Abstract
Understanding the neural mechanisms that underlie the function of central pattern generators (CPGs) presents a formidable challenge requiring sophisticated tools and well-chosen model systems. In this article, we describe recent work on vocalizations of the African clawed frog Xenopus laevis. These behaviors are driven by sexually differentiated CPGs and are exceptionally well suited to this objective. In particular, a simplified mechanism of vocal production (independent of respiratory musculature) allows straightforward interpretations of nerve activity with respect to behavior. Furthermore, the development of a fictively vocalizing isolated brain, together with the finding of rapid androgen-induced masculinization of female vocalizations, provides an invaluable tool for determining how new behaviors arise from existing circuits.
Introduction
Neuroscientists typically focus on traits (ranging from systems to molecules) that are reproducible among all individuals within a species, with the hope of elucidating fundamental principles that apply across species, including humans. Evolutionary biologists, by contrast, focus on phenotypes that are variable among individuals within a species because inter-individual variation is a substrate upon which selective pressures act. From the intersection of these two disciplines emerges an extremely interesting topic – the neural basis of individuality. Although it is important to understand the functional uniqueness of individual brains, the large sample size required to address this question makes it impractical. A fruitful alternative is to compare the nervous system of different subgroups of individuals divided by age, sex or endocrine profile within a species. These comparisons can point to strategies that the central nervous system (CNS) employs to generate behavioral variation among individuals.
Vocalizations of African clawed frogs, Xenopus laevis, have been studied for the past three decades as a model for understanding sexually differentiated behaviors [1]. The Xenopus vocal pathway offers three major advantages for investigating the neural basis of behavior. First, a simple sound production mechanism (a single pair of muscles, separate from the respiratory system and dedicated for vocal production [2]) makes the Xenopus vocal circuitry one of the most ‘approachable’ motor pathways in vertebrates; this mechanistic specialization is likely accompanied by a simpler neuronal network. Second, vocal patterns are generated by a central pattern generator (CPG; see Box 1) located in the brainstem which allows ‘calling’ to be studied in vitro – fictive vocalizations can be elicited in the isolated brain [3]. Third, vocalizations are sexually distinct, unlike other well-studied rhythmic motor patterns (e.g. breathing, chewing and locomotion). Therefore, Xenopus vocalizations provide a rare opportunity to study steroid-controlled sexual differentiation of CPGs in detail. In this review, we summarize recent progress in the Xenopus vocal system, emphasizing these attributes that make it an excellent model for exploring the neural logic of behavior.
Box 1. Central pattern generators.
Many rhythmic motor behaviors are produced by central pattern generators (CPGs), networks of neurons that are capable of generating coordinated output without sensory feedback [39]. Historically, rhythmic behaviors were believed to be generated as a result of chains of reflexes [40]. This view was overturned and replaced with the idea of endogenous oscillators: first with evidence provided by Graham-Brown [41], who demonstrated that rhythmic limb movements in mammals can be produced without central or peripheral rhythmic activity, and later by Wilson [42], who showed flight motor patterns in locust are generated without sensory input. Now CPGs that control various rhythmic motor behaviors in both vertebrates and invertebrates have been identified. One particularly useful attribute of CPGs is that many remain functional in completely isolated nervous systems, facilitating analyses at the level of neurons and synapses while functional output is monitored. Such analysis has led to the discovery of fundamental principles such as the interplay between membrane properties and synaptic strength in determining rhythmic output, dynamic roles of neuromodulators that reconfigure circuits and the homeostatic nature of neuronal networks [43–49]. These findings are of universal importance because oscillatory processes seen in networks other than motor systems (including thalamus, cortex and sensory pathways) might share the same organizational principle as motor CPGs [50,51].
Elucidating principles of CPG function continues to be bolstered by investigations of a wide range of model organisms and systems. For example, powerful molecular and genetic tools available in some species (e.g. mice, zebrafish) are promoting the discovery of specific genes and molecules that underlie CPG structure and function [48,52]. Likewise, comparative approaches using species with distinct attributes – such as sex differences in Xenopus vocal systems [3], or species-specific escape behaviors of nudibranch molluscs [53] – are offering crucial insights into the cellular and synaptic bases of the mechanisms of pattern generation. Ultimately, identifying the sources of sex- and species-specific variation might reveal mechanisms by which homologous neurons and circuits can diverge to generate novel motor outputs.
Vocalizations of Xenopus laevis and its androgen regulation
The vocal repertoire of adult Xenopus is rich; five calls are male specific (the advertisement call, amplectant call, answer call, chirping and growling), one call is female specific (rapping), and one call (ticking; also called release call in females) is shared by both sexes [4,5]. In this review, we concentrate on how the central vocal pathways generate sex-specific click rates, and how they are modified by androgen. The focus will be placed on advertisement call and ticking because they are the most common and best-studied vocalizations (Box 2).
Box 2. Sexually dimorphic calls differ in temporal and spectral traits.
Xenopus produces vocalizations under water, without breathing. The Xenopus vocal organ – the larynx – generates sounds (clicks) by a mechanism different from that of most vertebrates. Unlike terrestrial vertebrates, sound production does not require the flux of air across vocal folds. Instead, paired laryngeal muscles rapidly contract to snap apart two discs, which results in a click [2]. Thus, Xenopus calls are generated by a series of contractions and relaxations of laryngeal muscles at call-specific rates.
Xenopus vocalizations are made up of individual clicks produced in stereotyped temporal patterns. Two acoustic call features – temporal pattern and spectral frequency – are the sole elements that distinguish male and female calls. In male calls, clicks are repeated at wide-ranging rates (∼2–80 Hz), whereas in female calls, clicks are repeated only at slow rates (∼2–20 Hz). Dominant frequencies of male clicks are higher (∼2 kHz) than those of females (∼1 kHz; Figure Ic,d) [4]. A recent study showed that although the spectral structure of clicks elicits minor effects on behavioral responses, it is the rate, or temporal pattern, of clicks that provides the most salient information about the sex of the caller [54]. The advertisement call, produced exclusively by males, consists of two alternating click trills at ∼35 Hz and ∼70 Hz (slow trill and fast trill, respectively; Figure Ia). Ticking, predominantly produced by unreceptive females [4], is monophasic and slower (36 Hz; Figure Ib).
Figure I.
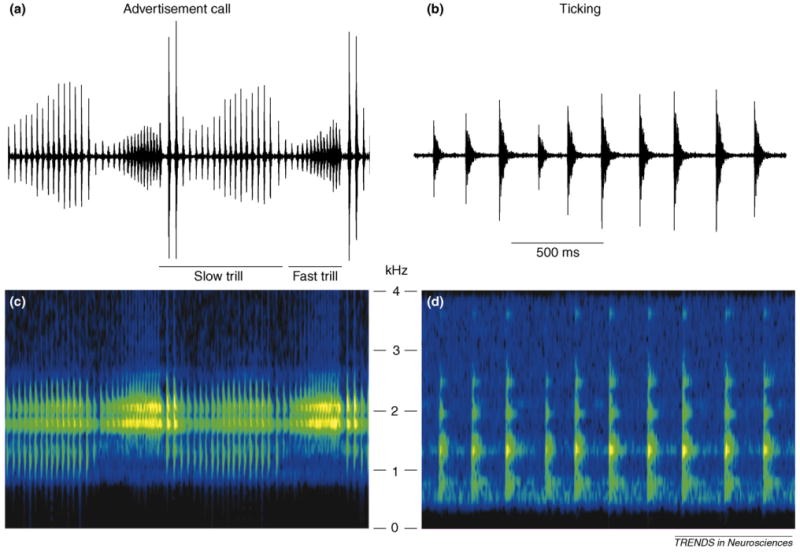
Temporal and spectral organization of advertisement calls and ticking. (a) Sound waveform of the male advertisement call that consists of alternating slow (∼35 Hz) and fast (∼70 Hz) trills. (b) Sound waveform of female ticking that consists of slow repetition of clicks (∼6 Hz). (c,d) Two dominant frequency bands (∼2 kHz) observed in male advertisement calls (c) are significantly higher than clicks produced by females (d).
Androgen regulates the production and sexual differentiation of Xenopus vocal traits, providing the chance to investigate sex-specific CPGs using a comparative approach. Wetzel and Kelley first demonstrated that androgens are necessary for male vocal production [6]. Calling is completely eliminated by castration (after approximately 1 month), but can be restored following several weeks of treatment with either testosterone (T) or dihydrotestosterone (DHT). These results reveal that androgen plays an ‘activational role’ in promoting male vocal behaviors [7].
Androgen also plays an ‘organizational role’ during development. Male and female frogs begin producing release calls as juveniles. Over time, males generate progressively faster trills that eventually become biphasic advertisement calls, whereas females produce release calls throughout development [8]. This behavioral differentiation correlates with postmetamorphic divergence of androgen levels in the sexes [9]. Because adult males are capable of release calling [4], androgen-induced vocal masculinization represents a gain of function – they acquire the ability to produce male-specific calls without losing the ability to produce release calls.
An exciting observation is that female Xenopus vocal patterns can also be masculinized by androgens in adulthood. Two studies have investigated the effects of androgen treatment on vocal behavior of adult females. In both cases, significant but incomplete vocal masculinization was observed [10,11] (Figure 1). Using intensive sampling methods, Potter et al. showed the temporal progression from feminine to de-feminized and then masculinized behavior between 1 and 13 weeks after commencing treatment [11]. In this study, testosterone-treated females (TFs) produced biphasic vocalizations with slow and fast trills (resembling male advertisement calls) as early as 8 weeks after treatment initiation (Figure 1). Although masculinization was not complete (TF fast trills remained somewhat slower than males), the qualitative change from slow monophasic rhythm to fast biphasic rhythm was dramatic. Therefore, unlike most mammals and birds, there is no ‘organizational critical period’ for androgen-mediated masculinization of the central vocal circuit in Xenopus.
Figure 1.
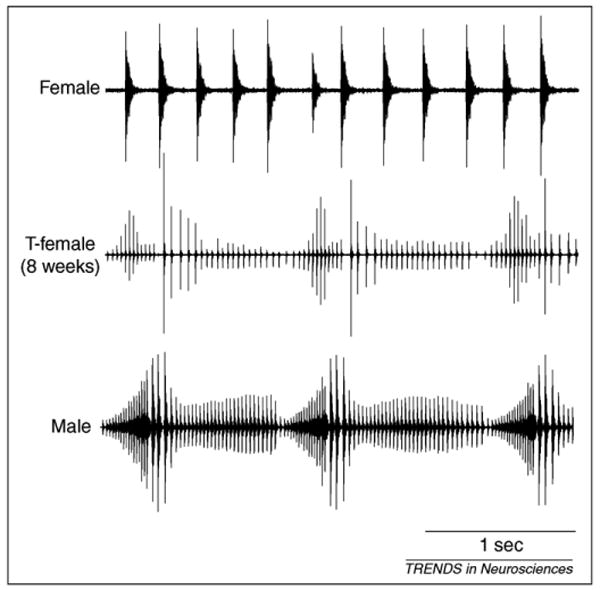
Androgen-induced masculinization of female vocalizations. A female that produced only ticking before testosterone treatment (top) began to produce biphasic advertisement calls within 8 weeks of the treatment (middle). The click rates are slightly slower than those of male advertisement calls (bottom). Figure reproduced, with permission, from [11], (2005) American Physiological Society.
Like males, TFs maintain the ability to produce ticking after masculinization. Thus, androgen endows male and female Xenopus with an ability to generate advertisement calls without preventing the production of ticking, either during development or in adulthood. As is the case for males [4], the types of vocalizations produced by TFs depend on social context [11]. After 12 weeks of testosterone treatment, TFs produce advertisement calls when paired with females, and release calls when paired with sexually receptive males. This result indicates that females also acquire the ability to select appropriate call types in response to sensory cues provided by the same or opposite sex. In summary, the rapid vocal masculinization in female adults provides an opportunity to investigate the strategies used by the CNS to introduce new behaviors.
Central pattern generators
The neural coding of sex-specific call patterns is straightforward in Xenopus. The Xenopus larynx has a single muscle pair which contracts synchronously to produce vocal clicks. The activity of laryngeal muscles is controlled by laryngeal motoneurons. When laryngeal nerve activity was monitored in vocalizing male and female frogs, one compound action potential (CAP; a synchronous volley of action potentials recorded on the nerve) immediately preceded the sound of each click [12]. Thus, sex-typical vocal rhythms are generated entirely by the central vocal pathways – action potentials generated by male and female laryngeal motoneurons are faithfully transduced into clicks by the larynx. Where are these patterns generated?
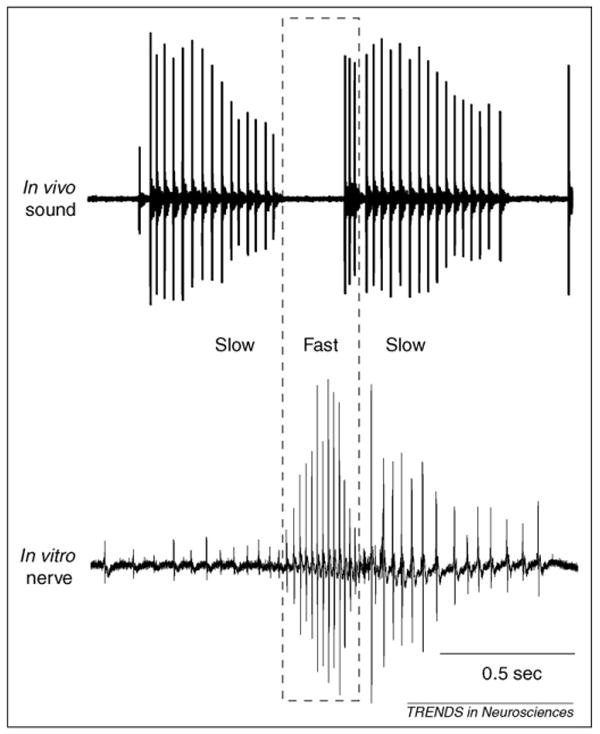
Taking advantage of the fact that laryngeal nerve activity provides a direct readout of behavior, a preparation that generates fictive vocalizations in vitro has been recently developed. Bath application of serotonin to isolated brains elicits activity resembling advertisement calls (from male brains) and ticking (from male and female brains) in the laryngeal nerves [3] (Figure 2). The finding is significant in two ways. First, the results prove that vocal production relies on a CPG – fictive calling does not require sensory feedback. Second, the development of a fictively calling isolated brain provides a powerful tool for investigating the neural basis of vocal pattern generation. In this section, we will first describe the central vocal pathways, and next examine suspected location of the vocal CPG in males and females.
Figure 2.
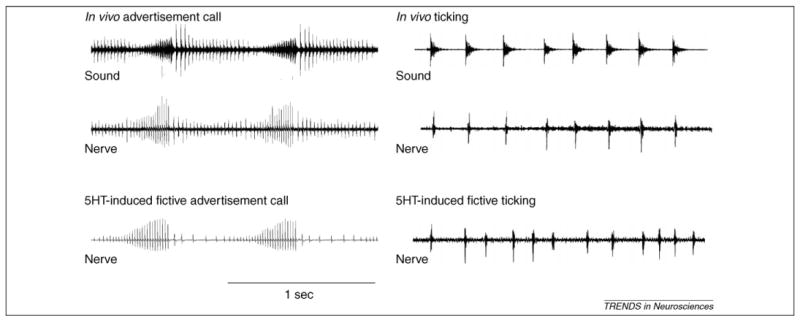
Laryngeal nerve activity as a direct readout of behavior. Simultaneous sound (top) and nerve recordings (middle) obtained from awake frogs show that each click is preceded by a compound action potential, indicating that the neuronal signals generated by the central vocal pathway are faithfully transduced into a series of clicks by the larynx. Serotonin-induced fictive vocalizations (bottom) are remarkably similar to nerve recordings obtained from awake frogs.
The original identification of Xenopus vocal nuclei was achieved by autoradiographic analysis of androgen-concentrating neurons throughout the brain. These areas include the thalamus, infundibulum, anterior preoptic area, ventral striatum, dorsal tegmental area of the medulla (DTAM) and the motor nucleus (n.) IX-X [13]. Later studies using dye injection revealed a robust reciprocal connection between DTAM and n.IX-X [14,15] (Figure 3a). These two areas are of particular interest because the hindbrain contains the neural circuits that generate call-specific vocal rhythms in both sexes (see below). DTAM might be homologous to mammalian parabrachial nucleus (PB), believed to regulate calling in primates [16] and bats [17], because of its connection to respiratory/vocal moto-neurons [18,19] and the basal forebrain [15,20] (Figure 3).
Figure 3.
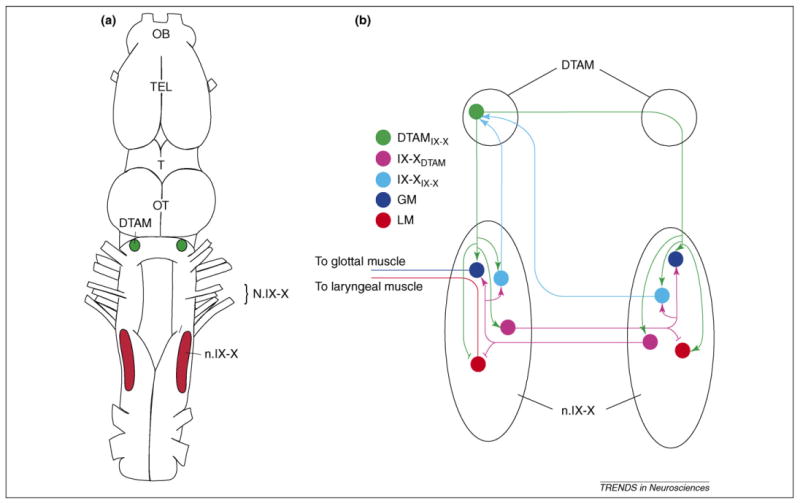
Brainstem vocal circuit of Xenopus laevis. (a) Dorsal view of the Xenopus CNS with key brainstem vocal nuclei. (b) Schematic diagram of the reciprocal connections between n.IX-X and DTAM. Four neuron populations – two motoneurons and two interneurons – have been identified in n.IX-X. Commissural neurons (magenta; IX-XIX-X) project to glottal (dark blue) and laryngeal (red) motoneurons and DTAM-projecting interneurons (light blue; IX-XDTAM). IX-XDTAM cells send robust inputs to DTAM, and all four n.IX-X neurons receive inputs from DTAM. Bars represent known excitatory synapses; arrows represent synapses with currently unknown valence [19]. Abbreviations: DTAM = dorsal tegmental area of medulla; OB = olfactory bulb; OT = optic tectum; T = thalamus; TEL = telencephalon; n.IX-X = motor nucleus IX-X; N.IX-X = cranial nerve IX-X; GM = glottal motoneuron; LM = laryngeal motoneuron; IX-XDTAM = DTAM-projecting n.IX-X interneuron; IX-XIX-X = n.IX-X-projecting commissural interneuron; DTAMIX-X = n.IX-X-projecting DTAM neuron.
A recent tract-tracing study has begun to identify specific neuronal types in the vocal circuit [18]. In n.IX-X, four neuron types were described: glottal (respiratory) and laryngeal (vocal) motoneurons, commissural (IX-XIX-X) neurons that connect the nuclei bilaterally and DTAM-projecting (IX-XDTAM) neurons with ascending inputs to DTAM (Figure 3b). In DTAM, three types of neurons were identified based on the patterns of projections to n.IX-X: ones that project ipsilaterally, contralaterally or bilaterally to n.IX-X (DTAMIX-X neurons). Double-labeling experiments showed that DTAMIX-X neurons project to all four identified neuron types in n.IX-X. Together, these results suggest that the robust reciprocal connection between n.IX-X and DTAM might be a vital feature in generating vocal patterns in Xenopus.
Where is the vocal CPG in Xenopus? Progressive transections using fictive preparation revealed that the brainstem contains the vocal CPG; isolated brainstems generate fictive advertisement calls in response to serotonin [3] (H.J. Rhodes and E.Z., unpublished). Motoneurons are not rhythmogenic themselves; intracellular recordings of laryngeal motoneurons during fictive calling showed that they are driven by temporally patterned synaptic inputs that are highly similar to motor output (E.Z., unpublished). Thus, the vocal CPG is upstream of the motoneurons. Two major candidates are the premotor neurons in DTAM and interneurons in n.IX-X. The importance of the two nuclei in vocal production was originally demonstrated in leopard frog, Rana pipiens; lesioning each nucleus abolished calling, and electrical stimulation of DTAM (referred to as pre-trigeminal nucleus) activated vocalizations in vivo [21]. Based on lesioning and transection experiments, Schmidt proposed that the nuclei are components of a two-part vocal CPG [22].
In Xenopus, the importance of DTAM in fictive advertisement calling is also apparent. When DTAM is removed bilaterally, 5-HT-induced fictive advertisement calls are entirely abolished [3]. Further, electrically stimulating DTAM evokes fictive fast trills in the absence of 5-HT [3]. Thus, DTAM is necessary for fictive advertisement calls, and might be specialized for generating the fast trill component (see below). The importance of n.IX-X interneurons, by contrast, has not been systematically examined to date. However, robust anatomical connections make it likely that DTAM and n.IX-X cooperate to generate advertisement calls in Xenopus, as they do in R. pipiens.
There is considerable evidence indicating that separate oscillatory networks generate the slow and fast trills of male advertisement calls. First, as mentioned above, electrical stimulation of DTAM triggers fast trills, but not slow trills, in male brains [3]. Second, bisecting the rostral brainstem – severing connections between left and right DTAM nuclei as well as decussating projections from DTAM to the contralateral motor nuclei [18] – degrades the fast trill whereas the slow trill remains intact [3]. Third, selective cooling of DTAM using cryoprobes modifies fast trill rates without affecting slow trill rates (A.Y., unpublished). Thus, separate neuronal populations might establish the slow and fast trills in males; DTAM is likely to contain the fast trill circuit. Interestingly, to evoke fast trills, electrical stimulation to DTAM must be delivered at rates that resemble slow trills (20–40 Hz); slower or faster stimulus trains failed to activate fictive fast trills. This observation suggests that a slow trill oscillator is strongly linked to, and might gate, the fast trill network in DTAM. Dense projections from n.IX-X to DTAM [18] seem to be ideal for this purpose, raising the possibility that the slow trill generator might reside in n.IX-X.
The ticking oscillator is also localized in the brainstem and includes DTAM; isolated male and female brainstems generate ticking in response to 5-HT, and bilateral removal of DTAM abolishes 5-HT-induced fictive ticking in both sexes [3] (A.Y., unpublished). Thus, ticking and advertisement call networks share the same premotor nucleus.
Androgen-induced masculinization of the vocal CPG
Steroid hormones exert their effect on target tissues either by modifying gene transcription (genomic effects) or by rapidly modifying cellular function by acting on membrane receptors (nongenomic effects [23]). Although androgens can rapidly alter Xenopus vocal pathways via nongenomic effects, as recently demonstrated in the vocal pathways of the midshipman fish [24,25], such short-term effects have yet to be explored. In this section, we review the long-term process of androgen-induced modulation of the vocal CPG, most likely mediated by genomic action.
Androgen receptors are found in laryngeal muscles [26,27] and in central vocal neurons [28]. Accordingly, masculinization can happen independently in each tissue at different rates and extents. One observation suggests that the CPG is masculinized faster and more completely than the larynx. Using TF isolated brains, Rhodes et al. [3] showed that the fictive nerve activity of an 8 week TF was more fully masculinized, in terms of CAP rates, than the actual behavior. The fully masculinized neuronal signal – alternating fast and slow CAP trills – generated by the vocal CPG could be low-pass filtered by an incompletely masculinized larynx – the muscle can tetanize during the fast portion, resulting in failure to produce clicks and periodic silent periods (Figure 4). This indicates that the vocal CPG is more ‘plastic’ and masculinizes more rapidly than the larynx.
Figure 4.
Peripheral constraints on vocal masculinization. Top: vocal recordings from a female after 8 weeks of testosterone exposure. Bottom: fictive recordings from the brain of the same animal. Slow and fast trill segments of vocal and fictive recordings are marked. The fictive call resembles male advertisement calls more closely than the sound produced in vivo, which shows an abnormal pause between slow and fast trills. The vocal CPG is likely to be more ‘plastic’ and masculinizes more rapidly than the larynx. Consequently, the fully masculinized neuronal signal generated by the vocal CPG could be low-pass filtered by an incompletely masculinized larynx. Figure reproduced, with permission, from [3], (2007) Society for Neuroscience.
An obvious question that arises from masculinization of the vocal CPG is the following. What elements of the CPG change in response to androgen? Androgen regulates several sexually distinct traits in the vocal circuit during development. For example, the number of neurons in the motor nucleus is larger [29–31], dendritic arborizations of n.IX-X neurons are more extensive [32] and motoneuron somata are larger in males than in females [11]. Functionally, laryngeal motoneurons are also sexually differentiated: for example, the hyperpolarization-activated inward current (Ih) is almost exclusively expressed in male motoneurons, and a low-threshold potassium conductance is more prominent in male than in female motoneurons [33]. Interestingly, vocal masculinization is not accompanied by complete morphological and physiological masculinization of the central vocal pathways. For instance, the number of motoneurons does not change even after extensive treatment with androgen in adulthood [30], whereas somata sizes become fully masculinized in as little as 1 week [11]. Non-masculinized characteristics such as motoneuron number are probably not necessary for the generation of advertisement calls. Instead, these features might be important for other traits unique to males, such as the production of other male-specific call types (e.g. growling) or the vocal endurance required to generate over 40 bouts of advertisement calls at a time without pause, neither of which has been observed in masculinized females. Identification of androgen-induced neuronal modifications that parallel vocal masculinization will lead to an understanding of key neural elements that underlie rhythm generation of advertisement calls.
Sexually differentiated CPGs
In vitro fictive studies indicate that the vocal CPG in females consists of a single network whereas the male CPG has two (or more) distinct components – one unique to males (advertisement call), and one shared with females (ticking). The ticking network is likely the default network, because juveniles and adults of both sexes share the call [8]. The CPG network generating advertisement calls, by contrast, seems to be a derived circuit added to the vocal pathways in response to androgen; increased androgen levels during development in males and exogenous application of androgen in juvenile or adult females both lead to the acquisition of advertisement calls. In this section, we will speculate on how ticking and advertisement call components of the CPG coexist in male and TF brains.
As discussed earlier, DTAM is essential for the production of both fictive ticking and advertisement calls. This means that in male and TF brains, the two oscillators are overlapping at the nucleus level. It is unclear, however, whether the same DTAM neuron population generates both calls, or whether separate populations are dedicated for each call. These two types of CPG organization, reorganizing (multifunctional) circuitry and dedicated circuitry, originally proposed by Morton and Chiel [34], are common architectural strategies employed by vertebrates and invertebrates in producing multiple motor patterns [35–38]. Although we do not have an answer to the question, the consequences of the two organizational schemes are quite different in TFs. If the TF CPG becomes multifunctional in response to androgen, then we expect that some neurons included in an existing ticking network undergo modifications and rewiring to execute new motor rhythms. If TFs acquire a dedicated CPG for advertisement calls de novo, then we expect that new circuits will be built, either by recruiting new neurons through neurogenesis, or latent neurons from existing pathways serving as a dedicated advertisement call CPG. Most likely, the new CPG will consist of two overlapping networks in which some neurons are dedicated to one pattern, whereas other neurons contribute to the production of both patterns, as seen in other systems (e.g. Ref. [36]). Analyses of single-neuron activity during ongoing rhythms (now possible with the fictive preparation) will allow us to answer this question in Xenopus males and TFs.
Although vocal masculinization induced by exogenous androgen in adult females might not be identical to male masculinization during development, the brevity of the masculinization process in adult females makes it more amenable to experimental procedures. Neuronal subtypes have been identified (for example, IX-XIX-X, IX-XDTAM and DTAMIX-X) that can be substrates for androgen-mediated transformation of vocal CPGs, although additional neurons undoubtedly await discovery. The fictive preparation affords easy access to vocal nuclei during physiological and imaging techniques, thus providing the perfect arena for this endeavor.
Conclusion
Acquisition of a novel behavioral repertoire during development is universal, but its neural basis is poorly understood. For example, most male vertebrates and invertebrates acquire courtship behavior when they reach sexual maturity, and all metabolous animals express new sets of behavioral patterns after metamorphosis. Understanding the neural processes that underlie androgen-induced vocal masculinization might shed light on the fundamental principles of the introduction of novel behavioral repertoires.
The Xenopus vocal system is a unique model for studying the neural basis of behavior. Of keen interest will be future research focused on understanding androgen-induced changes that take place at cellular, synaptic and network levels that underlie functional modifications. With the use of electrophysiological, anatomical and optical imaging techniques in fictive preparations, it should be possible to determine (i) the patterns of neuronal activity underlying two call patterns, (ii) whether networks are multifunctional or dedicated and (iii) how morphological and physiological properties of neurons and synapses are changed in response to androgen. Thus, by characterizing neural transformation that underlies functional modification of well-defined behavioral pathways, we can begin to understand how neural networks generate behavioral variation.
Acknowledgments
We thank Darcy Kelley and Matt Wachowiak for critical reading of the manuscript. The authors' research and preparation of this manuscript have been supported by National Institute of Neurological Disorders and Stroke grant RO1 NS048834, a Clare Boothe Luce Professorship and startup funds provided by Boston University (A.Y.).
References
- 1.Kelley DB. Generating sexually differentiated songs. Curr Opin Neurobiol. 1997;7:839–843. doi: 10.1016/s0959-4388(97)80144-4. [DOI] [PubMed] [Google Scholar]
- 2.Yager D. A unique sound production mechanism in the pipid anuran Xenopus. Zool J Linn Soc. 1992;104:351–375. [Google Scholar]
- 3.Rhodes HJ, et al. Xenopus vocalizations are controlled by a sexually differentiated hindbrain central pattern generator. J Neurosci. 2007;27:1485–1497. doi: 10.1523/JNEUROSCI.4720-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tobias ML, et al. Vocal communication between male Xenopus laevis. Anim Behav. 2004;67:353–365. doi: 10.1016/j.anbehav.2003.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tobias ML, et al. Rapping, a female receptive call, initiates male-female duets in the South African clawed frog. Proc Natl Acad Sci U S A. 1998;95:1870–1875. doi: 10.1073/pnas.95.4.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wetzel DM, Kelley DB. Androgen and gonadotropin effects on male mate calls in South African clawed frogs. Xenopus laevis. Horm Behav. 1983;17:388–404. doi: 10.1016/0018-506x(83)90048-x. [DOI] [PubMed] [Google Scholar]
- 7.Kelley DB, Pfaff DW. Hormone effects on male sex behavior in adult South African clawed frogs. Xenopus laevis Horm Behav. 1976;7:159–182. doi: 10.1016/0018-506x(76)90045-3. [DOI] [PubMed] [Google Scholar]
- 8.Oberlander J, et al. Sexual differentiation of vocal motoneurons in development of Xenopus laevis. Soc Neurosci Abstr. 2004 program no. 672.6. [Google Scholar]
- 9.Kang L, et al. Androgen biosynthesis and secretion in developing Xenopus laevis. Gen Comp Endocrinol. 1995;100:293–307. doi: 10.1006/gcen.1995.1160. [DOI] [PubMed] [Google Scholar]
- 10.Hannigan P, Kelley DB. Androgen-induced alterations in vocalizations of female Xenopus laevis: modifiability and constraints. J Comp Physiol [A] 1986;158:517–527. doi: 10.1007/BF00603797. [DOI] [PubMed] [Google Scholar]
- 11.Potter KA, et al. Androgen-induced vocal transformation in adult female African clawed frogs. J Neurophysiol. 2005;94:415–428. doi: 10.1152/jn.01279.2004. [DOI] [PubMed] [Google Scholar]
- 12.Yamaguchi A, Kelley DB. Generating sexually differentiated vocal patterns: laryngeal nerve and EMG recordings from vocalizing male and female African clawed frogs (Xenopus laevis) J Neurosci. 2000;20:1559–1567. doi: 10.1523/JNEUROSCI.20-04-01559.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelley DB. Auditory and vocal nuclei in the frog brain concentrate sex hormones. Science. 1980;207:553–555. doi: 10.1126/science.7352269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wetzel DM, et al. A proposed neural pathway for vocalization in South African clawed frogs, Xenopus laevis. J Comp Physiol [A] 1985;157:749–761. doi: 10.1007/BF01350072. [DOI] [PubMed] [Google Scholar]
- 15.Brahic CJ, Kelley DB. Vocal circuitry in Xenopus laevis: telencephalon to laryngeal motor neurons. J Comp Neurol. 2003;464:115–130. doi: 10.1002/cne.10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jurgens U. Neural pathways underlying vocal control. Neurosci Biobehav Rev. 2002;26:235–258. doi: 10.1016/s0149-7634(01)00068-9. [DOI] [PubMed] [Google Scholar]
- 17.Smotherman M, et al. A mechanism for vocal-respiratory coupling in the mammalian parabrachial nucleus. J Neurosci. 2006;26:4860–4869. doi: 10.1523/JNEUROSCI.4607-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zornik E, Kelley DB. Breathing and calling: neuronal networks in the Xenopus laevis hindbrain. J Comp Neurol. 2007;501:303–315. doi: 10.1002/cne.21145. [DOI] [PubMed] [Google Scholar]
- 19.Zornik E, Kelley DB. Regulation of respiratory and vocal motor pools in the isolated brain of Xenopus laevis. J Neurosci. 2008;28:612–621. doi: 10.1523/JNEUROSCI.4754-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreno N, Gonzalez A. Central amygdala in anuran amphibians: neurochemical organization and connectivity. J Comp Neurol. 2005;489:69–91. doi: 10.1002/cne.20611. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt RS. Central mechanisms of frog calling. Behaviour. 1966;26:251–285. doi: 10.1163/156853965x00228. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt RS. Neural correlates of frog calling: production by two semi-independent generators. Behav Brain Res. 1992;50:17–30. doi: 10.1016/s0166-4328(05)80284-0. [DOI] [PubMed] [Google Scholar]
- 23.Michels G, Hoppe UC. Rapid actions of androgens. Front Neuroendocrinol. 2007;29:182–198. doi: 10.1016/j.yfrne.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Remage-Healey L, Bass AH. Rapid, hierarchical modulation of vocal patterning by steroid hormones. J Neurosci. 2004;24:5892–5900. doi: 10.1523/JNEUROSCI.1220-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Remage-Healey L, Bass AH. Plasticity in brain sexuality is revealed by the rapid actions of steroid hormones. J Neurosci. 2007;27:1114–1122. doi: 10.1523/JNEUROSCI.4282-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelley D, et al. Development and hormone regulation of androgen receptor levels in the sexually dimorphic larynx of Xenopus laevis. Dev Biol. 1989;131:111–118. doi: 10.1016/s0012-1606(89)80042-9. [DOI] [PubMed] [Google Scholar]
- 27.Fischer LM, et al. Androgen-directed development of the Xenopus laevis larynx: control of androgen receptor expression and tissue differentiation. Dev Biol. 1995;170:115–126. doi: 10.1006/dbio.1995.1200. [DOI] [PubMed] [Google Scholar]
- 28.Perez J, et al. Androgen receptor mRNA expression in Xenopus laevis CNS: sexual dimorphism and regulation in laryngeal motor nucleus. J Neurobiol. 1996;30:556–568. doi: 10.1002/(SICI)1097-4695(199608)30:4<556::AID-NEU10>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 29.Kelley DB, Dennison J. The vocal motor neurons of Xenopus laevis: development of sex differences in axon number. J Neurobiol. 1990;21:869–882. doi: 10.1002/neu.480210605. [DOI] [PubMed] [Google Scholar]
- 30.Watson JT, et al. Laryngeal muscle and motor neuron plasticity in Xenopus laevis: testicular masculinization of a developing neuromuscular system. J Neurobiol. 1993;24:1615–1625. doi: 10.1002/neu.480241206. [DOI] [PubMed] [Google Scholar]
- 31.Kay JN, et al. Trophic effects of androgen: development and hormonal regulation of neuron number in a sexually dimorphic vocal motor nucleus. J Neurobiol. 1999;40:375–385. [PubMed] [Google Scholar]
- 32.Kelley DB, et al. Sex differences in the motor nucleus of cranial nerve IX-X in Xenopus laevis: a quantitative Golgi study. J Neurobiol. 1988;19:413–429. doi: 10.1002/neu.480190503. [DOI] [PubMed] [Google Scholar]
- 33.Yamaguchi A, et al. Functional specialization of male and female vocal motoneurons. J Neurosci. 2003;23:11568–11576. doi: 10.1523/JNEUROSCI.23-37-11568.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morton DW, Chiel HJ. Neural architectures for adaptive behavior. Trends Neurosci. 1994;17:413–420. doi: 10.1016/0166-2236(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 35.Ritter DA, et al. In vivo imaging of zebrafish reveals differences in the spinal networks for escape and swimming movements. J Neurosci. 2001;21:8956–8965. doi: 10.1523/JNEUROSCI.21-22-08956.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Briggman KL, Kristan WB., Jr Imaging dedicated and multifunctional neural circuits generating distinct behaviors. J Neurosci. 2006;26:10925–10933. doi: 10.1523/JNEUROSCI.3265-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nusbaum MP, et al. The roles of co-transmission in neural network modulation. Trends Neurosci. 2001;24:146–154. doi: 10.1016/s0166-2236(00)01723-9. [DOI] [PubMed] [Google Scholar]
- 38.Lieske SP, et al. Reconfiguration of the neural network controlling multiple breathing patterns: eupnea, sighs and gasps. Nat Neurosci. 2000;3:600–607. doi: 10.1038/75776. [DOI] [PubMed] [Google Scholar]
- 39.Marder E, Calabrese RL. Principles of rhythmic motor pattern generation. Physiol Rev. 1996;76:687–717. doi: 10.1152/physrev.1996.76.3.687. [DOI] [PubMed] [Google Scholar]
- 40.Sherrington CS. Flexion-reflex of the limb, crossed extension-reflex, and reflex stepping and standing. J Physiol. 1910;40:28–121. doi: 10.1113/jphysiol.1910.sp001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Graham-Brown T. The intrinsic factors in the act of progression in the mammal. Proc R Soc Lond B Biol Sci. 1911;84:308–319. [Google Scholar]
- 42.Wilson D. The central nervous control of locust flight. J Exp Biol. 1961;38:471–490. [Google Scholar]
- 43.Marder E, et al. Invertebrate central pattern generation moves along. Curr Biol. 2005;15:R685–R699. doi: 10.1016/j.cub.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 44.Grillner S. The motor infrastructure: from ion channels to neuronal networks. Nat Rev Neurosci. 2003;4:573–586. doi: 10.1038/nrn1137. [DOI] [PubMed] [Google Scholar]
- 45.Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lieske SP, Ramirez JM. Pattern-specific synaptic mechanisms in a multifunctional network. II. Intrinsic modulation by metabotropic glutamate receptors. J Neurophysiol. 2006;95:1334–1344. doi: 10.1152/jn.00506.2004. [DOI] [PubMed] [Google Scholar]
- 47.Lieske SP, Ramirez JM. Pattern-specific synaptic mechanisms in a multifunctional network. I. Effects of alterations in synapse strength. J Neurophysiol. 2006;95:1323–1333. doi: 10.1152/jn.00505.2004. [DOI] [PubMed] [Google Scholar]
- 48.Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci. 2006;29:279–306. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- 49.Bass AH, Remage-Healey L. Central pattern generators for social vocalization: androgen-dependent neurophysiological mechanisms. Horm Behav. 2008 doi: 10.1016/j.yhbeh.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuste R, et al. The cortex as a central pattern generator. Nat Rev Neurosci. 2005;6:477–483. doi: 10.1038/nrn1686. [DOI] [PubMed] [Google Scholar]
- 51.Grillner S, et al. Microcircuits in action – from CPGs to neocortex. Trends Neurosci. 2005;28:525–533. doi: 10.1016/j.tins.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 52.Fetcho JR, et al. Zebrafish and motor control over the last decade. Brain Res Rev. 2008;57:86–93. doi: 10.1016/j.brainresrev.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Newcomb JM, Katz PS. Homologues of serotonergic central pattern generator neurons in related nudibranch molluscs with divergent behaviors. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2007;193:425–443. doi: 10.1007/s00359-006-0196-4. [DOI] [PubMed] [Google Scholar]
- 54.Vignal C, Kelley D. Significance of temporal and spectral acoustic cues for sexual recognition in Xenopus laevis. Proc Biol Sci. 2007;274:479–488. doi: 10.1098/rspb.2006.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]


