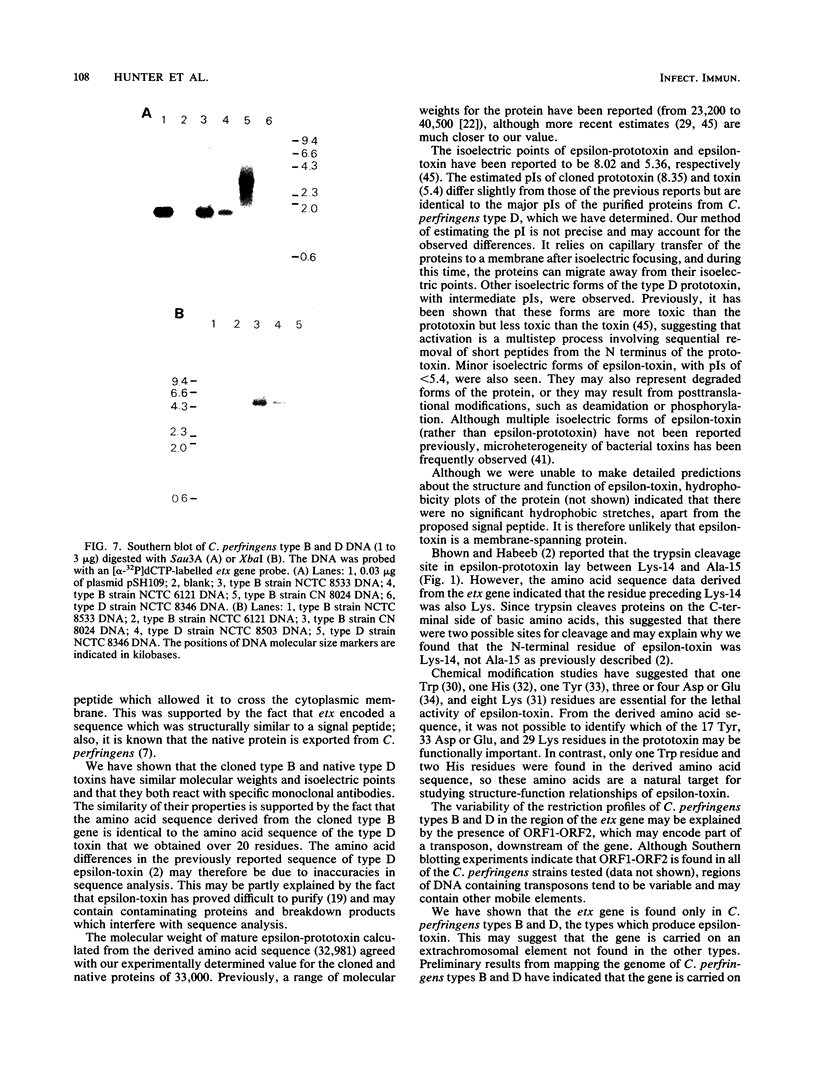
- von Heijne G. How signal sequences maintain cleavage specificity. J Mol Biol. 1984 Feb 25;173(2):243–251. doi: 10.1016/0022-2836(84)90192-x. [DOI] [PubMed] [Google Scholar]