Abstract
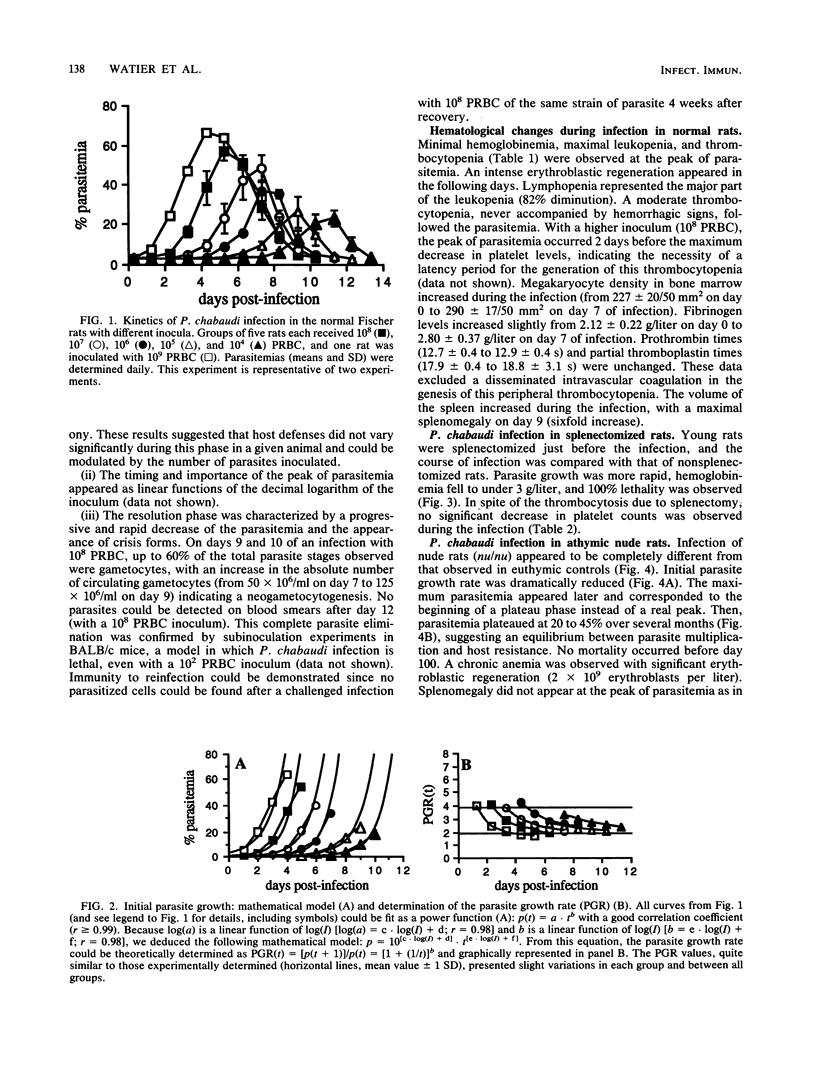
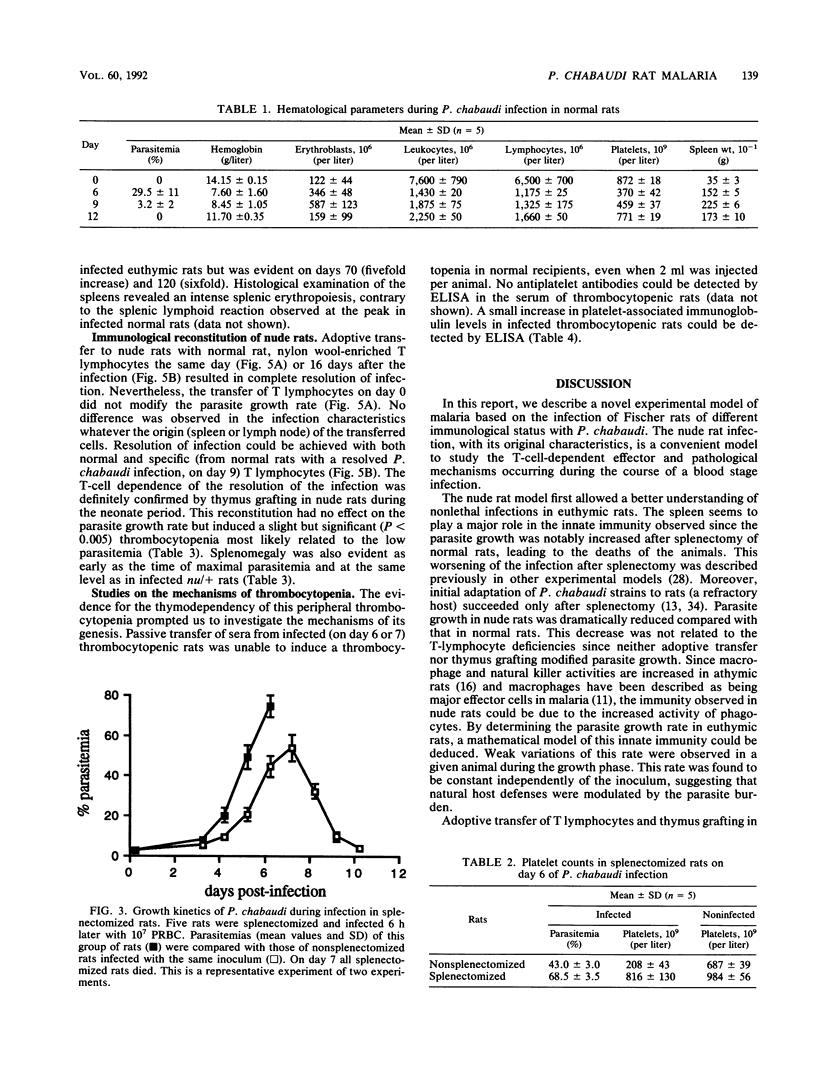
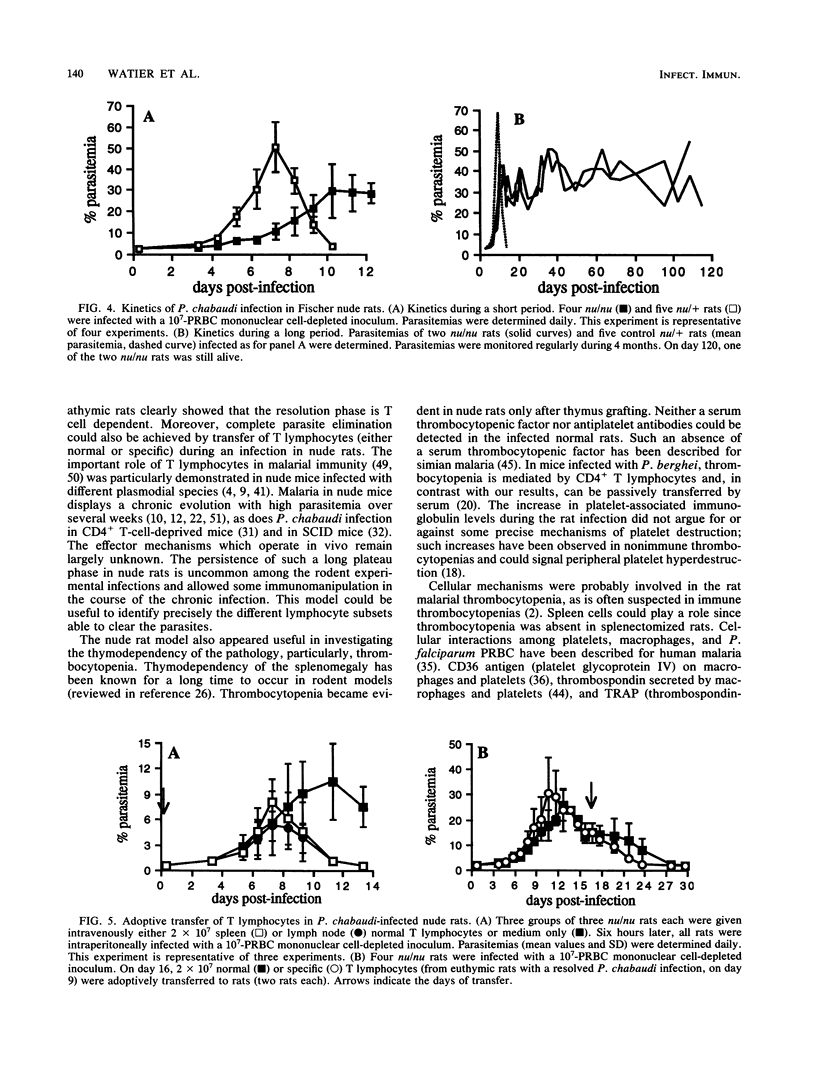
Normal, splenectomized, and athymic Fischer rats were infected with Plasmodium chabaudi. In normal rat infections, acute-phase infection resolved rapidly and completely. In splenectomized rats, infection resulted in high parasitemia and ultimately death. In nude rats, parasite growth was reduced compared with normal rats, and a persistent parasitemia (between 20 and 45%) was observed for several months. Complete resolution of the infection was achieved after adoptive transfer of T lymphocytes, even when transfer occurred during the course of infection. These results indicated that an acquired, T-lymphocyte-dependent immunity was necessary for the complete recovery observed in normal rats. In normal rats, thrombocytopenia and splenomegaly occurred during infection. By contrast, in nude rats, both of these pathological manifestations were only observed after thymus grafting. Thrombocytopenia was also absent in the splenectomized animals. Despite an increase in platelet-associated immunoglobulin levels during the infection, thrombocytopenia was not transferred by injection of infected rat serum to normal recipients. It has been concluded that the nude rat infection can be regarded as a novel and useful model for studying the T-cell-dependent effector and pathological mechanisms and to investigate the anti-P. chabaudi immune response.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asch A. S., Barnwell J., Silverstein R. L., Nachman R. L. Isolation of the thrombospondin membrane receptor. J Clin Invest. 1987 Apr;79(4):1054–1061. doi: 10.1172/JCI112918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auriault C., Damonneville M., Pancré V., Capron A. Effector functions of platelets in parasitic diseases and their regulation by cytokines. Diagn Microbiol Infect Dis. 1990 Sep-Oct;13(5):423–427. doi: 10.1016/0732-8893(90)90013-l. [DOI] [PubMed] [Google Scholar]
- Brake D. A., Long C. A., Weidanz W. P. Adoptive protection against Plasmodium chabaudi adami malaria in athymic nude mice by a cloned T cell line. J Immunol. 1988 Mar 15;140(6):1989–1993. [PubMed] [Google Scholar]
- Brandt S. J., Bodine D. M., Dunbar C. E., Nienhuis A. W. Dysregulated interleukin 6 expression produces a syndrome resembling Castleman's disease in mice. J Clin Invest. 1990 Aug;86(2):592–599. doi: 10.1172/JCI114749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown I. N., Allison A. C., Taylor R. B. Plasmodium berghei infections in thymectomized rats. Nature. 1968 Jul 20;219(5151):292–293. doi: 10.1038/219292a0. [DOI] [PubMed] [Google Scholar]
- Brown K. N. The parasitology of malaria and the study of protective immunity. Immunol Lett. 1990 Aug;25(1-3):97–99. doi: 10.1016/0165-2478(90)90098-b. [DOI] [PubMed] [Google Scholar]
- Carrington P. A., Hill R. J., Stenberg P. E., Levin J., Corash L., Schreurs J., Baker G., Levin F. C. Multiple in vivo effects of interleukin-3 and interleukin-6 on murine megakaryocytopoiesis. Blood. 1991 Jan 1;77(1):34–41. [PubMed] [Google Scholar]
- Cavacini L. A., Long C. A., Weidanz W. P. T-cell immunity in murine malaria: adoptive transfer of resistance to Plasmodium chabaudi adami in nude mice with splenic T cells. Infect Immun. 1986 Jun;52(3):637–643. doi: 10.1128/iai.52.3.637-643.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavacini L. A., Parke L. A., Weidanz W. P. Resolution of acute malarial infections by T cell-dependent non-antibody-mediated mechanisms of immunity. Infect Immun. 1990 Sep;58(9):2946–2950. doi: 10.1128/iai.58.9.2946-2950.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I. A., Allison A. C. Babesia microti and Plasmodium berghei yoelii infections in nude mice. Nature. 1974 Nov 22;252(5481):328–329. doi: 10.1038/252328a0. [DOI] [PubMed] [Google Scholar]
- Clark I. A. Cell-mediated immunity in protection and pathology of malaria. Parasitol Today. 1987 Oct;3(10):300–305. doi: 10.1016/0169-4758(87)90187-6. [DOI] [PubMed] [Google Scholar]
- Coombs G. H., Gutteridge W. E. Growth in vitro and metabolism of Plasmodium vinckei chabaudi. J Protozool. 1975 Nov;22(4):555–560. doi: 10.1111/j.1550-7408.1975.tb05232.x. [DOI] [PubMed] [Google Scholar]
- Cox J., Semoff S., Hommel M. Plasmodium chabaudi: a rodent malaria model for in-vivo and in-vitro cytoadherence of malaria parasites in the absence of knobs. Parasite Immunol. 1987 Sep;9(5):543–561. doi: 10.1111/j.1365-3024.1987.tb00529.x. [DOI] [PubMed] [Google Scholar]
- Duquesne V., Auriault C., Darcy F., Decavel J. P., Capron A. Protection of nude rats against Toxoplasma infection by excreted-secreted antigen-specific helper T cells. Infect Immun. 1990 Jul;58(7):2120–2126. doi: 10.1128/iai.58.7.2120-2126.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier W. A. Thrombospondin: a modular adhesive glycoprotein of platelets and nucleated cells. J Cell Biol. 1987 Aug;105(2):625–632. doi: 10.1083/jcb.105.2.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George J. N. Platelet immunoglobulin G: its significance for the evaluation of thrombocytopenia and for understanding the origin of alpha-granule proteins. Blood. 1990 Sep 1;76(5):859–870. [PubMed] [Google Scholar]
- Grau G. E., Frei K., Piguet P. F., Fontana A., Heremans H., Billiau A., Vassalli P., Lambert P. H. Interleukin 6 production in experimental cerebral malaria: modulation by anticytokine antibodies and possible role in hypergammaglobulinemia. J Exp Med. 1990 Nov 1;172(5):1505–1508. doi: 10.1084/jem.172.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau G. E., Piguet P. F., Gretener D., Vesin C., Lambert P. H. Immunopathology of thrombocytopenia in experimental malaria. Immunology. 1988 Dec;65(4):501–506. [PMC free article] [PubMed] [Google Scholar]
- Grau G. E., Piguet P. F., Vassalli P., Lambert P. H. Tumor-necrosis factor and other cytokines in cerebral malaria: experimental and clinical data. Immunol Rev. 1989 Dec;112:49–70. doi: 10.1111/j.1600-065x.1989.tb00552.x. [DOI] [PubMed] [Google Scholar]
- Grun J. L., Weidanz W. P. Immunity to Plasmodium chabaudi adami in the B-cell-deficient mouse. Nature. 1981 Mar 12;290(5802):143–145. doi: 10.1038/290143a0. [DOI] [PubMed] [Google Scholar]
- Ho M., Webster H. K. Immunology of human malaria. A cellular perspective. Parasite Immunol. 1989 Mar;11(2):105–116. doi: 10.1111/j.1365-3024.1989.tb00652.x. [DOI] [PubMed] [Google Scholar]
- Horstmann R. D., Dietrich M., Bienzle U., Rasche H. Malaria-induced thrombocytopenia. Blut. 1981 Mar;42(3):157–164. doi: 10.1007/BF01026385. [DOI] [PubMed] [Google Scholar]
- Inyang A. L., Sodeinde O., Okpako D. T., Essien E. M. Platelet reactions after interaction with cultured Plasmodium falciparum infected erythrocytes. Br J Haematol. 1987 Jul;66(3):375–378. doi: 10.1111/j.1365-2141.1987.tb06926.x. [DOI] [PubMed] [Google Scholar]
- Kelton J. G., Keystone J., Moore J., Denomme G., Tozman E., Glynn M., Neame P. B., Gauldie J., Jensen J. Immune-mediated thrombocytopenia of malaria. J Clin Invest. 1983 Apr;71(4):832–836. doi: 10.1172/JCI110836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzrock R., Quesada J. R., Talpaz M., Hersh E. M., Reuben J. M., Sherwin S. A., Gutterman J. U. Phase I study of multiple dose intramuscularly administered recombinant gamma interferon. J Clin Oncol. 1986 Jul;4(7):1101–1109. doi: 10.1200/JCO.1986.4.7.1101. [DOI] [PubMed] [Google Scholar]
- Langhorne J., Meding S. J., Eichmann K., Gillard S. S. The response of CD4+ T cells to Plasmodium chabaudi chabaudi. Immunol Rev. 1989 Dec;112:71–94. doi: 10.1111/j.1600-065x.1989.tb00553.x. [DOI] [PubMed] [Google Scholar]
- Meding S. J., Langhorne J. CD4+ T cells and B cells are necessary for the transfer of protective immunity to Plasmodium chabaudi chabaudi. Eur J Immunol. 1991 Jun;21(6):1433–1438. doi: 10.1002/eji.1830210616. [DOI] [PubMed] [Google Scholar]
- Musoke A. J., Cox H. W. Experimental infection with Plasmodium chabaudi in rats. Observations on adaptation and the immune responses to infection. J Parasitol. 1977 Jun;63(3):464–470. [PubMed] [Google Scholar]
- Neva F. A., Sheagren J. N., Shulman N. R., Canfield C. J. Malaria: host-defense mechanisms and complications. Ann Intern Med. 1970 Aug;73(2):295–306. doi: 10.7326/0003-4819-73-2-295. [DOI] [PubMed] [Google Scholar]
- Ockenhouse C. F., Magowan C., Chulay J. D. Activation of monocytes and platelets by monoclonal antibodies or malaria-infected erythrocytes binding to the CD36 surface receptor in vitro. J Clin Invest. 1989 Aug;84(2):468–475. doi: 10.1172/JCI114188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockenhouse C. F., Tandon N. N., Magowan C., Jamieson G. A., Chulay J. D. Identification of a platelet membrane glycoprotein as a falciparum malaria sequestration receptor. Science. 1989 Mar 17;243(4897):1469–1471. doi: 10.1126/science.2467377. [DOI] [PubMed] [Google Scholar]
- Pancré V., Monté D., Delanoye A., Capron A., Auriault C. Interleukin-6 is the main mediator of the interaction between monocytes and platelets in the killing of Schistosoma mansoni. Eur Cytokine Netw. 1990 Mar-Apr;1(1):15–19. [PubMed] [Google Scholar]
- Phillips S. M., Bentley A. G., Linette G., Doughty B. L., Capron M. The immunologic response of congenitally athymic rats to Schistosoma mansoni infection. I. In vivo studies of resistance. J Immunol. 1983 Sep;131(3):1466–1474. [PubMed] [Google Scholar]
- Prater C. A., Plotkin J., Jaye D., Frazier W. A. The properdin-like type I repeats of human thrombospondin contain a cell attachment site. J Cell Biol. 1991 Mar;112(5):1031–1040. doi: 10.1083/jcb.112.5.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich K. A., George F. W., 4th, Law J. L., Martin W. J. Cell-adhesive motif in region II of malarial circumsporozoite protein. Science. 1990 Sep 28;249(4976):1574–1577. doi: 10.1126/science.2120774. [DOI] [PubMed] [Google Scholar]
- Roberts D. W., Rank R. G., Weidanz W. P., Finerty J. F. Prevention of recrudescent malaria in nude mice by thymic grafting or by treatment with hyperimmune serum. Infect Immun. 1977 Jun;16(3):821–826. doi: 10.1128/iai.16.3.821-826.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson K. J., Hall J. R., Jennings M. W., Harris T. J., Marsh K., Newbold C. I., Tate V. E., Weatherall D. J. A highly conserved amino-acid sequence in thrombospondin, properdin and in proteins from sporozoites and blood stages of a human malaria parasite. Nature. 1988 Sep 1;335(6185):79–82. doi: 10.1038/335079a0. [DOI] [PubMed] [Google Scholar]
- Rupin A., Gruel Y., Poumier-Gaschard P., Chassaigne M., Leroy J., Bardos P. Thrombocytopenia in systemic lupus erythematosus: association with antiplatelet and anticardiolipin antibodies. Clin Immunol Immunopathol. 1990 Jun;55(3):418–426. doi: 10.1016/0090-1229(90)90128-d. [DOI] [PubMed] [Google Scholar]
- Sherwood J. A., Roberts D. D., Spitalnik S. L., Lawler J. W., Miller L. H., Howard R. J. Falciparum malaria parasitized erythrocytes bind to a carboxy-terminal thrombospondin fragment and not the amino-terminal heparin-binding region. Mol Biochem Parasitol. 1990 May;40(2):173–181. doi: 10.1016/0166-6851(90)90039-o. [DOI] [PubMed] [Google Scholar]
- Stechschulte D. J. Plasmodium berghei infection in thymectomized rats. Proc Soc Exp Biol Med. 1969 Jul;131(3):748–752. doi: 10.3181/00379727-131-33968. [DOI] [PubMed] [Google Scholar]
- Warrell D. A. Pathophysiology of severe falciparum malaria in man. Parasitology. 1987;94 (Suppl):S53–S76. doi: 10.1017/s0031182000085826. [DOI] [PubMed] [Google Scholar]
- Weidanz W. P., Long C. A. The role of T cells in immunity to malaria. Prog Allergy. 1988;41:215–252. [PubMed] [Google Scholar]
- Weidanz W. P., Melancon-Kaplan J., Cavacini L. A. Cell-mediated immunity to the asexual blood stages of malarial parasites: animal models. Immunol Lett. 1990 Aug;25(1-3):87–95. doi: 10.1016/0165-2478(90)90097-a. [DOI] [PubMed] [Google Scholar]
- Weinbaum F. I., Evans C. B., Tigelaar R. E. Immunity to Plasmodium Berghei yoelii in mice. I. The course of infection in T cell and B cell deficient mice. J Immunol. 1976 Nov;117(5 PT2):1999–2005. [PubMed] [Google Scholar]


