Abstract
Background
Multiple studies have shown that genetic variation in the α-2 subunit of the GABA-A receptor (GABRA2) is associated with risk for alcohol dependence. Recent reports have suggested that GABRA2 may exert its influence on dependence through factors such as sensitivity to alcohol's intoxicating effects and that GABRA2 may also contribute to a common underlying genetic vulnerability to both alcohol and drug dependence. The present study tested for association between GABRA2 and alcohol dependence, smoking and illicit drug use within the Australian population.
Methods
We genotyped 11 single nucleotide polymorphisms within or flanking GABRA2 in 4597 subjects (34.6% males) from 2618 families comprising 814 monozygotic pairs, 1177 dizygotic pairs and 627 twins whose co-twin did not participate. Family-based association tests were conducted for binary and quantitative measures of alcohol dependence, smoking, cannabis and other illicit drug use.
Results
We observed evidence of association (p < 0.05) between multiple GABRA2 SNPs and quantitative measures of alcohol dependence, including symptom scores and principal component factor scores from the 9 criteria for DSM-IV alcohol dependence, in the opposite direction to that previously reported. In contrast, GABRA2 was not associated overall with dichotomous measure of alcohol dependence, nor with smoking, cannabis or illicit drug use.
Conclusions
The GABRA2 allelic associations found in clinical case-control studies have detectable but minor effects on DSM-defined alcohol dependence in the general community. Systematic comparisons of allelic effects on alcohol dependence in clinical cases and in the general community are required.
Keywords: alcoholism, cannabis, drug dependence, GABRA2, smoking
INTRODUCTION
A combination of suggestive linkage findings (Long et al., 1998; Porjesz et al., 2002; Reich et al., 1998; Williams et al., 1999) and biological plausibility (Buck, 1996; Davies, 2003; Grobin et al., 1998; Koob, 2004; Krystal et al., 2006) led the Collaborative Study on the Genetics of Alcoholism (COGA) to conduct the first fine mapping association study of the GABAA receptor gene cluster, investigating alcohol dependence (AD) and β-EEG activity in a cohort of United States (U.S.) families enriched for alcoholism (Edenberg et al., 2004). The GABAA receptor gene cluster on chromosome 4 comprises four genes (GABRG1, GABRA2, GABRA4 and GABRB1) where the strongest linkage finding in relation to the AD phenotype was detected with markers (D4S3242, D4S2393) close to GABRB1 (Long et al., 1998; Williams et al., 1999). The family-based COGA study genotyped 69 single nucleotide polymorphisms (SNPs) across the gene cluster and only detected significant allelic and haplotypic association with SNPs in GABRA2, where the region of strongest association with alcohol dependence extended from intron 3 past the 3’ end of GABRA2. A recent study by Covault and colleagues (2008) has extended the observed association from the GABRA2 locus to potential regulatory regions immediately upstream of GABRG1 (downstream of GABRA2). Independent studies have confirmed the association between GABRA2 and AD in other Caucasian populations from the U.S. (Covault et al., 2004), Russia (Lappalainen et al., 2005) and Germany (Fehr et al., 2006; Soyka et al., (2008)) using a case-control approach, while others groups have found no association (Drgon et al., 2006; Matthews et al., 2007)
With respect to mechanisms for this effect, Pierucci-Lagha et al. (2005) and Haughey et al. (2008) have proposed that increased risk for alcohol dependence may be due to GABRA2 moderating the subjective effects of alcohol (an individual's susceptibility to intoxication). Other evidence suggests that GABRA2 may contribute to a common underlying genetic vulnerability to alcohol and drug dependence. It is known from twin studies that there are shared genetic risk factors between alcohol and nicotine dependence (Madden et al., 2000; True et al., 1999) as well as alcohol and drug dependence (Kendler et al., 2003). The COGA group recently reported an association between GABRA2 and drug dependence, including illicit drugs and cannabis (Agrawal et al., 2006). Importantly, subsequent analysis of the COGA dataset showed that the initial association between GABRA2 and AD was primarily driven by those individuals with co-occurring illicit drug dependence and when those individuals were excluded, evidence for association with AD disappeared (Agrawal et al., 2006). In contrast, excluding European American AD cases comorbid for drug (cocaine and/or opioid) dependence increased the strength of association between GABRA2 and AD (Covault et al., 2004). However, while Drgon et al. (2006) did not find association between six GABRA2 SNPs and AD in their European American case and control sample, they did observe a trend towards significance (p = 0.052) with polysubstance abuse among African Americans.
Other studies have revealed further complexity in the relationship between GABRA2 and AD risk where GABRA2 modulates risk for other co-occurring psychiatric disorders including conduct disorder (Dick et al., 2006) and anxiety (Enoch et al., 2006) and the presence of comorbidity within AD samples has been shown to affect the likelihood of observing an association between GABRA2 and AD. However, the interaction can result in conflicting findings. While Covault and colleagues (2004) observed stronger association with AD in a restricted sample of alcoholics without comorbid major depression, Matthews et al. (2007) found no evidence of association between GABRA2 and AD in alcoholic subjects selected for minimal primary comorbidity for drug dependence and other psychiatric disorders.
In the present study we primarily test for association between eleven SNPs spanning GABRA2, and alcohol dependence and alcohol consumption measures, in a sample of 4597 individuals representative of the general Australian population. With data available on tobacco, cannabis and illicit drug use measures, we then expanded our analyses to test whether the pathway from GABRA2 genetic variation to alcohol-related outcomes may also modulate risk for these frequently co-occurring substance-use disorders.
MATERIALS AND METHODS
Participants
Participants were recruited from 1992 to 1995 for a telephone interview based twin study conducted at the Queensland Institute of Medical Research (QIMR; Heath et al., 1997). This interview was based upon an Australian modified version (SSAGA-OZ) of the Semi-Structured Assessment for the Genetics of Alcoholism instrument (SSAGA; Bucholz et al., 1994) designed for genetic studies of alcoholism. The SSAGA is a psychiatric interview which retrospectively assesses physical, psychological and social manifestations of AD along with several other psychiatric disorders (American Psychiatric Association, 1994) and has undergone both reliability and validity testing (Bucholz et al., 1994; Hesselbrock et al., 1999). A total of 4597 subjects (34.6% males) from 2618 families comprising 814 (583 female and 231 male) monozygotic (MZ) pairs, 1177 (482 female, 198 male and 491 opposite sex) dizygotic (DZ) pairs and 627 twins (38.8% male) whose co-twin did not particpate were included in genetic analysis. The participants were predominantly of Northern European ancestry (>90%) and aged 26 to 89 years (mean age was 43.8 ± 11.5 years) at the time of testing. Subjects gave written informed consent and provided blood samples from which DNA was isolated using standard protocols. Genetic studies were approved by the QIMR Human Research Ethics committee. Zygosity of same-sex twins was assessed using nine polymorphic DNA microsatellite markers (AmpF1STR Profiler Plus Amplification Kit, Applied Biosystems, Foster City, CA) and three blood groups (ABO, MNS, and Rh), giving a probability of correct assignment greater than 99.99% (Nyholt, 2006a).
Phenotypes
Alcohol
Diagnostic assessment of lifetime history of AD was ascertained from the adapted SSAGA interview data. Alcohol dependence was diagnosed by computer algorithms based on the criteria of the Third Revised (DSM-IIIR) and Fourth (DSM-IV) Diagnostic and Statistical Manuals of the American Psychiatric Association (American Psychiatric Association, 1987; American Psychiatric Association, 1994). Similarly, DSM-IV alcohol abuse was assigned by computer algorithm. In addition to the dichotomous definition of AD, more informative (quantitative) measures were calculated and a summary of the alcohol-related traits examined in this study is given in Table1:
Number of criteria met for DSM-IIIR (sumAD3r) and DSM-IV (sumAD4) alcohol dependence
Principal component factor analysis of the 7 criteria for DSM-IV (one factor with an eigenvalue >1; factAD4) and 9 criteria for DSM-IIIR (two factors had an eigenvalue >1; factAD3r_1 and factAD3r_2): the factAD4 factor accounted for 37.7% of the variance in the symptom count variable while the factAD3r_1 and factAD3r_2 scores accounted for 34.5% and 13.0%, respectively.
Alcohol consumption: frequency and quantity of alcohol consumption; maximum number of alcoholic drinks consumed in a single day ever and in a single day within the past 12 months.
Table 1.
Summary of the sample demographics and alcohol-related traits, by sex.
| Item | Assessment | Male | Female |
|---|---|---|---|
| Sample | Number of participants, by sex | 1592 | 3005 |
| Age | Age at time of interview (years) | 42.6 ± 10.9 | 44.3 ± 11.8 |
| (Age Range, years) | (26−89) | (28−84) | |
| Abstainer | Has never consumed alcohol | 36 (2.2%) | 87 (2.9%) |
| Current | Has consumed alcohol in the previous 12 months | 1474 (92.6%) | 2784 (92.6%) |
| Freq | Drinking frequency during the past 12 months (days) | 124.6 ± 112.8 | 82.7 ± 105.2 |
| (percentage who drink on a daily basis) | (7.6%) | (5.5%) | |
| (percentage who drink on a weekly basis) | (67.0%) | (44.9%) | |
| TypDy | Number of drinks typically consumed on days when they drank alcohol in the past 12 months | 2.9 ± 2.3 | 1.9 ± 1.5 |
| (percentage of men who consume >6 drinks and women who consume >3 drinks) | (4.0%) | (9.5%) | |
| Quant | Quantity of drinks consumed in previous 12 months (Freq*TypDy) | 406.9 ± 537.8 | 181.1 ± 277.3 |
| MaxDr12 | Most drinks consumed in a single day in the previous 12 months | 7.6 ± 6.6 | 3.7 ± 3.2 |
| MaxDr | Most drinks ever consumed in a single day | 16.7 ± 11.5 | 7.9 ± 6.0 |
| sumAbuse | Number of DSM-IV alcohol abuse criteria met (out of four) | 0.55 ± 0.82 | 0.11 ± 0.40 |
| (percentage with no symptoms) | (61.4%) | (90.8%) | |
| sumAD3r | Number of DSM-IIIR alcohol dependence criteria met (out of seven) | 2.10 ± 2.12 | 0.78 ± 1.45 |
| (percentage with no symptoms) | (35.7%) | (70.1%) | |
| sumAD4 | Number of DSM-IV alcohol dependence criteria met (out of nine) | 1.30 ± 1.49 | 0.48 ± 0.96 |
| (percentage with no symptoms) | (39.6%) | (71.4%) | |
| Abuse | Diagnosed with DSM-IV alcohol abuse | 584 (38.8%) | 267 (9.2%) |
| AD3r | Diagnosed with DSM-IIIR alcohol dependence | 375 (23.6%) | 172 (5.7%) |
| AD4 | Diagnosed with DSM-IV alcohol dependence | 290 (18.2%) | 131 (4.4%) |
| factAbuse | Single principal component factor score from DSM-IV alcohol abuse criteria | 0.42 ± 1.44 | −0.20 ± 0.62 |
| factAD3r_1 | First principal component factor score from DSM-IIIR alcohol dependence criteria | 0.43 ± 1.26 | −0.20 ± 0.76 |
| factAD3r_2 | Second principal component factor score from DSM-IIIR alcohol dependence criteria | 0.45 ± 1.24 | −0.21 ± 0.77 |
| factAD4 | Single principal component factor score from DSM-IV alcohol dependence criteria | −0.28 ± 1.28 | 0.13 ± 0.81 |
Cigarette smoking
The SSAGA did not assess smoking related measures, but information on smoking-related phenotypes were available from a Health and Lifestyle Survey mailed questionnaire that the twins responded to in 1989 (Whitfield et al., 2000). Two smoking related measures were coded:
(d) Current smoking: a dichotomous item reflecting whether the participant was a current smoker at the time of questionnaire assessment, with never smokers set to missing;
(e) Pack years of cigarettes smoked: a quantitative measure calculated as: Pack years = (cigarettes smoked on an average day x total years smoked) / 20; the number of cigarettes smoked on an average day was calculated from a categorical variable ranging from 1 (never smoked) to 6 (smoked 40+ cigarettes per day).
Illicit drug use
Diagnostic criteria for abuse and dependence were not included in the SSAGA interview. Therefore, we used the following assessments of illicit drug use:
(f) Quantitative cannabis use: An ordinal measure of cannabis use was created from the participant's report of how often in their entire lifetime they had used cannabis. Participants could respond with any number, but were also given the option of responding “Too many times to count” which was assumed to be greater than 950. These responses were then collapsed into a five category ordinal scale, defined separately for males and females. For males these categories were 1−2 times, 3−9, 10−49, 50−499, 500+; for females they were 1 time, 2−4 times, 5−11, 12−99, 100+. Frequency was also coded in all participants, including lifetime abstainers, who were coded as 0.
(g) Other illicit drug use: A dichotomous item reflecting whether the participant had used an illicit drug other than cannabis, including cocaine, stimulants, sedatives, opiates, solvents, hallucinogens, phencyclidine (PCP) or other illicit drugs, even once in their lifetime.
(h) Drug problems: A dichotomous item assessing severe liability to drug abuse/dependence was coded positively if the respondent said that they had: ‘... ever discussed any other drug problem with any professional’ or if they had ‘... ever been treated for a drug problem’.
Genotyping
Fourteen single nucleotide polymorphisms (SNPs) across the GABRA2 locus were selected on the basis of previous work completed by our group (Lind et al., 2008). Briefly, 41 SNPs within or flanking GABRA2 were genotyped in an independent Australian twin sample (Alcohol Challenge Twin Study) that completed an alcohol challenge test (Martin et al., 1985). The 41 SNPs were selected on the basis of data available at the time from (1) association studies (e.g., Covault et al., 2004; Edenberg et al., 2004; Pierucci-Lagha et al., 2005) and (2) the International HapMap Project public database (http://www.hapmap.org/; Phase II dbSNP Build 124). Linkage disequilibrium data from this study indicated that a reduced set of fourteen haplotype-tagging SNPs would provide appropriate coverage (r2 ≥ 0.95) of GABRA2. Three SNPs (rs279871, rs279836, rs497068) failed during the assay design or provided unreliable genotype data and were excluded. The locations of the eleven remaining SNPs typed in the study are shown in Figure 1 and SNP information, including the observed minor allele frequency (MAF), is described in Table 2.
Figure 1. A schematic representation of GABRA2 gene structure, linkage disequilibrium and genotyped markers.
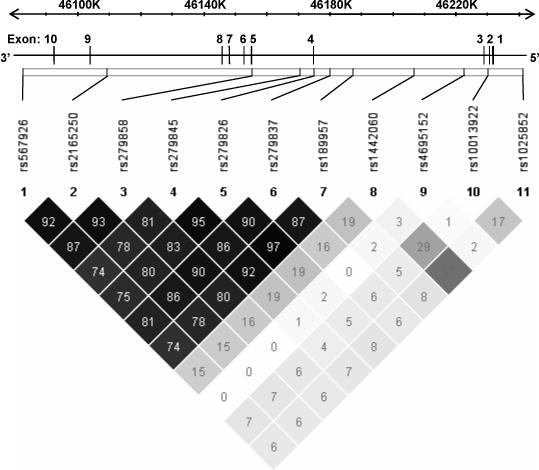
The gene structure of GABRA2 is shown with exons numbered and relative exon size denoted by the width of the vertical bars. The 11 single nucleotide polymorphisms analyzed in this study are shown in relation to their location across GABRA2 and regions of low to high pair-wise linkage disequilibrium (LD), as measured by the r2 statistic, are represented by light grey to black shading, respectively.
Table 2.
GABRA2 marker information, including genetic map position, location within GABRA2 and minor allele frequency.
| SNP No. | SNP | Chromosomal Locationa | Functional Locationb | Gene Position | Alleles |
MAF | |
|---|---|---|---|---|---|---|---|
| Minorc | Major | ||||||
| 1 | rs567926 | 45,936,526 | 150,176 | 3′UTR | C | T | 0.418 |
| 2 | rs2165250 | 45,963,241 | 123,461 | Intron 8 | C | T | 0.420 |
| 3 | rs279858 | 46,009,350 | 77,352 | Exon 5 | G | A | 0.433 |
| 4 | rs279845 | 46,024,480 | 62,222 | Intron 4 | T | A | 0.435 |
| 5 | rs279826 | 46,028,966 | 57,736 | Intron 4 | C | T | 0.452 |
| 6 | rs279837 | 46,034,080 | 52,622 | Intron 3 | C | T | 0.432 |
| 7 | rs189957 | 46,041,436 | 45,266 | Intron 3 | C | T | 0.449 |
| 8 | rs1442060 | 46,060,824 | 25,878 | Intron 3 | T | C | 0.489 |
| 9 | rs4695152 | 46,076,414 | 10,288 | Intron 3 | C | G | 0.039 |
| 10 | rs10013922 | 46,084,118 | 2,584 | Intron 2 | C | A | 0.261 |
| 11 | rs1025852 | 46,095,088 | −8,386 | Promoter | T | C | 0.368 |
MAF, minor allele frequency; SNP, single nucleotide polymorphism; UTR, untranslated region.
Position in nucleotides as estimated in dbSNP (Build 128).
Position relative to the transcription start site at 46,086,702 on chromosome 4 (dbSNP Build 128)
The allele with the lowest frequency.
Genotyping forward and reverse polymerase chain reaction (PCR) primers and a extension primer were designed using the Sequenom MassARRAY Assay Design (version 3.0) software (Sequenom Inc., San Diego, CA) and purchased from Bioneer Corporation (Daejeon, Korea). Genotyping was carried out in standard 384-well plates with 12.5 ng genomic DNA used per sample. We used a modified Sequenom protocol where half reaction volumes were used in each of the PCR, SAP and iPLEX stages giving a total reaction volume of 5.5 μL. The iPLEX reaction products were desalted by diluting samples with 18 μL of water and 3 μL SpectroCLEAN resin (Sequenom) and then were spotted on a SpectroChip (Sequenom), processed and analyzed on a Compact MALDI-TOF Mass Spectrometer by MassARRAY Workstation software (version 3.3) (Sequenom). Allele calls for each 384-well plate were reviewed using the cluster tool in the SpectroTyper software (Sequenom) to evaluate assay quality. Genotype error checking, sample identity and zygosity assessment and Hardy-Weinberg equilibrium analyses were completed in PEDSTATS (Wigginton and Abecasis, 2005).
Statistical Analyses
Association Analyses
Quantitative trait analysis was performed in QTDT (Abecasis et al., 2000a; Abecasis et al., 2000b), with 22 traits examined. All alcohol-related quantitative traits were first transformed to normality using a piecewise normal transformation and were corrected for sex and age effects by fitting covariates in the regression model. Drug-related quantitative traits (i.e. pack years and quantitative cannabis use) were log-transformed, the effects of sex, age (linear and quadratic) and interactions between sex and age (linear and quadratic) were tested in a regression framework, significant covariates were regressed out and the resulting residuals were used for quantitative transmission disequilibrium tests. Three types of association tests were performed. An analysis robust to population stratification was conducted using the orthogonal “within” test in QTDT. Since parents were not genotyped this analysis only included families with two DZ offspring. To account for the fact that transmissions were not independent in the DZ families due to the presence of linkage in DZ pairs, a permutation procedure (10,000 permutations) was used to correct the p-values for the orthogonal test. Secondly, analyses of all 2618 families were performed using a test of total association which considers transmissions between and within families. For the test of total association, families with either one twin or two MZ twins only contribute to the ‘between’ component and a simple permutation procedure can not be applied. Thus, non-independent transmissions were corrected for by modeling linkage and association within a maximum likelihood framework. MZ twin status was included in QTDT analyses by adding zygosity status to the data file. Thirdly, quantitative haplotypic association was tested in the QPDTPHASE module of the UNPHASED statistical package (Dudbridge, 2003) which only allows analysis of the ‘within’ component in DZ families. To reduce multiple testing, haplotype testing was only performed on four alcohol-related traits of interest (factAD3r_1, factAD4, sumAbuse, sumAD3r).
Binary trait analysis was performed for comparison with the above quantitative trait analysis and also for comparison with the work of other groups. Association of DSM-IIIR and DSMIV AD was examined using the UNPHASED statistical package in two stages. First, for DZ families, a pedigree disequilibrium (PDT) style test was used, in the PDTPHASE module, to test for over-transmission of allele to offspring (a ‘within’ family test that should be robust to any population stratification effects). Second, for MZ families, pairs with discordant phenotypes were set to phenotype unknown while pairs with concordant phenotypes were not changed. Subsequently, one MZ twin per family was included for each trait analyzed in COCAPHASE. Since no parents were genotyped, each MZ twin was treated as unrelated and a case-control test was implemented.
Pair-wise marker-marker linkage disequilibrium (LD) was assessed using the D' and r2 statistic in Haploview 3.31 (Barrett et al., 2005). Many of the traits studied were correlated and there was substantial LD across GABRA2 (see Figure 1). As a result the effective number of statistical tests done was substantially less than the actual number of tests. To take account of both of these factors we estimated the effective number of tests by utilizing permutations in QTDT. Since QTDT only implements permutations for the “within” test, we cannot do this for the “total” test (but see below). Two p-values are available from the permutation routine; one p-value which corrects for all tests done, p_corrected, and another p-value for each trait/marker combination singly, p_uncorrected. If all traits and markers were uncorrelated then a permutation procedure would yield a p-value equivalent to a Bonferroni correction; that is, p_corrected = 1 – (1-p_uncorrected)^n where n denotes the number of tests done. An estimate of the effective number of independent “within” tests is hence n_effective = log(1-p_corrected)/log(1-p_uncorrected), approximately 110 (compared with 242 non-independent tests in total). Making the simplifying assumptions that 1) the “total” tests exhibit similar correlation between tests as the “within” test and 2) that the “within” and “total” tests are independent, the total number of effectively independent tests is ∼220. Since the “within” and “total” tests are in fact correlated, the most appropriate correction for multiple testing based on the “effective number of independent tests” would be correction for between 110 and 220 tests. That is, a p-value smaller than 0.00045 (and perhaps as small as 0.000225) is required for study wide significance.
Survival analyses
Cox proportional hazards models were set up in STATA (StataCorp, 2003). Twin clustering was accounted for by allowing members of a twin pair to cluster on their family number, which allows standard errors to be adjusted using a robust variance estimator. The event of interest for the survival analysis was one or more symptoms of alcohol dependence and age of onset of the first DSM-IIIR alcohol dependence symptom was the time to event. Individuals who had never used alcohol even once in their lifetime and those who used alcohol but, at the time of interviews, reported no dependence symptoms, were censored and their time to event was set to their age at interview.
The hazard ratios for the association between genotype and age at first symptom of alcohol dependence were computed in a univariate model and then, in a model that adjusted for sex as well as an interaction between sex and genotype. Genotypes that were significantly associated with alcohol dependence symptoms in QTDT analyses (rs279858 and rs279845) were selected for the survival analyses. For the first series of analyses (Models 1−3), genotype was coded as 0 (no copies of risk allele from QTDT), 1 (1 copy of risk allele from QTDT) and 2 (2 copies of risk allele from QTDT). In a second series of analyses (Models 4−6), genotype was coded under a dominance model as 0 (no copies) and 1 (1 or 2 copies of risk allele). In models 1 and 4, only genotype, coded as 0, 1, 2 and 0,1 respectively, was entered into the model. In models 2 and 5, both genotype and sex were included while in models 3 and 6, the effects of genotype, sex and genotype*sex were examined.
RESULTS
Descriptive
A total of 4597 twin participants provided blood samples and completed the SSAGA-OZ questionnaire. 2618 families were included in the analysis. Since parents were not genotyped, only families containing a non-identical sibling pair contributed to the “within” test of association. 1177 families included a DZ pair and hence were potentially informative for both the “within” and the “total” test of association. 814 families included an MZ pair and hence contributed information to just the “total” test. Similarly, 627 families had just a single offspring (i.e. trios) and these contributed information to just the “total” test. The mean age of the study population was 43.8 ± 11.5 years, ranging from 26 to 89 years. The mean age of female participants was significantly higher (44.3 vs 42.6) than for males (p = 0.001) (see Table 1). While a small percentage (<3%) of the sample had always abstained from alcohol, the majority (∼93%) had consumed alcohol in the previous year, with 7.6% and 5.5% of male and females, respectively, reporting that they drank alcohol on a daily basis. With respect to alcohol dependence, male participants were approximately three fold more likely to meet a lifetime diagnosis of either DSM-IIIR (23.6%) or DSM-IV (18.2%) dependence than female twins. However, ∼37% of males and ∼70% of females did not report any symptoms of alcohol dependence. Similarly, most twins did not meet lifetime criteria for DSM-IV alcohol abuse.
36.4 and 44.5% of men and women who had ever smoked a cigarette reported current smoking. The mean pack years smoked was 12.7 [range 0−97] and 15 [range 0−89] in women and men respectively. Additionally, 19.4 and 34.7% of the women and men respectively reported lifetime cannabis use while 12.7% of women and 16.9% of men reported use of other illicit drugs. The mean lifetime number of times that the users had consumed cannabis was 19.5 and 50.6 in the women and men respectively.
Marker information including genetic map position, location within GABRA2 and the minor allele frequencies of the eleven SNPs genotyped within our Australian twin sample are listed in Table 2. Call rates of ≥98% were achieved for all SNPs except rs567926 where 96.9% of the sample was successfully genotyped. No SNPs showed significant deviations from Hardy-Weinberg equilibrium (HWE) at a p < 0.01 level. Discordant genotypes between MZ twins were identified using PEDSTATS and made up 0.12% of the data. The physical locations of, and linkage disequilibrium (LD) between, the 11 SNPs typed across the GABRA2 gene are presented schematically in Figure 1. Substantial LD was observed between seven markers spanning from intron 3 to the 3'UTR, with lower levels of LD (r2 < 0.3) observed 5’ to this major haplotype block. This is consistent with LD data (on a smaller number of people) from the HapMap database for CEPH families of European origin.
Single-SNP Association Analyses
Alcohol-related measures
Within-family association results utilizing 10,000 permutations for two quantitative measures of DSM-IV alcohol dependence are reported in Table 3. The strongest evidence of association was found for the synonymous SNP rs279858 located in exon 5 (p = 0.007), with the G allele conferring a lower DSM-IV factor score (factAD4). With a correlation of 0.978 between the DSM-IV factor score and the number of DSM-IV AD symptoms experienced (sumAD4), the G allele also reduced the sumAD4, where the average number of symptoms reported in the study were 0.78, 0.76 and 0.71 in individuals with 0, 1 and 2 G-alleles, respectively. The T-allele of rs279845 also conferred lower factAD4 and sumAD4 scores. The directions of effect for both rs279858 and rs279845 are opposite to that reported in previous studies (Covault et al., 2004; Edenberg et al., 2004; Fehr et al., 2006; Lappalainen et al., 2005). Three other SNPs located in intron-3 and intron-4 were nominally associated with the factAD4 and sumAD4 measures. Similar levels of association (p < 0.05) were observed for four of the SNPs with the closely related “sum of DSM-IIIR AD symptoms” phenotype (data not shown). The “total” association results for the quantitative measures shown in Table 3 were non-significant. Although the “within” association is not significant after taking account of multiple testing (with a threshold level of significance set at 0.00045, see Subjects and Methods), multiple studies reporting association between GABRA2 and AD have been published. Our results provide limited support for a role of GABRA2 in risk for alcoholism.
Table 3.
Permutation-based GABRA2 “within” association results for two quantitative measures of DSM-IV alcohol dependence.
| SNP No. | SNP | Gene Position |
p-valuea |
|
|---|---|---|---|---|
| sumAD4 | factAD4 | |||
| 1 | rs567926 | 3′UTR | 0.21 | 0.18 |
| 2 | rs2165250 | Intron 8 | 0.16 | 0.12 |
| 3 | rs279858 | Exon 5 | 0.01 | 0.007 |
| 4 | rs279845 | Intron 4 | 0.01 | 0.01 |
| 5 | rs279826 | Intron 4 | 0.02 | 0.02 |
| 6 | rs279837 | Intron 3 | 0.11 | 0.10 |
| 7 | rs189957 | Intron 3 | 0.03 | 0.03 |
| 8 | rs1442060 | Intron 3 | 0.04 | 0.04 |
| 9 | rs4695152 | Intron 3 | 0.52 | 0.80 |
| 10 | rs10013922 | Intron 2 | 0.24 | 0.24 |
| 11 | rs1025852 | Promoter | 0.52 | 0.45 |
factAD4, single principal component factor score from DSM-IV alcohol dependence criteria; SNP, single nucleotide polymorphism; sumAD4, number of DSM-IV alcohol dependence criteria met; UTR, untranslated region.
p-values reported after 10,000 permutations run to correct for family structure.
Finally, while the correlation between sumAD3r and factAD3r_1 is strong (0.911), the correlation falls to 0.370 with the second factor score (factAD3r_2) which primarily loads onto the two DSM-IIIR criteria that address alcohol withdrawal. We did not observe an association with factAD3r_1 and only detected nominal association (p = 0.03) between one SNP in intron 3 (rs4695152) and the second factor score. Previous association studies have found evidence that GABRA2 genetic variation modulates aspects of alcohol withdrawal (Fehr et al., 2006; Soyka et al., 2008) and this relationship may explain, to an extent, our finding with the factAD3r_2 score. For both “within” and “total” tests, no significant association (smallest p-value ∼0.01 before correction for multiple testing) was detected between the alcohol use measures, including quantity and frequency of consumption, and SNPs in GABRA2.
Subsequent binary analyses of DSM-IIIR and DSM-IV alcohol dependence (AD3r, AD4) diagnoses were run in DZ families to permit within-family tests of association to be performed. The purpose of these analyses was to be able to directly compare our findings with those of other groups who only analysed the dichotomous diagnoses of AD. The number of affected (case) individuals in the entire sample is as follows: AD3r (959 cases) and AD4 (421). Within DZ families only, however, the number was reduced to 540 (AD3r) and 224 (AD4) cases; we did not observe significant association between either binary measure and the five SNPs of interest previously identified. Similarly, we did not observe any association with AD3r or AD4 in our MZ sample of unrelated cases and controls.
Smoking and drug use measures
For both log-transformed pack years and quantitative cannabis use, sex and age were significantly associated and consequently, were regressed out. Men reported greater smoking in pack years (β=−0.11, se=0.05) and a higher frequency of cannabis use (β=−0.14, se=0.02) while older individuals reported more pack years smoked (β=0.03, se=0.002) but reported less cannabis use (β=−0.14, se=0.02). For the binary traits, with the exception of self-reported history of drug-related problems (N=2 affected MZ twins), we were able to use both DZ pairs and a single MZ from a concordant affected pair (in a case-control framework) to test for association. For current smoking status, 270 DZ (89 concordant affected) pairs and 176 (84 concordant affected) representative members from a concordant MZ pair were available. No significant association was noted in the within-family analysis of smoking or illicit drug use measures and SNPs in GABRA2. However, in the MZ only sample of unrelated cases and controls, current smoking status in people who have ever smoked was associated with 7 SNPs in GABRA2 (see Table 4), the same that were associated with quantitative measures of alcohol dependence in Table 3. Our most significant finding was with rs279826 (p = 0.007), however in the DZ only analyses, the corresponding p-value for this analysis was p = 0.69.
Table 4.
GABRA2 association results for current smoking status using DZ sibling pairs (PDTPHASE) and unrelated cases and controls from MZ pairs.
| SNP No. | SNP | Gene Position |
p-value |
|
|---|---|---|---|---|
| DZ siblings | Case-Control (from MZ pairs) | |||
| 1 | rs567926 | 3′UTR | 0.69 | 0.02 |
| 2 | rs2165250 | Intron 8 | 0.89 | 0.04 |
| 3 | rs279858 | Exon 5 | 0.89 | 0.02 |
| 4 | rs279845 | Intron 4 | 0.29 | 0.02 |
| 5 | rs279826 | Intron 4 | 0.69 | 0.007 |
| 6 | rs279837 | Intron 3 | 0.79 | 0.007 |
| 7 | rs189957 | Intron 3 | 0.79 | 0.009 |
| 8 | rs1442060 | Intron 3 | 0.66 | 0.23 |
| 9 | rs4695152 | Intron 3 | 0.32 | 0.27 |
| 10 | rs10013922 | Intron 2 | 0.27 | 0.55 |
| 11 | rs1025852 | Promoter | 0.99 | 0.56 |
DZ, dizygotic; MZ, monozygotic; SNP, single nucleotide polymorphism; UTR, untranslated region.
Haplotypic Association Analyses
Using a sliding window method, a series of two- and three-SNP haplotypes were analyzed in three quantitative phenotypes: factAD4, factAD3r, sumAbuse (the 1177 DZ families were used for this analysis). For factAD4, the smallest p-value for any 2-SNP haplotype was between rs279858 and rs279845 (p = 0.01, uncorrected for multiple testing). This is no better than the best single marker result (p = 0.007). Haplotypes for the other variables all produced less significant results than for the single markers. Haplotype association analysis between sumAD3r and the LD block spanning SNPs 1−7 (Table 2) was not significant, with two complementary common (>5%) haplotypes observed: T-T-A-A-T-T-T (59.1%), C-C-G-T-C-C-C (36.5%) where the rs279858 and rs279845 alleles are highlighted in bold.
Due to the association between current smoking status and SNPs in GABRA2, we also conducted haplotype tests on the unrelated MZ case-control sample (dropping haplotypes with frequencies < 0.01). Sliding windows revealed the most significant 2-SNP haplotype (p = 0.01) to be between rs2165250 and rs279858 with overtransmission of T-G (64%) to the controls. Haplotype association with the 7-SNP haplotype showed evidence for overtransmission of the T-T-G-A-T-T-T (63%) to the controls/ex-smokers and of the complementary C-C-A-T-C-C-C (47%) to the cases/current smokers was noted (global p-value of 0.03) where the rs279845 and rs279826 alleles are highlighted in bold.
Survival Analyses
As described in the methods section, the full set of twins was used in the survival analysis (4597 individuals). Two SNPs were selected for survival analyses: rs279858 and rs279845, our two strongest signals in the association analyses. For both rs279858 (A/G) and for rs279845 (A/T), A was the risk allele. With respect to rs279858, having 1 or more copies of the A allele was significantly associated with age at first alcohol dependence symptom (p = 0.014) with A/A individuals at increased risk when compared to A/G individuals, and the G/G individuals at least risk (Figure 2a). After adjustment for sex (models 2 and 5 in Table 5), which was significantly associated with age at onset, genotype was still associated with age at onset. However, an interaction with sex was not significant (Models 3 and 6, which included the effects of genotype, sex and their interaction in Table 5) and could be dropped from the model. An additive model fitted well – individuals with two copies of the risk allele were more likely to have an earlier age of onset of alcohol dependence symptoms, when compared with those carrying one copy of the risk allele.
Figure 2.
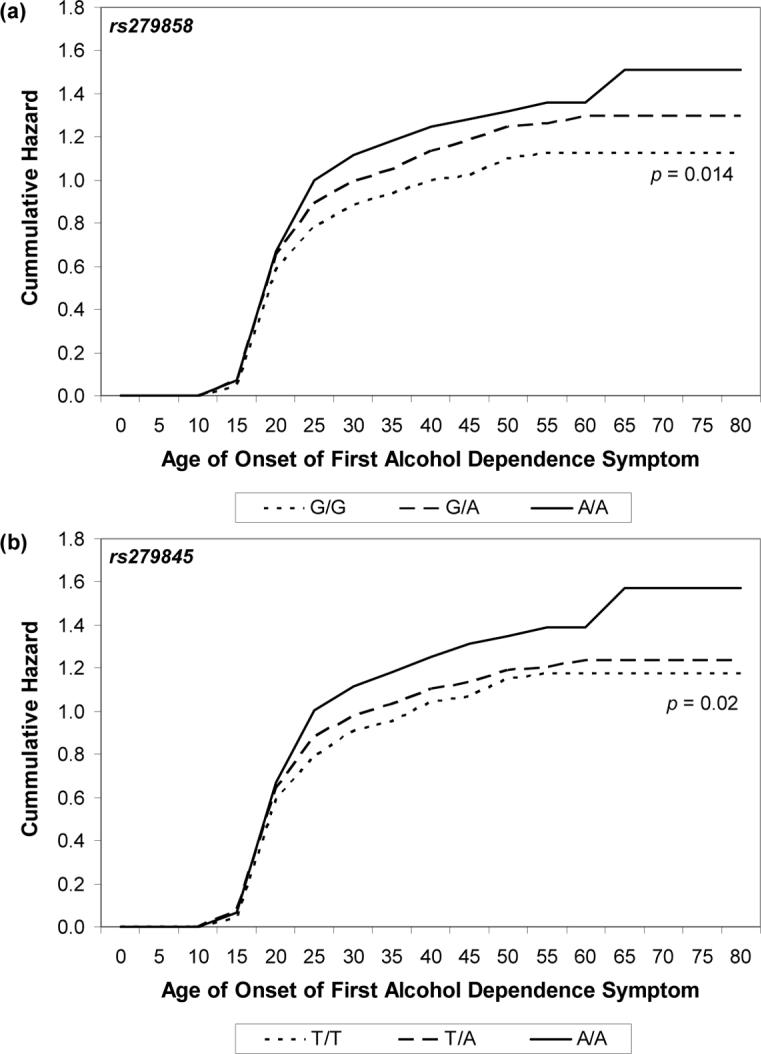
Nelson-Aalen cumulative hazard estimates for age of onset of the first DSM-IIIR alcohol dependence symptom across the lifespan for (a) rs279858 and (b) rs279845 genotype. Tests of genotypic differences: (a) p < 0.014, (b) p = 0.02.
Table 5.
Hazard ratios for the association between two GABRA2 single nucleotide polymorphisms and age at first symptom of DSM-IIIR alcohol dependence.
| Hazard Model | Single Nucleotide Polymorphism |
|||
|---|---|---|---|---|
| rs279858 | rs279845 | rs279845 recessive | ||
| Model 1 | ||||
| |
genotype (0/1/2) |
1.1 [1.02−1.19] |
1.1 [1.01−1.19] |
|
| Model 2 | ||||
| sex | 0.48 [0.43−0.53] | 0.47 [0.42−0.53] | ||
| |
genotype (0/1/2) |
1.09 [1.01−1.18] |
1.08 [1.00−1.16] |
|
| Model 3 | ||||
| sex | 0.44 [0.36−0.55] | 0.40 [0.32−0.49] | ||
| genotype (0/1/2) | 1.06 [0.96−1.18]ns | 1.01 [0.92−1.12]ns | ||
| |
sex* genotype (0/1/2) |
1.06 [0.91−1.22]ns |
1.14 [0.98−1.34]ns |
|
| Model 4 | ||||
| |
genotype (0/1) |
1.18 [1.03−1.36] |
1.14 [0.98−1.34]ns |
1.13 [1.01−1.27] |
| Model 5 | ||||
| sex | 0.47 [0.42−0.53] | 0.47 [0.42−0.52] | 0.47 [0.42−0.52] | |
| |
genotype (0/1) |
1.19 [1.03−1.37] |
1.13 [0.98−1.30]ns |
1.09 [0.97−1.22]ns |
| Model 6 | ||||
| sex | 0.46 [0.35−0.59] | 0.43 [0.33−0.55] | 0.43 [0.38−0.50] | |
| genotype (0/1) | 1.17 [0.96−1.42]ns | 1.07 [0.89−1.30]ns | 0.98 [0.84−1.15]ns | |
| sex* genotype (0/1) | 1.04 [0.78−1.39]ns | 1.12 [0.84−1.49]ns | 1.26 [1.0−1.58] | |
ns = non significant (p > 0.05)
While rs279845 was associated with age at onset of alcohol dependence symptoms (p = 0.02), the greatest risk was noted for this with two copies of the risk allele (A/A) (Figure 2b). Adjusting for sex reduced the effect of genotype. As shown in Figure 2b and in Table 5, coding the genotype for a dominance model did not fit the data well. In contrast, a recessive model for genotype (A/A versus A/T and T/T) fitted well. Interestingly, the interaction between genotype (recessive model) and sex was significant at p = 0.05 and rs279845 influenced age at onset of alcohol dependence symptoms in women alone (data not shown).
DISCUSSION
It is established that genetic variation in the alpha-2 subunit of the GABAA receptor (GABRA2) is associated with risk for alcohol dependence (Covault et al., 2004; Edenberg et al., 2004; Fehr et al., 2006; Lappalainen et al., 2005; Soyka et al., 2008). While the primary function of GABAA receptors is to mediate the activity of gamma-aminobutyric acid (GABA), the major inhibitory neurotransmitter in the central nervous system, GABAA receptors also play a role in the chronic and acute effects of alcohol including motor incoordination, anxiolysis, motivation for excessive drinking and alcohol withdrawal (Buck, 1996; Davies, 2003; Grobin et al., 1998; Koob, 2004; Krystal et al., 2006). Recently, GABRA2 has also been shown to exert its influence on anxiety (Enoch et al., 2006) and several disinhibitory traits such as conduct disorder (Dick et al., 2006), illicit drug dependence (Agrawal et al., 2006; Drgon et al., 2006) as well as nicotine dependence (Agrawal et al., 2008).
In the current study, we investigated the relationship between GABRA2 and a series of quantitative and binary measures of alcohol consumption and dependence in a large twin sample representative of the general Australian population. Extending the scope of the study to incorporate other substance-use disorders that frequently co-occur with alcoholism, we then examined the role of GABRA2 in smoking, cannabis and other illicit drug use.
Consistent with previous findings, the strongest evidence for GABRA2 SNP effects was with quantitative alcohol dependence variables, namely the number of DSM-IV criteria met for alcohol dependence (sumAD4) and the principal component factor score extracted from the DSM-IV alcohol dependence criteria variable (factAD4). Five SNPs, four of which are located within the major LD block that extends from intron 3 to the 3'UTR of GABRA2 in our sample, were nominally associated (p < 0.05, “within” test of association) with these two phenotypes. The fact that this result is not significant after correction for multiple testing, combined with the non-significant finding for these markers for the “total” test of association, means that the effect of these markers on quantitative alcohol dependence variables is modest at best.
Our most significantly associated SNP was the synonymous coding variant in exon 5 (rs279858) which has previously been found to be associated with AD status (Covault et al., 2004; Edenberg et al., 2004; Fehr et al., 2006; Lappalainen et al., 2005) and subjective intoxication (Haughey et al., 2008; Pierucci-Lagha et al., 2005). In our data, the frequency of the rs279858 G allele was lower in DSM-IV diagnosed alcoholics (41.5%) compared to non-alcoholic twins (43.7%) and was, as expected, related to a lower DSM-IV factor score and number of DSM-IV AD symptoms experienced by each twin. The direction of effect is opposite to that reported by Pierucci-Lagha and colleagues (2005) where individuals carrying G alleles were less sensitive to the effect of alcohol and hence more likely to become alcohol dependent and in three studies where the G allele was significantly over-represented among alcoholics (Covault et al., 2004; Fehr et al., 2006; Lappalainen et al., 2005). In each of these studies the frequency of the G-allele ranged from 34 to 42% in the control samples - a frequency similar to that observed in our population. The G-allele has also been associated with an increased probability of daily drinking and heavy drinking during 12-week treatment and 12-month post-treatment periods in alcoholics participating in the Project MATCH study (Bauer et al., 2007). Recently, Haughey and colleagues (2008) found that prefrontal cortex (PFC) GABRA2 mRNA levels in postmortem brains and an individual's sensitivity to the acute affects of alcohol were significantly influenced by the GABRA2 rs279858 SNP genotype. Heterozygous individuals exhibited less reward from alcohol than either homozygote, and postmortem brains of A/G individuals had significantly lower PFC mRNA levels compared to A/A homozygotes, suggesting that the A/G genotype may protect against developing alcohol dependence.
The direction of effect for rs279845 was also opposite to that reported by Edenberg et al. (2004) and Fehr et al. (2006). While both studies reported an at-risk haplotype for AD composed of three SNPs (rs279871-rs279845-rs279836) there is some confusion given that the haplotype may differ with Edenberg and colleagues and Fehr et al. reporting T-A-T and C-T-T, respectively. However, a recent review of the role of GABAA receptors in the development of alcohol dependence indicates that the same (more frequent) haplotype was identified by Edenberg et al. and Fehr and colleagues (Enoch, 2008). While we did not successfully genotype rs279871 and rs279836, the high degree of LD spanning intron 3 to the 3’ end of GABRA2 resulted in two common complementary haplotypes, accounting for 95.6% of the estimated chromosomes, and allows a comparison of previous studies with our rs279845 data given that the alleles are ‘A’ and ‘T’.
The at-risk rs279845 A-allele (along with the rs567926 T-allele, the at-risk rs279858 A-allele and the rs279837 T-allele) is carried on the more abundant haplotype in our population. In contrast, the at-risk (less frequent) C-C-G-T-T haplotype reported by Fehr et al. (2006) which includes rs567926 (C-allele), rs279858 (G) and rs279845 (T), and C-T-G-A-G-A-C haplotype identified by Covault et al. (2004) (including rs567926 C and rs279858 G), suggest we are observing an effect of the complementary haplotype on risk for AD in our population. Conversely, a recent case-control study of German treatment-seeking alcoholics, attempting to replicate the findings of Covault et al. (2004), identified the same (more frequent) at-risk haplotype as our study, observing T-A-T alleles at the rs567926, rs279858 and rs279837 loci (Soyka et al., 2008). Furthermore, the more frequent haplotype has been observed in Finnish alcoholics, albeit alcoholics with high tendency to be anxious (Enoch et al., 2006).
Since GABRA2 and another GABAA receptor gene (GABRG1) are in close proximity (128 Kb apart) with moderate LD reported to extend from the 3’ end of GABRA2 to potential regulatory regions upstream of GABRG1 (Covault et al., 2008), long range LD between the four GABRA2 SNPs of interest (rs279858, rs279845, rs279826 and rs189957) and variants in surrounding genes (including GABRA4, GABRB1) was investigated in ssSNPer (Nyholt, 2006b). In each analysis, only moderate LD (r2∼0.5) was observed in the region between GABRA2 and GABRG1 loci, with lower levels (r2<0.3) detected elsewhere, suggesting that the association signal is located within GABRA2.
None of the quantitative alcohol consumption measure showed significant associations, including the MaxDr and MaxDr12 phenotypes which have previously been shown to be highly correlated with alcohol use disorders (Saccone et al., 2000). Overall, there is little support for GABRA2 allelic effects on alcohol intake in our population. Furthermore, GABRA2 was not associated overall with smoking, cannabis or other illicit drug use. However, evidence in a case-control subset suggested an association between GABRA2 and current smoking status among ever-smokers (i.e., smoking persistence), including the synonymous polymorphism, rs279858, which has recently been found to be associated with nicotine dependence in a sample of U.S. and Australian adults (Agrawal et al., 2008).
Significant SNP associations with current smoking were seen in the case-control sample derived from MZ twins, but not when using a TDT-based analysis in the DZ sibling pairs. A reason for this may be the reduced power to detect association in the TDT framework when parental genotypes are missing and there is only one additional phenotyped (and genotyped) sibling (i.e. the DZ co-twin). Yang et al. (2003) demonstrated that for a common disease model, the absence of additional phenotyped siblings, affects power – this limitation could extend to our analyses of alcohol and illicit drug-related phenotypes as well. In addition, McGinnis et al. (2002) have argued that compared with the TDT (when including genotyped parents), case-control samples require fewer individuals to achieve a similar level of power – contrasting this with our TDT design, where parents are absent, it is plausible that the smaller case-control sample was sufficiently powered to detect the association signal. Notwithstanding these possibilities, our results with current smoking need careful replication and currently should be viewed with some caution. A number of emerging genomewide association studies (GWAS) may provide clues. To date, we are not aware of any GWAS that has implicated polymorphisms in GABRA2 – however, the candidate genes component of the NICSNP GWAS (Bierut et al., 2007; Saccone et al., 2007) reported evidence for association with GABRA4 with follow-up analyses also finding considerable support for the role of GABRA2 (data not shown).
Given that we found some evidence for association between GABRA2 and quantitative measures of DSM-IIIR and DSM-IV AD in our sample, we expected to detect significant associations with the diagnostic DSM-IIIR and DSM-IV AD phenotypes. However, only a trend towards association (p < 0.1) with DSM-IIIR dependence was observed. The low levels/absence of association for the binary measures of AD may be due to several factors. First, the binary trait (within-family) association analyses excluded both MZ families and families with only one twin available. The reduction in the number of informative (phenotyped) individuals included in our analyses resulted in an overall loss in power. Second, our community-based twin sample is not as severely affected by AD as other samples that have shown evidence for association between GABRA2 and AD, such as the COGA sample with multiple affected family members. Therefore, our sample may have a lower genetic load with respect to AD. Correspondingly, when Dick et al. (2006) compared the onset of AD across the lifespan in a combined sample of COGA families and control families with that of a reduced sample composed of control families alone, they observed less significant allelic effects among the controls (p = 0.013 vs 0.0035). Nevertheless, heritability for AD is high (47−75%) and the sibling relative risk is approximately 2 within our sample (Heath et al., 1997).
The allelic effects which we were able to detect are associated with relative risks of 1.1 to 1.2 for development of alcohol dependence, particularly dependence with onset of symptoms after the age of 25 (Table 5, Figure 2). This is consistent with recent estimates for detectable effects of other genes on risk of other diseases (Wellcome Trust Case Control Consortium., 2007). Detection of the expected large number of other polymorphisms producing small but collectively important effects on AD risk will require even larger individual studies, and meta-analysis across studies. In this connection it will also be important to test for heterogeneity across studies, and to seek explanations for any heterogeneity in areas such as study characteristics or populations studied. Given that GABRA2 variation has been shown to affect AD risk in multiple case-control studies based on clinical recruitment, it is notable that only small effects were found in our population-based cohort study. This emphasizes the need to evaluate allelic associations in diverse groups in order to form a full model of genetic effects on AD, and by implication for other complex diseases.
Acknowledgements
We would like to thank the twins and their parents for their co-operation; Genetic Epidemiology Laboratory staff for sample processing and DNA extraction; Dixie Statham for coordinating the SSAGA Study; and the clinical staff and many research interviewers for data collection. Sample and phenotype collection was funded by National Institute on Alcohol Abuse and Alcoholism (NIAAA) grants (AA007535, AA007728). Genotyping was funded by NIAAA grants (AA013321, AA013326, AA014041).
Footnotes
The authors wish it to be known that, in their opinion, the first two authors should be regarded as joint First Authors.
REFERENCES
- Abecasis GR, Cardon LR, Cookson WO. A general test of association for quantitative traits in nuclear families. Am J Hum Genet. 2000a;66:279–292. doi: 10.1086/302698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abecasis GR, Cookson WO, Cardon LR. Pedigree tests of transmission disequilibrium. Eur J Hum Genet. 2000b;8:545–551. doi: 10.1038/sj.ejhg.5200494. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Edenberg HJ, Foroud T, Bierut LJ, Dunne G, Hinrichs AL, Nurnberger JI, Crowe R, Kuperman S, Schuckit MA, Begleiter H, Porjesz B, Dick DM. Association of GABRA2 with drug dependence in the collaborative study of the genetics of alcoholism sample. Behav Genet. 2006;36:640–650. doi: 10.1007/s10519-006-9069-4. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Pergadia ML, Saccone SF, Hinrichs AL, Lessov-Schlaggar CN, Saccone NL, Neuman RJ, Breslau N, Johnson E, Hatsukami D, Montgomery GW, Heath AC, Martin NG, Goate AM, Rice JP, Bierut LJ, Madden PA. Gamma-aminobutyric acid receptor genes and nicotine dependence: evidence for association from a case-control study. Addiction. 2008;103:1027–1038. doi: 10.1111/j.1360-0443.2008.02236.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 3rd ed. APA; Washington, DC: 1987. [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. APA; Washington, DC: 1994. [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Covault J, Harel O, Das S, Gelernter J, Anton R, Kranzler HR. Variation in GABRA2 predicts drinking behavior in project MATCH subjects. Alcohol Clin Exp Res. 2007;31:1780–1787. doi: 10.1111/j.1530-0277.2007.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau OF, Swan GE, Rutter J, Bertelsen S, Fox L, Fugman D, Goate AM, Hinrichs AL, Konvicka K, Martin NG, Montgomery GW, Saccone NL, Saccone SF, Wang JC, Chase GA, Rice JP, Ballinger DG. Novel genes identified in a high-density genome wide association study for nicotine dependence. Hum Mol Genet. 2007;16:24–35. doi: 10.1093/hmg/ddl441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr., Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Buck KJ. New insight into the mechanisms of ethanol effects on GABAA receptor function and expression, and their relevance to behavior. Alcohol Clin Exp Res. 1996;20:198A–202A. doi: 10.1111/j.1530-0277.1996.tb01776.x. [DOI] [PubMed] [Google Scholar]
- Covault J, Gelernter J, Hesselbrock V, Nellissery M, Kranzler HR. Allelic and haplotypic association of GABRA2 with alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2004;129:104–109. doi: 10.1002/ajmg.b.30091. [DOI] [PubMed] [Google Scholar]
- Covault J, Gelernter J, Jensen K, Anton R, Kranzler HR. Markers in the 5'-Region of GABRG1 Associate to Alcohol Dependence and are in Linkage Disequilibrium with Markers in the Adjacent GABRA2 Gene. Neuropsychopharmacology. 2008 doi: 10.1038/sj.npp.1301456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M. The role of GABAA receptors in mediating the effects of alcohol in the central nervous system. J Psychiatry Neurosci. 2003;28:263–274. [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Bierut L, Hinrichs A, Fox L, Bucholz KK, Kramer J, Kuperman S, Hesselbrock V, Schuckit M, Almasy L, Tischfield J, Porjesz B, Begleiter H, Nurnberger J, Jr., Xuei X, Edenberg HJ, Foroud T. The role of GABRA2 in risk for conduct disorder and alcohol and drug dependence across developmental stages. Behav Genet. 2006;36:577–590. doi: 10.1007/s10519-005-9041-8. [DOI] [PubMed] [Google Scholar]
- Drgon T, D'Addario C, Uhl GR. Linkage disequilibrium, haplotype and association studies of a chromosome 4 GABA receptor gene cluster: candidate gene variants for addictions. Am J Med Genet B Neuropsychiatr Genet. 2006;141:854–860. doi: 10.1002/ajmg.b.30349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudbridge F. Pedigree disequilibrium tests for multilocus haplotypes. Genet Epidemiol. 2003;25:115–121. doi: 10.1002/gepi.10252. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer LO, Crowe RR, Goate A, Hesselbrock V, Jones K, Kwon J, Li TK, Nurnberger JI, Jr., O'Connor SJ, Reich T, Rice J, Schuckit MA, Porjesz B, Foroud T, Begleiter H. Variations in GABRA2, Encoding the alpha 2 Subunit of the GABAA Receptor, Are Associated with Alcohol Dependence and with Brain Oscillations. Am J Hum Genet. 2004;74:705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA. The role of GABA(A) receptors in the development of alcoholism. Pharmacol Biochem Behav. 2008 doi: 10.1016/j.pbb.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, Schwartz L, Albaugh B, Virkkunen M, Goldman D. Dimensional anxiety mediates linkage of GABRA2 haplotypes with alcoholism. Am J Med Genet B Neuropsychiatr Genet. 2006141:599–607. doi: 10.1002/ajmg.b.30336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr C, Sander T, Tadic A, Lenzen KP, Anghelescu I, Klawe C, Dahmen N, Schmidt LG, Szegedi A. Confirmation of association of the GABRA2 gene with alcohol dependence by subtype-specific analysis. Psychiatr Genet. 2006;16:9–17. doi: 10.1097/01.ypg.0000185027.89816.d9. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Matthews DB, Devaud LL, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol. Psychopharmacology (Berl) 1998;139:2–19. doi: 10.1007/s002130050685. [DOI] [PubMed] [Google Scholar]
- Haughey HM, Ray LA, Finan P, Villanueva R, Niculescu M, Hutchison KE. Human gamma-aminobutyric acid A receptor alpha2 gene moderates the acute effects of alcohol and brain mRNA expression. Genes Brain Behav. 2008;7:447–454. doi: 10.1111/j.1601-183X.2007.00369.x. [DOI] [PubMed] [Google Scholar]
- Heath AC, Bucholz KK, Madden PA, Dinwiddie SH, Slutske WS, Bierut LJ, Statham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychol Med. 1997;27:1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- Hesselbrock M, Easton C, Bucholz KK, Schuckit M, Hesselbrock V. A validity study of the SSAGA--a comparison with the SCAN. Addiction. 1999;94:1361–1370. doi: 10.1046/j.1360-0443.1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for GABA mechanisms in the motivational effects of alcohol. Biochem Pharmacol. 2004;68:1515–1525. doi: 10.1016/j.bcp.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Staley J, Mason G, Petrakis IL, Kaufman J, Harris RA, Gelernter J, Lappalainen J. Gamma-aminobutyric acid type A receptors and alcoholism: intoxication, dependence, vulnerability, and treatment. Arch Gen Psychiatry. 2006;63:957–968. doi: 10.1001/archpsyc.63.9.957. [DOI] [PubMed] [Google Scholar]
- Lappalainen J, Krupitsky E, Remizov M, Pchelina S, Taraskina A, Zvartau E, Somberg LK, Covault J, Kranzler HR, Krystal JH, Gelernter J. Association between alcoholism and gamma-amino butyric acid alpha2 receptor subtype in a Russian population. Alcohol Clin Exp Res. 2005;29:493–498. doi: 10.1097/01.alc.0000158938.97464.90. [DOI] [PubMed] [Google Scholar]
- Lind PA, Macgregor S, Montgomery GW, Heath AC, Martin NG, Whitfield JB. Effects of GABRA2 Variation on Physiological, Psychomotor and Subjective Responses in the Alcohol Challenge Twin Study. Twin Res Hum Genet. 2008;11:174–182. doi: 10.1375/twin.11.2.174. [DOI] [PubMed] [Google Scholar]
- Long JC, Knowler WC, Hanson RL, Robin RW, Urbanek M, Moore E, Bennett PH, Goldman D. Evidence for genetic linkage to alcohol dependence on chromosomes 4 and 11 from an autosome-wide scan in an American Indian population. Am J Med Genet. 1998;81:216–221. doi: 10.1002/(sici)1096-8628(19980508)81:3<216::aid-ajmg2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Madden PA, Bucholz KK, Martin NG, Heath AC. Smoking and the genetic contribution to alcohol-dependence risk. Alcohol Res Health. 2000;24:209–214. [PMC free article] [PubMed] [Google Scholar]
- Martin NG, Oakeshott JG, Gibson JB, Starmer GA, Perl J, Wilks AV. A twin study of psychomotor and physiological responses to an acute dose of alcohol. Behav Genet. 1985;15:305–347. doi: 10.1007/BF01070893. [DOI] [PubMed] [Google Scholar]
- Matthews AG, Hoffman EK, Zezza N, Stiffler S, Hill SY. The role of the GABRA2 polymorphism in multiplex alcohol dependence families with minimal comorbidity: within-family association and linkage analyses. J Stud Alcohol Drugs. 2007;68:625–633. doi: 10.15288/jsad.2007.68.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis R, Shifman S, Darvasi A. Power and efficiency of the TDT and case-control design for association scans. Behav Genet. 2002;32:135–144. doi: 10.1023/a:1015205924326. [DOI] [PubMed] [Google Scholar]
- Nyholt DR. On the probability of dizygotic twins being concordant for two alleles at multiple polymorphic loci. Twin Res Hum Genet. 2006a;9:194–197. doi: 10.1375/183242706776382383. [DOI] [PubMed] [Google Scholar]
- Nyholt DR. ssSNPer: identifying statistically similar SNPs to aid interpretation of genetic association studies. Bioinformatics. 2006b;22:2960–2961. doi: 10.1093/bioinformatics/btl518. [DOI] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Covault J, Feinn R, Nellissery M, Hernandez-Avila C, Oncken C, Morrow AL, Kranzler HR. GABRA2 alleles moderate the subjective effects of alcohol, which are attenuated by finasteride. Neuropsychopharmacology. 2005;30:1193–1203. doi: 10.1038/sj.npp.1300688. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H, Wang K, Almasy L, Chorlian DB, Stimus AT, Kuperman S, O'Connor SJ, Rohrbaugh J, Bauer LO, Edenberg HJ, Goate A, Rice JP, Reich T. Linkage and linkage disequilibrium mapping of ERP and EEG phenotypes. Biol Psychol. 2002;61:229–248. doi: 10.1016/s0301-0511(02)00060-1. [DOI] [PubMed] [Google Scholar]
- Reich T, Edenberg HJ, Goate A, Williams JT, Rice JP, Van Eerdewegh P, Foroud T, Hesselbrock V, Schuckit MA, Bucholz K, Porjesz B, Li TK, Conneally PM, Nurnberger JI, Jr., Tischfield JA, Crowe RR, Cloninger CR, Wu W, Shears S, Carr K, Crose C, Willig C, Begleiter H. Genome-wide search for genes affecting the risk for alcohol dependence. Am J Med Genet. 1998;81:207–215. [PubMed] [Google Scholar]
- Saccone NL, Kwon JM, Corbett J, Goate A, Rochberg N, Edenberg HJ, Foroud T, Li TK, Begleiter H, Reich T, Rice JP. A genome screen of maximum number of drinks as an alcoholism phenotype. Am J Med Genet. 2000;96:632–637. doi: 10.1002/1096-8628(20001009)96:5<632::aid-ajmg8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau O, Swan GE, Goate AM, Rutter J, Bertelsen S, Fox L, Fugman D, Martin NG, Montgomery GW, Wang JC, Ballinger DG, Rice JP, Bierut LJ. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet. 2007;16:36–49. doi: 10.1093/hmg/ddl438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyka M, Preuss UW, Hesselbrock V, Zill P, Koller G, Bondy B. GABA-A2 receptor subunit gene (GABRA2) polymorphisms and risk for alcohol dependence. J Psychiatr Res. 2008;42:184–191. doi: 10.1016/j.jpsychires.2006.11.006. [DOI] [PubMed] [Google Scholar]
- StataCorp . Stata Statistical Software: Release 8.0. Stata Corporation; College Station, TX: 2003. [Google Scholar]
- True WR, Xian H, Scherrer JF, Madden PA, Bucholz KK, Heath AC, Eisen SA, Lyons MJ, Goldberg J, Tsuang M. Common genetic vulnerability for nicotine and alcohol dependence in men. Arch Gen Psychiatry. 1999;56:655–661. doi: 10.1001/archpsyc.56.7.655. [DOI] [PubMed] [Google Scholar]
- Wellcome Trust Case Control Consortium Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield JB, Pang D, Bucholz KK, Madden PA, Heath AC, Statham DJ, Martin NG. Monoamine oxidase: associations with alcohol dependence, smoking and other measures of psychopathology. Psychol Med. 2000;30:443–454. doi: 10.1017/s0033291799001798. [DOI] [PubMed] [Google Scholar]
- Wigginton JE, Abecasis GR. PEDSTATS: descriptive statistics, graphics and quality assessment for gene mapping data. Bioinformatics. 2005;21:3445–3447. doi: 10.1093/bioinformatics/bti529. [DOI] [PubMed] [Google Scholar]
- Williams JT, Begleiter H, Porjesz B, Edenberg HJ, Foroud T, Reich T, Goate A, Van Eerdewegh P, Almasy L, Blangero J. Joint multipoint linkage analysis of multivariate qualitative and quantitative traits. II. Alcoholism and event-related potentials. Am J Hum Genet. 1999;65:1148–1160. doi: 10.1086/302571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Xu X, Laird N. Power evaluations for family-based tests of association with incomplete parental genotypes. Genetics. 2003;164:399–406. doi: 10.1093/genetics/164.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]


