Abstract
This study was undertaken to determine the functional consequences following tongue volume reduction on tongue internal kinematics during mastication and neuromuscular stimulation in a pig model. Six ultrasonic-crystals were implanted into the tongue body in a wedge-shaped configuration which allows recording distance changes in the bilateral length (LENG) and posterior thickness (THICK), as well as anterior (AW), posterior dorsal (PDW), and ventral (PVW) widths in 12 Yucatan-minipigs. Six animals received a uniform mid-sagittal tongue volume reduction surgery (reduction), and the other six had identical incisions without tissue removal (sham). The initial-distances among each crystal-pairs were recorded before, and immediately after surgery to calculate the dimensional losses. Referring to the initial-distance there were 3−66% and 1−4% tongue dimensional losses by the reduction and sham surgeries, respectively. The largest deformation in sham animals during mastication was in AW, significantly larger than LENG, PDW, PVW, and THICK (P < 0.01−0.001). In reduction animals, however, these deformational changes significantly diminished and enhanced in the anterior and posterior tongue, respectively (P < 0.05−0.001). In both groups, neuromuscular stimulation produced deformational ranges that were 2−4 times smaller than those occurred during chewing. Furthermore, reduction animals showed significantly decreased ranges of deformation in PVW, LENG, and THICK (P < 0.05−0.01). These results indicate that tongue volume reduction alters the tongue internal kinematics, and the dimensional losses in the anterior tongue caused by volume reduction can be compensated by increased deformations in the posterior tongue during mastication. This compensatory effect, however, diminishes during stimulation of the hypoglossal nerve and individual tongue muscles.
Keywords: tongue kinematics, tongue volume reduction, mas tication, neuromuscular stimulation, pig
Tongue morphology and function are associated with various clinical diseases such as malocclusion, obstructive sleeping apnea, dysphagia, Beckwith-Wiedemann and Down's syndromes, and cerebral palsy. Tongue volume reduction is a valuable approach for treating symptomatic macroglossia and some of these functional disorders (Herren et al., 1981; Ruff, 1985; Deguchi, 1993; Wolford and Cottrell, 1996; Davalbhakta and Lamberty, 2000; Li et al., 2002; Stuck et al., 2005). Given that the tongue is a volume-dependent muscular organ due to the nature of its hydrostat (Kier and Smith, 1985; Kier et al., 1989; Bailey and Fregosi, 2004), significant changes of tongue movement and regional deformation in various dimensions during function are most likely expected when the tongue volume is reduced surgically. We have previously examined regional deformation and volumetric changes of the tongue during natural feeding (Shcherbatyy and Liu, 2007; Liu et al., 2008) and under stimulation of the hypoglossal nerve as well as individual tongue muscles (Liu et al., 2006) in animals with an intact tongue. These studies revealed that regional volumetric change of the tongue occurs during feeding, and chewing requires larger volumetric changes than do ingestion and drinking, and dimensional expansions–contractions of the tongue are dominant in the transverse and sagittal planes during chewing and ingestion, respectively, but are smaller and more symmetrically distributed across various dimensions during drinking. These studies further demonstrated that the ranges of dimensional changes produced by stimulation are much less than those resulted from natural feeding including ingestion, chewing and drinking. However, it is unknown what functional consequences occur following the clinical application of tongue volume reduction, and how the internal kinematics of the tongue responds to surgically reducing its volume during feeding and under various stimulations.
This cohort study was designed to address these questions by examining the deformational changes in the tongue body during natural chewing and under the stimulation of the hypoglossal nerve and individual tongue muscles following a uniform mid-sagittal tongue volume reduction in a pig model. We hypothesized that (1) reducing the volume of the tongue would decrease its capacity for internal kinematics by diminishing the functional deformation in multiple dimensions, (2) this loss of deformational changes in one region might be compensated by proportional increases of deformational changes in other regions during natural chewing, and (3) this compensating effect would diminish under the stimulation of the hypoglossal nerve and individual tongue muscle.
MATERIALS AND METHODS
Animals and Device Placement
Twelve 12-week-old Yucatan miniature pigs (six sibling pairs of each gender, Sinclair Research Co., Columbia, MO) were first acclimated to the laboratory and experimental environment by daily training and handling for 5−7 days. Each pig was subjected to a 12-hr starvation period before experimental day. All procedures were approved by Institutional Animal Care and Use Committee of the University of Washington.
On the experimental day, the pig was anesthetized with isoflurane and nitrous oxide through a mouth mask initially and a nostril mask subsequently. Ringer's solution was given intravenously, and a heated pad was administered to provide warmth. The submandibular region was exposed through a midline incision and the two major extrinsic tongue muscles (genioglossus [GG] and styloglossus [SG]), as well as the hypoglossal nerve trunk (HGT) with its medial (HGM) and lateral (HGL) branches were identified and marked using 3−0 sutures. The bilateral tunnels that communicated between the submandibular region and the oral cavity proper were first made by penetrating the floor of the mouth along the inner surface of each mandibular corpus. Six piezoelectric ultrasonic crystals with B-barb (2 mm in diameter, Sonometrics Corporation, London, Canada), three on each side, were led into the oral cavity proper through these tunnels and implanted into the tongue to form a wedge-shaped configuration (Fig. 1). This configuration allows the following dimensional measurements to be made: #1−2: anterior width (AW); #3−4: posterior dorsal width (PDW); #5−6: posterior ventral width (PVW); #1−3 and #2−4: right and left length (RL and LL); and #3−5 and #4−6: right and left thickness (RT and LT). The detailed locations of each crystal were described elsewhere (Liu et al., 2006, 2007; Shcherbatyy and Liu, 2007). The Silastic-coated leads of these crystals were sutured onto their adjacent soft tissues to stabilize crystals, guided out of the mouth from the tunnel, and connected to a Sonometrics system.
Fig. 1.
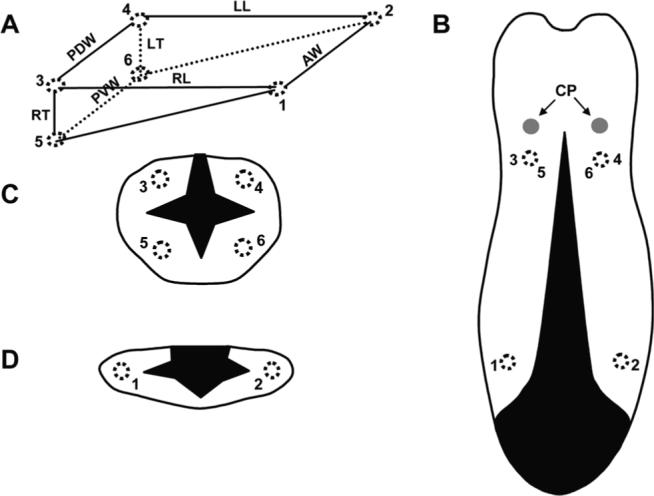
Array of six ultrasonic crystals and diagram of tongue volume reduction surgery. A: Wedge-shaped configuration of implanted crystals array. Numbers indicate the location of crystals. #1−2: anterior width (AW); #3−4: posterior dorsal width (PDW); #5−6: posterior ventral width (PVW); #1−3 and #2−4: right and left length (RL and LL); and #3−5 and #4−6: right and left thickness (RT and LT). B: Dorsal view of the tongue. C,D: Cross-sectional views of the posterior and the anterior tongue. Dotted circles indicate implanted crystal sites. Black-shaded areas represent surgical incisions and removed tongue tissue. Arrows indicate two circumvallate papillae (CP).
Immediately after crystal implantation, the tongue was placed in a rest position and the “initial lengths” of each dimension (distance between each crystal pairs) were first recorded as a baseline. The actual linear distances of these crystal pairs were also measured using a needle compass, and re-measured postmortem upon killing.
Tongue Surgery and Mastication
After crystal implantation and baseline recording, each animal underwent a uniform mid-sagittal tongue volume reduction or sham surgery, which was performed within the region circumscribed by the six implanted crystals using the revised procedure introduced by Davalbhakta and Lamberty (2000). This procedure allows the tissue resections occurring in the anterior two-thirds of the tongue, and involving not only length and width, but also thickness (Fig. 1B–D).
With the mouth opening separated 35−40 mm by a bite block, the surgery began with making the initial incisions at the circumvallate papillae after a local infiltration of lidocaine HCl 0.5% and epinephrine 1:200,000 solution (3−5 ml) by using an electrosurgical catery unit (ValleyLab SSE2, Colorado Biomedicals, Evergreen, CO). The bilateral incisions diverged to meet the lateral margin anteriorly. Cutting diathermy was used to undermine and create lateral mucomuscular flaps and to excise a conical wedge (above the tongue neurovascular bundles) from the central tongue. The removed tongue muscular tissue uniformly reduced tongue volume in three dimensions (length, width, and thickness). After hemostasis, the incision was closed in layers with absorbable sutures (Vicryl 4.0). The removed tongue tissue was preserved in a 50% alcohol solution. For the sham surgery, identical incisions were made and sutures placed, but without tissue removal. Each sibling pair (one reduction vs. one sham) underwent surgery and the experimental procedures on consecutive days. After surgery, the tongue was placed in the rest position, and the distances between each crystal pairs were recorded again to calculate the dimensional losses.
Anesthesia was ceased, and a regular diet of pellet chow was offered after the animal had regained consciousness. Ultrasound signals from implanted crystals during mastication were acquired at the sampling rate of 250Hz and recorded using the SonoLab program (Ver. 3.4.26-RC3).
Neuromuscular Stimulation
The pig was re-anesthetized and placed in a supine position after 15−20 min of feeding or when the pig stopped eating. The marked bilateral HGT, HGM, and HGL nerves were isolated from the lingual arteries and surrounding fascia. The proximal stumps of the HGT were cauterized to prevent current spread. Bipolar stainless stimulation hook electrodes (diameter 0.05 mm and interpolar distance 5 mm) were placed around the paired HGT, 5 mm proximal to their bifurcations. Similar methods were used for the placement of the electrodes around the paired HGM and HGL, which took place 3−4 mm close to their bifurcation where no secondary branches could be identified. In addition, wire stimulation electrodes (0.05 mm in diameter and interpolar distance 10 mm) were inserted into the bellies of bilateral GG and SG muscles under direct visualization using 27-gauge hypodermic needles (Fig. 2).
Fig. 2.
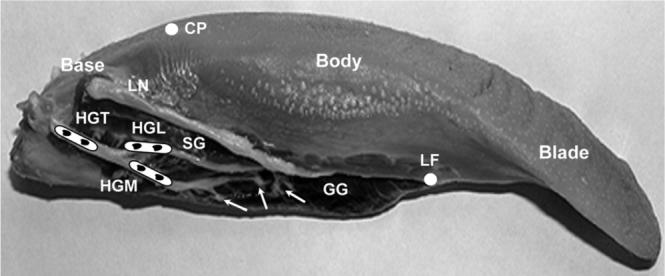
Scheme for the hypoglossal nerve and the extrinsic muscles stimulation. White solid circles indicate the anatomical landmarks for dividing the tongue into three segments: Blade: tongue tip to lingual frenum (LF); Body: lingual frenum to circumvallate papillae (CP); Base: papillae to tongue posterior border. HGT, hypoglossal trunk; HGM, medial branch; HGL, lateral branch; GG, genioglossus; SG, styloglossus; LN, lingual nerve. Arrows indicate secondary nerve branches of the HGM to GG. Black dots indicate the sites of stimulation electrodes.
Mineral oil was applied to the nerve and muscle preparation to keep them moist and to minimize current from spreading during stimulation. The electrode wires were connected to a stimulation isolation unit (Model S48; Grass Instrument, RI).
The tetanic-induced stimulating pulses were delivered to paired HGT, HGM, HGL, GG, and SG separately. The stimulation protocol was set as follows: Nerve: 600-msec trains, 0.5-msec pulse, 6−9 V; Muscle: 600-msec trains, 5-msec pulse, and 20−35 V. Each setting of stimulation was repeated three to five times and the resulting signals from ultrasonic crystals were recorded along with the stimulation pulses. Tongue and jaw movements were visualized and noted under stimulation conditions. A tetanic or a supramaximal condition was established by progressively increasing the voltage until the visible movement/deformation of the tongue reached a plateau or began to decrease despite the increase in voltage.
After completing the neuromuscular stimulation, the animals were euthanized by cardiac injection of Beuthanasia. The tongue was then excised from the harvested heads; the mucosa below the tongue was removed; and the transverse plane between the genioglossal and geniohyoid muscles was defined. The posterior portion of the tongue was sectioned flush with the superior surface of the hyoid bone. The tongue was then artificially divided into three segments (Bressmann et al., 2005; Sanders and Mu, 2005): the tongue blade, the anterior segment defined from the tip to lingual frenum; the tongue body, the middle segment defined from lingual frenum to circumvallate papillae; and the tongue base, the posterior segment defined from the papillae to the posterior border (Fig. 2). The width, length, and thickness of each segment were measured using a digital caliper. The weights of the entire tongue and the surgically removed portion were also recorded using a digital scale, and their volumes were measured using the method of water overflow.
Crystal Signal Analyses
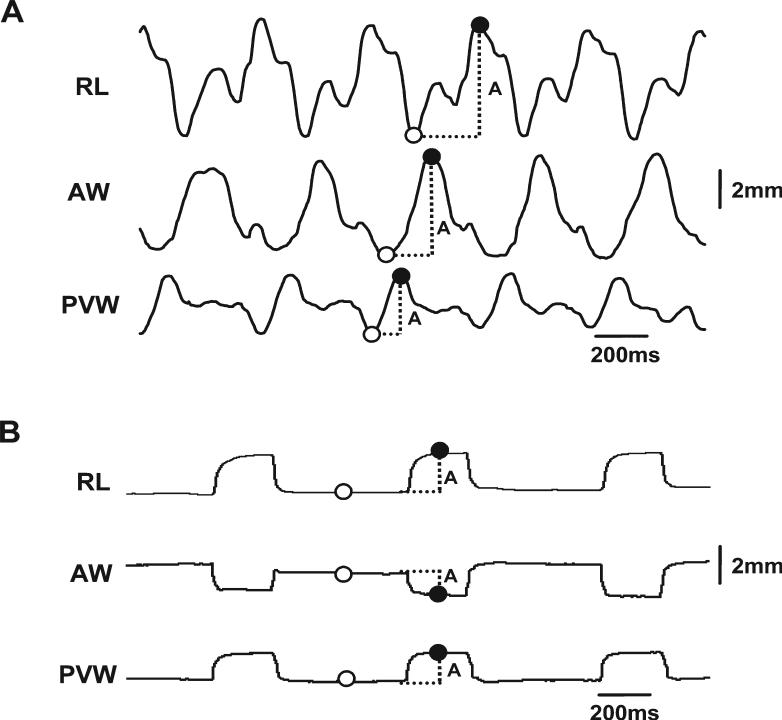
Off-line analyses were performed by selecting stable and consecutive 15−20 chewing cycles from the mastica-tory sequence of each animal using the SonoView program. Selection standards included signal rhythmicity, regularity, and consecutiveness. Outputs of wave data of selected crystal pairs acquired during chewing were exported to an Excel spreadsheet (Microsoft Co., Redmond, WA) where a macros program established the following values from each chewing cycle at any given crystal pair: (1) minimal value (valley), the amplitude measured at the starting of distance increase; (2) maximal value (peak), the amplitude measured at the starting of distance decrease (Fig. 3A). For neuromuscular stimulation, the distance changes were calculated by the peak value (upward or downward) during stimulation subtracted by the baseline value (Fig. 3B). All measurements of distance changes in various dimensions were converted to % of their initial lengths (chewing) or baseline values (stimulation) measured as described above to avoid the small disparities engendered by variation in the crystal implantations (initial distances among crystal pairs) in different animals. Due to the fragility of ultrasonic crystals and uncooperativeness of animals, not all crystal pairs were operational. The actual sample sizes for successful recordings were summarized in Table 1. Captured jaw movement images before and after the device placements were reviewed and digitized for verifying the possible functional interference caused by crystal implantations.
Fig. 3.
Calculation methods for sonomicrometric waves. A: Chewing: five chewing cycles are shown. B: Neuromuscular stimulation: three stimulation pulses are shown. Upward and downward waves indicate increase and decrease of the distance between a given crystal pair (dimensional change), respectively. Solid and empty circles indicate the maximal (peak) and minimal (valley for chewing and baseline for stimulation) values. A, amplitude of dimensional change. Refer to Figure 1 for other captions.
TABLE 1.
Sample Sizes of Successful Recordings
| Width |
Length |
Thickness |
||||||
|---|---|---|---|---|---|---|---|---|
| |
|
AW |
PDW |
PVW |
RL |
LL |
RT |
LT |
| Sham |
6 (114) |
6 (114) |
5 (97) |
5 (106) |
5 (97) |
6 (114) |
4 (78) |
|
| Chewing | Reduction | 5 (93) | 4 (76) | 5 (93) | 5 (93) | 4 (75) | 5 (93) | 4 (75) |
| Stimulation | ||||||||
| HG | Sham | 6 (18) | 6 (18) | 5 (15) | 6 (18) | 5 (15) | 6 (18) | 5 (15) |
| Reduction | 5 (15) | 5 (15) | 5 (15) | 6 (18) | 6 (18) | 5 (15) | 5 (15) | |
| HGM | Sham | 5 (15) | 6 (18) | 5 (15) | 6 (18) | 5 (15) | 6 (18) | 4 (12) |
| Reduction | 5 (15) | 5 (15) | 5 (15) | 6 (18) | 6 (18) | 6 (18) | 5 (15) | |
| HGL | Sham | 6 (18) | 6 (18) | 6 (18) | 6 (18) | 4 (12) | 6 (18) | 4 (12) |
| Reduction | 6 (18) | 5 (15) | 6 (18) | 6 (18) | 6 (18) | 5 (15) | 5 (15) | |
| GG | Sham | 6 (18) | 5 (15) | 6 (18) | 5 (15) | 5 (15) | 6 (18) | 4 (12) |
| Reduction | 5 (15) | 5 (15) | 5 (15) | 6 (18) | 6 (18) | 5 (15) | 5 (15) | |
| SG | Sham | 5 (15) | 6 (18) | 6 (18) | 6 (18) | 6 (18) | 6 (18) | 4 (12) |
| Reduction | 6 (18) | 5 (15) | 5 (15) | 6 (18) | 6 (18) | 5 (15) | 5 (15) | |
HGT: hypoglossal trunk; HGM and HGL: hypoglossal medial and lateral branches; GG: genioglossus; SG: styloglossus.
Numbers in parentheses present sampled chewing cycles or stimulation pulses.
Statistics
SPSS (Version 11.0.1) for Windows was used for the statistical analysis. Descriptive statistic was performed for all means, standard deviations (SD), and ranges of distance changes. Further exploration was done to examine their normal distribution through skewness calculations. One-way analysis of variance was used to compare amplitudes of various dimensions during mastication and under neuromuscular stimulation, followed by Tukey post hoc tests for pair-wise comparisons. Non-paired t-test was performed to compare distance changes of each dimension between sham and reduction animals. The significance level was set as P < 0.05.
RESULTS
Linear and Volumetric Reduction
Referring to the region circumscribed by six crystals as illustrated in Figure 1, the most significant dimensional loss caused by the surgery was found in AW (65.5% ± 4.6; P < 0.001), followed by PDW (15.7% ± 3.7; P < 0.001), and RT/LT (7.0%/7.4%; P < 0.01). Length (RL/LL) was reduced only by 3.7%/4.4%, and there was no significant change found in PVW. Certain minor negative or positive dimensional changes were seen in sham animals in which only the loss in AW showed significance (3.8% ± 1.4; P < 0.001, Table 2).
TABLE 2.
% of Dimensional Changes Pre and Post Surgery
| AW | PDW | PVW | RL | LL | RT | LT | |
|---|---|---|---|---|---|---|---|
| Sham | −3.8±1.4*** | −1.1±2.9 | 0.7±2.3 | −1.2±1.3 | −1.5±1.1 | 0.7±2.6 | 0.9±1.4 |
| Reduction | −65.5±4.6*** | −15.7±3.7*** | −3.1±1.7** | −3.7±0.8*** | −4.4±1.1*** | −7.4±4.7** | −7.0±3.1** |
AW: anterior width; PDW and PVW: posterior dorsal and ventral widths; RL and LL: right and left body lenghts; RT and LT: right and left posterior thicknesses. Positive and negative values indicate increase and decrease, respectively.
Asterisks indicate significant changes.
p < 0.01.
p < 0.001.
Although measuring volume and weight changes of the tongue was not possible in living animals, postmortem examination revealed that the surgery decreased the volume and weight up to 17.2% ± 0.9 and 17.1% ± 1.0, respectively, in reduction animals. Measurements of these postmortem samples further indicated that the surgery decreased the total length of the tongue by 22.5%, the combined width (sum of blade, body, and base widths) by 27.9% and the combined thickness (blade, body, and base thicknesses) by 7.1%.
Tongue Deformation during Chewing
Because statistical analyses did not find any significant differences between right and left lengths (RL and LL) and thicknesses (RT and LT), values from the two sides were combined (LENG and THICK).
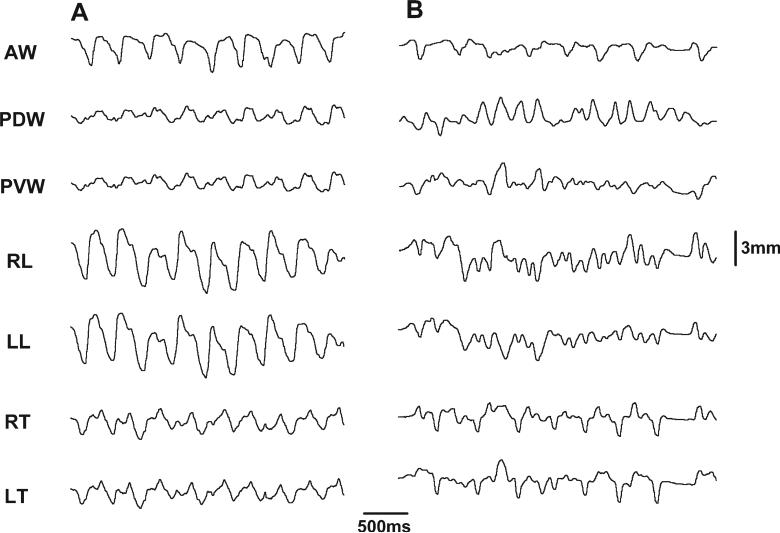
In sham animals, the deformation pattern during chewing was similar to those animals with an intact tongue (Shcherbatyy and Liu, 2007), and waves of deformational changes were more regular and stereotypical than those in reduction animals (compare Fig. 4A and B). In sham animals, the largest deformational change relative to the initial distances was seen in AW (25.2%; P < 0.001), and the smallest in PVW (15.7%; P < 0.001) and LENG (15.5%; P < 0.001). After surgery, these dimensional changes became distorted and were less stereotypical, although the basic pattern remained (Fig. 4). As compared to sham animals, changes in AW (14.4%) and LENG (10.7%) were diminished significantly by 30−44% (P < 0.01−0.001), but changes in the posterior tongue body (PDW, PVW, and THICK) were significantly enhanced by 10−20% (P < 0.05; Fig. 5). These alterations resulted in a significantly larger deformation in the posterior (PDW, PVW, and THICK) than anterior (AW, LENG) tongue, opposite to those found in the sham and intact control animals (Shcherbatyy and Liu, 2007).
Fig. 4.
Raw sonomicrometric tracing of chewing. A: Sham. B: Reduction. Refer to Figure 1 for all captions.
Fig. 5.
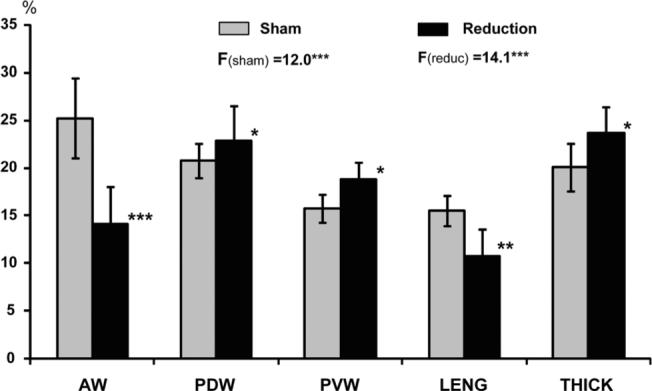
Comparison of dimensional changes during chewing between sham and reduction groups. F values and superscripted asterisks indicate the results of analysis of variance tests among all measured dimensions for sham (Fsham) and reduction (Freduc) groups. AW, anterior width; PDW and PVW, posterior dorsal and ventral width; LENG, combined body length; THICK, combined posterior thickness. Asterisks by vertical bars indicate significant difference between the two groups in each measured dimensions. *P < 0.05; **P < 0.01; ***P < 0.001.
Tongue Deformation under Neuromuscular Stimulation
Various dimensional changes produced by stimulation occurred simultaneously without a timing delay in both sham and reduction animals (Fig. 6). In sham animals, stimulation of paired hypoglossal nerve and its branches caused narrowing in AW and widening in both PDW and PVW with a similar range of changes, regardless of which part was stimulated (HGT, HGM, or HGL). However, LENG decrease (shortening) and THICK increase (thickening) were seen when HGT and HGL were stimulated, opposite to the changes that occurred when HGM was stimulated (lengthening and thinning; Fig. 7A). These features are similar to the previous findings in animals with an intact tongue (Liu et al., 2006). Contrast to the findings during chewing, the deformational ranges in AW and LENG were significantly smaller than those in PDW, PVW, and THICK (4−6% vs. 9−13%; P < 0.05−0.01).
Fig. 6.
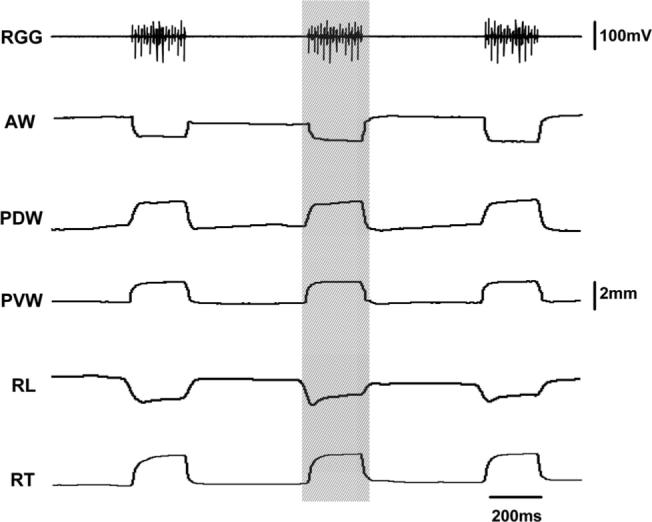
Raw sonomicrometric tracing during stimulation of the hypoglossal trunk (HGT). Hatched region indicates one stimulation pulse. RGG, right genioglossus. Refer Figure 1 for other captions. 63 × 50 mm (600 × 600 DPI).
Fig. 7.
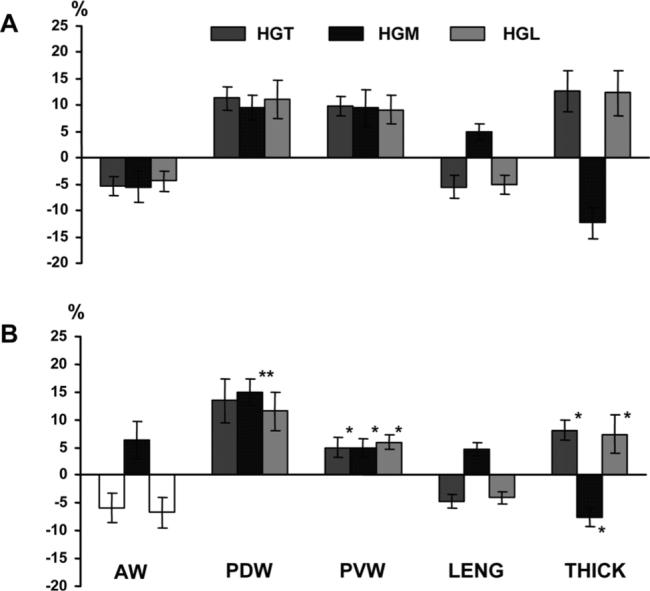
Tongue dimensional changes under stimulation of the hypoglossal nerve trunk (HGT), its medial (HGM) and lateral (HGL) branches in sham (A) and reduction (B) groups. Note that the bars for AW during HGT and HGL stimulation (A) are intentionally left blank because the deformational direction varied across animals (see text for the details). Asterisks by the vertical bars indicate significant difference between the two groups. *P < 0.05; **P < 0.01. Refer to Figure 2 for other captions.
In reduction animals, deformations produced by stimulation were less constant, along with altered directions and ranges. While dimensions of the posterior tongue body (PDW, PVW, and THICK) showed a similar direction of deformational changes to these in sham animals when paired HGT, HGM, and HGL were stimulated, the ranges of deformation in PVW and THICK were significantly diminished (P < 0.05). In contrast, the range of PDW increase (widening) significantly enhanced (P < 0.01) when HGM was stimulated. It must be mentioned that the deformation direction of AW varied across animals when HGT and HGL were stimulated. Of six reduction animals, three showed an increase and three a decrease in AW. Furthermore, AW widening, rather than narrowing as seen in sham animals, was identified when HGM was stimulated, and the absolute magnitudes of AW changes were slightly larger than those in sham animals (6−7% vs. 4−5%). There were no apparent differences in both direction and magnitude of LENG change, but the deformational magnitude in THICK were significantly smaller in reduction than sham animals (P < 0.05; Fig. 7B).
Stimulation of the extrinsic tongue muscles (GG or SG) in sham animals produced deformational changes with opposite direction in all dimensions except for PVW which showed widening under both stimulations (Fig. 8A). In general, contraction of GG increased AW, PVW, and LENG while decreased PDW and THICK. On the other hand, contraction of SG increased PDW, PVW, and THICK while decreased AW and LENG. Similar to the deformation produced by the hypoglossal nerve stimulation, changes were smaller in the anterior (AW and LENG) than the posterior (PDW, PVW, and THICK) tongue body (P < 0.05; compare Figs. 7A and 8A).
Fig. 8.
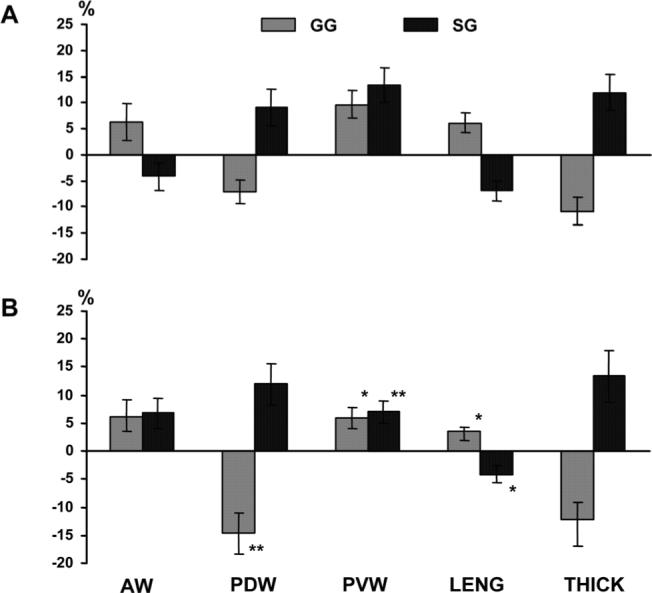
Tongue dimensional changes under stimulation of the extrinsic tongue muscles in sham (A) and reduction (B) groups. Asterisks by the vertical bars indicate significant difference between the two groups. *P < 0.05; **P < 0.01. GG, genioglossus; SG, styloglossus. Refer to Figure 2 for other captions.
Unlike the nerve stimulation, directions of deformational change in AW were consistent across reduction animals when GG or SG was stimulated. However, similar to the stimulation of HGM, the direction of deformational changes in AW showed an increase, opposite to that seen in sham animals, with an enhanced magnitude (4% vs. 7%). In addition, the deformational magnitudes were significantly reduced in LENG (lengthening) and PVW (widening) when paired GG was stimulated as compared to sham animals (P < 0.05), whereas the magnitude of PDW decrease (narrowing) was significantly enhanced (P < 0.01). When paired SG was stimulated, on the other hand, the ranges of PVW and LENG were significantly smaller (P < 0.05−0.01). Unlike the nerve stimulation, changes in THICK showed no difference between the sham and reduction animals (compare Figs. 7 and 8).
DISCUSSION
The tongue is an extremely flexible muscular hydro-static organ, and its musculature both creates motions and supplies a structural support for that motion. The tongue promptly and efficiently alters its shape in various dimensions and regions during function while keeping the entire volume unchanged, and in turn forms its local mechanical environment (Kier and Smith, 1985; Napadow et al., 1999; Gilbert et al., 2007). There has been a long-term controversy over whether the tongue adapts to an existing morphological situation rather than actively molding its surrounding tissue (Ingervall and Schmoker, 1990; Frohlich et al., 1991, 1993). Clinically, a surgically reducing tongue volume is a mainstay to treat true (tongue enlargement) and relative (insufficient space in oral cavity) macroglossia. These pathological conditions may be congenital, such as idiopathic muscular hypertrophy, or acquired, such as after mandibular set-back for correcting Class III skeletal malocclusion (Vogel et al., 1986; Ring, 1999). Because the tongue is composed of entire musculature, volume reduction actually results in the loss of muscular mass, that is, a source of tongue kinematics. Therefore, this intervention must lead to changes in the tongue internal kinematics during function. Unfortunately, no study has been done to address this important issue.
Through this study, the present results demonstrated that: (1) the pattern and amplitudes of internal kinematics during chewing are not significantly affected by sham surgery (without removal of tongue mass) as compared to the results from intact animals previously published (Shcherbatyy and Liu, 2007); (2) the basic features of internal kinematics during chewing still remain after surgically reducing tongue volume, but the regularity and amplitude of these kinematics are diminished; (3) while the major dimensional losses by the uniform mid-sagittal volume reduction were identified in AW and PDW (∼66% and 16%; Table 2), the major decrease in tongue internal kinematics during chewing were found in LENG and AW (31% and 44%; Fig. 5); and (4) changes in posterior widths (both PDW and PVW) and THICK were significantly enhanced to compensate for the loss in AW and LENG (Fig. 5). It must be emphasized that the tongue volume reduction surgery applied in the present study involves only the tongue blade and body. It is well known that the orthogonally oriented intrinsic muscle fibers are major components of the anterior two-thirds of the tongue (including both tongue blade and body), and extrinsic fibers are mainly inserted laterally (hyoglossus and styloglossus) and composing a large central region of the tongue base (genioglossus; Sicher, 1960; Odeh et al., 1995; Peter, 1995; Napadow et al., 1999; Gray, 2000), Therefore, intrinsic tongue musculature is the major component of the removed tongue tissue by the surgery, although muscle fibers from extrinsic tongue musculature, particularly genioglossus might be also included. From this point of view, such a compensatory effect by the posterior tongue might suggest that there is a mutual interaction and adaptation between these two schemes of tongue musculature: intrinsic and extrinsic. The previous notion that the extrinsic muscles are to position the tongue and the intrinsic muscles to shape the tongue (Sutlive et al., 1999; Gray, 2000) may no longer be true. In fact, internal kinematics of the tongue is driven by both intrinsic and extrinsic tongue musculature, and these two muscle groups are not only structurally interwoven but also functionally interacting. It can also be reasonably inferred that these changes in tongue internal kinematics that are induced by the volume reduction surgery may not be a transient effect but a permanent consequence, as the chance of full recovery through myore-generation of the tongue is unlikely and the fibrosis would be the most likely healing outcome. This inference is being tested in our ongoing chronic studies using the same animal model and approaches.
Theoretically, stimulation of the entire hypoglossal nerve should produce a larger deformation of the tongue than chewing because all tongue muscles are activated, and stimulation of the nerve would cause larger deformation than the stimulation of single tongue muscle. However, under nerve stimulation, the ranges of tongue dimensional changes were 2−4 times smaller as compared to those during chewing in sham animals (4−13% vs. 16−25%, compare Fig. 5 and Fig. 7A), and the ranges of deformation under the nerve and muscle stimulation were quite similar (compare Figs. 7A and 8A). Chewing is a repetitive sequence of movements involving jaw, tongue, hyoid, epiglottis, and other structures, and depends upon the coordination and synergy of musculatures controlling these structures. Therefore, the deformation of the tongue during chewing is the result of a combination of the contraction of tongue muscles and jaw/hyoid movement, whereas hypoglossal nerve stimulation evokes only the contraction of tongue muscles. This finding may explain why the tongue deforms more during chewing than under the stimulation of the paired hypoglossal nerves. The similar amplitudes of deformation seen with the nerve and single muscle stimulation may further suggest that the strength of a motor output from a supramaximally activated nerve is analogous to the tetanic contraction of its innervated muscle in the hypoglossal motor system.
Due to the lack of interaction and coordination between muscles under neuromuscular stimulation, it was expected that the compensation for the loss of tongue mass by the reduction surgery would be diminished. However, this effect could still be found when HGM (PDW widening) and GG (PDW narrowing) were stimulated (Figs. 7, 8). Because the dimensional loss of PDW by the surgery was the second largest after that of AW, the initial distance of PDW became smaller. Thus, a larger percentage of deformation of PDW occurred when paired HGM (the nerve that supplies motor output to GG, which is the largest tongue muscle responsible for altering tongue width) was stimulated. This finding may explain this exceptional finding of PDW enhancement under stimulation. As indicated in Table 2, the least dimensional loss after the surgery was in PVW (3.1%). Therefore, this region could be considered close to intact and should act similarly to what is observed in sham animals under stimulation. However, while the direction of deformation of PVW (widening) in reduction animals stayed the same as in sham animals, significant decreases in the deformational range were found under all stimulation conditions (Figs. 7, 8). This contradictory finding suggests that the mass or dimensional loss might not be the major factor causing the decrease in deformational range in the tongue. This notion can be further supported by the fact that the deformational range of PDW, the second largest dimensional loss (∼16%) by the reduction surgery (Table 2), adversely increased, rather than decreased, during chewing (Fig. 5).
Although the reduction surgery resulted in alterations of deformation in various dimensions under neuromuscular stimulation, the direction of deformation remained the same as seen in sham animals except for AW. This further demonstrated that the basic function of the hypoglossal nerve and the extrinsic tongue muscles were not destroyed by the reduction surgery. With regard to AW, it must be emphasized that this region was located in the tongue blade and underwent as much as ∼66% of dimensional loss after the reduction surgery (Table 2). Therefore, these inconsistent and distorted dimensional changes under stimulation in AW may be attributed to a major loss of the tongue blade (Fig. 1). The largest decrease in the range of dimensional changes in AW (∼44%; Fig. 5) during chewing may further demonstrate the functional distortion of the tongue blade after the reduction surgery. However, with the nature of muscular plasticity and the process of motor learning (Liu et al., 1998; Baldwin and Haddad, 2002), this damaged capacity of internal kinematics in the tongue blade is expected to be partially restored in the long-term. Our ongoing chronic studies will verify this expectation.
In summary, the present study support our hypotheses that tongue volume reduction alters the tongue internal kinematics and the dimensional losses in the anterior tongue caused by the volume reduction can be compensated by increased deformations in the posterior tongue during mastication. This compensatory effect, however, diminishes under the stimulation of the hypoglossal nerve and individual tongue muscles. Further studies are ongoing to verify if such an altered internal kinematics of the tongue would be persistent or subject to modification over time.
ACKNOWLEDGMENTS
The authors thank Drs. Mustafa Kayalioglu, a visiting scholar from Turkey, and Amir Seifi, a PhD student, and Ms. Xian-Qin Bai, a research technologist, for help with experiments. Z.J.L. was funded by NIDCR. A portion of this study was presented at the 87th General Session of the International Association for Dental Research (IADR), New Orleans, LA, March, 21−24, 2007.
Grant sponsor: National Institute of Dental and Craniofacial Research; Grant number: R01DE15659.
LITERATURE CITED
- Bailey EF, Fregosi RF. Coordination of intrinsic and extrinsic tongue muscles during spontaneous breathing in the rat. J Appl Physiol. 2004;96:440–449. doi: 10.1152/japplphysiol.00733.2003. [DOI] [PubMed] [Google Scholar]
- Baldwin KM, Haddad F. Skeletal muscle plasticity: cellular and molecular responses to altered physical activity paradigms. Am J Phys Med Rehabil. 2002;81:S40–S51. doi: 10.1097/01.PHM.0000029723.36419.0D. [DOI] [PubMed] [Google Scholar]
- Bressmann T, Uy C, Irish JC. Analysing normal and partial glossectomee tongues using ultrasound. Clin Linguist Phon. 2005;19:35–52. doi: 10.1080/02699200410001669834. [DOI] [PubMed] [Google Scholar]
- Davalbhakta A, Lamberty BG. Technique for uniform reduction of macroglossia. Br J Plast Surg. 2000;53:294–297. doi: 10.1054/bjps.1999.3311. [DOI] [PubMed] [Google Scholar]
- Deguchi T. Case report: three typical cases of glossectomy. Angle Orthod. 1993;63:199–207. doi: 10.1043/0003-3219(1993)063<0199:CRTTCO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Frohlich K, Ingervall B, Schmoker R. Influence of surgical tongue reduction on pressure from the tongue on the teeth. Angle Orthod. 1993;63:191–198. doi: 10.1043/0003-3219(1993)063<0191:IOSTRO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Frohlich K, Thuer U, Ingervall B. Pressure from the tongue on the teeth in young adults. Angle Orthod. 1991;61:17–24. doi: 10.1043/0003-3219(1991)061<0017:PFTTOT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Gilbert RJ, Napadow VJ, Gaige TA, Wedeen VJ. Anatomical basis of lingual hydrostatic deformation. J Exp Biol. 2007;210:4069–4082. doi: 10.1242/jeb.007096. [DOI] [PubMed] [Google Scholar]
- Gray H. Anatomy of the human body. 20th ed. Lea & Febiger; Philadelphia: 2000. 1918. Revised and re-edited. New York: Bartleby.Com. [Google Scholar]
- Herren P, Muller-Boschung P, Stutz G. Macroglossia and partial resection of the tongue out of orthodontic indication. Proc Finn Dent Soc. 1981;77:45–55. [PubMed] [Google Scholar]
- Ingervall B, Schmoker R. Effect of surgical reduction of the tongue on oral stereognosis, oral motor ability, and the rest position of the tongue and mandible. Am J Orthod Dentofacial Orthop. 1990;97:58–65. doi: 10.1016/S0889-5406(05)81710-X. [DOI] [PubMed] [Google Scholar]
- Kier WM, Smith KK. Tongues, tentacles and trunks: the biomechanics of movement in muscular-hydrostats. Zool J Linn Soc. 1985;83:307–324. [Google Scholar]
- Kier WM, Smith KK, Miyan JA. Electromyography of the fin musculature of the cuttlefish Sepia officinalis. J Exp Biol. 1989;143:17–31. doi: 10.1242/jeb.143.1.17. [DOI] [PubMed] [Google Scholar]
- Li KK, Powell NB, Riley RW, Guilleminault C. Temperature-controlled radiofrequency tongue base reduction for sleep-disordered breathing: long-term outcomes. Otolaryngol Head Neck Surg. 2002;127:230–234. doi: 10.1067/mhn.2002.126900. [DOI] [PubMed] [Google Scholar]
- Liu ZJ, Ikeda K, Harada S, Kasahara Y, Ito G. Functional properties of jaw and tongue muscles in rats fed a liquid diet after being weaned. J Dent Res. 1998;77:366–376. doi: 10.1177/00220345980770020501. [DOI] [PubMed] [Google Scholar]
- Liu ZJ, Shcherbatyy V, Kayalioglu M, Seifi A. Dimensional changes of the tongue during activation of hypoglossal nerves and contraction of tongue muscles. Harvard Society for the Advancement of Orthodontics; Boston: 2006. pp. 305–312. [Google Scholar]
- Liu ZJ, Kayalioglu M, Shcherbatyy V, Seifi A. Tongue deformation, jaw movement and muscle activity during mastication in pigs. Arch Oral Biol. 2007;52:309–312. doi: 10.1016/j.archoralbio.2006.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZJ, Yamamura B, Shcherbatyy V, Green JR. Regional volumetric changes of the tongue during mastication in pigs. J Oral Rehabil. 2008 doi: 10.1111/j.1365-2842.2008.01862.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napadow VJ, Chen Q, Wedeen VJ, Gilbert RJ. Intramural mechanics of the human tongue in association with physiological deformations. J Biomech. 1999;32:1–12. doi: 10.1016/s0021-9290(98)00109-2. [DOI] [PubMed] [Google Scholar]
- Odeh M, Schnall R, Gavriely N, Oliven A. Dependency of upper airway patency on head position: the effect of muscle contraction. Respir Physiol. 1995;100:239–244. doi: 10.1016/0034-5687(94)00135-m. [DOI] [PubMed] [Google Scholar]
- Peter LW. Alimentary system. In: Lawrence HB, editor. Gray's anatomy. 38th ed. Churchill Livingstone; New York: 1995. pp. 1721–1724. [Google Scholar]
- Ring ME. The treatment of macroglossia before the 20th century. Am J Otolaryngol. 1999;20:28–36. doi: 10.1016/s0196-0709(99)90047-9. [DOI] [PubMed] [Google Scholar]
- Ruff RM. Orthodontic treatment and tongue surgery in a class III open-bite malocclusion. A case report. Angle Orthod. 1985;55:155–166. doi: 10.1043/0003-3219(1985)055<0155:OTATSI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Sanders I, Mu LC. 2005 www.upperairway.com. In.
- Shcherbatyy V, Liu ZJ. Internal kinematics of the tongue during feeding in pigs. Anat Rec. 2007;290:1288–1299. doi: 10.1002/ar.20582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicher H. Oral anatomy. 3rd ed. CV Mosby Co.; St. Louis: 1960. [Google Scholar]
- Stuck BA, Kopke J, Hormann K, Verse T, Eckert A, Bran G, Duber C, Maurer JT. Volumetric tissue reduction in radiofrequency surgery of the tongue base. Otolaryngol Head Neck Surg. 2005;132:132–135. doi: 10.1016/j.otohns.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Sutlive TG, McClung JR, Goldberg SJ. Whole-muscle and motor-unit contractile properties of the styloglossus muscle in rat. J Neurophysiol. 1999;82:584–592. doi: 10.1152/jn.1999.82.2.584. [DOI] [PubMed] [Google Scholar]
- Vogel JE, Mulliken JB, Kaban LB. Macroglossia: a review of the condition and a new classification. Plast Reconstr Surg. 1986;78:715–723. [PubMed] [Google Scholar]
- Wolford LM, Cottrell DA. Diagnosis of macroglossia and indications for reduction glossectomy. Am J Orthod Dentofacial Orthop. 1996;110:170–177. doi: 10.1016/s0889-5406(96)70105-1. [DOI] [PubMed] [Google Scholar]