Abstract
In bone fracture healing, the extent to which the injured bone regains stability and strength depends on the mechanical properties of the tissues that are formed during healing. While many techniques have been used to quantify the overall mechanical behavior of fracture calluses, few data exist on the material properties of individual callus tissues. The overall goal of this study was to quantify these material properties. Nanoindentation was performed at multiple locations across thin (200 μm), longitudinal sections of rat fracture callus at 35 days post fracture. Following indentation, sections were stained with alizarin red S and alcian blue to obtain semi-quantitative estimates of tissue mineral content and proteoglycan content, respectively. Indentation moduli varied over three orders of magnitude (0.61–1010 MPa) throughout the callus. Much of this variation was due to the presence of multiple tissue types: the indentation moduli of granulation tissue, chondroid tissue and woven bone ranged 0.61–1.27 MPa (median = 0.99 MPa), 1.39–4.42 MPa (median = 2.89 MPa) and 26.92–1010.00 MPa (median = 132.00 MPa), respectively. In regions of alizarin red staining, the indentation modulus was correlated (r = 0.62, P = 0.04) with stain intensity, suggesting a positive correlation between modulus and mineral content in woven bone. In addition, the indentation modulus of woven bone along the periosteal aspect of the cortex increased with distance from the fracture gap (P = 0.004). These results demonstrate the usefulness of nanoindentation in characterizing the elastic properties of the heterogeneous mixture of tissues present in bone fracture callus.
Keywords: Fracture healing, Indentation, Modulus, Woven bone, Cartilage
1. Introduction
In bone fracture healing, the extent to which the injured bone regains stability and strength depends on the mechanical properties of the tissues that are formed during healing. Fracture healing is a regenerative process that consists of several phases, each involving the formation of a different type of tissue [1]. In the initial phase, the inflammatory response results in the formation of a hematoma and granulation tissue. The second phase is characterized by the formation of a soft callus consisting of cartilaginous or chondroid tissue, while the third phase involves ossification of the soft callus to form a hard or bony callus consisting primarily of woven bone tissue. Finally, the callus enters the remodeling phase, during which the woven bone is gradually replaced by lamellar bone tissue. Although these four phases are temporally sequential, the healing process is not spatially uniform. Thus, at any given time during healing, the fracture callus consists of a highly heterogeneous mixture of tissues. Further, factors such as disease [2,3] and drug treatment [4-8] can alter one or more of the phases of healing, leading to differences in composition and distribution of tissues throughout the callus. Quantifying callus tissue material properties and the relationships between these properties and the tissue composition, therefore provides a means of linking the underlying biology of the repair process to the gradual regaining of mechanical function experienced by the healing bone.
In contrast to the wealth of literature on the mechanical behavior of fracture calluses, few data are available on the material properties of individual callus tissues [9]. Indeed, callus tissue material properties are often estimated based on information regarding tissue composition or on the material properties of similar tissues [10,11]. Nanoindentation is a promising tool for probing callus tissue properties, because of the high spatial resolution that this technique affords. With typical indent depths <5 μm and contact areas on the order of ~10–100 μm2, nanoindentation is well suited for studying fracture healing in small animals such as rats and mice. Moreover, the length scale of the indents is comparable with that of individual cells, suggesting that the resulting measurements reflect aspects of the local, cellular mechanical environment. Many studies to date have demonstrated the use of nanoindentation on skeletal tissues, including bone [12-17] and cartilage [18-22]. Together, these studies have indicated that nanoindentation can be used to detect changes or differences in tissue properties due to disease or genetic variations [16,23-28], to measure regional variations in tissue properties [12,18,29], and to relate tissue stiffness to mineral content [21,30].
The high spatial resolution of nanoindentation and the potential for relating indentation measurements to tissue composition suggest strongly that this testing method can be used to track the spatial and temporal progression of fracture healing. This progression has been well characterized through histological analyses with respect to the distribution of tissues present in the callus at various time points during healing. For example, intramembranous ossification, or direct bone formation, occurs along the periosteal surface of the cortex, progressing along this surface from points remote to the injury towards the fracture gap. Within and just outside the fracture gap, however, endochondral ossification—bone formation that occurs via a cartilage intermediary—predominates. The extent to which these patterns of bone formation are reflected in regional variations in callus tissue properties is not known and yet is highly relevant for defining the mechanisms by which the stiffness and strength of the bone are restored.
The overall goal of this study was to characterize the material properties of fracture callus tissues. Thin serial sections of rat fracture callus were prepared, and nanoindentation was performed at multiple locations across each section. The specific objectives were (1) to quantify the elastic properties of various tissues present in the callus; and (2) to compare the measured elastic properties with histological assessments of the types and composition of tissues present.
2. Materials and method
2.1. Harvest of callus and sample preparation
All animal care and experimental procedures were in compliance with NIH guidelines and the authors’ institution’s Animal Care and Use Committee. A closed, unstabilized fracture was created in the mid-diaphysis of the right femur in a retired breeder, male Sprague–Dawley rat (weight ~500 g; age ~5 months) according to an established protocol [31]. The animal was killed 35 days after the fracture was created, and the harvested femur was stored frozen at −20 °C in PBS-soaked gauze until preparation for nanoindentation.
To prevent damage or alterations to the tissue properties, no polishing, formalin fixation, decalcification or paraffn embedding was involved in any of the sample preparation phases. The entire length of the portion of the femur containing the callus was excised using a dental saw. The sample was mounted on the freeze-stage of a sliding microtome (HM 450 Richard Allan, Kalamazoo, MI) and embedded in freezing medium (Histo Prep, Fisher Scientific, Pittsburgh, PA). A technique was developed in the laboratory to provide additional support to the callus tissues during sectioning. Briefly, a thin polyester membrane was coated with freezing medium and placed on the exposed surface of the callus. The membrane supported the entire face of the section during cutting, thus allowing the overall morphology of the callus to be kept intact in each 200 μm thick section. Each section was mounted on a microscope slide using a small amount of cyanoacrylate glue, and the membrane was removed easily from the top surface of the section once the freezing medium had melted. Excess freezing medium was washed away with abundant distilled water.
2.2. Nanoindentation
All nanoindentation experiments were performed at room temperature using a Hysitron Triboindenter (Minneapolis, MN, USA) and a 50 μm conospherical tip. During indentation, tissue sections were kept moist with water containing protease inhibitors and penicillin–streptomycin. Saline was not used, in order to avoid salt crystallization on the surface of sections, which would interfere with the contact between the tip and the sample. The reduced modulus was calculated using the Oliver–Pharr method [32], as
| (1) |
where S is the unloading stiffness, and Ac is the contact area. S was calculated as the initial slope (slope at 95%) of a polynomial function fit over 95–20% of the unloading curve. The elastic modulus of the tissue, Es, is related to the reduced modulus by
| (2) |
where vs and vt are Poisson’s ratio of the tissue and indenter tip (diamond-coated stainless titanium), respectively. The elastic modulus and Poisson’s ratio of the indenter tip were 1140 GPa and 0.07, respectively, as given by the manufacturer. Because of the diffculties in measuring Poisson’s ratio of the individual callus tissues, the reduced modulus values were not converted into elastic modulus. The reduced modulus values are reported as indentation moduli in this paper.
Regions on each callus section were chosen for nanoindentation based on tissue morphology and anatomic location. Regions of granulation tissue, chondroid tissue and woven bone within and surrounding the fracture gap were identified using Triboindenter optics. Granulation tissue was identified as tissue that was pink in color and located in the fracture gap. Chondroid tissue was identified by its glassy appearance, and woven bone by its opaque, mild yellow color. Subsequent histological staining confirmed the identities of these tissues.
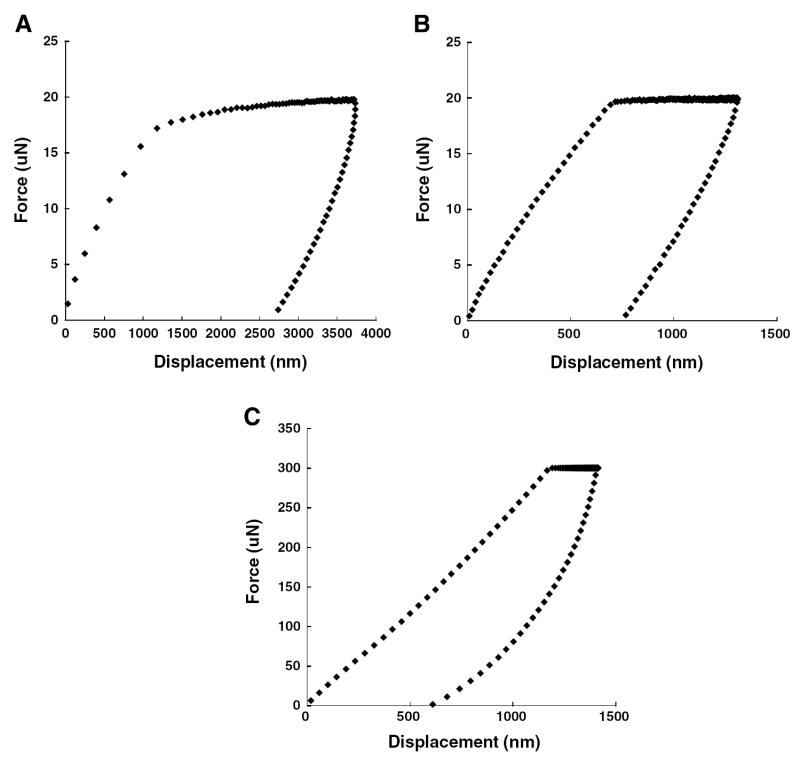
Each indent used a trapezoid-shaped load function: a preload for 6 s that allowed measurement of the system drift rate; a ramp of 2 s until the maximum peak force was reached; a hold of 3 s (for granulation tissue) or 15 s (for all other soft tissues); and finally an unloading ramp of 2 s until zero force was reached. The peak forces for granulation tissue, chondroid tissue and woven bone were 20, 20 and 300 μN, respectively (Fig. 1). These peak forces were chosen such that the indentation depth would be at least 10 times the surface roughness [17] of the sections (33 ± 12 nm as determined by atomic force microscopy). The hold period at the peak force was inserted in order to reduce the effect of tissue viscoelasticity on the initial unloading response [33]. The hold period for granulation tissue was limited to 3 s, because of the displacement limit (5 μm) of the system. Initial validation experiments using the above testing protocol indicated good measurement precision: repeated indents performed on rat cortical bone and rat articular cartilage produced coeffcients of variation (standard deviation divided by mean) of 0.078 and 0.21, respectively.
Fig. 1.
Representative force–displacement curves for indents performed on: (A) granulation tissue, (B) chondroid tissue, and (C) woven bone.
2.3. Histological staining
Following the indentation procedure described above, tissue sections were kept frozen at −20 °C until staining. Once thawed, the sections were fixed in 4% paraformaldehyde for 20 min, rinsed thoroughly, and then incubated in 1% alcian blue pH 2.5 (Fisher Scientific, Pittsburgh, PA) for 12 h. The sections were then incubated in alizarin red S (Fisher Scientific, Pittsburgh, PA) for 8 min, dehydrated briefly in xylene and coverslipped in Permount (Fisher Scientific, Pittsburgh, PA). Alcian blue is commonly used to identify the presence of sulfated proteoglycans in cartilage tissues, because it is a cationic dye that forms electrostatic bonds with negatively charged moieties on mucopolysaccharide chains. Alizarin red is a calcium-sensitive dye used to identify tissues containing calcium-rich hydroxyapatite crystals. The uptake of alizarin red has been shown to be approximately proportional to calcification activity [34-36]. After staining was complete, digital images of each section were captured at 40× magnification using an inverted microscope and CCD camera (Olympus BX 51, Tokyo, Japan).
Regions of tissue throughout each section were identified as stained with alcian blue, alizarin red or both. This categorization was performed by two independent observers who did not perform the nanoindentation experiments or see the nanoindentation results. For each region that corresponded to an indent location, the tissue was rated on a scale of 1–5, where 1 is the blue, 2 is the predominantly blue, 3 is the equally blue and red, 4 is the predominantly red, and 5 is the red. The inter-observer disagreement was very low (2 out of 27 regions), with the only discrepancies occurring between categories 1 and 2 and between categories 4 and 5. Thus, regions that received a rating of 1 or 2 by either observer were classified as alcian blue regions, those that received a rating of 3 were classified as mixed regions, and those that received a rating or 4 or 5 by either observer were classified as alizarin red regions. The average pixel intensity of each region was then quantified using image processing software (ImageJ, version 1.38, National Institute of Health).
2.4. Statistical analysis
Regression analysis was used to determine the dependence of indentation modulus in the periosteal region of the callus on distance from the fracture gap. This analysis was motivated by the results of histological analyses of fracture healing indicating that bone formation proceeds along the periosteal surface of the cortex towards the fracture gap. Pearson correlation analyses were carried out to determine the association between indentation modulus and alizarin red or alcian blue staining intensity.
3. Results
Indentation moduli varied over three orders of magnitude (0.61–1010 MPa) throughout the callus. Much of this variation was due to the presence of multiple tissue types: the indentation moduli of granulation tissue, chondroid tissue and woven bone ranged from 0.61 to 1.27 MPa (median = 0.99 MPa), 1.39–4.42 MPa (median = 2.89 MPa), and 26.92–1010.00 MPa (median = 132.00 MPa), respectively (Table 1). Notably, heterogeneity in moduli for a given tissue type was observed even within a single region of the callus (Fig. 2). The modulus of chondroid tissue varied by as much as threefold over a distance of 500 μm, and the modulus of woven bone by as much as twofold over the same distance.
Table 1.
Indentation moduli for three tissue types in an unstabilized fracture (SD = standard deviation)
| Tissue type
|
|||
|---|---|---|---|
| Woven bone (n = 41) | Chondroid tissue (n = 18) | Granulation tissue (n = 10) | |
| Mean (MPa) | 201.16 | 3.10 | 0.99 |
| Median (MPa) | 132.00 | 2.89 | 0.99 |
| SD (MPa) | 200.85 | 1.30 | 0.20 |
| Max (MPa) | 1010.00 | 4.42 | 1.27 |
| Min (MPa) | 26.92 | 1.39 | 0.61 |
Fig. 2.
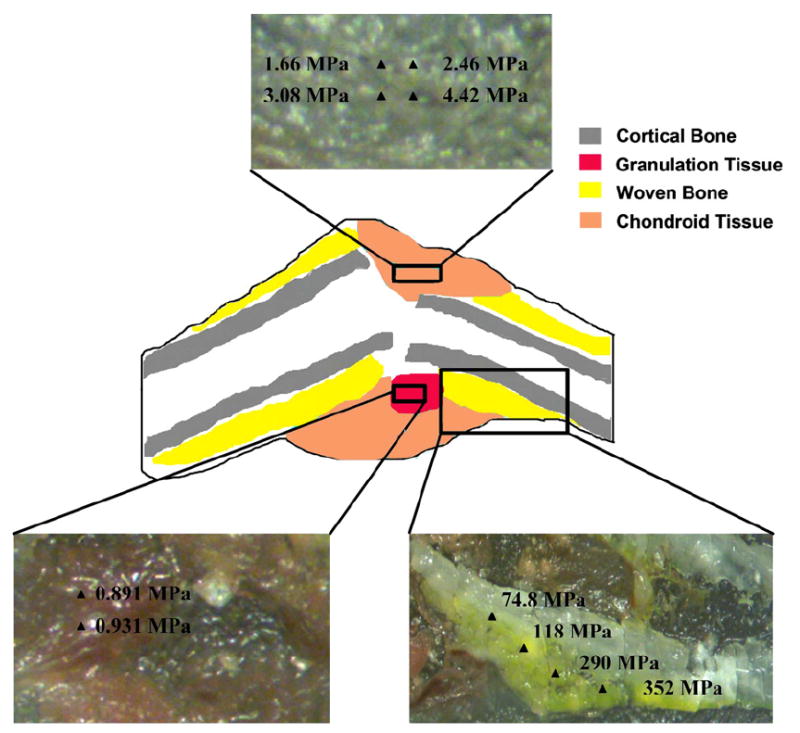
Schematic of a representative callus section depicting the location of the different tissue types with respect to the fracture gap and pre-existing bone. Indentation moduli at multiple positions throughout regions of each tissue type are shown in the insets.
Regions within and just outside the fracture gap consisted exclusively of chondroid tissue and granulation tissue, and woven bone was restricted to regions along the periosteal surface of the cortex (Fig. 2). For this woven bone tissue, the spatial variation in indentation modulus followed a consistent gradient. In each callus section in which periosteal woven bone was found and was sampled with nanoindentation, the indentation modulus increased with the distance from the fracture gap (P = 0.004, Fig. 3).
Fig. 3.
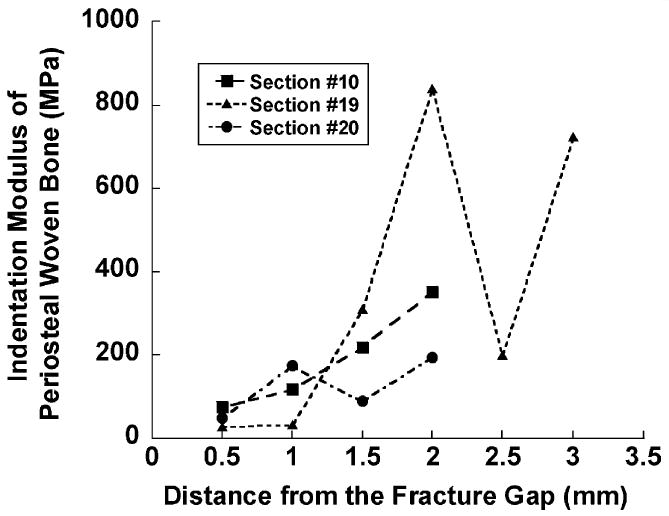
Plot of indentation modulus of periosteal woven bone tissue against distance from the fracture gap on three tissue sections. An increase in modulus with distance was found (P = 0.004).
Indentation moduli were correlated with staining intensity in regions stained with alizarin red. For regions classified as alizarin red, the indentation modulus increased with staining intensity (P ≤ 0.04, Fig. 4). No association between indentation modulus and staining intensity was found in alcian blue regions (P = 0.13).
Fig. 4.
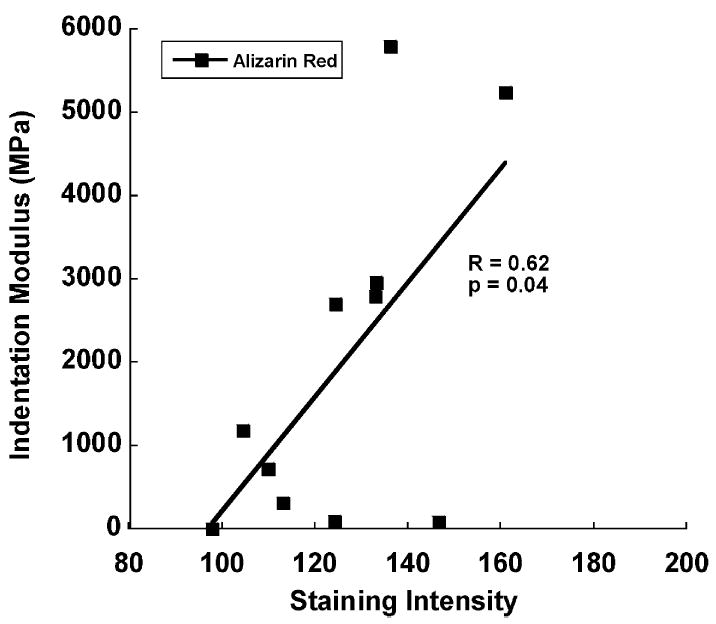
Indentation modulus and staining intensity in regions of alizarin red uptake. A positive correlation was found, indicating an increase in indentation modulus with tissue mineral content.
4. Discussion
Motivated by the importance of callus tissue material properties in governing the mechanical behavior of the fracture callus, the overall goal of this study was to characterize the elastic properties of fracture callus tissues. The results reveal that the callus exhibits a wide spectrum of indentation moduli, which is due in large part to the heterogeneous mixture of tissues present. However, a substantial range in modulus was also found for each tissue, indicating that each tissue type within the callus is not itself mechanically homogeneous. Pairing the nanoindentation measurements with semi-quantitative histological assessment provided insight into the heterogeneity in indentation moduli both within and among tissue types. In particular, it was found that the indentation modulus was positively correlated with alizarin red uptake. Taken together, these results demonstrate the usefulness of nanoindentation in quantifying the material properties of the mechanically disparate tissues present in fracture calluses.
To the authors’ knowledge, this is the first study to report direct measurements of the material properties of individual fracture callus tissues. In a previous study using a canine model of fracture healing, Markel et al. [9] reported indentation stiffness values at multiple locations throughout the callus but did not determine the corresponding indentation moduli or Young’s moduli. Moreover, the sampled tissues were identified solely by anatomic location and not by the type of tissue. In the present study, the identification of tissue type was based on appearance (e.g., color, opacity and texture) and was confirmed by subsequent histological staining. This enabled clear classification of tissue type such that, even if a mixture of tissues was present in a given indent location, this mixture was heavily dominated by one tissue type. The process of identifying the tissue at each indent location enabled assessment of the variability in indentation modulus both within and among different callus tissues.
Specific specimen preparation methods were used in this study in order to enable characterization of multiple tissue types within the callus. A tissue sectioning technique was developed, designed to minimize damage to the callus tissues and allow indents on fresh, as opposed to formalin fixed, tissue sections. Formalin fixation, because it involves cross-linking in the tissue, is known to alter the mechanical properties of soft tissues [19]. No polishing was performed, as this would either obliterate the granulation tissue and chondroid tissue present in a tissue section or leave polishing residue within these tissues that would confound the nanoindentation measurements. With these specimen preparation protocols, values of indentation modulus for chondroid tissue (3.10 ± 1.30 MPa) were obtained that are within the range of what has been reported for various types of cartilage, including articular cartilage (~0.5–5 MPa [19,37-40]) and fibrocartilage (~0.5 MPa [19]). This good agreement also lends confidence to the values of indentation modulus obtained for granulation tissue and woven bone. The data on granulation tissue moduli are particularly significant, given the interest in determining how stresses and strains in this tissue promote its differentiation into various skeletal tissues such as bone, cartilage and fibrous tissue [41-44].
Of the three types of tissues investigated, the largest amount of variation in indentation modulus was seen in woven bone (Table 1). It is likely that this variation reflects the wide range in mineralization of this tissue. During fracture healing, woven bone forms first in regions remote to the fracture gap and subsequently progresses along the periosteal aspect of the cortex towards the fracture gap. Consequently, at any given time during healing, the regions furthest from the gap are expected to have the highest mineral content. This biological paradigm of fracture healing is consistent with results on indentation modulus and mineral content of woven bone. The indentation modulus of woven bone was found to be correlated with an indicator of mineral content (Fig. 4) and increased along the periosteal aspect with distance from the fracture gap (Fig. 3).
There are several technical limitations that are relevant to this study. First, the use of unfixed tissue sections limited the duration of nanoindentation experiments for each section. Second, owing to the relatively uneven surface topography of the sections, the tissue surface had to be located for each indent prior to applying the defined force. Omitting the initial surface detection step would risk placing excessive force on the Triboindenter transducer. Together, these two limitations restricted the number of indents that could be performed on a given callus section. Third, only one fracture callus was investigated. The focus of this study was to develop experimental techniques that allow measurement of callus tissue material properties. Although it is not possible to generalize the results to other calluses, the robust nature of fracture healing [1,45] suggests that the results should provide an indication of the types of indentation modulus values—and the ranges in these values—that can be expected for callus tissues. Fourth, in using the Oliver–Pharr method to calculate the reduced modulus, the authors modeled each of the callus tissues as an elastic material. Although the effects of tissue viscoelasticity were reduced by using a trapezoidal load function and quick unloading ramp [46,47], it is still possible that the measurements obtained are not truly representative of the instantaneous elastic response of the tissue. Future work will be focused on accounting for the time-dependence of the tissues by analyzing the nanoindentation data using viscoelastic [48,49] or poroelastic [40] models. Lastly, tissue mineralization and proteoglycan content were not measured directly, but were instead estimated through a semi-quantitative histological approach that allowed a paired comparison of tissue composition and indentation modulus. Despite this limitation, the correlation between alizarin red uptake and indentation modulus is consistent with previous nanoindentation studies that used quantitative backscattered electron imaging [21], atomic emission spectroscopy [9] and micro-computed tomography [30] to quantify mineral content.
This study extends the current body of literature on nanoindentation of biological materials (reviewed in [50]) by applying this testing technique to the heterogeneous mixture of tissues comprising a bone fracture callus. The results demonstrate that nanoindentation can be used to quantify callus tissue properties in situ on a scale that is compatible with small animal models of fracture healing. Moreover, the spatial distribution of indentation moduli were consistent with current understanding of the biology of fracture healing, indicating the feasibility of using nanoindentation to elucidate further the structure–function relationships in healing and regenerating tissues.
Acknowledgments
Funding for this study was provided by NIH AR053353 (EFM). Use of the Hysitron Triboindenter was graciously provided by Dr. Catherine Klapperich. We should also like to thank Gregory J. Miller and Lauren N.M. Hayward for performing the color classification, and Louis C. Gerstenfeld for providing guidance on the histological staining.
References
- 1.Einhorn TA. The cell and molecular biology of fracture healing. Clin Orthop. 1998;355(Suppl):S7–S21. doi: 10.1097/00003086-199810001-00003. [DOI] [PubMed] [Google Scholar]
- 2.McCann RM, et al. Effect of osteoporosis on bone mineral density and fracture repair in a rat femoral fracture model. J Orthop Res. 2007 doi: 10.1002/jor.20505. [DOI] [PubMed] [Google Scholar]
- 3.Kayal RA, et al. Diminished bone formation during diabetic fracture healing is related to the premature resorption of cartilage associated with increased osteoclast activity. J Bone Miner Res. 2007;22(4):560–8. doi: 10.1359/jbmr.070115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Endo K, et al. Cyclooxygenase-2 inhibitor delays fracture healing in rats. Acta Orthop. 2005;76(4):470–4. doi: 10.1080/17453670510041439. [DOI] [PubMed] [Google Scholar]
- 5.Komatsubara S, et al. Human parathyroid hormone (1–34) accelerates the fracture healing process of woven to lamellar bone replacement and new cortical shell formation in rat femora. Bone. 2005;36(4):678–87. doi: 10.1016/j.bone.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Kakar S, et al. Enhanced chondrogenesis and Wnt-signaling in parathyroid hormone treated fractures. J Bone Miner Res. 2007 doi: 10.1359/jbmr.070724. [DOI] [PubMed] [Google Scholar]
- 7.Gerstenfeld LC, et al. Differential inhibition of fracture healing by non-selective and cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs. J Orthop Res. 2003;21(4):670–5. doi: 10.1016/S0736-0266(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 8.Li J, et al. Effect of bisphosphonate (incadronate) on fracture healing of long bones in rats. J Bone Miner Res. 1999;14(6):969–79. doi: 10.1359/jbmr.1999.14.6.969. [DOI] [PubMed] [Google Scholar]
- 9.Markel MD, Wikenheiser MA, Chao EY. A study of fracture callus material properties: relationship to the torsional strength of bone. J Orthop Res. 1990;8(6):843–50. doi: 10.1002/jor.1100080609. [DOI] [PubMed] [Google Scholar]
- 10.Gardner TN, et al. The influence of mechanical stimulus on the pattern of tissue differentiation in a long bone fracture – an FEM study. J Biomech. 2000;33(4):415–25. doi: 10.1016/s0021-9290(99)00189-x. [DOI] [PubMed] [Google Scholar]
- 11.Lacroix D, et al. Biomechanical model to simulate tissue differentiation and bone regeneration: application to fracture healing. Med Biol Eng Comput. 2002;40(1):14–21. doi: 10.1007/BF02347690. [DOI] [PubMed] [Google Scholar]
- 12.Rho JY, Tsui TY, Pharr GM. Elastic properties of human cortical and trabecular lamellar bone measured by nanoindentation. Biomaterials. 1997;18(20):1325–30. doi: 10.1016/s0142-9612(97)00073-2. [DOI] [PubMed] [Google Scholar]
- 13.Turner CH, et al. The elastic properties of trabecular and cortical bone tissues are similar: results from two microscopic measurement techniques. J Biomech. 1999;32(4):437–41. doi: 10.1016/s0021-9290(98)00177-8. [DOI] [PubMed] [Google Scholar]
- 14.Zysset PK, et al. Elastic modulus and hardness of cortical and trabecular bone lamellae measured by nanoindentation in the human femur. J Biomech. 1999;32(10):1005–12. doi: 10.1016/s0021-9290(99)00111-6. [DOI] [PubMed] [Google Scholar]
- 15.Hoffer CE, et al. Heterogeneity of bone lamellar-level elastic moduli. Bone. 2000;26(6):603–9. doi: 10.1016/s8756-3282(00)00268-4. [DOI] [PubMed] [Google Scholar]
- 16.Silva MJ, et al. Nanoindentation and whole-bone bending estimates of material properties in bones from the senescence accelerated mouse SAMP6. J Biomech. 2004;37(11):1639–46. doi: 10.1016/j.jbiomech.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 17.Donnelly E, et al. Effects of surface roughness and maximum load on the mechanical properties of cancellous bone measured by nanoindentation. J Biomed Mater Res A. 2006;77(2):426–35. doi: 10.1002/jbm.a.30633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li C, Pruitt LA, King KB. Nanoindentation differentiates tissue-scale functional properties of native articular cartilage. J Biomed Mater Res A. 2006;78(4):729–38. doi: 10.1002/jbm.a.30751. [DOI] [PubMed] [Google Scholar]
- 19.Franke O, et al. Mechanical properties of hyaline and repair cartilage studied by nanoindentation. Acta Biomater. 2007;3(6):873–81. doi: 10.1016/j.actbio.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Tomkoria S, Patel RV, Mao JJ. Heterogeneous nanomechanical properties of superficial and zonal regions of articular cartilage of the rabbit proximal radius condyle by atomic force microscopy. Med Eng Phys. 2004;26(10):815–22. doi: 10.1016/j.medengphy.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Gupta HS, et al. Two different correlations between nanoindentation modulus and mineral content in the bone–cartilage interface. J Struct Biol. 2005;149(2):138–48. doi: 10.1016/j.jsb.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Ebenstein D, et al. A nanoindentation technique for functional evaluation of cartilage repair tissue. J Mater Res. 2004;19(1):273–81. [Google Scholar]
- 23.Akhter MP, et al. Bone biomechanical properties in LRP5 mutant mice. Bone. 2004;35(1):162–9. doi: 10.1016/j.bone.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 24.Kavukcuoglu NB, et al. Osteopontin deficiency and aging on nanomechanics of mouse bone. J Biomed Mater Res A. 2007;83(1):136–44. doi: 10.1002/jbm.a.31081. [DOI] [PubMed] [Google Scholar]
- 25.Guo XE, Goldstein SA. Vertebral trabecular bone microscopic tissue elastic modulus and hardness do not change in ovariectomized rats. J Orthop Res. 2000;18(2):333–6. doi: 10.1002/jor.1100180224. [DOI] [PubMed] [Google Scholar]
- 26.Jamsa T, et al. Mechanical properties in long bones of rat osteopetrotic mutations. J Biomech. 2002;35(2):161–5. doi: 10.1016/s0021-9290(01)00203-2. [DOI] [PubMed] [Google Scholar]
- 27.Wang XM, et al. Variation of nanomechanical properties of bone by gene mutation in the zebrafish. Biomaterials. 2002;23(23):4557–63. doi: 10.1016/s0142-9612(02)00201-6. [DOI] [PubMed] [Google Scholar]
- 28.Chen Q, et al. Congenital lack of COX-2 affects mechanical and geometric properties of bone in mice. Calcif Tissue Int. 2003;73(4):387–92. doi: 10.1007/s00223-002-0009-x. [DOI] [PubMed] [Google Scholar]
- 29.Rho JY, et al. Variations in the individual thick lamellar properties within osteone by nanoindentation. Bone. 1999;25(3):295–300. doi: 10.1016/s8756-3282(99)00163-5. [DOI] [PubMed] [Google Scholar]
- 30.Mulder L, et al. Relationship between tissue stiffness and degree of mineralization of developing trabecular bone. J Biomed Mater Res A. 2007 doi: 10.1002/jbm.a.31474. [DOI] [PubMed] [Google Scholar]
- 31.Bonnarens F, Einhorn TA. Production of a standard closed fracture in laboratory animal bone. J Orthop Res. 1984;2(1):97–101. doi: 10.1002/jor.1100020115. [DOI] [PubMed] [Google Scholar]
- 32.Oliver WC, Pharr GM. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J Mater Res. 1992;7(6):1564–83. [Google Scholar]
- 33.Briscoe BJ, Fiori L, Pelillo E. Nanoindentation of polymeric surfaces. J Phys D: Appl Phys. 1998;31(19):2395–405. [Google Scholar]
- 34.Zhao Y, et al. The effect of 3-hydroxybutyrate on the in vitro differentiation of murine osteoblast MC3T3-E1 and in vivo bone formation in ovariectomized rats. Biomaterials. 2007;28(20):3063–73. doi: 10.1016/j.biomaterials.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Gregory CA, et al. An Alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal Biochem. 2004;329(1):77–84. doi: 10.1016/j.ab.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Puchtler H, Meloan SN, Terry MS. On the history and mechanism of alizarin and alizarin red S stains for calcium. J Histochem Cytochem. 1969;17(2):110–24. doi: 10.1177/17.2.110. [DOI] [PubMed] [Google Scholar]
- 37.Athanasiou KA, et al. Effects of aging and dietary restriction on the structural integrity of rat articular cartilage. Ann Biomed Eng. 2000;28(2):143–9. doi: 10.1114/1.238. [DOI] [PubMed] [Google Scholar]
- 38.Wang L, et al. Effects of ageing on the biomechanical properties of rat articular cartilage. Proc Inst Mech Eng [H] 2006;220(4):573–8. doi: 10.1243/09544119H04404. [DOI] [PubMed] [Google Scholar]
- 39.Setton LA, et al. Mechanical properties of canine articular cartilage are significantly altered following transection of the anterior cruciate ligament. J Orthop Res. 1994;12(4):451–63. doi: 10.1002/jor.1100120402. [DOI] [PubMed] [Google Scholar]
- 40.Cao L, et al. Compressive properties of mouse articular cartilage determined in a novel micro-indentation test method and biphasic finite element model. J Biomech Eng. 2006;128(5):766–71. doi: 10.1115/1.2246237. [DOI] [PubMed] [Google Scholar]
- 41.Claes LE, Heigele CA. Magnitudes of local stress and strain along bony surfaces predict the course and type of fracture healing. J Biomech. 1999;32(3):255–66. doi: 10.1016/s0021-9290(98)00153-5. [DOI] [PubMed] [Google Scholar]
- 42.Lacroix D, Prendergast PJ. A mechano-regulation model for tissue differentiation during fracture healing: analysis of gap size and loading. J Biomech. 2002;35(9):1163–71. doi: 10.1016/s0021-9290(02)00086-6. [DOI] [PubMed] [Google Scholar]
- 43.Isaksson H, et al. Bone regeneration during distraction osteogenesis: mechano-regulation by shear strain and fluid velocity. J Biomech. 2007;40(9):2002–11. doi: 10.1016/j.jbiomech.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 44.Le AX, et al. Molecular aspects of healing in stabilized and non-stabilized fractures. J Orthop Res. 2001;19(1):78–84. doi: 10.1016/S0736-0266(00)00006-1. [DOI] [PubMed] [Google Scholar]
- 45.McKibbin B. The biology of fracture healing in long bones. J Bone Joint Surg Brit. 1978;60B(2):150–62. doi: 10.1302/0301-620X.60B2.350882. [DOI] [PubMed] [Google Scholar]
- 46.Ngan AHW, Tang B. Viscoelastic effects during unloading in depth-sensing indentation. J Mater Res. 2002;17(10):2604–10. [Google Scholar]
- 47.Fan Z, Rho JY. Effects of viscoelasticity and time-dependent plasticity on nanoindentation measurements of human cortical bone. J Biomed Mater Res A. 2003;67(1):208–14. doi: 10.1002/jbm.a.10027. [DOI] [PubMed] [Google Scholar]
- 48.Cheng L, et al. Spherical-tip indentation of viscoelastic material. Mech Mat. 2005;37:213–26. [Google Scholar]
- 49.Oyen ML. Spherical indentation creep following ramp loading. J Mater Res. 2005;20(8):2094–100. [Google Scholar]
- 50.Ebenstein DM, Pruitt LA. Nanoindentation of soft hydrated materials for application to vascular tissues. J Biomed Mater Res A. 2004;69(2):222–32. doi: 10.1002/jbm.a.20096. [DOI] [PubMed] [Google Scholar]