Abstract
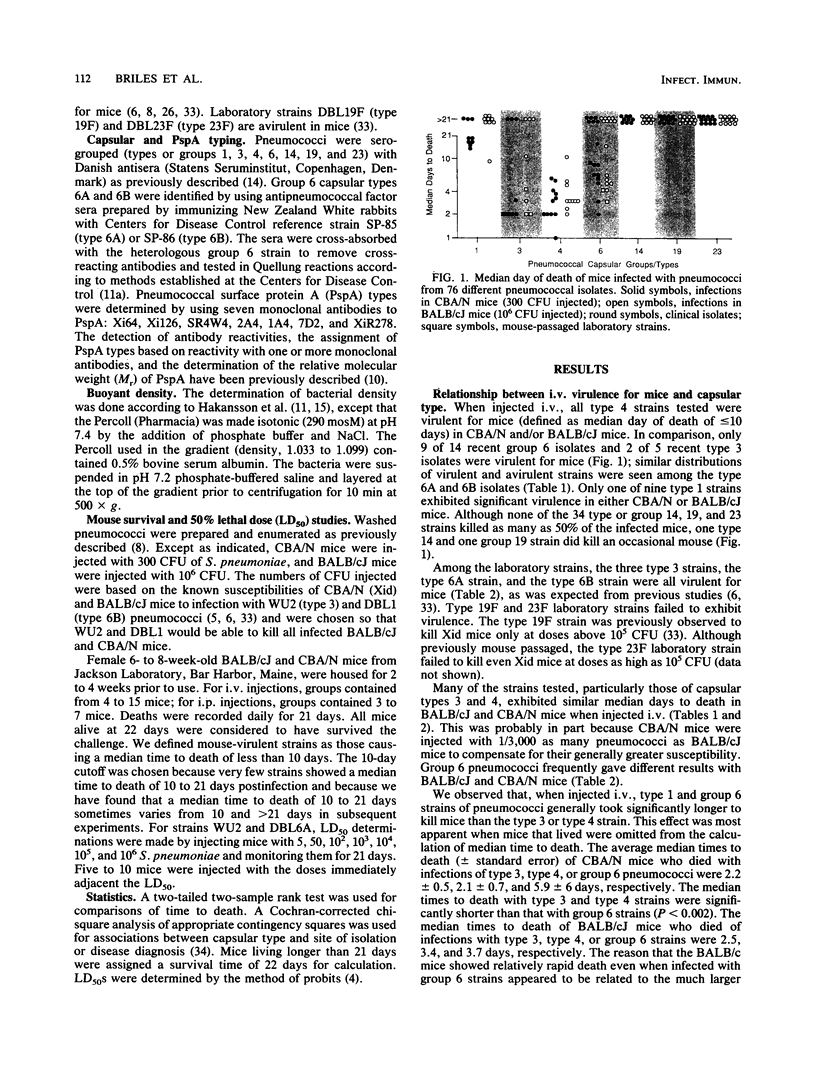
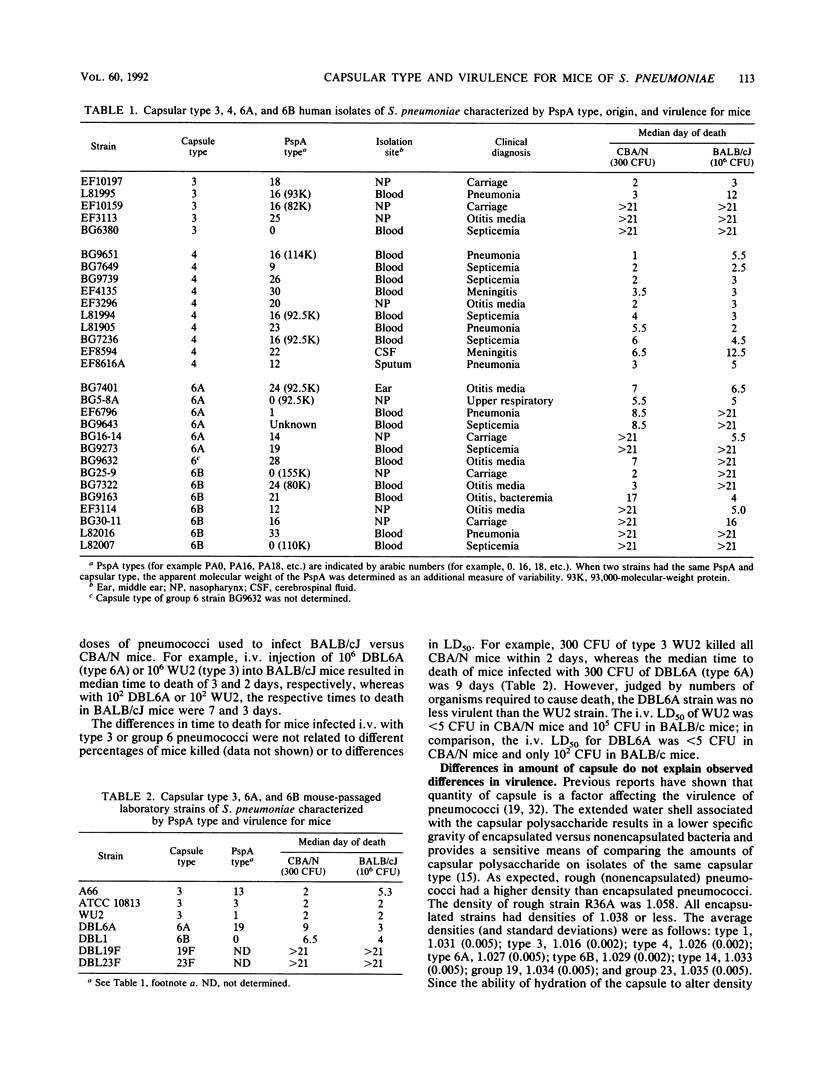
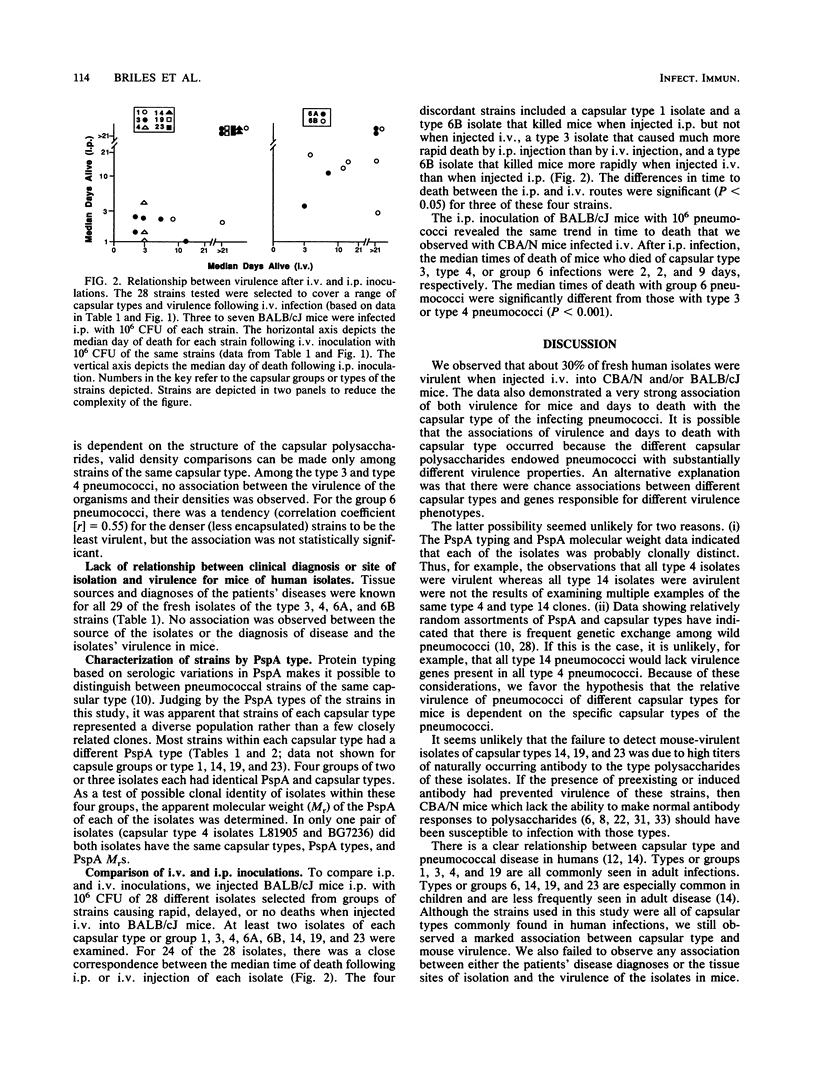
The relationship between capsular type and virulence for mice was examined with 69 fresh human isolates of Streptococcus pneumoniae. These isolates represented eight capsular types or groups. Serologic and molecular weight differences in PspA (pneumococcal surface protein A) indicated that the strains were clonally distinct. Mice were infected intravenously with washed bacteria of all 69 isolates in sterile salt solutions. Twenty-eight of the isolates were also injected intraperitoneally to permit comparisons between the intravenous and intraperitoneal routes. With a few exceptions, there was concordance between the ability of strains to cause fatal infections by the two routes. About 30% of the 69 isolates were virulent for mice. The abilities of the isolates to kill mice and the length of time between inoculation and death were strongly associated with capsular type. All type 4 isolates, 40% of type 3 isolates, and 60% of group 6 isolates were virulent for mice; type 1 isolates were marginally virulent; and all type or group 14, 19, and 23 isolates were avirulent. Times to death were generally longer for mice infected with group 6 or type 1 than for those infected with type 3 or 4 pneumococci. There was no relationship between clinical diagnosis or tissue source of the isolates and virulence for mice.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amsbaugh D. F., Hansen C. T., Prescott B., Stashak P. W., Barthold D. R., Baker P. J. Genetic control of the antibody response to type 3 pneumococcal polysaccharide in mice. I. Evidence that an X-linked gene plays a decisive role in determining responsiveness. J Exp Med. 1972 Oct 1;136(4):931–949. doi: 10.1084/jem.136.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry A. M., Yother J., Briles D. E., Hansman D., Paton J. C. Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect Immun. 1989 Jul;57(7):2037–2042. doi: 10.1128/iai.57.7.2037-2042.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles D. E., Forman C., Hudak S., Claflin J. L. Anti-phosphorylcholine antibodies of the T15 idiotype are optimally protective against Streptococcus pneumoniae. J Exp Med. 1982 Oct 1;156(4):1177–1185. doi: 10.1084/jem.156.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles D. E., Horowitz J., McDaniel L. S., Benjamin W. H., Jr, Claflin J. L., Booker C. L., Scott G., Forman C. Genetic control of the susceptibility to pneumococcal infection. Curr Top Microbiol Immunol. 1986;124:103–120. doi: 10.1007/978-3-642-70986-9_7. [DOI] [PubMed] [Google Scholar]
- Briles D. E., Nahm M., Marion T. N., Perlmutter R. M., Davie J. M. Streptococcal group A carbohydrate has properties of both a thymus-independent (TI-2) and a thymus-dependent antigen. J Immunol. 1982 May;128(5):2032–2035. [PubMed] [Google Scholar]
- Briles D. E., Nahm M., Schroer K., Davie J., Baker P., Kearney J., Barletta R. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 streptococcus pneumoniae. J Exp Med. 1981 Mar 1;153(3):694–705. doi: 10.1084/jem.153.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Converse G. M., 3rd, Dillon H. C., Jr Epidemiological studies of Streptococcus pneumoniae in infants: methods of isolating pneumococci. J Clin Microbiol. 1977 Mar;5(3):293–296. doi: 10.1128/jcm.5.3.293-296.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain M. J., Waltman W. D., 2nd, Turner J. S., Yother J., Talkington D. F., McDaniel L. S., Gray B. M., Briles D. E. Pneumococcal surface protein A (PspA) is serologically highly variable and is expressed by all clinically important capsular serotypes of Streptococcus pneumoniae. Infect Immun. 1990 Oct;58(10):3293–3299. doi: 10.1128/iai.58.10.3293-3299.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finland M., Barnes M. W. Changes in occurrence of capsular serotypes of Streptococcus pneumoniae at Boston City Hospital during selected years between 1935 and 1974. J Clin Microbiol. 1977 Feb;5(2):154–166. doi: 10.1128/jcm.5.2.154-166.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray B. M., Dillon H. C., Jr Clinical and epidemiologic studies of pneumococcal infection in children. Pediatr Infect Dis. 1986 Mar-Apr;5(2):201–207. doi: 10.1097/00006454-198603000-00009. [DOI] [PubMed] [Google Scholar]
- Håkansson S., Holm S. E., Wagner M. Density profile of group B streptococci, type III, and its possible relation to enhanced virulence. J Clin Microbiol. 1987 Apr;25(4):714–718. doi: 10.1128/jcm.25.4.714-718.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht J. C., Schiffman G., Austrian R. Some biological properties of Pneumococcus type 37 and the chemistry of its capsular polysaccharide. J Exp Med. 1970 Sep 1;132(3):475–487. doi: 10.1084/jem.132.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock R. A., Paton J. C., Hansman D. Comparative efficacy of pneumococcal neuraminidase and pneumolysin as immunogens protective against Streptococcus pneumoniae. Microb Pathog. 1988 Dec;5(6):461–467. doi: 10.1016/0882-4010(88)90007-1. [DOI] [PubMed] [Google Scholar]
- MacLEOD C. M., KRAUS M. R. Relation of virulence of pneumococcal strains for mice to the quantity of capsular polysaccharide formed in vitro. J Exp Med. 1950 Jul 1;92(1):1–9. doi: 10.1084/jem.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel L. S., Scott G., Kearney J. F., Briles D. E. Monoclonal antibodies against protease-sensitive pneumococcal antigens can protect mice from fatal infection with Streptococcus pneumoniae. J Exp Med. 1984 Aug 1;160(2):386–397. doi: 10.1084/jem.160.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel L. S., Yother J., Vijayakumar M., McGarry L., Guild W. R., Briles D. E. Use of insertional inactivation to facilitate studies of biological properties of pneumococcal surface protein A (PspA). J Exp Med. 1987 Feb 1;165(2):381–394. doi: 10.1084/jem.165.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mond J. J., Lieberman R., Inman J. K., Mosier D. E., Paul W. E. Inability of mice with a defect in B-lymphocyte maturation to respond to phosphorycholine on immunogenic carriers. J Exp Med. 1977 Oct 1;146(4):1138–1142. doi: 10.1084/jem.146.4.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin L. G., Zwahlen A., Moxon E. R. Role of intravascular replication in the pathogenesis of experimental bacteremia due to Haemophilus influenzae type b. J Infect Dis. 1985 Aug;152(2):307–314. doi: 10.1093/infdis/152.2.307. [DOI] [PubMed] [Google Scholar]
- Wallick S., Claflin J. L., Briles D. E. Resistance to Streptococcus pneumoniae is induced by a phosphocholine-protein conjugate. J Immunol. 1983 Jun;130(6):2871–2875. [PubMed] [Google Scholar]
- Waltman W. D., 2nd, McDaniel L. S., Andersson B., Bland L., Gray B. M., Eden C. S., Briles D. E. Protein serotyping of Streptococcus pneumoniae based on reactivity to six monoclonal antibodies. Microb Pathog. 1988 Sep;5(3):159–167. doi: 10.1016/0882-4010(88)90018-6. [DOI] [PubMed] [Google Scholar]
- Watson D. A., Musher D. M. Interruption of capsule production in Streptococcus pneumonia serotype 3 by insertion of transposon Tn916. Infect Immun. 1990 Sep;58(9):3135–3138. doi: 10.1128/iai.58.9.3135-3138.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker L. S., Scher I. X-linked immune deficiency (xid) of CBA/N mice. Curr Top Microbiol Immunol. 1986;124:87–101. doi: 10.1007/978-3-642-70986-9_6. [DOI] [PubMed] [Google Scholar]
- Yother J., Forman C., Gray B. M., Briles D. E. Protection of mice from infection with Streptococcus pneumoniae by anti-phosphocholine antibody. Infect Immun. 1982 Apr;36(1):184–188. doi: 10.1128/iai.36.1.184-188.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]




