Abstract
Dietary carotenoids show numerous biological activities, including antioxidant activity, induction of apoptosis, and inhibition of mammary cell proliferation. Studies examining the role of carotenoid consumption in relation to breast cancer recurrence are limited and report mixed results. We tested the hypothesis that breast cancer survivors with high dietary and plasma carotenoids would show significantly lower levels of oxidative stress than breast cancer survivors with low dietary and plasma carotenoid levels. Two hundred seven postmenopausal breast cancer survivors from the Women's Healthy Eating and Living Study volunteered for this ancillary study. Dietary data were analyzed by the Arizona Food Frequency Questionnaire and plasma carotenoids α-carotene, β-carotene, lutein plus zeaxanthin, lycopene, and β-cryptoxanthin and quantified with high-performance liquid chromatography, and immunoaffinity chromatography-monoclonal antibody–based ELISAs were used to analyze the urine samples for 8-hydroxy-2′-deoxyguanosine (8-OhdG) and 8-isoprostaglandin-F2α (8-iso-PGF2α). The correlations between dietary and plasma carotenoids were 0.34 for β-carotene, 0.46 for α-carotene, 0.39 for β-cryptoxanthin, 0.27 for lycopene, 0.30 for lutein plus zeaxanthin, and 0.30 for total carotenoids. The 8-OHdG oxidative stress biomarker was significantly reduced at the highest quartile of total plasma carotenoid concentrations (P = 0.001) and 8-iso-PGF2α was moderately reduced (P = 0.088). Dietary carotenoid levels were not significantly associated with oxidative, stress indicators, although dietary lycopene and lutein/zeaxanthin were modestly associated with 8-OHdG levels (P = 0.054 and 0.088, respectively). Key findings include a significant inverse association between total plasma carotenoid concentrations and oxidative stress as measured by urinary 8-OHdG and a moderately significant inverse association with 8-iso-PGF2α, a protective association that was not shown for dietary carotenoid intake.
Introduction
Breast cancer remains the leading cancer among women in the United States, and although survival rates have improved considerably in the past decade, efforts to continue to reduce the burden of breast cancer are needed. Studies investigating the role of fruit and vegetable consumption in relation to breast cancer risk have provided inconsistent findings (1-3). Those relating fruit and vegetable consumption to breast cancer recurrence are limited in number but generally are suggestive of a protective association with increased intake (4-6). For example, McEligot et al. (7) reported that a higher intake of vegetables was associated with a 40% to 50% reduced risk of dying after breast cancer diagnosis.
One issue of relevance to assessing the relationship between fruit and vegetable intake and breast cancer risk is the need to look beyond total intake to assess the role of specific plant foods. Specifically, carotenoids, found in fruits and vegetables, show numerous biological activities, including antioxidant activity (8, 9) and induction of apoptosis and inhibition of mammary cell proliferation, making them plausible candidates for modifying breast cancer risk (10-13). Previous cohort studies have reported that plasma total carotenoids were inversely associated with risk for recurrence of breast cancer or a new primary cancer (14, 15); however, other studies have found null results (16). Therefore, the importance of carotenoids, as assessed by diet or plasma concentrations, in breast cancer prevention and recurrence remains unclear.
In an earlier analysis assessing the relationship between total plasma carotenoid concentrations and breast cancer recurrence among 1,551 breast cancer survivors enrolled in the Women's Healthy Eating and Living (WHEL) study, Rock et al. (14) showed an estimated 43% reduction in a new breast cancer event in women showing higher versus lower plasma carotenoid concentrations. In addition, analysis of lifestyle factors shown to be protective against breast cancer recurrence in these same women enrolled in the comparison arm of the WHEL study, high fruit and vegetable intake combined with greater reported physical activity levels was associated with significantly reduced mortality (17). To follow-up on these findings in terms of possible biological mechanisms of recurrence protection, in this research study we tested the hypothesis that breast cancer survivors with high intake of dietary carotenoids and high concentrations of plasma carotenoids would show significantly lower levels of oxidative stress, as measured by urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG) and 8-iso-prostaglandin-F2α (8-iso-PGF2α), than breast cancer survivors with low dietary and low plasma carotenoid levels.
Higher intake of carotenoid-rich fruit and vegetables has been associated with a significant reduction in oxidative stress biomarkers, both in relation to DNA damage and lipid peroxidation (18-20). Diets low in antioxidants, particularly antioxidant carotenoids provided as vegetables and fruit in the diet, may contribute to increased oxidative stress (20, 21) and thus indirectly contribute to breast cancer recurrence.
Materials and Methods
Study Population
A subgroup of breast cancer survivors from the Portland, San Diego, and Tucson/Phoenix recruitment sites, who were previously enrolled in the parent WHEL Study, volunteered to participate in this ancillary study between 1998 and 2000. The WHEL study design has been described previously (22). Briefly, between 1995 and 2000, 3,088 women ages 18 to 70 years who had been successfully treated for early-stage breast cancer (stages I, II, or IIIA disease that had been diagnosed within the previous 4 years) were enrolled into the parent WHEL study. Enrollees were at least 6 months after diagnosis, accessible by telephone, and able to commit to the study intervention activities. Women were recruited from private oncology practices, tumor registries, advertisements, and community outreach programs. Eligible subjects completed the informed consent process in accordance with the ethical standards of the Human Subjects Committee at the participating research institutions and were randomized to either an intensive dietary intervention or comparison group. Exclusion criteria specific to participation in this ancillary study of oxidative stress included current smoking, hepatic or renal dysfunction, and inability or unwillingness to collect a 24-h urine sample. This article represents a cross-sectional study of baseline carotenoid intake, plasma carotenoid concentrations, and oxidative stress biomarkers among the 207 WHEL women who consented to participate in this ancillary study.
Data Collection
All data for this ancillary study analysis were specific to baseline measures collected before randomization into the parent WHEL study treatment groups. A standardized questionnaire developed for cancer prevention research was used to obtain demographic data at baseline. Clinical data were collected by questionnaire as well as selected extracts from the participant's medical record (e.g., mammogram report, cancer therapy, and pathology reports). Anthropometric measures, including height, weight, calculated body mass index (BMI), and waist and hip circumferences and ratio, and information about personal/lifestyle habits were collected at the baseline clinic visit. Collection of dietary data, and collection and processing of oxidative biomarkers, is described below.
Dietary Data
The Arizona Food Frequency Questionnaire was used to measure self-reported dietary intake at the time of study entry (23). The Arizona Food Frequency Questionnaire is a 153-food/beverage item questionnaire that measures age- and gender-specific serving sizes and frequency of usual intake of the previous 3months using a Likert-type scale. The Arizona Food Frequency Questionnaire has been shown to exhibit modest correlations with true carotenoid intake ranging from 0.39 to 0.44, which together with plasma marker correlations of 0.75 to 0.86 provides a more accurate estimate of intake (24). The Behavioral Measurement Shared Service at the Arizona Cancer Center completed nutrient analyses of the Arizona Food Frequency Questionnaire using proprietary software programming “Metabolize,” which was developed and is updated annually at the University of Arizona, Arizona Cancer Center. Nutrient values were derived from the Continuing Survey of Food Intake by Individuals 1994 to 1996 and 1998 U.S. Department of Agriculture-Nutrition Coordinating Center Carotenoid Database for U.S. Foods and the United States Department of Agriculture Food Composition Database and the Nutrient Database for Standard Reference, versions 11 to 13(25). The analysis determined intake of energy, macronutrients, micronutrients, quantification of fruit and vegetable servings, and carotenoid intake (α-carotene, β-carotene, lutein plus zeaxanthin, lycopene, and β-cryptoxanthin) defined as diet, dietary supplement, and dietary plus supplement values. If five or more items were missing from the questionnaire, the data were considered to be incomplete and were not included in the final analysis.
Dietary supplementation data were collected using an inventory of manufacturer-specific products reported as being consumed over the previous month. Supplement labels from all products taken by study participants were photocopied for accuracy of entry into the dietary supplement database, developed specifically for the WHEL study. Participants reported supplement type, manufacturer, dose, and frequency information, which were reviewed by the study coordinator during the initial study enrollment clinic visit.
Plasma Carotenoids
Fasting blood samples were collected during the baseline clinic visit and protected from light during processing. Samples were shipped on dry ice to the University of California at San Diego Nutrition Shared Resource laboratory. Plasma carotenoids: α-carotene, β-carotene, lutein plus zeaxanthin, lycopene, and β-cryptoxanthin; were separated and quantified with high-performance liquid chromatography methodology (26). The values presented as lutein are assumed to be lutein plus zeaxanthin as they elute together with this method. The total plasma carotenoid concentration is calculated as the sum of the individual concentrations. Accuracy was assessed by periodic analysis of National Institute of Standards and Technology standard reference material, and a pooled plasma sample was analyzed with batches of study samples to monitor analytic precision, with a day-to-day coefficient of variation of ∼7%. In addition, the laboratory participates in the National Institute of Standards and Technology round robin quality assurance program to monitor variability and reliability of these carotenoid measurements.
Total plasma cholesterol concentrations were determined with the Kodak Ektachem Analyzer system (Johnson & Johnson Clinical Diagnostics) and used in the interpretation of plasma carotenoid data. Carotenoids are transported in the plasma nonspecifically by cholesterol-rich lipoproteins. Thus, the size of the pool in which these compounds exist in the circulation is a nondietary influencing factor that is considered in the analysis of the relationship between plasma carotenoid concentration (as an indicator of dietary intake) and recurrence-free survival (27). Standard reference materials from the manufacturer were used to validate analytic precision of these procedures. The laboratory also participates in the American College of Pathologists quality assurance program to monitor variability and reliability for these lipid measures.
Oxidative Stress Biomarkers
Twenty-four–hour urine samples were collected at baseline to measure oxidative damage biomarkers. Subjects were asked to refrain from alcohol, all dietary supplements, heavy exercise, and sun exposure for 48 h before and during the 24-h urine collection. All urine was preserved under refrigeration or on ice in a closed container to protect from light exposure throughout the collection. Samples were transported to the clinic within 3 h of completion in a study provided cooler. Once at the clinic, the urine sample was gently and thoroughly vortexed for 90 s and samples were placed in −80°C storage. Samples were shipped on dry ice by overnight delivery to Genox Corp. (Baltimore, MD) for analysis. To standardize the levels of oxidative stress, urinary creatinine analysis was completed using a colorimetric assay kit (Sigma-Aldrich). Immunoaffinity chromatography-monoclonal antibody–based ELISAs were used to analyze the urine samples for 8-OHdG (8-OHdG Check ELISA kit, Genox) and 8-iso-PGF2α (Correlate-EIA 8-iso-Prostaglandin F2α Immunoassay kit, Assay Designs). The urine samples were analyzed in duplicate under light-protected conditions. The ELISA assays were completed and analyzed in a single batch at Genox using an automated immunochemical analyzer to reduce measurement error and pooled urine samples from healthy adults were used as a quality control. The mean, SD, and coefficient of variation were calculated, and any sample with a coefficient of variation of ≥ 10% was reanalyzed. Coefficients of variation for 8-OHdG and 8-iso-PGF2α were <7%.
Statistical Analysis
Measures of central tendency and distributions were computed for demographic characteristics of study participants and checked for outliers. Due to some missing data, the demographics of those participants with incomplete urine, plasma, or dietary intake data were compared with those of participants with complete data using the Mann-Whitney independent sample comparison tests (for continuous variables) and m2 analyses (for categorical variables).
Means and SDs for urine, plasma, and dietary intake data from the Arizona Food Frequency Questionnaire were produced and checked for normalcy. Total plasma and dietary carotenoids were calculated by summing the individual carotenoids measured. For correlation and multivariate analyses, urine measures of 8-OHdG (ng/mL) and 8-iso-PGF2α (pg/mL) were divided by urine creatinine values (mg/dL) producing 8-OHdG (ng/mg creatinine) and 8-iso-PGF2α (pg/mg creatinine), respectively. To correlate plasma and dietary carotenoids, those variables were log transformed to the base 10 (log10) to normalize distributions. Pearson correlations were carried out on these log-transformed variables. In addition, to test the effect of BMI on dietary carotenoids in correlations with plasma carotenoids, dietary carotenoids were regressed on BMI values and residuals were used in correlations. Because these residuals did not transform to normally distributed variables, Spearman correlations were conducted.
To examine the relationships of urine oxidative stress biomarkers with plasma and dietary carotenoids, carotenoid measures were ranked into quartiles. In analysis of covariance models with 8-OHdG (ng/mL) and 8-iso-PGF2α (pg/mL) as the dependent variables, quartiles of either plasma or dietary individual carotenoids and their totals were entered as independent factors along with the covariates, age, BMI, physical activity, and plasma or dietary cholesterol (depending on whether plasma or dietary carotenoids were used). The physical activity measure was included only in models of 8-iso-PGF2α regressed on plasma carotenoids where it was independently associated with the dependent variable and had an effect on the relationship between the plasma carotenoid/biomarker relationship. From these models, adjusted oxidative stress biomarker means were calculated for each quartile of plasma or dietary carotenoid. Adjusted mean levels of oxidative stress biomarkers were tested for significance using Bonferroni post hoc tests. Because the urinary oxidative stress biomarkers were not normally distributed, analyses of covariance were repeated using transformed (log10) dependent variables and results were similar. Sensitivity analyses were also done excluding participants who were taking β-carotene supplements (n = 20).
Results
Of the 207 women recruited and consented for participation in this ancillary study, 200 had complete baseline dietary and urinary data, and 192 had complete urinary, dietary, and plasma data, for analysis (Fig. 1). Selected demographic and lifestyle characteristics are presented in Table 1. In brief, the subjects were predominantly white/non-Hispanic (89.6%) with an average age of 53.5 years (SD, ±9.1). The majority of the subjects reported having some level of education after high school. The average BMI would classify subjects as overweight (mean BMI, 27.1 kg/m2). A few significant differences were found between the study group and the analytic group (those participants with incomplete urine, plasma, or dietary intake data) for the analysis presented. Overall, subjects without plasma for assessment of carotenoid concentrations were more likely to have had a smoking history and reported a longer mean pack-year history of smoking (7.7 versus 5.5 years; P = 0.02) and also reported lower educational achievement (P = 0.04).
Figure 1.
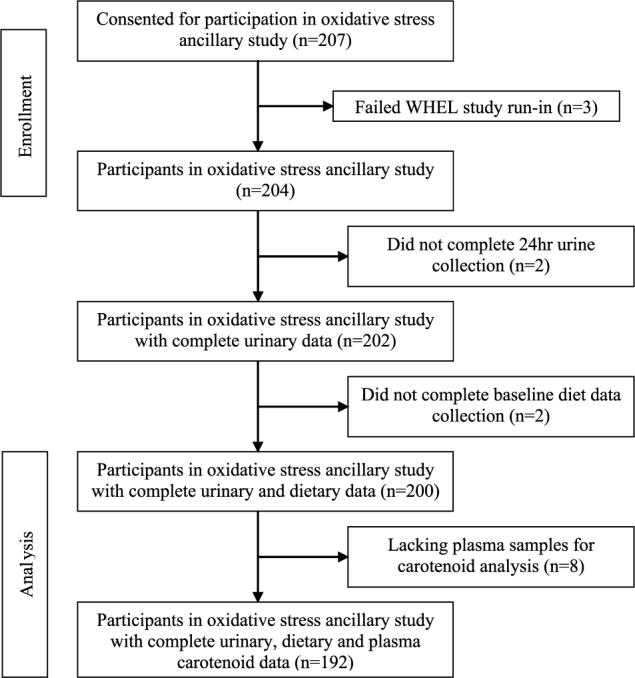
Consolidated Standards of Reporting Trials diagram.
Table 1.
Baseline demographics and clinical and lifestyle characteristics of breast cancer survivors participating in the WHEL study (n = 204)
| Characteristic | Study cohort |
|---|---|
| Age at randomization (y) | |
| Mean ± SD | 53.5 ± 9.1 |
| Height | |
| Mean ± SD | 164.0 ± 6.4 |
| Weight | |
| Mean ± SD | 72.7 ± 16.7 |
| BMI (kg/m2) | |
| Mean ± SD | 27.1 ± 6.0 |
| Exercise (metabolic equivalents/wk) | 825.3 ± 840.9 |
| Alcohol consumption (g/d) | 3.7 ± 7.7 |
| Ethnicity (%) | |
| White (non-Hispanic) | 89.6 |
| Hispanic | 4.9 |
| Asian/Pacific Islander | 1.5 |
| Other | 4.0 |
| Education | |
| Postcollege, college | 53.0 |
| Some college | 31.8 |
| High school graduate | 15.2 |
| Breast cancer stage, n (%) | |
| I | 35.8 |
| II | 58.8 |
| IIIA | 5.4 |
| Breast cancer treatment, n (%) | |
| Chemotherapy | 74.5 |
| Radiation therapy | 66.7 |
| Smoking status | |
| Past | 44.8 |
| Never | 54.7 |
| Current | 0.0 |
| Unknown | 0.5 |
Dietary intake of fruit, vegetables, and carotenoids (mean ± SD) is presented in Table 2A, along with measured plasma carotenoid concentrations of the study sample population (Table 2B). Mean total fruit and vegetable intake for the study population was reported to be 708 ± 462 g/d, whereas mean total daily dietary carotenoid intake, excluding supplements, was estimated to be 16,712 μg/d. The correlations between dietary and plasma carotenoids (log transformed), including individual and total carotenoid values, were 0.34 for β-carotene, 0.46 for α-carotene, 0.39 for β-cryptoxanthin, 0.27 for lycopene, 0.30 for lutein plus zeaxanthin, and 0.30 for total carotenoids. On average, study participants reported greater consumption of β-carotene and lycopene compared with other carotenoids, and this was reflected in the higher plasma concentrations of these carotenoids.
Table 2.
Dietary Intake of Fruits, Vegetables, and Carotenoids (n = 200) and Plasma Carotenoid Concentrations (n = 192)
| Mean ± SD | |
|---|---|
| A. Dietary intake of fruit, vegetables, and carotenoids (n = 200) | |
| Food group intake (g·d−1) | |
| Fruit | 268 ± 225 |
| Fruit juice | 184 ± 270 |
| Fruit + fruit juice | 452 ± 352 |
| Vegetables | 238 ± 179 |
| Vegetable juice | 18 ± 75 |
| Vegetables + vegetable juice | 255 ± 205 |
| Fruits and vegetables (including juices) | 708 ± 462 |
| Dietary carotenoid intake (μg·d−1) | |
| β-Carotene | 5,781 ± 7,882 |
| α-Carotene | 1,412 ± 3,076 |
| β-Cryptoxanthin | 253 ± 201 |
| Lycopene | 5,730 ± 4,537 |
| Lutein + zeaxanthin | 3,536 ± 4,080 |
| Total carotenoid intake | 16,712 ± 14,960 |
| Supplemental β-carotene (IU/d) | 1,551 ± 6,143 |
| B. Baseline plasma carotenoid concentrations (n = 192) | |
| Plasma carotenoids (μmol·L−1) | |
| β-Carotene | 1.1 ± 1.7 |
| α-Carotene | 0.2 ± 0.3 |
| β-Cryptoxanthin | 0.2 ± 0.2 |
| Lycopene | 0.7 ± 0.3 |
| Lutein | 0.4 ± 0.3 |
| Total carotenoid concentration | 2.5 ± 2.0 |
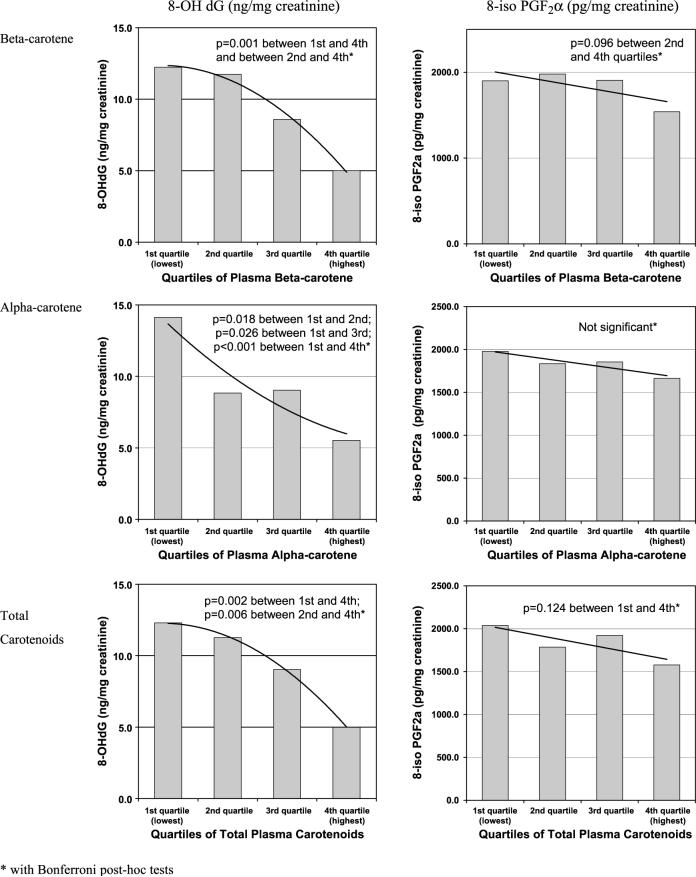
Table 3 represents the analysis of covariance analysis assessing the relationship between dietary and plasma carotenoid levels and measured levels of oxidative stress (for 8-OHdG and 8-iso-PGF2α, independently). As shown, the 8-OHdG oxidative stress biomarker was significantly reduced at the highest quartile of total plasma carotenoid concentrations (P = 0.001) and the 8-iso-PGF2α oxidative stress biomarker was marginally significantly reduced (P = 0.088). This protective association was most strongly associated with plasma α-carotene and β-carotene concentrations in relation to 8-OHdG–associated oxidative stress and with β-carotene alone for 8-iso-PGF2α. Dietary carotenoid levels were generally not significantly associated with oxidative stress indicators, regardless of the biomarker assessed, although dietary lycopene and lutein/zeaxanthin were modestly associated with 8-OHdG levels (P = 0.054 and 0.088, respectively).
Table 3.
Adjusted mean urine biomarkers for quartiles of plasma and dietary intake levels of carotenoids
| 1st quartile (lowest) |
2nd quartile |
3rd quartile |
4th quartile (highest) |
P* | P for trend | ||
|---|---|---|---|---|---|---|---|
| Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | ||||
| Plasma (n = 192) | |||||||
| 8-OHdG (ng)† | β-Carotene | 12.2 ± 1.3 | 11.7 ± 1.3 | 8.6 ± 1.2 | 5.0 ± 1.3 | 0.001 | <0.001 |
| α-Carotene | 14.1 ± 1.3 | 8.8 ± 1.2 | 9.0 ± 1.2 | 5.5 ± 1.3 | <0.001 | <0.001 | |
| β-Cryptoxanthin | 11.6 ± 1.3 | 9.7 ± 1.3 | 8.6 ± 1.3 | 7.8 ± 1.3 | 0.229 | 0.041 | |
| Lycopene | 11.1 ± 1.4 | 8.1 ± 1.3 | 8.0 ± 1.3 | 10.5 ± 1.3 | 0.279 | 0.053‡ | |
| Lutein + zeaxanthin | 10.4 ± 1.4 | 9.4 ± 1.3 | 10.0 ± 1.4 | 7.8 ± 1.3 | 0.551 | 0.237 | |
| Total | 12.3 ± 1.4 | 11.3 ± 1.3 | 9.0 ± 1.4 | 5.0 ± 1.3 | 0.001 | <0.001 | |
| 8-iso PGFα (pg)† | β-Carotene | 1,899.3 ± 128.3 | 1,980.6 ± 122.3 | 1,906.4 ± 119.0 | 1,539.7 ± 128.9 | 0.072 | 0.066 |
| α-Carotene | 1,977.9 ± 122.6 | 1,834.2 ± 121.3 | 1,853.7 ± 119.2 | 1,663.4 ± 128.1 | 0.393 | 0.115 | |
| β-Cryptoxanthin | 1,825.5 ± 125.1 | 1,775.3 ± 123.4 | 1,878.1 ± 119.8 | 1,859.6 ± 127.7 | 0.941 | 0.728 | |
| Lycopene | 2,004.4 ± 127.8 | 1,862.4 ± 120.0 | 1,621.5 ± 121.7 | 1,854.5 ± 121.2 | 0.197 | 0.231 | |
| Lutein + zeaxanthin | 1,840.4 ± 130.9 | 1,938.1 ± 121.6 | 1,933.8 ± 121.8 | 1,629.6 ± 126.2 | 0.254 | 0.103‡ | |
| Total | 2,038.2 ± 129.3 | 1,785.0 ± 121.3 | 1,921.2 ± 120.3 | 1,578.3 ± 130.7 | 0.088 | 0.051 | |
| Dietary intake, μg (n = 200) | |||||||
| 8-OHdG (ng)† | β-Carotene | 9.9 ± 1.3 | 9.6 ± 1.3 | 9.6 ± 1.3 | 8.3 ± 1.3 | 0.825 | 0.384 |
| α-Carotene | 10.6 ± 1.3 | 10.5 ± 1.3 | 8.8 ± 1.2 | 7.5 ± 1.3 | 0.265 | 0.039 | |
| β-Cryptoxanthin | 11.3 ± 1.3 | 8.2 ± 1.3 | 8.7 ± 1.2 | 9.2 ± 1.2 | 0.324 | 0.350 | |
| Lycopene | 8.5 ± 1.3 | 8.5 ± 1.3 | 12.3 ± 1.2 | 8.1 ± 1.2 | 0.054 | 0.559 | |
| Lutein + zeaxanthin | 11.8 ± 1.3 | 7.3 ± 1.2 | 9.4 ± 1.2 | 9.0 ± 1.3 | 0.088 | 0.244 | |
| Total | 9.5 ± 1.3 | 8.9 ± 1.3 | 10.6 ± 1.2 | 8.4 ± 1.3 | 0.618 | 0.731 | |
| 8-iso PGFa (pg)† | β-Carotene | 1,872.7 ± 119.7 | 1,812.1 ± 120.3 | 1,965.6 ± 118.7 | 1,698.4 ± 121.1 | 0.460 | 0.436 |
| α-Carotene | 1,949.4 ± 119.4 | 1,832.2 ± 120.4 | 1,908.0 ± 118.3 | 1,662.2 ± 120.6 | 0.345 | 0.212 | |
| β-Cryptoxanthin | 1,729.6 ± 121.4 | 1,843.4 ± 120.9 | 1,840.1 ± 117.8 | 1,938.8 ± 119.4 | 0.687 | 0.533 | |
| Lycopene | 1,791.0 ± 124.1 | 1,777.6 ± 121.3 | 1,863.3 ± 118.5 | 1,918.2 ± 124.8 | 0.858 | 0.690 | |
| Lutein + zeaxanthin | 1,875.0 ± 122.1 | 1,831.8 ± 119.3 | 1,796.5 ± 118.3 | 1,850.1 ± 122.2 | 0.973 | 0.840 | |
| Total | 1,844.9 ± 120.4 | 1,711.4 ± 118.9 | 2,063.1 ± 116.8 | 1,728.0 ± 122.2 | 0.128 | 0.783 | |
NOTE: For age, BMI, and plasma or dietary cholesterol, and for plasma carotenoids and 8-iso PGFα, physical activity additionally.
Overall value for analysis of covariance.
Urine biomarker (/mg creatinine).
Quadratic trend.
Figure 2 further illustrates the relationship between selected plasma carotenoid concentrations as well as total carotenoids and the two oxidative stress biomarkers assessed in this study, 8-OHdG and 8-iso-PGF2α. As illustrated, among women in the highest quartile of plasma α-carotene and β-carotene as well as total plasma carotenoid concentrations, there was a significant inverse association with 8-OHdG but not with 8-iso-PGF2α.
Figure 2.
Biomarkers of oxidative stress by quartiles of plasma carotenoid levels among WHELcancer survivors (n = 192).
Twenty subjects reported daily intake of β-carotene supplementation at levels >5,000 IU/d (mean, 21,893 ± 7,269 IU/d). The inverse association between β-carotene and 8-OHdG was evident with or without the inclusion of women taking β-carotene supplements, but a significant inverse association was not shown between β-carotene and 8-iso-PGF2α when women taking β-carotene supplements were removed from the model (data not shown).
Discussion
This study is unique in providing an assessment of the relationship between oxidative stress biomarkers and plasma and dietary carotenoid levels in a sample of breast cancer survivors. Key findings from this study include a significant inverse association between total plasma carotenoid concentrations and oxidative stress as measured by urinary 8-OHdG and a modestly significant inverse association with 8-iso-PGF2α. These protective associations were not shown for dietary carotenoid intake, perhaps due to well-established measurement error that occurs with self-report instruments, such as food frequency questionnaires. Notably, the significant correlations between dietary and plasma carotenoid levels were relatively low (ranging from 0.27 to 0.46), however these values are comparable with other validation studies (24, 28).
Breast cancer is thought to occur through a multistep carcinogenesis pathway that includes damage to DNA (29). This damage may lead to structural modifications in DNA (30), which over time and without repair can lead to increased cancer incidence. Additionally, radiation therapy and several chemotherapeutic agents exert their antitumor effects through increased formation of reactive oxygen species (31), leading to increased oxidative stress and provoking cell death as a result of massive cellular damage (32). So, although the malignant neoplasms undergo apoptosis, damage may also occur to normal, healthy cells. Further, high oxidative stress may be associated with increased recurrence, and thus, one mechanism by which diet may help to prevent cancer recurrence is through modulation of DNA damage to these healthy cells.
A recent cohort analysis of the comparison group participants in the WHEL study suggested that breast cancer survivors presenting at the highest quartile of total plasma carotenoid concentration are at a significantly reduced risk for breast cancer recurrence, whereas women at the bottom quartile of total plasma carotenoid concentration are at significantly increased risk for recurrence (14). Our findings suggest that the protective association between higher total plasma carotenoid concentrations and breast cancer recurrence could, at least in part, be explained by the biological antioxidant activity of circulating carotenoids. Yet, our data did not support a protective relationship between dietary carotenoids and oxidative stress despite a recent analysis from this same study population that suggested that high fruit and vegetable intake in combination with greater physical activity was associated with reduced mortality (17). A plausible explanation could be that fruits and vegetables, even those rich in carotenoids, act to improve health through a wide variety of biological mechanisms and not antioxidant activity alone (33).
There is a paucity of research evaluating the role of plasma carotenoids and/or carotenoid intake and breast cancer survival or recurrence. In one study of 516 postmenopausal breast cancer survivors from California, McEligot et al. (7) provided evidence that greater intake of carotenoids was associated with a significant trend toward reduced mortality (P < 0.05), consistent with the significant 43% reduction in all-cause mortality (hazard ratio, 0.57; 95% confidence interval, 0.35−0.94) associated with vegetable intake. High vegetable intake was also shown to reduce mortality among 103women previously diagnosed with breast cancer residing in Australia (5). Similarly, the Nurses Health Study has shown a moderately significant reduction in all-cause mortality among nurses diagnosed with breast cancer who report greater vegetable intake (P = 0.07; ref. 34), an association that was statistically significant only among the subgroup of women diagnosed with node-negative disease. Further, a recent survival analysis from the Long Island Breast Cancer Study by Fink et al. (35) suggested that a pattern of high fruit and vegetable intake among 1,235 women previously diagnosed with breast cancer did not significantly reduce all-cause mortality. Likewise, another recent analysis from the WHEL study population found no evidence that adoption of a diet high in fruit, vegetables, and fiber and low in fat compared with a 5-a-day fruit and vegetable diet prevents breast cancer recurrence or death among women with previously treated early-stage breast cancer (36). One noteworthy difference between the Fink study and the present study is that the dietary fruit and vegetable intake in the Long Island study subjects was assessed one year before diagnosis, whereas in the WHEL study population diet was assessed, on average, two years after breast cancer diagnosis and, as such, may have reflected change in intake after diagnosis (37). Together, these data may indicate that prediagnostic diet may not significantly influence postdiagnosis survival, especially for premenopausal women. However, postdiagnosis increases in fruit and vegetable intake, which result in greater total plasma carotenoid concentrations, may offer protection against recurrent disease, partially due to a reduction in oxidative stress (14).
Our findings suggesting that increased intake of fruits and vegetables is associated with reduced oxidative stress are supported by some controlled feeding trials (20) but not others (38, 39). In a study conducted among 28 women assessed to be at greater risk for breast cancer than the general population, Thompson et al. (20) showed a significant inverse relationship between increased fruit and vegetable intake during a 14-day intervention and change in urinary 8-iso-PGF2α. In that study, plasma carotenoid concentrations were used to assess dietary change and all five carotenoids (α-carotene, β-carotene, lutein plus zeaxanthin, lycopene, and β-cryptoxanthin) measured increased significantly from baseline levels. Of interest, a secondary analysis from that study suggested that the largest and most significant changes in oxidative stress indicators were observed among those showing the lowest baseline plasma α-carotene concentrations. Similarly, we also found plasma α-carotene concentrations to be a significant predictor of oxidative stress, but in the present study, this was specific to urinary 8-OHdG levels. Null associations were shown in two other feeding studies, one by Moller et al. (38) providing 600 g/d fruit and vegetables daily for 24 days to 43 healthy young adults and another providing a fruit and vegetable concentrate daily to 22 adult male smokers using a crossover design that compared consumption of a fruit-vegetable concentrate to control food/beverage over a 3-week feeding period (39). The latter study was likely underpowered to see a significant effect and it is possible that the 2-week washout period was insufficient for plasma carotenoid concentrations to return to baseline levels. Further, the study populations were markedly different from those in the present study in that a significant percentage of subjects were male, many were smokers, and in the Moller study the mean study subject was >3 decades younger than our participants. The levels of oxidative stress in our study group were not as high as has been suggested for breast cancer patients in other studies (40-43) and may be related to both the fact that our subjects were recruited, on average, just over two years after breast cancer diagnosis and are considered to be consuming relatively healthy diets since the time of breast cancer diagnosis (37). In fact, the plasma carotenoid concentrations observed in the present study sample are generally higher than those observed in the general population, as reflected in National Health and Nutrition Examination Survey III (44).
Although these findings are important, and may indicate one specific protective association of carotenoids, there are several other hypotheses as to why greater plasma carotenoid concentrations may be associated with reduced risk for breast cancer or recurrence. Carotenoids have been shown to inhibit proliferation of transformed cells (45, 46), to reduce inflammatory response (31), and, indirectly through conversion to retinol, to induce epithelial cell differentiation (47, 48). In addition, although greater intake of fruits and vegetables is consistently associated with higher plasma carotenoid concentrations (49-51) and has been shown in the parent WHEL cohort (24), it may be the combination of carotenoids and other bioactive food components and nutrients found in these plant foods that affords protection. In the present study, the mean dietary intake of fruits and vegetables was approximately seven servings per day, well above the average U.S. intake (52). Yet, despite this relatively well-nourished study sample population (36), a range of plasma carotenoid concentrations was observed and linked with a protective effect for breast cancer recurrence.
Supplemental carotenoids also contribute to elevated plasma levels independent of dietary intake. In our sample, 20 subjects reported use of dietary supplements containing β-carotene (one reported use of lutein- and one reported used of lycopene-containing dietary supplementation). Analysis inclusive or exclusive of subjects supplementing with β-carotene suggested that supplementation was important to showing a protective reduction in 8-iso-PGF2α in relation to plasma β-carotene concentrations, whereas 8-OHdG levels were significantly reduced in relation to plasma β-carotene concentrations regardless of the inclusion or exclusion of women taking supplemental β-carotene. Though of interest, the loss of association between plasma concentrations and 8-iso-PGF2α may simply be related to a reduction in sample size. Another plausible explanation may be that a certain “threshold concentration” of plasma β-carotene exists above which reduction in oxidative stress can be expected. This “modifying” plasma concentration (in terms of oxidative stress reduction) may be higher to induce significant reductions in lipid peroxidation than for DNA-associated oxidative stress.
Breast cancer remains the second most common cause of cancer death among women in the United States and thus the continued interest in identifying modifiable risk factors to reduce the incidence of recurrent disease remains. Diet offers significant potential to reduce oxidative stress and thus reduce breast cancer recurrence.
Acknowledgments
Grant support: National Cancer Institute grants CA93658, CA69375, CA23074 and HATCH grant 126660.
References
- 1.Gandini S, Merzenich H, Robertson C, Boyle P. Meta-analysis of studies on breast cancer risk and diet: the role of fruit and vegetable consumption and the intake of associated micronutrients. Eur J Cancer. 2000;36:636–46. doi: 10.1016/s0959-8049(00)00022-8. [DOI] [PubMed] [Google Scholar]
- 2.La Vecchia C, Altieri A, Tavani A. Vegetables, fruit, antioxidants and cancer: a review of Italian studies. Eur J Nutr. 2001;40:261–7. doi: 10.1007/s394-001-8354-9. [DOI] [PubMed] [Google Scholar]
- 3.Lagiou P, Olsen J, Trichopoulos D. Consumption of vegetables and fruits and risk of breast cancer. JAMA. 2005;293:2209–10. doi: 10.1001/jama.293.18.2209-a. [DOI] [PubMed] [Google Scholar]
- 4.Demark-Wahnefried W, Rock C. Nutrition-related issues for the breast cancer survivor. Semin Oncol. 2003;30:789–98. doi: 10.1053/j.seminoncol.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 5.Ingram D. Diet and subsequent survival in women with breast cancer. Br J Cancer. 1994;69:592–5. doi: 10.1038/bjc.1994.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rock C, Demark-Wahnefried W. Nutrition and survival after the diagnosis of breast cancer: a review of the evidence. J Clin Oncol. 2002;20:3302–16. doi: 10.1200/JCO.2002.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McEligot A, Largent J, Ziogas A, Peel D, Anton-Culver H. Dietary fat, fiber, vegetable, and micronutrients are associated with overall survival in postmenopausal women diagnosed with breast cancer. Nutr Cancer. 2006;55:132–40. doi: 10.1207/s15327914nc5502_3. [DOI] [PubMed] [Google Scholar]
- 8.Borek C. Antioxidants and cancer. Sci Med (Phila) 1997;4:51–62. [Google Scholar]
- 9.Pool-Zobel B, Bub L, Liegibel U, Lishaut S, Rechkemmer G. Mechanism by which vegetable consumption reduces genetic damage in humans. Cancer Epidemiol Biomarkers Prev. 1998;7:891–9. [PubMed] [Google Scholar]
- 10.Sumantran V, Zhang R, Lee D, Wicha M. Differential regulation of apoptosis in normal versus transformed mammary epithelium by lutein and retinoic acid. Cancer Epidemiol Biomarkers Prev. 2000;9:257–63. [PubMed] [Google Scholar]
- 11.Prakash P, Krinsky N, Russell R. Retinoids, carotenoids, and human breast cancer cell cultures: a review of differential effects. Nutr Rev. 2000;58:17–76. doi: 10.1111/j.1753-4887.2000.tb01856.x. [DOI] [PubMed] [Google Scholar]
- 12.Terry P, Terry J, Wolk A. Fruit and vegetable consumption in the prevention of cancer: an update. J Intern Med. 2001;250:280–90. doi: 10.1046/j.1365-2796.2001.00886.x. [DOI] [PubMed] [Google Scholar]
- 13.Borek C. Dietary antioxidants and human cancer. Integr Cancer Ther. 2004;3:333–41. doi: 10.1177/1534735404270578. [DOI] [PubMed] [Google Scholar]
- 14.Rock C, Flatt S, Natarajan L, et al. Plasma carotenoids and recurrence-free survival in women with a history of breast cancer. J Clin Oncol. 2005;23:6631–8. doi: 10.1200/JCO.2005.19.505. [DOI] [PubMed] [Google Scholar]
- 15.Bohlke K, Spiegelman D, Trichopoulou A, et al. Vitamins A, C, and E and the risk of breast cancer: results from a case-control study in Greece. Br J Cancer. 1999;79:23–9. doi: 10.1038/sj.bjc.6690006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Potischman N, Swanson C, Coates R, et al. Intake of food groups and associated micronutrients in relation to risk of early-stage breast cancer. Int J Cancer. 1999;82:315–21. doi: 10.1002/(sici)1097-0215(19990730)82:3<315::aid-ijc1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 17.Pierce J, Stephanick M, Flatt S, et al. Greater survival after breast cancer in physically active women with high vegetable-fruit intake regardless of obesity. J Clin Oncol. 2007;25:2345–51. doi: 10.1200/JCO.2006.08.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verhagen H, Poulsen H, Loft S, et al. Reduction of oxidative DNA-damage in humans by Brussels sprouts. Carcinogenesis. 1995;16:969–70. doi: 10.1093/carcin/16.4.969. [DOI] [PubMed] [Google Scholar]
- 19.Giovannelli L, Saieva C, Masala G, et al. Nutritional and lifestyle determinants of DNA oxidative damage: a study in a Mediterranean population. Carcinogenesis. 2002;23:1483–9. doi: 10.1093/carcin/23.9.1483. [DOI] [PubMed] [Google Scholar]
- 20.Thompson H, Heimendinger J, Haegele A, et al. Effect of increased vegetable and fruit consumption on markers of oxidative cellular damage. Carcinogenesis. 1999;20:2261–6. doi: 10.1093/carcin/20.12.2261. [DOI] [PubMed] [Google Scholar]
- 21.Steinmetz K, Potter J. Vegetables, fruit and cancer prevention: a review. J Am Diet Assoc. 1996;96:1027–39. doi: 10.1016/S0002-8223(96)00273-8. [DOI] [PubMed] [Google Scholar]
- 22.Pierce J, Faerber S, Wright F, et al. A randomized trial of the effect of a plant-based dietary pattern on additional breast cancer events and survival: the Women's Healthy Eating and Living WHEL Study. Control Clin Trials. 2002;23:728–56. doi: 10.1016/s0197-2456(02)00241-6. [DOI] [PubMed] [Google Scholar]
- 23.Ritenbaugh C, Aickin M, Tared D, et al. Use of food frequency questionnaire to screen for dietary eligibility in a randomized cancer prevention phase III trial. Cancer Epidemiol Biomarkers Prev. 1997;6:347–54. [PubMed] [Google Scholar]
- 24.Natarajan L, Flatt S, Sun X, et al. Validity and systematic error in measuring carotenoid consumption with dietary self-report instruments. Am J Epidemiol. 2006;163:770–8. doi: 10.1093/aje/kwj082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.United States Department of Agriculture, ARS [2007 Mar 30]; USDA Nutrient Database for Standard Reference, Release 13. Nutrient Data Laboratory Home Page 1999. Available from: http://www.ars.usda.gov/main/site_main.htm?modecode=12354500.
- 26.Gamboa-Pinto A, Rock C, Ferruzzi M, et al. Cervical tissue and plasma concentrations of a-carotene and β-carotene are correlated. J Nutr. 1998;128:1933–6. doi: 10.1093/jn/128.11.1933. [DOI] [PubMed] [Google Scholar]
- 27.Thomson C, Giuliano A, Rock C, et al. Measuring dietary change in a diet intervention trial: comparing food frequency questionnaire and dietary results. Am J Epidemiol. 2003;157:754–62. doi: 10.1093/aje/kwg025. [DOI] [PubMed] [Google Scholar]
- 28.Eliassen A, Colditz G, Peterson K, et al. Biomarker validation of dietary intervention in two multiethnic populations. Prev Chronic Dis. 2006;3:A44–57. [PMC free article] [PubMed] [Google Scholar]
- 29.Fearon E, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–67. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 30.Olinski R, Gackowski D, Foksinski M, et al. Oxidative DNA damage: assessment of the role in carcinogenesis, atherosclerosis, and acquired immunodeficiency syndrome. Free Radic Biol Med. 2002;33:192–200. doi: 10.1016/s0891-5849(02)00878-x. [DOI] [PubMed] [Google Scholar]
- 31.Hozawa A, Jacobs D, Steffes M, Gross M, Steffen L, Lee D. Relationships of circulating carotenoid concentrations with several markers of inflammation, oxidative stress, and endothelial dysfunction: the Coronary Artery Risk Development in Young Adults (CARDIA)/Young Adult Longitudinal Trends in Antioxidants (YALTA) study. Clin Chem. 2007;53:447–55. doi: 10.1373/clinchem.2006.074930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mignotte B, Vayssiere J. Mitochondria and apoptosis. Eur J Biochem. 1998;252:1–15. doi: 10.1046/j.1432-1327.1998.2520001.x. [DOI] [PubMed] [Google Scholar]
- 33.WHO . WHO Technical Report 916. WHO; Geneva: 2003. Diet, nutrition and the prevention of chronic diseases. Report of a joint FAO/WHO expert consultation. [PubMed] [Google Scholar]
- 34.Holmes M, Hunter D, Colditz G, et al. Association of dietary intake of fat and fatty acids with risk of breast cancer. JAMA. 1999;281:914–20. doi: 10.1001/jama.281.10.914. [DOI] [PubMed] [Google Scholar]
- 35.Fink B, Gaudet M, Britton J, et al. Fruits, vegetables, and micronutrient intake in relation to breast cancer survival. Breast Cancer Res Treat. 2006;98:199–208. doi: 10.1007/s10549-005-9150-3. [DOI] [PubMed] [Google Scholar]
- 36.Pierce J, Natarajan L, Caan B, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer. JAMA. 2007;298:289–98. doi: 10.1001/jama.298.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomson C, Flatt S, Rock C, Ritenbaugh C, Newman V, Pierce J. Increased fruit, vegetable and fiber intake and lower fat intake reported among women previously treated for invasive breast cancer. J Am Diet Assoc. 2002;102:801–8. doi: 10.1016/s0002-8223(02)90180-x. [DOI] [PubMed] [Google Scholar]
- 38.Moller P, Vogel U, Pedersen A, Dragsted L, Sandstrom B, Loft S. No effect of 600 grams fruit and vegetables per day on oxidative DNA damage and repair in healthy nonsmokers. Cancer Epidemiol Biomarkers Prev. 2003;12:1016–22. [PubMed] [Google Scholar]
- 39.van den Berg R, van Vliet T, Broekmans W, et al. A vegetable/fruit concentrate with high antioxidant capacity has no effect on biomarkers of antioxidant status in male smokers. J Nutr. 2001;131:1714–22. doi: 10.1093/jn/131.6.1714. [DOI] [PubMed] [Google Scholar]
- 40.Polat M, Taysi S, Gul M, et al. Oxidant/antioxidant status in blood of patients with malignant breast tumor and benign breast disease. Cell Biochem Funct. 2002;20:327–31. doi: 10.1002/cbf.980. [DOI] [PubMed] [Google Scholar]
- 41.Ray G, Batra S, Shukla N, et al. Lipid peroxidation, free radical production and antioxidant status in breast cancer. Breast Cancer Res Treat. 2000;59:163–70. doi: 10.1023/a:1006357330486. [DOI] [PubMed] [Google Scholar]
- 42.Huang Y, Sheu J, Lin T. Association between oxidative stress and changes of trace elements in patients with breast cancer. Clin Biochem. 1999;32:131–6. doi: 10.1016/s0009-9120(98)00096-4. [DOI] [PubMed] [Google Scholar]
- 43.Hietanen E, Bartsch H, Bereziat J, et al. Diet and oxidative stress in breast, colon, and prostate cancer patients: a case-control study. Eur J Clin Nutr. 1994;48:575–86. [PubMed] [Google Scholar]
- 44.Wei W, Kim Y, Boudreau N. Association of smoking with serum and dietary levels of antioxidants in adults: NHANES III, 1988−1994. Am J Public Health. 2001;91:258–64. doi: 10.2105/ajph.91.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Czeczuga-Semeniuk E, Wolczynski S, Dabrowska M, Dzieciol J, Anchim T. The effect of doxorubicin and retinoids on proliferation, necrosis and apoptosis in MCF-7 breast cancer cells. Folia Histochem Cytobiol. 2004;42:221–7. [PubMed] [Google Scholar]
- 46.Olsson M, Gustavsson K, Andersson S, Nilsson A, Duan R. Inhibition of cancer cell proliferation in vitro by fruit and berry extracts and correlations with antioxidant levels. J Agric Food Chem. 2004;52:7264–71. doi: 10.1021/jf030479p. [DOI] [PubMed] [Google Scholar]
- 47.Sporn M, Roberts A. Role of retinoids in differentiation and carcinogenesis. Cancer Res. 1983;43:3034–40. [PubMed] [Google Scholar]
- 48.Calaf G, Emenakar N, Hei T. Effect of retinol on radiation- and estrogen-induced neoplastic transformation of human breast epithelial cells. Oncol Rep. 2005;13:1017–27. doi: 10.3892/or.13.6.1017. [DOI] [PubMed] [Google Scholar]
- 49.Al-Delaimy W, Ferrari P, Slimani N, et al. Plasma carotenoids as biomarkers of intake of fruits and vegetables: individual-level correlations in the European Prospective Investigation into Cancer and Nutrition (EPIC). Eur J Clin Nutr. 2005;59:1387–96. doi: 10.1038/sj.ejcn.1602252. [DOI] [PubMed] [Google Scholar]
- 50.Block G, Norkus E, Hudes M, Mandel S, Helzlsouer K. Which plasma antioxidants are most related to fruit and vegetable consumption? Am J Epidemiol. 2001;154:1113–8. doi: 10.1093/aje/154.12.1113. [DOI] [PubMed] [Google Scholar]
- 51.van Kappel A, Steghens J, Zeleniuch-Jacquotte A, Chajes V, Toniolo P, Riboli E. Serum carotenoids as biomarkers of fruit and vegetable consumption in the New York Women's Health Study. Public Health Nutr. 2001;4:829–35. doi: 10.1079/phn2000115. [DOI] [PubMed] [Google Scholar]
- 52.Johnston C, Taylor C, Hampl J. More Americans are eating “5 a day” but intakes of dark green and cruciferous vegetables remain low. J Nutr. 2000;130:3063–7. doi: 10.1093/jn/130.12.3063. [DOI] [PubMed] [Google Scholar]