Abstract
We utilized data from the comparison group of the Women's Healthy Eating and Living randomized trial to investigate an “a priori” hypothesis suggested by CYP2D6 studies that hot flashes may be an independent predictor of tamoxifen efficacy. A total of 1551 women with early stage breast cancer were enrolled and randomized to the comparison group of the WHEL multi-institutional trial between 1995 and 2000. Their primary breast cancer diagnoses were between 1991 and 2000. At study entry, 864 (56%) of these women were taking tamoxifen, and hot flashes were reported by 674 (78%). After 7.3 years of follow-up, 127 of those who took tamoxifen at baseline had a confirmed breast cancer recurrence. Women who reported hot flashes at baseline were less likely to develop recurrent breast cancer than those who did not report hot flashes (12.9% vs 21%, P = 0.01). Hot flashes were a stronger predictor of breast cancer specific outcome than age, hormone receptor status, or even the difference in the stage of the cancer at diagnosis (Stage I versus Stage II). These findings suggest an association between side effects, efficacy, and tamoxifen metabolism. The strength of this finding suggests that further study of the relationship between hot flashes and breast cancer progression is warranted. Additional work is warranted to clarify the mechanism of hot flashes in this setting.
Keywords: Breast cancer, Hot flashes, Survival, Tamoxifen
Introduction
Tamoxifen is metabolized through the cytochrome P450 pathway to 4-hydroxy tamoxifen (4OH-TAM) and endoxifen, which are more potent antiestrogens than the parent drug. Because steady state serum concentrations of endoxifen are 5−10 fold higher than 4OH-TAM, endoxifen is believed to be the most important metabolite [1]. The activity of the CYP2D6 enzyme correlates with endoxifen levels [2, 3]. Women with polymorphisms of the CYP2D6 enzyme appear to derive less benefit from adjuvant tamoxifen and experience hot flashes less often than women with normal enzyme activity [4]. Thus, having hot flashes may be an indicator of better prognosis.
Hot flashes are the subjective sensation of heat that is thought to be related to a narrowing of the thermoneutral zone [5]. It is generally understood that the onset of symptoms occurs as a result of decreased estrogen or increased gonadotropin concentrations [6]. However, other reported precipitators include psychological stress, hot weather, alcohol and caffeine [7]. The physiological mechanism is not completely understood [8]. Nearly two-thirds of breast cancer survivors report that hot flashes compromise their quality of life [9, 10]. The most common request for additional treatment from breast cancer survivors is for relief of hot flashes [11].
In this report, we investigate the association of reported hot flashes with prognosis in the no-treatment comparison group of the Women's Healthy Eating and Living (WHEL) study, a large scale trial of the role of dietary pattern in breast cancer recurrence. Women in this trial were enrolled after completion of their primary therapy for breast cancer.
Methods
Participants
Between 1995 and 2000, the WHEL trial enrolled women who had been treated for Stages I (T1c)-IIIA breast cancer, who were 2−48 months from initial breast cancer diagnosis and were between 18 and 70 years at diagnosis [12]. Information about the primary tumor and cancer treatment data were abstracted from the medical record, and the trial documented and confirmed subsequent reported breast cancer recurrences or new primary breast cancers. A total of 3,088 eligible participants were randomized to a dietary intervention (n = 1,537) or a control group (n = 1,551). The participating institutions included: University of California, San Diego and Davis, Stanford University, Kaiser Permanente in Oakland and Portland, University of Arizona at Tucson and the MD Anderson Cancer Center. The study was approved by each institution's review board and participants signed an informed consent.
Measures
At baseline, women reported their dietary intake, menopausal status, and anti-estrogen use. They also completed the lengthy “Thoughts and Feelings” questionnaire developed for the Women's Health Initiative [13] and used with their permission. This questionnaire includes a 34-item self-report symptom inventory that assesses a variety of physical and psychological symptoms, including ‘vasomotor symptoms’ [14]. Participants were asked about the occurrence and severity of these vasomotor symptoms during the previous 4 weeks: not occurring, mild but not interfering with usual activities, moderate and interfering somewhat with usual activities, or severe such that usual activities could not be performed (scored 0−3)[13]. Tumor stage, grade, and hormone receptor status were derived from medical records.
Each 6 months, clinical site staff conducted a brief telephone interview with each study participant and sought information on any breast cancer recurrence or new primary breast cancer that may have developed. All reports were investigated and the medical record was reviewed by two oncologists blinded to the study group of the participant for confirmation of the breast cancer diagnosis. As of December 1, 2005 breast cancer status was confirmed in 96% of this study cohort.
Unadjusted cancer event proportions for the presence of hot flashes were compared with those not reporting hot flashes. Bivariate associations of vasomotor symptoms with age, race/ethnicity, menopausal status, cancer stage, estrogen and progesterone receptor status, and time since diagnosis were tested using chi-square tests for categorical and t-tests for continuous variables. After testing the validity of the proportional hazards assumption, a delayed-entry Cox proportional hazards model tested the association between recurrence-free survival and hot flashes, adjusting for tumor stage and grade and patient age. This model adjusts for the fact that women enter the study at 2−48 months beyond their initial diagnosis and are not under observation for a possible recurrence before study entry. Odds ratios and 95% confidence intervals are reported. Women who died without a new breast cancer event were censored at their date of death; those without a new breast cancer event were censored at the earlier of December 1, 2006 or the date of their most recent self-report of their breast cancer status. The average length of observation was 24 months between diagnosis and study entry, and 89 months after study entry.
Kaplan–Meier curves demonstrating overall survival, and survival in 2 age strata, as a function of hot flashes reported at study entry, were created. Analyses were conducted in SAS version 9.1 (Cary NC).
Results
Among the 1551 women randomized to the WHEL comparison group, 637 were not taking an anti-estrogen drug at baseline; one was on anastrazole, 16 on raloxifene and 897 (58%) were on tamoxifen. This study focuses on these women taking tamoxifen. Thirty-three of them had missing baseline data on hot flashes or night sweats. Excluding these, the final sample size for women taking tamoxifen for this study was 864. Among this group, 127 had a confirmed cancer event during the observation period.
Most of the women (78%) reported hot flashes, and 69% of those reporting hot flashes also reported night sweats. Only 4% of the women reported night sweats in the absence of hot flashes, and 18% reported neither hot flashes nor night sweats. The incidence of hot flashes was not significantly associated with patient age; mean age was 54 years in both groups, with a range from 27 to 73 years in the hot flash group and a range from 28 to 73 years in the no symptom group.
Unadjusted cancer recurrence/new primary rates among women who reported mild, moderate, and severe hot flashes were similar at 13.9, 12.2, and 11.9% respectively, compared with 21.6% for women not reporting hot flashes. Thus we collapsed the hot flashes data into a binary variable.
Unadjusted recurrence rates for a subsequent breast cancer event were 14.3% among those reporting both hot flashes and night sweats, 9.6% among those reporting only hot flashes, 22.2% among those reporting only night sweats, and 21.4% among those reporting neither vasomotor symptom. Thus, the recurrence effect appears to be specific to reporting of any hot flashes (versus none).
Although participants who reported hot flashes did not vary by age, race/ethnicity, use of chemotherapy, or cancer stage (Table 1), reporting did vary by time since diagnosis and by time on tamoxifen. Hot flashes developed more in women who were closer to diagnosis and who were closer to their commencement of tamoxifen treatment. Eighty-one percent of those who had been on tamoxifen for a year or less at baseline reported hot flashes, compared with 72% of those who had been on tamoxifen more than 3 years. Women who had not had a recurrence by the conclusion of the observation period were more likely to have reported hot flashes at baseline (80%), compared with those who subsequently metastasized (72%), had a contralateral recurrence (62%) or a local/regional recurrence (50%).
Table 1.
Distribution of demographic and cancer characteristics by hot flash reporting
| Hot flashes n(%) 674(78) |
No hot flashes n(%) 190(22) |
P-value | |
|---|---|---|---|
| Age in years Mean(SD) | 54(8) | 54(10) | 0.66 |
| Race/Ethnicity | 0.43 | ||
| Caucasian | 580(78) | 161(22) | |
| Hispanic | 38(83) | 8(17) | |
| Asian | 21(70) | 9(30) | |
| African American | 19(83) | 4(17) | |
| Other | 16(67) | 8(33) | |
| Stage of breast cancer | 0.81 | ||
| I | 253(78) | 70(22) | |
| II | 300(77) | 89(23) | |
| III | 121(80) | 31(20) | |
| Hormone receptor status | 0.07 | ||
| ER+/PR+ | 525(79) | 143(21) | |
| ER+/PR− | 88(73) | 32(27) | |
| ER−/PR+ | 27(96) | 1(4) | |
| ER−/PR− | 23(72) | 9(28) | |
| Unknown PR | 11(69) | 5(31) | |
| Adjuvant therapy | 0.22 | ||
| Chemotherapy + Tamoxifen | 427(79) | 111(21) | |
| Tamoxifen only | 247(76) | 79(24) | |
| Months on tamoxifen, Mean(SD) | 19(13) | 21(13) | 0.01 |
| Months since diagnosis, Mean(SD) | 23(12) | 26(13) | 0.01 |
| Recurrence of Cancer | 0.02 | ||
| None | 587(80) | 150(20) | |
| Local/Regional | 7(50) | 7(50) | |
| Contralateral | 8(62) | 5(38) | |
| Distant | 72(72) | 28(28) |
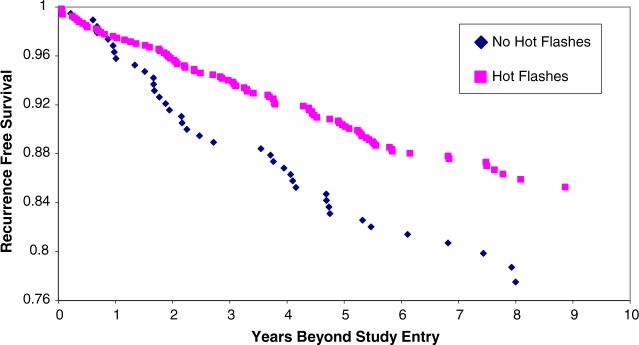
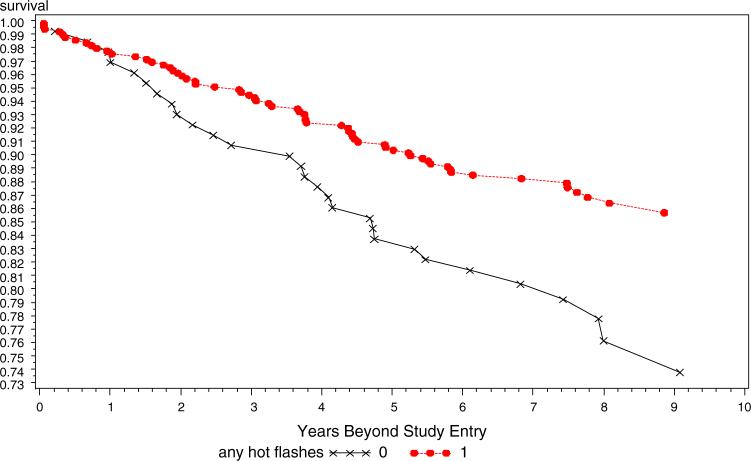
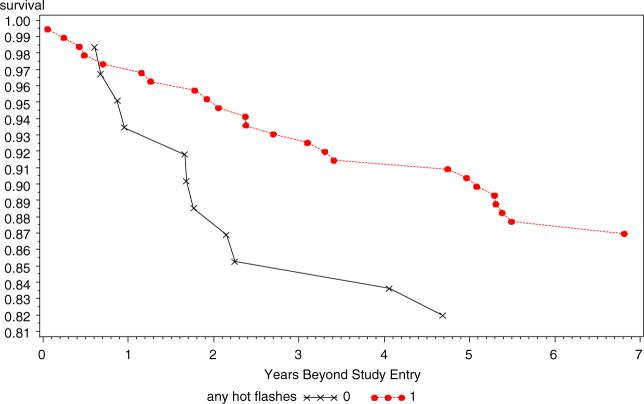
A delayed entry Cox proportional hazards model adjusting for tumor stage and grade (Table 2) showed that those reporting hot flashes had a hazard ratio of 0.50 of recurrence during the follow-up period (95% CI 0.36−0.69) and that hot flashes were more predictive of outcome for tamoxifen treated patients than were age, grade, hormone receptor status, or stage of the initial cancer (I vs II). Because the interval between diagnosis and study entry was significantly shorter in those who reported hot flashes compared to those who did not, the Cox model was also run with the addition of time between diagnosis and study entry as an independent variable. However, this additional covariate did not alter the hazard ratio for hot flashes as a predictor of recurrence. Kaplan–Meier curves for all with and without hot flashes, and stratified by age group are displayed in Figs. 1–3. Figure 1 presents the overall data for the study and demonstrates that the study effect was clear and consistent across all years of follow-up. Figure 2 presents the data only for the 248 women who were under the age of 50 years at initial diagnosis. Figure 3 presents the data for women who were aged over 50 years at the time of diagnosis. The strong and consistent effect over time is observed in each figure, regardless of age or presumably menopausal status.
Table 2.
Cox proportional hazards model for breast cancer end point in WHEL Study women on tamoxifen at baseline (n = 864)
| Predictor | Hazard ratio | 95% CI |
|---|---|---|
| Stage of breast cancer* | ||
| I (reference) | 1.00 | - |
| II | 1.61 | (0.92−2.83) |
| III | 4.26 | (2.36−7.68) |
| Grade of cancer | ||
| I (reference) | 1.00 | |
| II | 2.02 | (0.98−4.14) |
| III | 1.97 | (0.91−4.26) |
| Missing grade | 0.72 | (0.22−2.38) |
| Hot Flashes at baseline | ||
| No (reference) | 1.00 | - |
| Yes | 0.50 | (0.36−0.69) |
Tumor stage was originally reported using AJCC 4th staging criteria, and then recalculated using AJCC 6th staging
Model is also adjusted for age, time interval between diagnosis and study entry, tumor hormone receptor status, and baseline dietary fiber intake
Fig. 1.
Kaplan–Meier recurrence-free survival as a function of reported hot flashes in all tamoxifen users
Fig. 3.
Recurrence-free survival as a function of hot flashes in tamoxifen, age ≥50 years
Fig. 2.
Recurrence-free survival as a function of hot flashes in tamoxifen users, age <50 years
Discussion
This study tested an “a priori” hypothesis suggested from published research on the role of CYP2D6 in the efficacy of tamoxifen as adjuvant therapy for breast cancer. The WHEL study comparison group was an ideal study to test this hypothesis as it only enrolled women diagnosed with early stage breast cancer between the years 1991 and 2000. The majority of the study participants were prescribed tamoxifen and were still taking it at the time of study entry (<4 years post diagnosis). The study also included a detailed self-reported symptom inventory as part of its baseline quality of life measures and vasomotor symptoms were included.
This study provides the first evidence that self-reported hot flashes may be predictive of the efficacy of tamoxifen and long term survival in women with early stage breast cancer. The marked effect observed was not restricted to younger women as might be expected were the symptoms simply reflective of menopausal status.
A pharmacogenetic mechanism for hot flashes is suggested by Goetz and colleagues [15]. They have reported that women taking adjuvant tamoxifen who were genotyped to be extensive metabolizers of CYP2D6 had a significant improvement in relapse free survival compared with those who had intermediate or absent enzyme activity. In Goetz's study, hot flashes were reported by 36/177 women with normal enzyme activity, compared with 0/13 of the others [4]. This finding raises the possibility that side effects are an indirect measure of CYP2D6 activity. The importance of CYP2D6 activity was further supported by a re-analysis of the aforementioned data reclassifying women according to genotype and concomitant medications that might inhibit CYP2D6 activity. Individuals who were taking inhibitors of CYP2D6 were designated as “decreased metabolizers,” even if their genotype was consistent with extensive metabolism. After reclassification of patients, the impact of enzyme activity on relapse free survival was more significant [15].
Our data support the possibility of a significant association between hot flashes and disease outcome. The study needs to go further, however, in testing this hypothesis. Fortunately, baseline blood was collected on all WHEL study participants and stored at −80°C. Analysis of this stored blood should be undertaken to correlate genotype CYP2D6 genotype with plasma concentration of tamoxifen metabolites and incidence of vasomotor symptoms in this population. However, the pathophysiology of hot flashes is not well understood and it is possible that it may be unrelated to tamoxifen and its metabolism [16]. Nevertheless, the strength of this finding for an “a priori” analysis suggests the need for further studies of the relationship between hot flashes and disease outcome.
Acknowledgements
The Women's Healthy Eating and Living (WHEL) Study was initiated with the support of the Walton Family Foundation and continued with funding from NCI grant CA 69375.
Biography
The Women's Healthy Eating and Living (WHEL) Study Group: WHEL Study Coordinating Center: University of California, San Diego, Cancer Prevention and Control Program, San Diego, California: Dr. John P. Pierce (Principal Investigator), Dr. Barbara A. Parker (Medical Director), Dr. Wayne Bardwell, Susan Faerber, Shirley W. Flatt, Sheila Kealey, Dr. Loki Natarajan, Vicky Newman, Dr. Cheryl L. Rock; Whel Study Clinic Sites: Center For Health Research, Portland, Oregon: Dr. Njeri Karanja, Dr. Mark Rarick; Kaiser Permanente Northern California, Oakland, California: Dr. Bette J. Caan, Dr. Lou Fehrenbacher; Northern California Cancer Center, Palo Alto, California: Dr. Marcia L. Stefanick, Dr. Robert Carlson; University Of Arizona, Tucson And Phoenix, Arizona: Dr. Cynthia Thomson, Dr. James Warnecke; University Of California, Davis, Davis, California: Dr. Ellen B. Gold, Dr. Sidney Scudder; University Of California, San Diego Cancer Center, San Diego, California: Dr. Kathryn A. Hollenbach, Dr. Linda Wasserman; University Of Texas M. D. Anderson Cancer Center, Houston, Texas: Dr. Lovell A. Jones, Dr. Richard Theriault.
References
- 1.Stearns V, Johnson MD, Rae JM, et al. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst. 2003;95(23):1758–1764. doi: 10.1093/jnci/djg108. [DOI] [PubMed] [Google Scholar]
- 2.Borges S, Desta Z, Li L, et al. Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: implication for optimization of breast cancer treatment. Clin Pharmacol Ther. 2006;80(1):61–74. doi: 10.1016/j.clpt.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Desta Z, Ward BA, Soukhova NV, et al. Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6. J Pharmacol Exp Ther. 2004;310(3):1062–1075. doi: 10.1124/jpet.104.065607. [DOI] [PubMed] [Google Scholar]
- 4.Goetz MP, Rae JM, Suman VJ, et al. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol. 2005;23(36):9312–9318. doi: 10.1200/JCO.2005.03.3266. [DOI] [PubMed] [Google Scholar]
- 5.Freedman RR, Blacker CM. Estrogen raises the sweating threshold in postmenopausal women with hot flashes. Fertil Steril. 2002;77(3):487–490. doi: 10.1016/s0015-0282(01)03009-6. [DOI] [PubMed] [Google Scholar]
- 6.Stearns V, Ullmer L, Lopez JF, et al. Hot flushes. Lancet. 2002;360(9348):1851–1861. doi: 10.1016/s0140-6736(02)11774-0. [DOI] [PubMed] [Google Scholar]
- 7.Kronenberg F. Hot flashes: epidemiology and physiology. Ann NY Acad Sci. 1990;592:52–86. doi: 10.1111/j.1749-6632.1990.tb30316.x. discussion 123–133. [DOI] [PubMed] [Google Scholar]
- 8.Carpenter JS, Andrykowski MA, Cordova M, et al. Hot flashes in postmenopausal women treated for breast carcinoma: prevalence, severity, correlates, management, and relation to quality of life. Cancer. 1998;82(9):1682–1691. [PubMed] [Google Scholar]
- 9.Couzi RJ, Helzlsouer KJ, Fetting JH. Prevalence of menopausal symptoms among women with a history of breast cancer and attitudes toward estrogen replacement therapy. J Clin Oncol. 1995;13(11):2737–2744. doi: 10.1200/JCO.1995.13.11.2737. [DOI] [PubMed] [Google Scholar]
- 10.Avis NE, Brockwell S, Colvin A. A universal menopausal syndrome?. Am J Med. 2005;118(12 Suppl 2):37–46. doi: 10.1016/j.amjmed.2005.09.057. [DOI] [PubMed] [Google Scholar]
- 11.Hickey M, Saunders CM, Stuckey BG. Management of menopausal symptoms in patients with breast cancer: an evidence-based approach. Lancet Oncol. 2005;6(9):687–695. doi: 10.1016/S1470-2045(05)70316-8. [DOI] [PubMed] [Google Scholar]
- 12.Pierce JP, Faerber S, Wright FA, et al. A randomized trial of the effect of a plant-based dietary pattern on additional breast cancer events and survival: the Women's Healthy Eating and Living (WHEL) Study. Control Clin Trials. 2002;23(6):728–756. doi: 10.1016/s0197-2456(02)00241-6. [DOI] [PubMed] [Google Scholar]
- 13.Matthews KA, Shumaker SA, Bowen DJ, et al. Women's health initiative. Why now? What is it? What's new? Am Psychol. 1997;52(2):101–116. doi: 10.1037//0003-066x.52.2.101. [DOI] [PubMed] [Google Scholar]
- 14.Bardwell WA, Major JM, Rock CL, Newman VA, Thomson CA, Chilton JA, Dimsdale JE, Pierce JP. Health-related quality of life in women previously treated for early-stage breast cancer. Psychooncology. 2004;13(9):595–604. doi: 10.1002/pon.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goetz MP, Knox SK, Suman VJ, et al. The impact of cytochrome P450 2D6 metabolism in women receiving adjuvant tamoxifen. Breast Cancer Res Treat. 2007;101(1):113–121. doi: 10.1007/s10549-006-9428-0. [DOI] [PubMed] [Google Scholar]
- 16.Casper RF, Yen SS. Neuroendocrinology of menopausal flushes: an hypothesis of flush mechanism. Clin Endocrinol (Oxf) 1985;22(3):293–312. doi: 10.1111/j.1365-2265.1985.tb03243.x. [DOI] [PubMed] [Google Scholar]