Abstract
Most cardiac Na+ channels open transiently within milliseconds upon membrane depolarization and are responsible for the excitation propagation. However, some channels remain active during hundreds of milliseconds, carrying the so-called persistent or late Na+ current (INaL) throughout the action potential plateau. INaL is produced by special gating modes of the cardiac-specific Na+ channel isoform. Experimental data accumulated over the past decade show the emerging importance of this late current component for the function of both normal and especially failing myocardium, where INaL is reportedly increased. Na+ channels represent a multi-protein complex and its activity is determined not only by the pore-forming α subunit but also by its auxiliary β subunits, cytoskeleton, and by Ca2+ signaling and trafficking proteins. Remodeling of this protein complex and intracellular signaling pathways may lead to alterations of INaL in pathological conditions. Increased INaL and the corresponding Na+ influx in failing myocardium contribute to abnormal repolarization and an increased cell Ca2+ load. Interventions designed to correct INaL rescue normal repolarization and improve Ca2+ handling and contractility of the failing cardiomyocytes. New therapeutic strategies to target both arrhythmias and deficient contractility in HF may not be limited to the selective inhibition of INaL but also include multiple indirect, modulatory (e.g. Ca2+- or cytoskeleton- dependent) mechanisms of INaL function.
Keywords: Late sodium current, heart failure, calcium, action potential, numerical model, sodium-calcium exchanger
INTRODUCTION
Chronic HF is associated with profound abnormalities in both cardiac rhythm and contractile function. Despite recent progress in treatment of congestive HF, mortality remains high. Approximately 40% of these patients die suddenly due to the sudden cardiac death syndrome. Ventricular tachycardia and fibrillation have been documented in ∼ 80% of patients with congestive HF in whom ECG Holter recordings were being obtained at the time of sudden death [1, 2].
Despite intensive research, the electrophysiological mechanisms leading to these arrhythmias are not completely clear. It is widely appreciated that perturbation in control of the action potential (AP) duration (APD) and its propagation, is the proximate cases of arrhythmia. Among numerous proteins involved in the cardiac cell remodeling in HF, the voltage-gated Na+ channels (NaCh) deserve special consideration, as they seem to be critically involved in abnormal conduction, repolarization, and Ca2+ handling [3, 4]. Most NaCh open only transiently and are quickly inactivated resulting in the peak transient current, INaT, which determines excitation and conduction. However, some NaCh remain active, carrying so-called persistent or late Na+ current (INaL) throughout the AP plateau (reviews [5, 6]).
A growing body of evidence shows that INaL provides a major contribution to the AP plateau duration in ventricular cardiomyocytes (VCs) in a variety of mammalian species including humans [7-11].
Since late openings of NaCh generate both electric current and Na+ influx during the AP plateau, INaL is expected to contribute to at least two known HF cellular mechanisms: 1) electrophysiological remodeling and 2) altered cell Na+ cycling. The latter mechanism is tightly integrated with Ca2+ cycling, as Na+ modulates the Na+/Ca2+ exchanger (NCX) operation [4]. These anticipated INaL contributions could be amplified at the state of chronic HF that reportedly increases the whole cell INaL [10, 12, 13].
The importance of INaL contribution into HF mechanisms has been demonstrated in experiments where “correction” of INaL in failing cardiomyocytes resulted in: 1) rescue of normal repolarization, 2) decrease beat-to-beat APD variability, 3) improvement of Ca2+ handling and contractility [10, 13-15]. Accordingly INaL has emerged as a novel possible target for cardioprotection to treat the failing heart [6, 16, 17].
COMPLEX IONIC MECHANISMS OF IMPAIRED REPOLARIZATION IN CHRONIC HEART FAILURE
Studies with microelectrodes in ventricular myocardial fibers obtained from failing human hearts [18, 19] and patch-clamped ventricular cardiomyocytes (VCs) isolated from failing hearts have demonstrated a prolongation of APD [10, 11, 20-22] indicating impaired repolarization in HF. The prolongation was less prominent at higher rates [18] and varied depending on the etiology of HF [22]. Experimental and clinical studies suggest that dispersion of repolarization and EADs are two major mechanisms underlying torsade de pointes [23, 24]. This type of arrhythmia is induced by a pause or bradycardia [25]. Indeed, the APD prolongation and dispersion of duration, as well as the incidence of EADs, were advanced at lower pacing rates in VCs of humans and dogs with chronic HF [10, 11, 14]. EADs, in turn, can be a substrate for triggered arrhythmias described in patients with HF [23, 26].
A delicate balance between inward and outward currents maintains the cardiac AP plateau, and repolarization occurs as activating outward currents prevail over inactivating inward currents. Accordingly, APD increase can be explained by reduction of hyperpolarizing outward currents and/or by an increase of depolarizing inward currents. The balance of ion currents is substantially altered in HF as a result of the remodeling of ion channels [3, 27]. Decrease of the transient outward potassium current (Ito) has been reported by many investigators and is now accepted as a common feature of ischemia and HF (review [3]). However, some studies also showed that Ito in human VCs shows no dramatic differences between cells derived from failing and non-failing hearts [28]. Remodeling of other potassium currents (IKr, IKs, and IK1) is also variable and seems to be related to the HF etiology (ischemic vs. non-ischemic)[27]. While numerous studies tested HF-related changes of L- type Ca2+ channel expression and function, the data remain controversial: ICaL was found decreased, unchanged or increased in HF. In addition, alterations in expression of the electrogenic Na+/Ca2+ exchanger (NCX) [29] and Ca2+ cycling by sarcoplasmic reticulum (SR) [4] contribute to the complex AP remodeling. Numerous pharmacological approaches targeting different players/contributors of abnormal electrical heart function have been tested to treat arrhythmias in HF, however the problem is still far from being solved. A novel perspective target could be INaL because this inward current greatly contributes to AP duration, especially in human myocardium [11, 13, 16], and it is reportedly increased by chronic HF [10, 12, 13].
DEFINITION AND MAJOR CHARACTERISTICS OF THE LATE SODIUM CURRENT
Voltage clamp studies have identified several types of single NaCh activity and whole cell Na+ currents that could contribute to APD in cardiomyocytes. The variety of NaCh activities identified until the present time was classified (see review [6]) in terms of the late (or persistent) Na+ current i.e. INaL (or IpNa), and background Na+ currents. Our review is focused on properties and targeting INaL rather than background Na+ currents. In contrast to INaL, background Na+ currents have been poorly characterized to date and have no clear molecular identity.
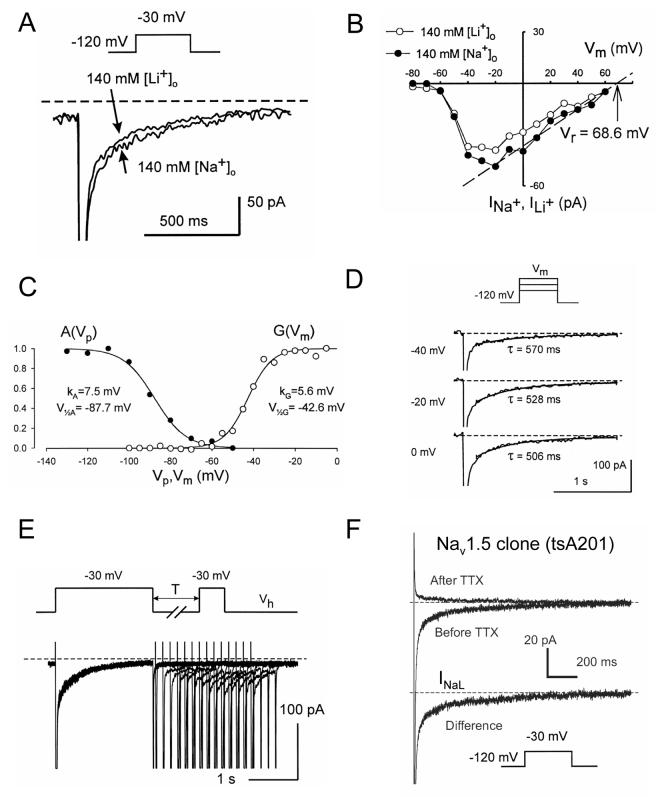
Major biophysical and pharmacological characteristics of the whole-cell INaL have been studied in great detail in human VCs by our research group [11, 13, 16] (Fig. (1)) and can be summarized as follows: 1) slow potential-independent inactivation and re-activation (∼0.5 s), 2) steady-state activation and inactivation similar to that for INaT, 3) low sensitivity to the specific toxins TTX and STX similar to the cardiac NaCh isoform Nav1.5. A slowly inactivating INaL with aforementioned biophysical characteristics has been identified in VCs of dogs [10, 13, 15, 30], guinea pigs [31-33], rabbits [34] and rats [35]. INaL is also produced by heterologously expressed cardiac NaCh isoform main α-subunit Nav1.5 (Fig. (1F)).
Figure (1).
Biophysical properties of the slowly inactivating, late Na+ current (INaL) evaluated by whole cell patch clamp in human ventricular cardiomyocytes (A-E) and human cloned Nav1.5 expressed in tsA201 cells (F). A-B: INaL can be carried either by Na+ or Li+. B: I-V relation for INaL. C: examples of steady-state activation and availability curves, G(Vm) and A(Vp), respectively. D: Examples of original traces illustrating voltage-independent INaL decay. E: slow reactivation of INaL. F: INaL produced by Nav1.5 was assessed as difference current before application of a selective Na+ channel blocker tetrodotoxin (TTX, 30 μM) and after TTX. Voltage protocols are shown at the traces. Recording was performed at 24°C. Modified from [11] (A-E) and [65](F), used with permission.
It is important to note, however, that INaL is not a “window” current that was suggested long time ago as a theoretical mechanism to explain the persistent Na+ current in cardiac cells [36, 37]. According to the Hodgkin-Huxley formalism [38], the “window” current is a non-inactivating component of INaT, resulting from the crossover of its steady-state activation and inactivation curves. The overlap of steady-state activation and inactivation curves occurs within a relatively narrow region of voltages close to INaT activation threshold (Fig 1C). However, INaL exhibit time-dependent inactivation and is present even at positive voltages (Fig.1B, filled circles, Fig. (3C)) [11, 31], where the calculated “window” current is negligible.
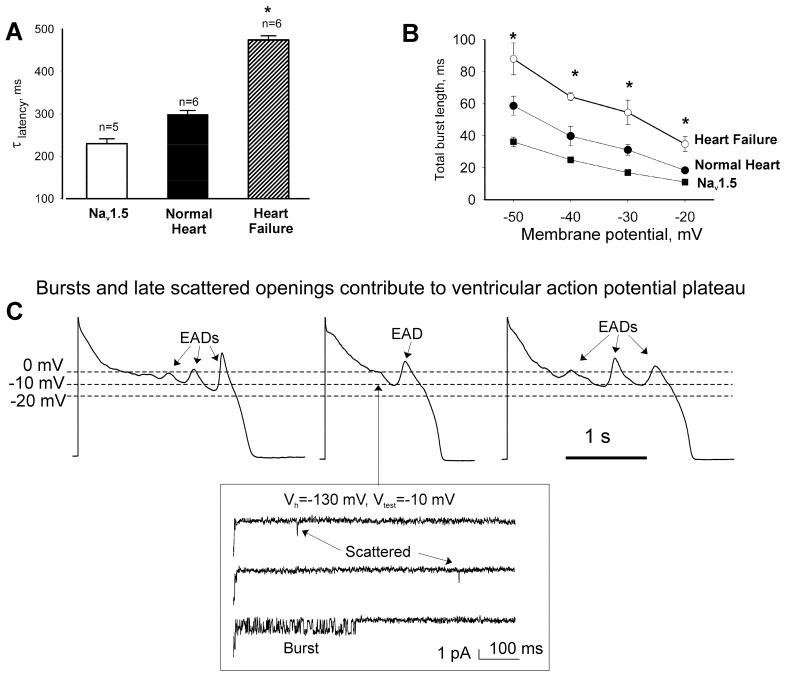
Figure (3).
Inactivation of both late scattered mode (A) or burst mode (B) of the late openings of Na+ channel was slowest in failing human cardiomyocytes compared with those from normal human hearts or heterologously expressed Nav1.5. *P<0.05, heart failure vs. normal heart or clone (Mean±SEM). Cell-attached patches, 24°C. (Adapted from [12]). C: recordings of action potentials in failing human cardiomyocytes are shown along with the late scattered mode and burst mode openings occurring at -10 mV, i.e. within the voltages of the action potential plateau. Adapted from [12, 40], used with permission.
INAL IS INCREASED IN FAILING HUMAN AND CANINE VENTRICULAR MYOCARDIUM
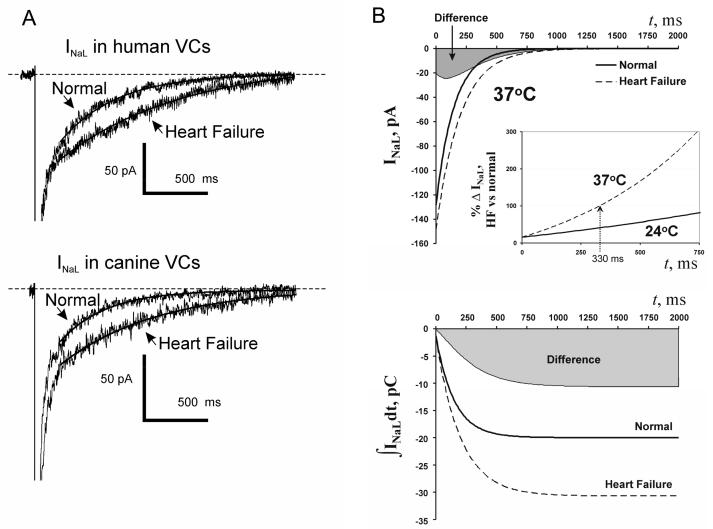
The first evidence of INaL importance in electrophysiological remodeling of cardiomyocytes in HF was found in 1995 in an experimental dog model of chronic HF [39]. Further patch clamp studies in human and canine hearts have conclusively shown that chronic HF increases INaL density and significantly slows inactivation kinetics of INaL in VCs [10, 13] (Fig. (2A)). Analysis of idealized INaL time course (Fig. (2B)) in canine normal and failing VCs shows that 1) absolute INaL difference between normal and failing canine VCs at 37°C (top panel in Fig. (2B), gray area) has a maximum at 90 ms after membrane depolarization, i.e. within the time of AP plateau duration; 2) the relative difference between normal and failing cells increases progressively with membrane depolarization, doubling after 330 ms (Fig. (2B), inset)); 3) The integral of INaL reflecting Na+ influx transferred by INaL, is much greater in HF (bottom panel in Fig. (2B), gray area) [13]. Accordingly, INaL may disturb cell Na+ balance and have different dynamic impacts on the balance of the ionic currents at the different phases of AP plateau in failing heart. The importance of these phenomena is discussed below.
Figure (2).
A: Chronic heart failure slows and increases INaL. A: examples of whole cell INaL recordings in human and dog ventricular cardiomyocytes. B: Idealized INaL and their integrals in normal and failing dog cardiomyocytes of same size (200 pF) calculated using Q10 factors (37°C) and average parameters of INaL density and decay time constant measured in normal and HF canine VCs. Larger and slower INaL in failing cardiomyocytes results in substantial increase in total charge (or Na+ influx) transfer by INaL. Gray areas illustrate difference between failing and normal cells. Adapted from [13], used with permission.
THE INaL FINE STRUCTURE AND RELATED SODIUM FLUXES: PARADOXES AND THEIR MECHANISMS
In cell-attached patches from heterologously expressed Nav1.5 and native human cardiomyocytes, the late NaCh activity is arranged in two major gating modes: late scattered mode (LSM) and “burst” mode (BM) (Fig. (3C) inset)). A detailed analysis of the properties of these modes and its contributions to whole cell INaL lead to some unexpected, paradoxical conclusions [12, 40]. Paradox #1: The late scattered openings were previously reported in guinea pig and considered to represent “background” activity [41]; however, they actually possess ultra slow (hundreds of ms) inactivation, similar to that of whole-cell current decay in human and dog cardiomyocytes [13, 40]. Paradox #2: While inactivation of INaT is voltage-dependent, the inactivation time constant of LSM is potential-independent. Paradox #3: Bursts are often considered as a persistent activity, but they do inactivate and cease well within AP duration (Fig.3B). Paradox #4: While bursts represent abundant NaCh activity, a numerical evaluation of the contributions of BM and LSM to INaL based on the Markov model of single channel data unexpectedly pointed to LSM openings, but not the bursts, as a major contributor to whole cell INaL. Paradox #5: While INaT amplitude is about 3 orders of magnitude larger than that of INaL (50 nA in comparison to 50 pA), the total charge (reflecting Na+ influx) transferred across the membrane by INaT and INaL is almost the same! In fact, INaT span is about 3 orders of magnitude shorter than that of INaL (2 ms in comparison to 2 s), resulting in an almost equal total charge transfer by these currents.
INaL transfers significantly more Na+ into failing vs. normal VCs. The total charge transferred by INaL is predicted to be 28.5 and 45 pC for normal and failing VCs, respectively, or a ∼58% increase. This model estimate is in line with a 53.6% HF-induced increase of Na+ influx via INaL evaluated from whole cell patch clamp measurements in canine normal and failing VCs (gray area in Fig. (2 B) bottom panel; [13]). Taking into account that INaT is reduced by 30-40% in HF VCs [42-44], the role of INaL in Na+ homeostasis should be even more substantial in the failing cells, in line with the recent finding that [Na]i is significantly increased in failing paced cardiomyocytes [45].
One possibility for the increased INaL in HF could be an additional, HF-specific NaCh activity. However, no qualitative changes in NaCh gating were found in failing human VCs in comparison to normal VCs and Nav1.5 clone [40]; they exhibit early openings and the two modes of late gating (LSM and BM) with the same single NaCh conductance [40]. The inconspicuous differences turn out to be quantitative: 1) inactivation of LSM (Fig. (3A)) was significantly slower and 2) burst length was significantly larger in failing compared to normal myocytes or Nav1.5 clone (Fig. 3B)) [13].
MOLECULAR IDENTITY OF INAL IN NORMAL AND FAILING VENTRICULAR MYOCARDIUM
The problem of molecular identity of INaL has been approached in many different ways including methods of biophysics, pharmacology, and molecular biology. Late NaCh openings show similar gating modes and Na+ conductance in normal human cardiomyocytes and in heterologously expressed Nav1.5. Furthermore, the heterologously expressed Nav1.5 produces the slowly inactivating whole cell INaL in tsA201 cells (Fig. (1F)) similar to that observed in human cardiomyocytes (Fig. (1A-D)). These data indicate the common molecular origin of the late channel openings i.e., they are produced by Nav1.5 in human cardiomyocytes. Dose-response curves for blockade of INaL by TTX and STX reveal only a single-site binding with the half-concentration that is to say typical for the Nav1.5 in both dog and human failing hearts (IC50 was 1.2 vs. 1.53 μM for TTX and 62 vs. 98 nM for STX comparing dog vs. human cardiomyocytes) [11, 13]. Furthermore, INaL is sensitive to Cd2+ (IC50=104 μM) [13], what is typical for cardiac but not neuronal Na+ channel isoforms [46]. Silencing the SCN5A gene responsible for Nav1.5 expression with siRNA decreases INaL by 75% in wide range of membrane potentials (including AP plateau), resulting in a significant reduction of AP duration and variability in dogs with chronic HF [47]. Thus, while contributions of other NaCh isoforms to INaL are still not excluded [17], Nav1.5 likely provides a major contribution to INaL in both normal and failing canine and human VCs.
MECHANISMS FOR INaL ALTERATIONS IN PATHOLOGICAL CONDITIONS: POSSIBLE ROLE OF THE CHANNEL MICROENVIRONMENT
Since results of multiple studies (described above) indicate that HF alters INaL likely via modulation(s) of Nav1.5 gating, the question of which specific modulatory mechanisms may underlie INaL alterations, deserve special consideration, because these might represent new indirect targets for INaL-related therapies.
NaCh and associated modulatory proteins
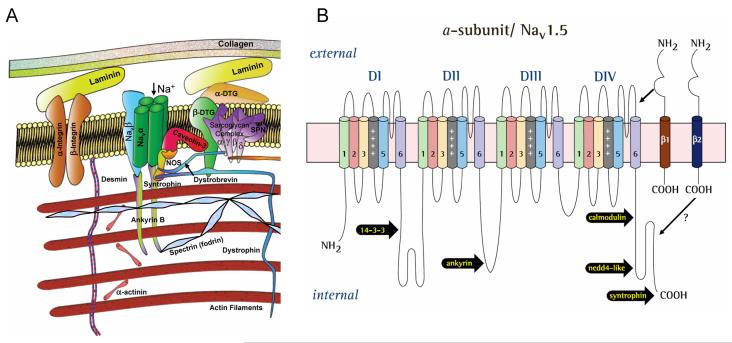
The function of the NaCh is not fully determined by its protein structure, but also depends on its environment. Na+ channels represent a multi-protein complex comprising not only the main pore-forming α subunit and its auxiliary β subunits, but also components of cytoskeleton, Ca2+-sensitive protein calmodulin, regulatory kinases and phosphatases, trafficking proteins, and extracellular matrix proteins embedded into lipid bilayer plasma membrane. A diagram of Nav1.5 and its interacting proteins is shown in Fig. (4 A). For in-depth reviews on this matter see [48-50]. Here we highlight only the components that seem to modulate INaL in different pathological conditions, including hypoxia and HF. The III-IV linker (see Fig. (4 B)) is responsible for NaCh inactivation [51], and mutations in this region disrupt Nav1.5 inactivation causing persistent Na+ current linked to inherited LQT3 syndrome [52]. Recently, COOH terminal that has binding sites for abundant regulatory proteins has been implicated in Nav1.5 inactivation [53, 54].
Figure (4).
Schematic illustration of Na+ channel macromolecular complex. A: The pore forming α subunit of the channel interacts with β-subunits, cytoskeleton and the extracellular matrix (Modified after [50], used with permission). B: schematic presentation of the α subunit of the cardiac Na+ channel isoform (Nav1.5) with reported sites of interaction with β subunits (restricted only to β1 and β2) and other regulatory proteins. Reprinted from [17], used with permission.
Modulation of INaL by β subunits
Mammalian voltage gated NaCh are associated with auxiliary β subunits. The β-subunit gene family has four members β1(SCN1B), β2 (SCN2B), β3 (SCN3B) β4 (SCN4B) (see for review [48, 50]). All these β-subunits are expressed in rodent hearts and are differently localized to specific sub cellular domains and cell types. β1 subunit is non-covalently attached to α subunit, and β2 subunit is covalently linked to α subunit by a disulfide bond [55]. The protein of these β subunits contains an extracellular amino-terminus, a single transmembrane segment, and an intracellular COOH terminus (C-terminus) (Fig. (4B)). The extracellular N-terminus of all β subunits contain immunoglobulin domain found in cell adhesion molecules. Particularly, immunoglobulin domain for β2 (and probably for β4) is similar to contactin, whereas for β1 and its splice variant β1A it is similar to myelin Po. This unique property allows interactions with variety of signaling molecules and components of the extracellular matrix. With the regard to the intracellular domain, direct interactions of β1 subunit C terminus and ankyrin B in rat brain membranes have been demonstrated, indicating a role of this subunit in main α subunit (Nav) localization. Direct interaction between cytoplasmic C-terminus domain of Nav1.1 with β1 and β3 has been recently demonstrated [56]. β subunits do not form an ion-conducting pore, but modulate NaCh function, NaCh protein expression at the plasma membrane (trafficking), and cell adhesion [48, 50]. More specifically, β1-subunit: 1) is involved in abnormal NaCh activity associated with the LQT3 mutation [57], 2) aggravates NaCh dysfunction in Brugada syndrome [58], 3) modifies the blockade of NaCh by fatty acids [59] and lidocaine [60], 4) modulates the trafficking of Nav1.5 [61], and 5) affects the burst mode of the heterologously expressed skeletal muscle NaCh isoform [62].
Very few reports are currently available about modulation by β subunits of late openings of the cardiac Na+ channel. Heterologous co-expression of β1 subunit with Nav1.5 in HEK293 cells diminishes IbNa [63], but increases INaL amplitude and significantly slows INaL decay in tsA201 cells [64]. At the same time, β2 subunit does not affect INaL parameters [64]. The potency of β1 subunit to modulate INaL has been confirmed in preliminary studies in native cell environment. In normal dog VCs, knocking down of SCN1B by antisense nucleotides significantly accelerated INaL decay [65]. In HF, Nav1.5 protein level is down regulated, whereas β1 remains unchanged, indicating a relatively higher membrane content of β1 [43]. This suggests potential involvement of β1 in the reported INaL alterations in HF.
Modulation of INaL by cytoskeleton
The cytoskeleton proteins form a framework within the cytoplasm with links to the membrane that include attachments to integral membrane proteins. This framework maintains cell shape, plasma membrane integrity, and localization of membrane proteins such as ion exchange carriers and ion channels (Fig 7, see for review [48-50, 66]). Multiple experimental studies demonstrated that the sub-membrane cytoskeleton modulate INaL in cardiomyocytes.
Figure (7).
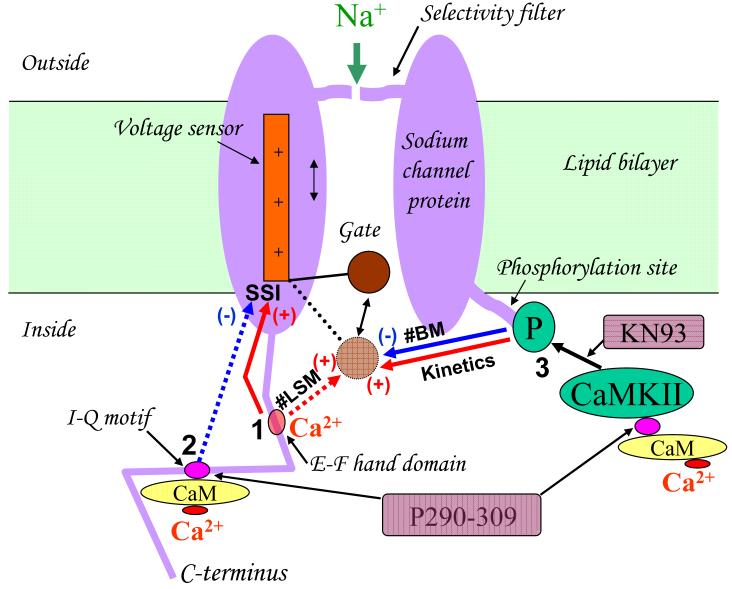
Simplified hypothetical diagram of the intracellular Ca2+ signaling pathways modulating late sodium current in normal and failing ventricular cardiac myocytes. Pathways 1, 2 and 3 (marked by black digits) represent Ca2+ binding to: the E-F domain, CaM-binding site (IQ motif) on C-terminus of NaCh as well as CaM/CaMKII complex, respectively. In addition, shown are the inhibitory sites of the CaM antagonist peptide P290-309 and KN93 - inhibitor of CaMKII, which were used in our original study [110] to discover the INaL modulation depicted in the diagram. Dashed line arrows indicate the regulation pathways which are operational only in heart failure. Reprinted from [110], used with permission.
Ankyrin-B
An adapter protein ankyrin-B, which is expressed in heart, links Nav1.5 to the cytoskeleton. A knockout of ankyrin B in affects late Na+ channel openings in mouse cardiomyocytes [67]. Furthermore, mutations in ankyrin B cause the LQT4 syndrome in one French family [68], indicating the importance of channel environment for the channel function. Accordingly, a disruption of any member of this multi-protein complex by pathological conditions may lead to alterations of INaL.
F-actin
Cytochalasin-D, an agent that interferes with F-actin polymerization as well as anti-actin antibody [69], slows inactivation of cardiac Na+ channel by inducing bursts of openings. It also affects coupling of steady state activation and availability of Na+ current [70, 71].
Fodrin
Fodrin (spectrin)-based cytoskeleton, another element of the NaCh microenvironment in heart, is a dynamic structure, which is altered under a variety of pathological conditions (e.g. ischemia or heart failure [72-74]). The role of the fodrin-based cytoskeleton in INaL modulation has been suggested in experiments with the antisense oligonucleotides targeting mRNA’s encoding α- and β-fodrin in dog VCs [75].
The fodrin breakdown that happens in some disease states featuring poor Ca2+ handling can be mediated by the Ca2+ - activated enzyme calpain and caspase [72, 76, 77]. Therefore, disturbances of the ankyrin and/or fodrin-based cytoskeleton may affect the Na+ channel inactivation process. Cytoskeleton elements such as ankyrin may bind directly to α and β subunits (see above). The link to β subunits may thus modulate INaL indirectly.
Tubular cytoskeleton
also may be involved in NaCh gating regulation. For example, antitumor agent taxol, that stabilizes microtubules, shifts NaCh activation threshold towards more negative membrane potentials [78] and thus may increase the pool of NaCh that underlies INaL.
Modulation of INaL by the metabolites of membrane phospholipids
Lysophosphatidylcholine (LPC) is the endogenous amphiphilic lipid metabolite that rapidly accumulates in myocardium during ischemia and represents a major factor causing the electrophysiological alterations that contribute to cardiac arrhythmia [79, 80]. Recently, a significant prolongation of QTc interval has been found during early transmural ischemia in patients undergoing balloon angioplasty [81]. Experimentally, LPC causes membrane depolarization, reduction of the maximal upstroke velocity of AP, sustained abnormal rhythmic activity in Purkinje fibers, and delayed afterpotentials (DADs) in isolated tissue [79, 82]. The cellular mechanisms of the LPC effects include specific modifications of Na+ current: significant decrease of INaT and an emergence of late NaCh openings that produce a sustained Na+ current [35, 83-86]. Interestingly, the late NaCh openings caused by LPC form clusters of the synchronized multiple channel openings [83, 85]. One of the mechanisms underlying these LPC-induced modifications might be an integration of LPC into the lipid membrane, which would increase the membrane fluidity [87]. This, in turn, enhances motility and interaction of proteins within the membrane. On the other hand, LPC can activate neuromodulation signaling via PKA and PKC [88, 89], affecting NaCh slow inactivation [90].
Recently emerged modulators
A novel protein partner of Nav1.5 has been discovered using a yeast 2-hybrid screen [91]. This protein, called 14-3-3η, interacts with the cytoplasmic I inter-domain of NaCh (see Fig. (4 B)). Although its direct effect on INaL properties has not yet been studied, it was shown that this protein influences the inactivation process by delaying recovery from inactivation[91]. Additionally, Src family tyrosine kinase Fyn has the phosphorylation site on Nav1.5A III-IV linker [92], which is known to be responsible for the inactivation. The most recently emerged modulator is a membrane micro domain protein Caveolin-3. This protein was previously linked to NaCh trafficking, [93] but now is implicated in LQT syndrome [94]. Implementation of these new players in INaL modulation in different pathological conditions awaits further studies.
Modulation of INaL by the Ca2+signaling pathways
Structurally, the carboxyl terminus of NaCh has binding sites for Ca2+ itself [95] and for Ca2+-binding protein calmodulin (CaM) that acts as a Ca2+ sensor translating changes in cytoplasmic Ca2+ into cellular responses [96] (Fig. (7)). Discovery of these sites inspired multiple studies in heterelogously expressed NaCh, including a brain, skeletal and cardiac muscle isoforms [97-101]. It was found that some inactivation states of INaT of heterologously expressed cardiac and skeletal NaCh isoforms may be modulated directly by Ca2+, CaM and/or via Ca2+ /CaM/CaM-kinase signaling cascade. There are only a few studies to date performed in cardiomyocytes that addressed the question about INaL modulation by Ca2+ signaling specifically in NaCh native environment. It was shown that over expression of CaMKIIδc enhances INaL and increases [Na]i [102]. Our recent detailed studies in normal and failing dog cardiomyocytes have demonstrated multiple, complex effects of Ca2+, CaM, and CaMKII on INaL properties (summarized in Fig.7), including the fine structure of INaL, described by fast (originated from bursts) and slow (originated from LSM) exponentials. More specifically, INaL greatly enhances as [Ca2+]i increase: its maximum density increases, decay of both exponentials describing INaL decay slows, and steady-state inactivation curve (SSI) shifts towards more positive potentials. The latter means that more available NaCh generate a larger INaL. Testing inhibition of CaMKII and CaM revealed similarities and differences of INaL modulation in failing vs. normal dog myocytes. Similarities were as follows: 1) CaMKII slows INaL decay and decreases the amplitude of fast exponential; 2) Ca2+ shifts SSI rightward. The following differences in failing vs. normal myocytes were found: 1) slowing INaL by CaMKII is greater; 2) CaM shifts SSI leftward; 3) Ca2+ increases the amplitude of slow exponential. These data suggest that Ca2+/CaM/CaMKII signaling increases INaL and Na+ influx in both normal and failing myocytes by slowing inactivation kinetics and shifting SSI. This Na+ influx provides a novel Ca2+ positive feedback mechanism (via Na+/Ca2+ exchanger), enhancing contractions at higher beating rates, but worsening cardiomyocytes contractile and electrical performance in conditions of poor Ca2+ handling in heart failure (multiple INaL-mediated Na+ - Ca2+ interactions in HF are discussed in detail below).
EXPERIMENTAL EVIDENCE OF INaL IMPORTANCE FOR ABNORMAL REPOLARIZATION, Ca2+ HANDLING, AND CONTRACTILITY IN FAILING MYOCARDIUM
Inhibition of INaL normalizes AP duration and beat-to-beat variability and eliminates EADs in HF VCs
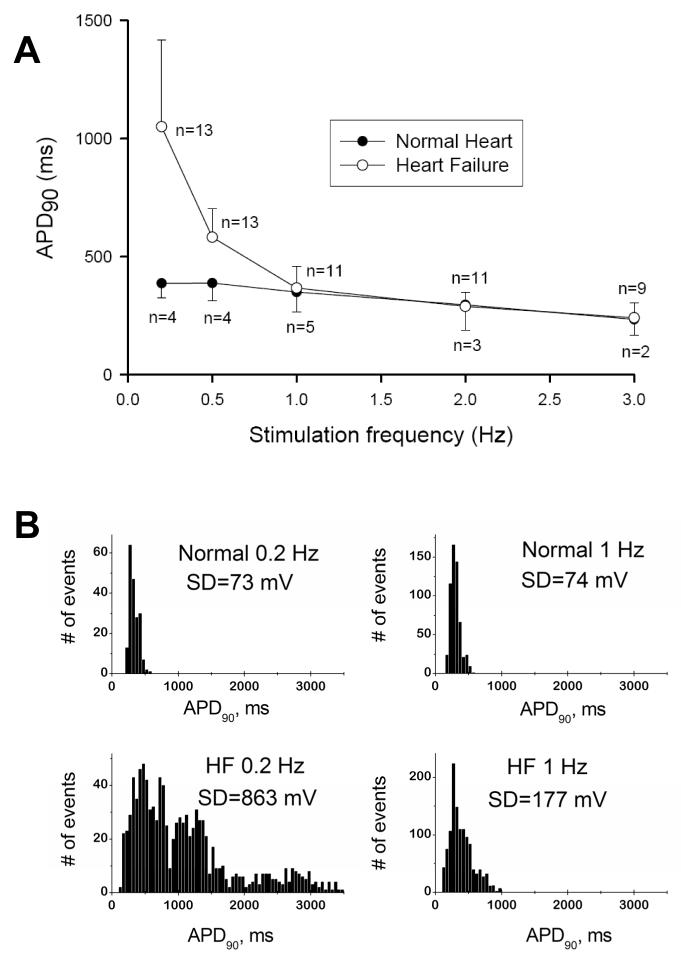
APD is extremely frequency dependent in failing VCs [10]. At low pacing rates of 0.2-0.5 Hz the mean APD is significantly larger in failing VCs (Fig. (5 A)), and failing cells eventually exhibit EADs (Fig. (3 C)) [10, 11, 13]. In addition to the prolongation, APD also exhibits significant beat-to-beat variability in failing VCs. Although AP prolongation is not evident at physiological frequencies (1Hz and higher), variability of APD in failing cells is substantially larger than in normal cells (compare APD distributions and their SD values in Fig. (5 B)) [10, 13]. Increased and slowed INaL (Fig. (2)) greatly contributes to APD prolongation, variability, and EADs. Partial reduction in the magnitude of INaL caused by specific NaCh blockers (TTX, 1.5 μM, STX, 100 nM), a new antianginal drug ranolazine (that turned out to be a specific INaL blocker), or injection of external current opposite to INaL during the AP plateau significantly shorten the prolonged APD, decrease beat-to-beat variability of APD, and abolish EADs [10, 11, 13, 15].
Figure (5).
A: Frequency-dependence of action potential duration in ventricular cardiomyocytes of normal dogs and dogs with chronic heart failure. Note that largest difference occurs at low pacing rates. B: at the low (0.2 Hz) and the physiologic (1 Hz) pacing rates, AP duration in failing myocytes exhibits significant beat-to beat variability (see respective SD values in the APD90 distribution histograms) Adapted from [10] with permission.
Blockade of INaL improves Ca2+ transient and contractility in HF
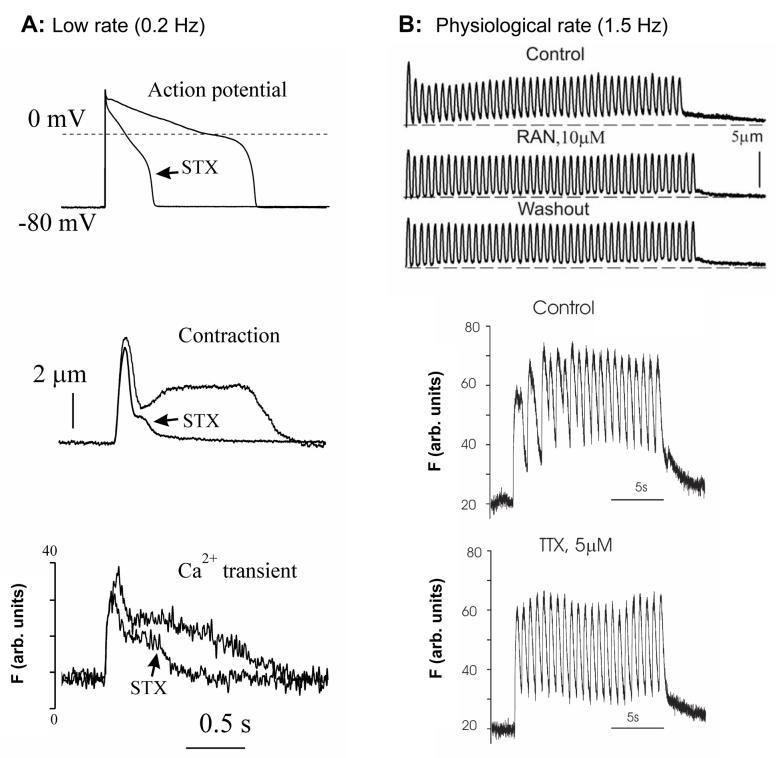
The contractile dysfunction in HF is related not only to ongoing loss of functional cardiac units, but also to abnormal function of cardiomyocytes. HF is characterized by systolic dysfunction as a result of depressed Ca2+ transients [4] and by abnormal relaxation of cardiac myocytes [103, 104]. At low pacing rates, the prolonged relaxation is associated with the spike-dome configuration of contractile response of cardiomyocytes (Fig. (6 A)). The ratio between amplitudes of the spike and dome phases was suggested to be an index for the severity of HF [103]. Similar to the shape of abnormal contraction, abnormally prolonged Ca2+ transients have also been observed in both ventricular muscle strips [103] and cardiomyocytes isolated from failing hearts [14, 105] (Fig. (6 A)). At the higher frequencies, these abnormalities account for the reversal of the force-frequency relationship in failing myocardium, leading to an increase in diastolic [Ca2+]i and diastolic tension (Fig. (6 B) “Control”) [15, 106]. A partial blockade of INaL by STX, TTX or ranolazine greatly improves performance of failing VCs; it abolishes the dome phase of both contraction-relaxation cycle and Ca2+ transient at low pacing rates and prevents the rising diastolic tension and [Ca2+]i at the higher pacing rates in failing VCs [14, 15] (Fig.(6)).
Figure (6).
A: Examples of effects of a specific Na+ channel blocker saxitoxin (STX) on AP duration, contraction and Ca2+ transient in ventricular cardiomyocytes of dogs with chronic heart failure at a low pacing rate of 0.2 Hz STX reduces AP duration, abolishes “dome” phase of contraction and of Ca2+ transient in failing cells. B: At higher pacing rates a specific INaL blockers ranolazine reduces diastolic tension, and a specific Na+ channel blocker tetrodotoxin (TTX) reduces Ca2+ accumulation (Fluo-4 signals). Adapted from [14, 15, 17], used with permission.
INTEGRATION OF INaL INTO ELECTROPHYSIOLOGICAL AND Ca2+ MECHANISMS IN HF
Dramatic improvement of cardiomyocyte function by INaL inhibition described above provides an evidence for a substantial INaL contribution to the functional remodeling in the failing heart. However, the interpretation of these effects needs extreme care because electrophysiology, contraction, and Ca2+ dynamics in cardiomyocytes are interrelated via multiple feedback mechanisms. The extent of deterioration of cardiomyocyte function and these feedback mechanisms vary greatly with the progression of HF and etiology. Since late openings of NaCh generate both an electric current and a Na+ influx, INaL is expected to contribute to at least two established HF mechanisms: 1) electrophysiological remodeling [3], and 2) altered cell Na+ and Ca2+ cycling [4].
The role of INaL in AP prolongation and EADs
Given the high membrane resistance during the AP plateau, INaL provides a critical contribution to the altered delicate balance of ion currents and thus to the APD. The relative contribution of INaL to the AP plateau in failing VCs is amplified by the reduced K+ currents in HF [3]. Since HF simultaneously increases and slows INaL it is expected to contribute directly to AP prolongation in HF, thus explaining APD normalization with INaL inhibition described above. Prolonged APD allows more time for ICaL reactivation and thus facilitates EADs [107]. Since INaL contributes to AP prolongation, it thus indirectly contributes to EADs. Accordingly, INaL inhibition eliminates EADs likely as a result of APD shortening.
INaL and elevated [Na+]i increase Ca2+ entry via NCX and limit depression of systolic function
A major problem in HF is systolic dysfunction, which is associated with smaller Ca2+ transient and sarcoplasmic reticulum (SR) Ca2+ content. The extent of deterioration of systolic function is limited by multiple compensatory mechanisms (listed below), which are indirectly linked to INaL and elevated Na+.
NCX function depends on Na+ and Ca2+ concentrations and membrane voltage. In HF VCs, increased Na+ influx (including that via INaL) shifts the NCX operation from the predominant forward mode to the reverse mode i.e. from Ca2+ efflux to Ca2+ entry [108]. Elevated Na+ in HF thus limits SR unloading and provides additional Ca2+ influx during the AP [4]. Interestingly, in addition to preserving SR Ca2+ load, this operational shift in failing human myocardium results in the direct activation of contraction during the terminal phases of the AP via the reverse mode NCX Ca2+ influx [109]. Thus, a larger cellular Na+ load (contributed by INaL) may be also important to drive contractions via this HF-specific mechanism.
The vast majority of studies demonstrated that NCX is upregulated in HF [29], therefore the above effects could be amplified by the enhanced NCX function.
INaL contributes to APD prolongation and thus indirectly prolongs Ca2+ influxes via ICaL and the reverse mode NCX.
INaL in HF is positively modulated by intracellular Ca2+ [110] (Fig.7), which could yield a new possible amplification mechanism of the Ca2+ entry. This creates a positive feedback loop from INaL via NCX to larger cell Ca2+ load, then from the larger Ca2+ load back to INaL.
Adverse effects of increased [Ca2+]i in HF. Role of INaL and NCX in diastolic dysfunction
While the increased INaL boosts a cell Ca2+ load and thereby limits the depression of systolic function in HF, it also leads to diastolic dysfunction, especially at high rates as described above. Relaxation of cardiac myocytes occurs when [Ca2+]i declines, allowing Ca2+ dissociation from the myofilaments. Ca2+ is removed from cytosol, mainly via SERCA, which takes Ca2+ back into the SR, and, by NCX operating in the forward mode during diastole [111]. It is believed that the diastolic dysfunction in HF is mainly due to a reduced SERCA function in HF. At the same time, increased expression and function of NCX in HF tends to offset the deficiency of Ca2+ removal by SERCA (review [4]). The contribution of the increased INaL to the Ca2+ removal could be twofold. First, as discussed above, INaL and related increase of [Na+]i facilitate Ca2+ influx. Secondly, higher [Na+]i during diastole partially offsets the function of the forward mode NCX and thus worsens the problems of Ca2+ removal from the cytosol and diastolic dysfunction. The improvement of diastolic function by the inhibition of INaL (Fig.10B) can be attributed both to a decrease in Ca2+ load during the AP plateau and to improve removal of Ca2+ by forward mode NCX during diastole. Indeed partial blockade of the NCX improves EC coupling in HF [112] and reduces both EADs and DADs [113, 114].
Ca2+ overload and increased diastolic Na+: potential importance of INaL for DADs
DADs occur as a result of spontaneous Ca2+ releases during diastole via the activation of the forward mode of NCX. INaL does not occur at low diastolic potentials in humans and dogs (see Fig. 2B, filled circles), so it cannot have a direct contribution to DADs. However, as discussed above, in HF VCs a larger Na+ influx via INaL increases Ca2+ influx via NCX during AP leading to SR Ca2+ overload, which, in turn, is critical for initiation of spontaneous Ca2+ release (such as Ca2+ waves) during diastole. INaL involvement in DADs is thus limited to the contribution of the INaL to the Ca2+ overload. On the other hand, high diastolic [Na+]i in HF [45] decreases the forward mode NCX current and, hence, attenuates the amplitude of DADs. This positive factor of INaL can be counterbalanced by down regulation of IK1 in HF [115], facilitating membrane excitations.
Role of INaL in dispersion of repolarization: a feedback from abnormal Ca2+ cycling?
The mechanisms of dispersion of repolarization in HF remain unclear. A possible mechanism involves beat-to-beat alternations and/or fluctuations in intracellular Ca2+ cycling transduced to abnormal repolarization by electrogenic feedback mechanisms (review [116]). As discussed above, there are at least three possible indirect contributions of INaL to the beat-to-beat variability problem.
The INaL contribution to the abnormal Ca2+ cycling.
The INaL dependence on Ca2+/CaM/CaMKII. Thus INaL can serve as one of the electrogenic Ca2+ feedback mechanisms along with other Ca2+ -dependent ion currents, such as ICaL inactivation, IKs, Ito, Ca2+-activated Cl--current, and NCX.
The synchronicity of Ca2+ release depends on the state of phosphorylation of Ca2+ cycling proteins [117] and on the AP shape [118] (including the AP shape of failing VCs, [119]). Thus INaL contributes to the fluctuations of Ca2+ transients to the extent to which INaL contributes to the AP shape. More specifically, the contribution of INaL could be important for the abnormal early repolarization phase in HF via its increased burst mode [12] (Fig. (3 B)), especially when Ito is decreased [3]. Further, the activity of LSM of NaCh also increased in human HF VCs [12] (Fig. (3 A)); they persist on the AP plateau (Fig. (3 C)) and, hence, tend to prevent further repolarization.
Summary of INaL role in failing myocardium: friend or foe?
INaL and its Na+ influx can directly and indirectly contribute to several important HF mechanisms related to electrophysiological remodeling and ion homeostasis. These contributions could either improve or worsen performance of HF myocardium, i.e. being “friend” or “foe”, respectively (Fig.1). INaL is “friend” as it contributes to 1) APD prolongation as an adaptive and an anti-arrhythmic (anti-re-entry) response [3], and 2) Ca2+ entry to limit depression of systolic function. The latter mechanism is an intrinsic, adaptive, digitalis-like effect with all corresponding risks and benefits. Interestingly, a large slow component of the Na+ current decay (burst mode) has been identified in post-myocardial infarction-remodeled myocytes [120], i.e. in the transitional period from an infarction to HF. The increased INaL may indeed serve as an initial mechanism of adaptation to match an increased contractility demand for the survived VCs.
Although APD prolongation can be beneficial in HF, the temporal and spatial dispersion of repolarization that accompanies AP prolongation is critical for arrhythmia and sudden cardiac death [3]. Additionally, DADs and EADs have critical importance for non-reentrant arrhythmia or triggered activity (recent review [4]). Accordingly, INaL is “foe” as it contributes to EADs, DADs, dispersion of repolarization, and diastolic dysfunction.
INaL IS A NOVEL TARGET FOR CARDIOPROTECTION
Na+ channel: To block or not to block?
After negative outcomes of Cardiac Arrhythmia Suppression Trial (CAST) [121, 122] Class I antiarrhythmic drugs [123] targeting NaCh became close to a “taboo”. The trial tested whether the suppression of ventricular arrhythmias by encainide, flecainide, moricizine after myocardial infarction improves survival. The conclusion was that the “treatment strategies designed solely to suppress these arrhythmias should no longer be followed” [122]. However, the discoveries of inherited mutations in SCN5A gene that lead to an increased INaL (LQT3 syndrome, see for review [124]) and of increased and slowed INaL in acquired, chronic HF (discussed in this review) give rise to a revival of NaCh as a therapeutic target. However, these studies suggest that not all NaCh must be equally targeted. The emerging paradigm for Na+ channels in HF is that INaT is decreased [42-44] but simultaneously INaL is increased (see above). Blockers of INaT are proarrhythmic in HF because they slow conduction, thus worsening conduction problems (review [124]) and facilitating development of re-entry. Accordingly, new strategies for treatment must be considered: the new type of “smart” drugs should preferentially block INaL over INaT. This requirement calls for a new classification of Class 1 drugs in the future.
As discussed above increased INaL likely contributes to both electrical and contractile abnormalities of failing VCs, the potential benefits of a preferential INaL blockade in HF could be both an antiarrhythmic effect and an improvement of diastolic function. A preferential blockade of INaL over INaT in failing human VCs was reported for amiodarone [16], suggesting an explanation of why amiodarone, classified as Class III anti-arrhythmic drug, shows a remarkable efficiency among K+-channel blockers. Accordingly a new index of “smart drug” has been recently suggested as a ratio of the drug potencies to block INaL over of INaT (i.e. (IC50_INaT/IC50_INaL)) [15]. Based on this new index three different drugs were compared and fell in the following sequence: ranolazine (37.8) > amiodarone (12.9) > lidocaine (2.7), suggesting that the most promising drug is a new antianginal drug ranolazine [15].
The potential great benefits (preventing arrhythmias and Ca2+ overload) of the preferential INaL blockade can be expected not only in HF but also in other cardiac diseases, such as hypoxia and ischemia, in which INaL increase and Na+-Ca2+ overload are major features. A hypothesis that Na+ initiates Ca2+ overload in hypoxia, ischemia and reperfusion was based on the experimental finding that Na+ accumulation precedes the Ca2+ overload [125-127]. NaCh are critically involved in this process because NaCh blockers or activators reduce or increase Ca2+ overload in these pathological conditions, respectively [128-130]. In ischemia, targeting INaL is especially encouraged because LPC dramatically increases INaL [84, 85]. Indeed, in recent clinical trial ranolazine has been effective to reduce the incidence of arrhythmias in patients with non ST-segment elevation acute coronary syndrome [131]. On the other hand, the therapeutic strategy of a preferential INaL blockade should be exercised with care. The balanced and effective therapy targeting INaL should take into account that INaL might be involved in adaptive response at different states of cardiac disease, making the question whether INaL is “friend” or “foe” vitally important.
Alternative targets delineated by multiple mechanisms of INaL modulation
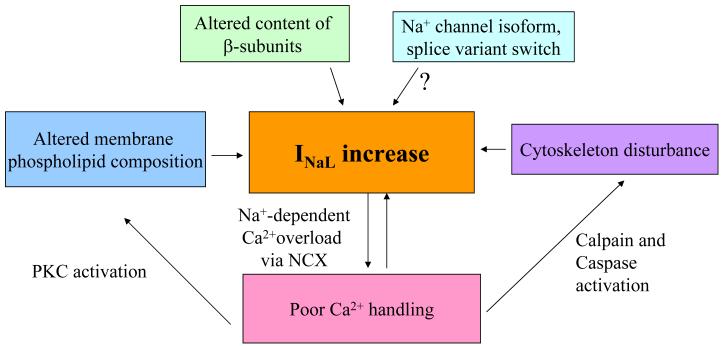
Figure 8 summarizes discussed above NaCh modulatory mechanisms, which could be involved in the INaL increase in HF. Thus, INaL increase in HF can be prevented, at least in part, in different ways: i. Stabilizing membrane phospholipid composition by preventing LPC accumulation, ii. Normalizing β-subunits membrane content, iii. Stabilizing subsarcolemmal cytoskeleton, iv. Normalizing Ca2+ homeostasis.
Figure (8).
Simplified diagram of the modulatory mechanisms that lead to the INaL increase in HF. These mechanisms may serve as a road map to develop new strategies for HF treatment (see text for detail).
Acknowledgments
Sources of funding
This research was supported by grants from the National Institute of Health HL-53819, HL074238, American Heart Association 0350472Z, research grant from CV Therapeutics, Palo Alto, CA (AU), and the Intramural Research Program of the National Institutes of Health, National Institute on Aging (VAM).
List of abbreviations
- AP
action potential
- APD
action potential duration
- CaM
calmodulin
- CaMKII
Ca2+-calmodulin activated protein kinase II
- BM
burst mode of NaCh gating
- DADs
delayed afterdeplarizations
- DHP
dihydropiridine
- EADs
early afterdepolarizations
- HF
heart failure
- I-V
current-voltage relationship
- INaT
fast transient sodium current
- INaL
late sodium current
- LPC
lysophosphatilylcholine, the membrane phosholipid metabolite
- LSM
late scattered mode of NaCh gating
- NaCh
voltage sensitive sodium channels
- NCX
Na+/Ca2+ exchanger
- PKA
protein kinase A
- PKC
protein kinase C
- SR
sarcoplasmic reticulum
- TTX
tetrodotoxin, a specific blocker of NaCh
- STX
saxitoxin, a specific blocker of NaCh
- VCs
left ventricular cardiomyocytes
- (dV/dt)max
maximal AP upstroke velocity
References
- [1].Bayes de Luna A, Coumel P, Leclercq JF. Am Heart J. 1989;117:151. doi: 10.1016/0002-8703(89)90670-4. [DOI] [PubMed] [Google Scholar]
- [2].Nikolic G, Bishop RL, Singh JB. Circulation. 1982;66:218. doi: 10.1161/01.cir.66.1.218. [DOI] [PubMed] [Google Scholar]
- [3].Tomaselli GF, Zipes DP. Circ Res. 2004;95:754. doi: 10.1161/01.RES.0000145047.14691.db. [DOI] [PubMed] [Google Scholar]
- [4].Bers DM, Despa S, Bossuyt J. Ann N Y Acad Sci. 2006;1080:165. doi: 10.1196/annals.1380.015. [DOI] [PubMed] [Google Scholar]
- [5].Carmeliet E. J Cardiovasc Electrophysiol. 2006;17:S2. doi: 10.1111/j.1540-8167.2006.00378.x. [DOI] [PubMed] [Google Scholar]
- [6].Noble D, Noble PJ. Heart. 2006;92:iv1. doi: 10.1136/hrt.2005.078782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Coraboeuf E, Deroubaix E, Coulombe A. Am J Physiol. 1979;236:H561. doi: 10.1152/ajpheart.1979.236.4.H561. [DOI] [PubMed] [Google Scholar]
- [8].Gintant GA, Datyner NB, Cohen IS. Biophysical Journal. 1984;45:509. doi: 10.1016/S0006-3495(84)84187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Carmeliet E. Pflugers Archiv - European Journal of Physiology. 1987;408:18. doi: 10.1007/BF00581835. [DOI] [PubMed] [Google Scholar]
- [10].Undrovinas AI, Maltsev VA, Sabbah HN. Cell Mol Life Sci. 1999;55:494. doi: 10.1007/s000180050306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Maltsev VA, Sabbah HN, Higgins RSD, Silverman N, Lesch M, Undrovinas AI. Circulation. 1998;98:2545. doi: 10.1161/01.cir.98.23.2545. [DOI] [PubMed] [Google Scholar]
- [12].Maltsev VA, Undrovinas AI. Cardiovasc Res. 2006;69:116. doi: 10.1016/j.cardiores.2005.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Maltsev VA, Silverman N, Sabbah HN, Undrovinas AI. Eur J Heart Fail. 2007;9:219. doi: 10.1016/j.ejheart.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Maltsev VA, Sabbah HN, Tanimura M, Lesch M, Goldstein S, Undrovinas AI. Cell Molec Life Sci. 1998;54:597. doi: 10.1007/s000180050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Undrovinas AI, Belardinelli L, Undrovinas NA, Sabbah HN. J Cardiovasc Electrophysiol. 2006;17:S169. doi: 10.1111/j.1540-8167.2006.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Maltsev VA, Sabbah HN, Undrovinas AI. J Mol Cell Cardiol. 2001;33:923. doi: 10.1006/jmcc.2001.1355. [DOI] [PubMed] [Google Scholar]
- [17].Maltsev VA, Undrovinas A. Progr Biohys Molec Biol. 2008;96:421. doi: 10.1016/j.pbiomolbio.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Vermeulen JT, McGuire MA, Opthof T, Coronel R, de Bakker JM, Klopping C, Janse MJ. Cardiovascular Research. 1994;28:1547. doi: 10.1093/cvr/28.10.1547. [DOI] [PubMed] [Google Scholar]
- [19].Chang CY, Yeh TC, Chiu HC, Huang JH, Lin CI. International Journal of Cardiology. 1995;50:43. doi: 10.1016/0167-5273(95)02328-t. [DOI] [PubMed] [Google Scholar]
- [20].Beuckelmann DJ, Nabauer M, Erdmann E. Circ Res. 1993;73:379. doi: 10.1161/01.res.73.2.379. [DOI] [PubMed] [Google Scholar]
- [21].Nabauer M, Beuckelmann DJ, Erdmann E. Circ Res. 1993;73:386. doi: 10.1161/01.res.73.2.386. [DOI] [PubMed] [Google Scholar]
- [22].Koumi S, Backer CL, Arentzen CE. Circulation. 1995;92:164. doi: 10.1161/01.cir.92.2.164. [DOI] [PubMed] [Google Scholar]
- [23].Surawicz B. Journal of the American College of Cardiology. 1989;14:172. doi: 10.1016/0735-1097(89)90069-7. [DOI] [PubMed] [Google Scholar]
- [24].Cranefield PF, Aronson RS. Cardiovascular Drugs & Therapy. 1991;5:531. doi: 10.1007/BF03029780. [DOI] [PubMed] [Google Scholar]
- [25].Cranefield PF, Aronson RS. Pacing & Clinical Electrophysiology. 1988;11:670. doi: 10.1111/j.1540-8159.1988.tb06016.x. [DOI] [PubMed] [Google Scholar]
- [26].Aronson RS, Ming Z. Circulation. 1993;87:VII76. [Google Scholar]
- [27].Akar FG, Tomaselli GF. Ann Med. 2005;37:44. doi: 10.1080/07853890510007214. [DOI] [PubMed] [Google Scholar]
- [28].Wettwer E, Amos G, Gath J, Zerkowski HR, Reidemeister JC, Ravens U. Cardiovasc Res. 1993;27:1662. doi: 10.1093/cvr/27.9.1662. [DOI] [PubMed] [Google Scholar]
- [29].Studer R, Reinecke H, Bilger J, Eschenhagen T, Bohm M, Hasenfuss G, Just H, Holtz J, Drexler H. Circ Res. 1994;75:443. doi: 10.1161/01.res.75.3.443. [DOI] [PubMed] [Google Scholar]
- [30].Zygmunt AC, Eddlestone GT, Thomas GP, Nesterenko VV, Antzelevitch C. Am J Physiol. 2001;281:H689. doi: 10.1152/ajpheart.2001.281.2.H689. [DOI] [PubMed] [Google Scholar]
- [31].Sakmann BF, Spindler AJ, Bryant SM, Linz KW, Noble D. Circ Res. 2000;87:910. doi: 10.1161/01.res.87.10.910. [DOI] [PubMed] [Google Scholar]
- [32].Belardinelli L, Shryock JC, Fraser H. Heart. 2006;92:iv6. doi: 10.1136/hrt.2005.078790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].La C, You Y, Zhabyeyev P, Pelzer DJ, McDonald TF. J Membr Biol. 2006;210:43. doi: 10.1007/s00232-005-0844-6. [DOI] [PubMed] [Google Scholar]
- [34].Wu L, Shryock JC, Song Y, Belardinelli L. J Pharmacol Exp Ther. 2006;316:718. doi: 10.1124/jpet.105.094862. [DOI] [PubMed] [Google Scholar]
- [35].Chattou S, Coulombe A, Diacono J, Le Grand B, John G, Feuvray D. J Mol Cell Cardiol. 2000;32:1181. doi: 10.1006/jmcc.2000.1151. [DOI] [PubMed] [Google Scholar]
- [36].Gadsby DC, Cranefield PF. J Gen Physiol. 1977;70:725. doi: 10.1085/jgp.70.6.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Attwell D, Cohen I, Eisner D, Ohba M, Ojeda C. Pflügers Arch. 1979;379:137. doi: 10.1007/BF00586939. [DOI] [PubMed] [Google Scholar]
- [38].Hodgkin AL, Huxley AF. J Physiol. 1952;117:500. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Maltsev VA, Lesch M, Undrovinas AI. Circulation. 1995;92:I. doi: 10.1161/01.cir.98.23.2545. [DOI] [PubMed] [Google Scholar]
- [40].Undrovinas AI, Maltsev VA, Kyle JW, Silverman NA, Sabbah HN. J Mol Cell Cardiol. 2002;34:1477. doi: 10.1006/jmcc.2002.2100. [DOI] [PubMed] [Google Scholar]
- [41].Kiyosue T, Arita M. Circ Res. 1989;64:389. doi: 10.1161/01.res.64.2.389. [DOI] [PubMed] [Google Scholar]
- [42].Maltsev VA, Sabbah HN, Undrovinas AI. Cell Mol Life Sci. 2002;59:1561. doi: 10.1007/s00018-002-8529-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zicha S, Maltsev VA, Nattel S, Sabbah HN, Undrovinas AI. J Mol Cell Cardiol. 2004;37:91. doi: 10.1016/j.yjmcc.2004.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Valdivia CR, Chu WW, Pu J, Foell JD, Haworth RA, Wolff MR, Kamp TJ, Makielski JC. J Mol Cell Cardiol. 2005;38:475. doi: 10.1016/j.yjmcc.2004.12.012. [DOI] [PubMed] [Google Scholar]
- [45].Despa S, Islam MA, Weber CR, Pogwizd SM, Bers DM. Circulation. 2002;105:2543. doi: 10.1161/01.cir.0000016701.85760.97. [DOI] [PubMed] [Google Scholar]
- [46].Satin J, Kyle JW, Chen M, Bell P, Cribbs LL, Fozzard HA, Rogart RB. Science. 1992;256:1202. doi: 10.1126/science.256.5060.1202. [DOI] [PubMed] [Google Scholar]
- [47].Undrovinas AI, Mishra S, Undrovinas NA. Circulation. 2005;112:II. [Google Scholar]
- [48].Meadows LS, Isom LL. Cardiovasc Res. 2005;67:448. doi: 10.1016/j.cardiores.2005.04.003. [DOI] [PubMed] [Google Scholar]
- [49].Abriel H, Kass RS. Trends Cardiovasc Med. 2005;15:35. doi: 10.1016/j.tcm.2005.01.001. [DOI] [PubMed] [Google Scholar]
- [50].Nerbonne JM, Kass RS. Physiol Rev. 2005;85:1205. doi: 10.1152/physrev.00002.2005. [DOI] [PubMed] [Google Scholar]
- [51].Patton DE, West JW, Catterall WA, Goldin AL. Proc. Natl. Acad. Sci. USA. 1992;89:10905. doi: 10.1073/pnas.89.22.10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bennett PB, Yazawa K, Makita N, George AL., Jr. Nature. 1995;376:683. doi: 10.1038/376683a0. [DOI] [PubMed] [Google Scholar]
- [53].Cormier JW, Rivolta I, Tateyama M, Yang AS, Kass RS. J Biol Chem. 2002;277:9233. doi: 10.1074/jbc.M110204200. [DOI] [PubMed] [Google Scholar]
- [54].Motoike HK, Liu H, Glaaser IW, Yang AS, Tateyama M, Kass RS. J Gen Physiol. 2004;123:155. doi: 10.1085/jgp.200308929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Messner DJ, Catterall WA. J Biol Chem. 1986;261:211. [PubMed] [Google Scholar]
- [56].Spampanato J, Kearney JA, de Haan G, McEwen DP, Escayg A, Aradi I, MacDonald BT, Levin SI, Soltesz I, Benna P, Montalenti E, Isom LL, Goldin AL, Meisler MH. J Neurosci. 2004;24:10022. doi: 10.1523/JNEUROSCI.2034-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].An RH, Wang XL, Kerem B, Benhorin J, Medina A, Goldmit M, Kass RS. Circulation Research. 1998;83:141. doi: 10.1161/01.res.83.2.141. [DOI] [PubMed] [Google Scholar]
- [58].Makita N, Shirai N, Wandg DW, Sasaki K, George ALJ, Kanno M, Kitabatake A. Circulation. 2000;101:54. doi: 10.1161/01.cir.101.1.54. [DOI] [PubMed] [Google Scholar]
- [59].Xiao YF, Wright SN, Wang GK, Morgan JP, Leaf A. Am. J. Physiol. 2000;279:H35. doi: 10.1152/ajpheart.2000.279.1.H35. [DOI] [PubMed] [Google Scholar]
- [60].Makielski JC, Limberis J, Fan Z, Kyle JW. Cardiovasc Res. 1999;42:503. doi: 10.1016/s0008-6363(99)00024-3. [DOI] [PubMed] [Google Scholar]
- [61].Zhou J, Yui J, Hu NN, George ALJ, Murray KT. Circ. Res. 2000;87:33. doi: 10.1161/01.res.87.1.33. [DOI] [PubMed] [Google Scholar]
- [62].Chang SY, Satin J, Fozzard HA. Biophys J. 1996;70:2581. doi: 10.1016/S0006-3495(96)79829-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Valdivia C, Nagatomo T, Makielski J. J Mol Cell Cardiol. 2002;34:1029. doi: 10.1006/jmcc.2002.2040. [DOI] [PubMed] [Google Scholar]
- [64].Undrovinas AI, Maltsev VA. Pacing Clin Electrophysiol. 2001;24:622. [Google Scholar]
- [65].Undrovinas AI, Maltsev VA. Biophys J. 2002;82:89a. [Google Scholar]
- [66].Bennett V, Baines AJ. Physiol Rev. 2001;81:1353. doi: 10.1152/physrev.2001.81.3.1353. [DOI] [PubMed] [Google Scholar]
- [67].Chauhan VS, Tuvia S, Buhusi M, Bennett V, Grant AO. Circ Res. 2000;86:441. doi: 10.1161/01.res.86.4.441. [DOI] [PubMed] [Google Scholar]
- [68].Mohler PJ, Schott JJ, Gramolini AO, Dilly KW, Guatimosim S, duBell WH, Song LS, Haurogne K, Kyndt F, Ali ME, Rogers TB, Lederer WJ, Escande D, Le Marec H, Bennett V. Nature. 2003;421:634. doi: 10.1038/nature01335. [DOI] [PubMed] [Google Scholar]
- [69].Undrovinas A, Dubreuil R, Makielski JC. Circulation. 1993;88:I. [Google Scholar]
- [70].Undrovinas AI, Shander GS, Makielski JC. American Journal of Physiology. 1995;269:H203. doi: 10.1152/ajpheart.1995.269.1.H203. [DOI] [PubMed] [Google Scholar]
- [71].Maltsev VA, Undrovinas AI. J. Mol. Cell. Cardiol. 1996;28:A162. doi: 10.1006/jmcc.1998.0715. [DOI] [PubMed] [Google Scholar]
- [72].Yoshida K, Inui M, Harada K, Saido TC, Sorimachi Y, Ishihara T, Kawashima S, Sobue K. Circ Res. 1995;77:603. doi: 10.1161/01.res.77.3.603. [DOI] [PubMed] [Google Scholar]
- [73].Heling A, Zimmermann R, Kostin S, Maeno Y, Hein S, Devaux B, Bauer E, Klovekorn WP, Schlepper M, Schaper W, Schaper J. Circ Res. 2000;86:846. doi: 10.1161/01.res.86.8.846. [DOI] [PubMed] [Google Scholar]
- [74].Hein S, Kostin S, Heling A, Maeno Y, Schaper J. Cardiovasc Res. 2000;45:273. doi: 10.1016/s0008-6363(99)00268-0. [DOI] [PubMed] [Google Scholar]
- [75].Undrovinas AI, Maltsev VA. Biophys. J. 2003;84:25A. [Google Scholar]
- [76].Matsumura Y, Saeki E, Otsu K, Morita T, Takeda H, Kuzuya T, Hori M, Kusuoka H. J Mol Cell Cardiol. 2001;33:1133. doi: 10.1006/jmcc.2001.1373. [DOI] [PubMed] [Google Scholar]
- [77].Rotter B, Kroviarski Y, Nicolas G, Dhermy D, Lecomte MC. Biochem J. 2004;378:161. doi: 10.1042/BJ20030955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Maltsev VA, Undrovinas A. J Mol Cell Cardiol. 1997;26:A175. [Google Scholar]
- [79].Corr PB, Yamada KA, Creer MH, Sharma AD, Sobel BE. J Mol Cell Cardiol. 1987;19:45. doi: 10.1016/s0022-2828(87)80609-0. [DOI] [PubMed] [Google Scholar]
- [80].DaTorre SD, Creer MH, Pogwizd SM, Corr PB. Journal of Molecular & Cellular Cardiology. 1991;23:11. doi: 10.1016/0022-2828(91)90019-i. [DOI] [PubMed] [Google Scholar]
- [81].Kenigsberg DN, Khanal S, M. K, Krishnan SC. J Am Coll Cardiol. 2007 doi: 10.1016/j.jacc.2006.11.035. in press. [DOI] [PubMed] [Google Scholar]
- [82].Arnsdorf MF, Sawicki GJ. Circulation Research. 1981;49:16. doi: 10.1161/01.res.49.1.16. [DOI] [PubMed] [Google Scholar]
- [83].Burnashev NA, Undrovinas AI, Fleidervish IA, Rosenshtraukh LV. Pflugers Arch. 1989;415:124. doi: 10.1007/BF00373151. [DOI] [PubMed] [Google Scholar]
- [84].Burnashev NA, Undrovinas AI, Fleidervish IA, Makielski JC, Rosenshtraukh LV. J Mol Cell Cardiol. 1991;23:23. doi: 10.1016/0022-2828(91)90020-m. [DOI] [PubMed] [Google Scholar]
- [85].Undrovinas AI, Fleidervish IA, Makielski JC. Circ Res. 1992;71:1231. doi: 10.1161/01.res.71.5.1231. [DOI] [PubMed] [Google Scholar]
- [86].Shander GS, Undrovinas AI, Makielski JC. J Mol Cell Cardiol. 1996;28:743. doi: 10.1006/jmcc.1996.0069. [DOI] [PubMed] [Google Scholar]
- [87].Fink KL, Gross RW. Circ Res. 1984;55:585. doi: 10.1161/01.res.55.5.585. [DOI] [PubMed] [Google Scholar]
- [88].Obata T. Life Sci. 2002;71:2083. doi: 10.1016/s0024-3205(02)01993-8. [DOI] [PubMed] [Google Scholar]
- [89].Scott GA, Arioka M, Jacobs SE. J Invest Dermatol. 2006;5:5. doi: 10.1038/sj.jid.5700567. [DOI] [PubMed] [Google Scholar]
- [90].Chen Y, Yu FH, Surmeier DJ, Scheuer T, Catterall WA. Neuron. 2006;49:409. doi: 10.1016/j.neuron.2006.01.009. [DOI] [PubMed] [Google Scholar]
- [91].Allouis M, Le Bouffant F, Wilders R, Peroz D, Schott JJ, Noireaud J, Le Marec H, Merot J, Escande D, Baro I. Circ Res. 2006;98:1538. doi: 10.1161/01.RES.0000229244.97497.2c. [DOI] [PubMed] [Google Scholar]
- [92].Ahern CA, Zhang JF, Wookalis MJ, Horn R. Circ Res. 2005;96:991. doi: 10.1161/01.RES.0000166324.00524.dd. [DOI] [PubMed] [Google Scholar]
- [93].Yarbrough TL, Lu T, Lee HC, Shibata EF. Circ Res. 2002;90:443. doi: 10.1161/hh0402.105177. [DOI] [PubMed] [Google Scholar]
- [94].Vatta M, Ackerman MJ, Ye B, Makielski JC, Ughanze EE, Taylor EW, Tester DJ, Balijepalli RC, Foell JD, Li Z, Kamp TJ, Towbin JA. Circulation. 2006;114:2104. doi: 10.1161/CIRCULATIONAHA.106.635268. [DOI] [PubMed] [Google Scholar]
- [95].Wingo TL, Shah VN, Anderson ME, Lybrand TP, Chazin WJ, Balser JR. Nat Struct Mol Biol. 2004;11:219. doi: 10.1038/nsmb737. [DOI] [PubMed] [Google Scholar]
- [96].Mori M, Konno T, Ozawa T, Murata M, Imoto K, Nagayama K. Biochemistry. 2000;39:1316. doi: 10.1021/bi9912600. [DOI] [PubMed] [Google Scholar]
- [97].Tan HL, Kupershmidt S, Zhang R, Stepanovic S, Roden DM, Wilde AA, Anderson ME, Balser JR. Nature. 2002;415:442. doi: 10.1038/415442a. [DOI] [PubMed] [Google Scholar]
- [98].Deschenes I, Neyroud N, DiSilvestre D, Marban E, Yue DT, Tomaselli GF. Circ Res. 2002;90:E49. doi: 10.1161/01.res.0000012502.92751.e6. [DOI] [PubMed] [Google Scholar]
- [99].Herzog RI, Liu C, Waxman SG, Cummins TR. J Neurosci. 2003;23:8261. doi: 10.1523/JNEUROSCI.23-23-08261.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Kim J, Ghosh S, Liu H, Tateyama M, Kass RS, Pitt GS. J Biol Chem. 2004;279:45004. doi: 10.1074/jbc.M407286200. [DOI] [PubMed] [Google Scholar]
- [101].Young KA, Caldwell JH. J Physiol. 2005;565:349. doi: 10.1113/jphysiol.2004.081422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Wagner S, Dybkova N, Rasenack EC, Jacobshagen C, Fabritz L, Kirchhof P, Maier SK, Zhang T, Hasenfuss G, Brown JH, Bers DM, Maier LS. J Clin Invest. 2006;22:22. doi: 10.1172/JCI26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Gwathmey JK, Copelas L, MacKinnon R, Schoen FJ, Feldman MD, Grossman W, Morgan JP. Circ Res. 1987;61:70. doi: 10.1161/01.res.61.1.70. [DOI] [PubMed] [Google Scholar]
- [104].Davies CH, Davia K, Bennett JG, Pepper JR, Poole-Wilson PA, Harding SE. Circulation. 1995;92:2540. doi: 10.1161/01.cir.92.9.2540. [DOI] [PubMed] [Google Scholar]
- [105].Beuckelmann DJ, Erdmann E. Basic Res Cardiol. 1992;87:235. doi: 10.1007/978-3-642-72474-9_19. [DOI] [PubMed] [Google Scholar]
- [106].Feldman MD, Gwathmey JK, Phillips P, Schoen F, Morgan JP. J Applied Cardiol. 1988;3:273. [Google Scholar]
- [107].January CT, Riddle JM. Circ Res. 1989;64:977. doi: 10.1161/01.res.64.5.977. [DOI] [PubMed] [Google Scholar]
- [108].Baartscheer A, Schumacher CA, Belterman CN, Coronel R, Fiolet JW. Cardiovasc Res. 2003;57:986. doi: 10.1016/s0008-6363(02)00848-9. [DOI] [PubMed] [Google Scholar]
- [109].Weisser-Thomas J, Piacentino V, 3rd, Gaughan JP, Margulies K, Houser SR. Cardiovasc Res. 2003;57:974. doi: 10.1016/s0008-6363(02)00732-0. [DOI] [PubMed] [Google Scholar]
- [110].Maltsev VA, Reznikov V, Undrovinas NA, Sabbah HN, Undrovinas A. Am J Physiol Heart Circ Physiol. 2008;294:H1597. doi: 10.1152/ajpheart.00484.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Bers DM. Developments in cardiovascular medicine. Vol. 122. Kluwer Academic Publishers; Dordrecht, Netherlands: 1991. Excitation-contraction coupling and cardiac contractile force. [Google Scholar]
- [112].Hobai IA, Maack C, O’Rourke B. Circ Res. 2004;95:292. doi: 10.1161/01.RES.0000136817.28691.2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Pogwizd SM, Bers DM. Ann N Y Acad Sci. 2002;976:454. doi: 10.1111/j.1749-6632.2002.tb04775.x. [DOI] [PubMed] [Google Scholar]
- [114].Nagy ZA, Virag L, Toth A, Biliczki P, Acsai K, Banyasz T, Nanasi P, Papp JG, Varro A. Br J Pharmacol. 2004;143:827. doi: 10.1038/sj.bjp.0706026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Kaab S, Nuss HB, Chiamvimonvat N, O’Rourke B, Pak PH, Kass DA, Marban E, Tomaselli GF. Circ Res. 1996;78:262. doi: 10.1161/01.res.78.2.262. [DOI] [PubMed] [Google Scholar]
- [116].Wilson LD, Wan X, Rosenbaum DS. Ann N Y Acad Sci. 2006;1080:216. doi: 10.1196/annals.1380.018. [DOI] [PubMed] [Google Scholar]
- [117].Song LS, Wang SQ, Xiao RP, Spurgeon H, Lakatta EG, Cheng H. Circ Res. 2001;88:794. doi: 10.1161/hh0801.090461. [DOI] [PubMed] [Google Scholar]
- [118].Sah R, Ramirez RJ, Backx PH. Circ Res. 2002;90:165. doi: 10.1161/hh0202.103315. [DOI] [PubMed] [Google Scholar]
- [119].Harris DM, Mills GD, Chen X, Kubo H, Berretta RM, Votaw VS, Santana LF, Houser SR. Circ Res. 2005;96:543. doi: 10.1161/01.RES.0000158966.58380.37. [DOI] [PubMed] [Google Scholar]
- [120].Huang B, El-Sherif T, Gidh-Jain M, Qin D, El-Sherif N. J Cardiovasc Electrophysiol. 2001;12:218. doi: 10.1046/j.1540-8167.2001.00218.x. [DOI] [PubMed] [Google Scholar]
- [121].Epstein AE, Bigger JT, Jr., Wyse DG, Romhilt DW, Reynolds-Haertle RA, Hallstrom AP. Journal of the American College of Cardiology. 1991;18:14. doi: 10.1016/s0735-1097(10)80210-4. [DOI] [PubMed] [Google Scholar]
- [122].Epstein AE, Hallstrom AP, Rogers WJ, Liebson PR, Seals AA, Anderson JL, Cohen JD, Capone RJ, Wyse DG. Jama. 1993;270:2451. [PubMed] [Google Scholar]
- [123].Vaughan Williams EM. J Clin Pharmacol. 1984;24:129. doi: 10.1002/j.1552-4604.1984.tb01822.x. [DOI] [PubMed] [Google Scholar]
- [124].Shah M, Akar FG, Tomaselli GF. Circulation. 2005;112:2517. doi: 10.1161/CIRCULATIONAHA.104.494476. [DOI] [PubMed] [Google Scholar]
- [125].Murphy JG, Smith TW, Marsh JD. Am J Physiol. 1988;254:H1133. doi: 10.1152/ajpheart.1988.254.6.H1133. [DOI] [PubMed] [Google Scholar]
- [126].Tani M. Annu Rev Physiol. 1990;52:543. doi: 10.1146/annurev.ph.52.030190.002551. [DOI] [PubMed] [Google Scholar]
- [127].Malloy CR, Buster DC, Castro MM, Geraldes CF, Jeffrey FM, Sherry AD. Magn Reson Med. 1990;15:33. doi: 10.1002/mrm.1910150105. [DOI] [PubMed] [Google Scholar]
- [128].Haigney MC, Lakatta EG, Stern MD, Silverman HS. Circulation. 1994;90:391. doi: 10.1161/01.cir.90.1.391. [DOI] [PubMed] [Google Scholar]
- [129].Ver Donck L, Borgers M. Am J Physiol. 1991;261:H1828. doi: 10.1152/ajpheart.1991.261.6.H1828. [DOI] [PubMed] [Google Scholar]
- [130].Ver Donck L, Verellen G, Geerts H, Borgers M. J Mol Cell Cardiol. 1992;24:977. doi: 10.1016/0022-2828(92)91864-2. [DOI] [PubMed] [Google Scholar]
- [131].Scirica BM, Morrow DA, Hod H, Murphy SA, Belardinelli L, Hedgepeth CM, Molhoek P, Verheugt FW, Gersh BJ, McCabe CH, Braunwald E. Circulation. 2007;116:1647. doi: 10.1161/CIRCULATIONAHA.107.724880. [DOI] [PubMed] [Google Scholar]