Abstract
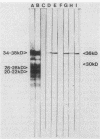
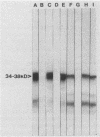
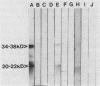
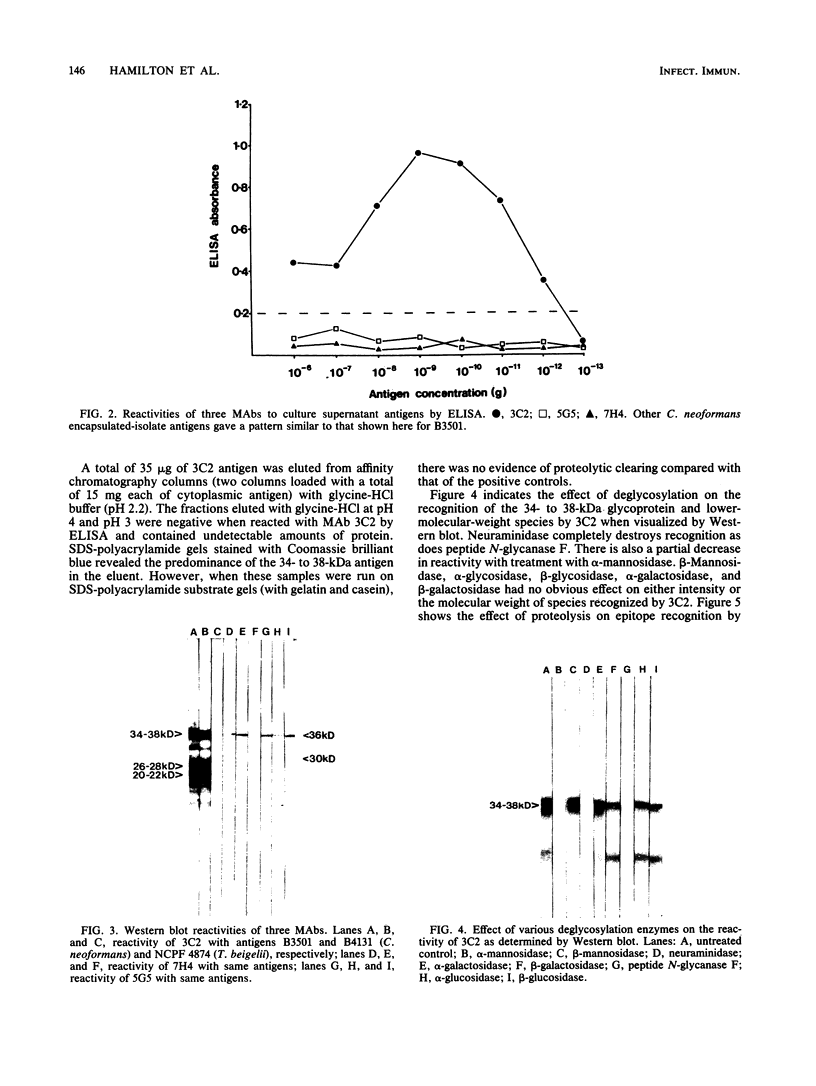
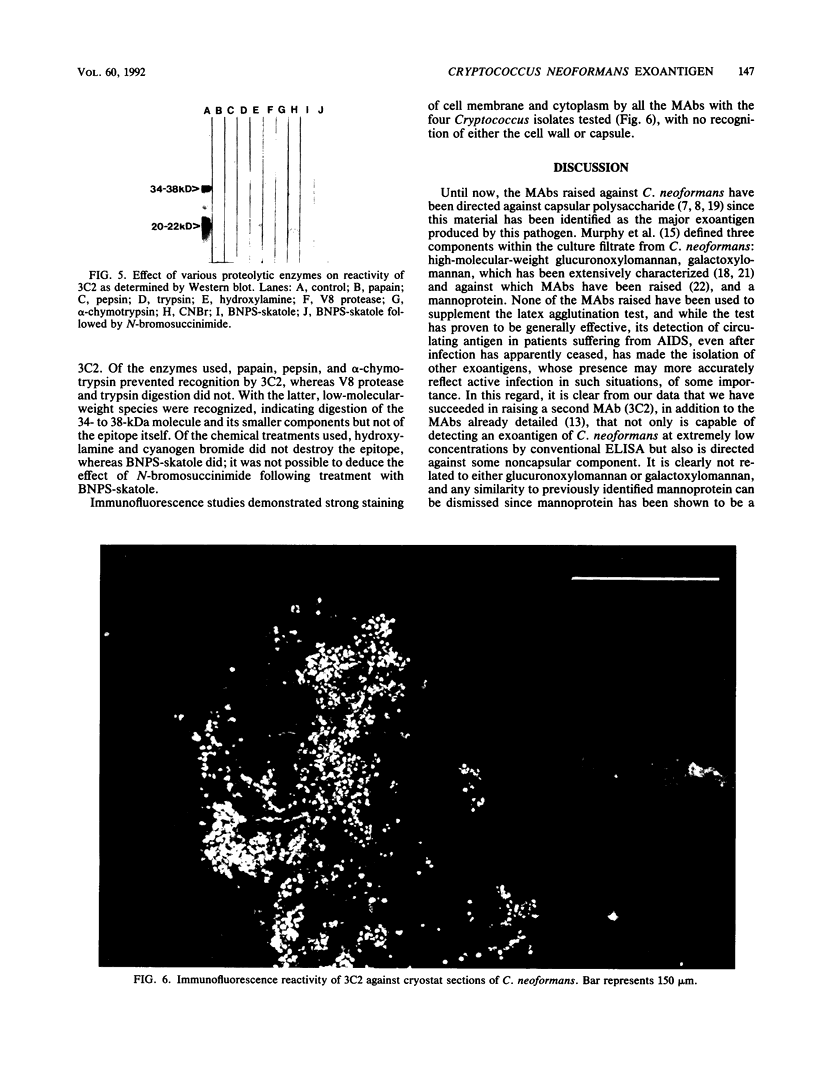
Three monoclonal antibodies (MAbs), all of the immunoglobulin G1 subclass, were raised against Cryptococcus neoformans by using the technique of cyclophosphamide ablation of B-cell responses against shared epitopes of the cross-reactive fungus Trichosporon beigelii. MAb 3C2 was reactive against the encapsulated and nonencapsulated isolates of C. neoformans var. neoformans by enzyme-linked immunosorbent assay (ELISA) and Western blot (immunoblot), and in addition to a 34- to 38-kDa determinant, it recognized a series of lower-molecular-weight species. 3C2 also reacted strongly with culture supernatant preparations of C. neoformans var. neoformans by ELISA. 3C2 showed no recognition of either T. beigelii or C. neoformans var. gattii antigens. Enzymatic deglycosylation followed by reaction with 3C2 on Western blots revealed that sialic acid was an integral part of the determinant, together with N-acetylglucosaminyl-asparagine and alpha-mannose. Proteolytic digestion showed that the epitope was pepsin sensitive and that it also contained tryptophan and glycine and/or leucine as determinants of recognition by 3C2. The pI of the glycoprotein was 7.1. Affinity chromatography-purified antigen did not exhibit proteolytic activity on sodium dodecyl sulfate-polyacrylamide substrate gels. Indirect fluorescence antibody tests revealed that 3C2 labelling was confined to the cell membrane and cytoplasm of yeasts. The remaining MAbs, 7H4 and 5G5, recognized both capsulated and nonencapsulated strains of C. neoformans var. neoformans by both ELISA and Western Blot, identifying linear determinants with molecular masses of 36 and 30 kDa. They were unreactive against culture supernatant antigen (exoantigen) from either variant of C. neoformans.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burkot T. R., Zavala F., Gwadz R. W., Collins F. H., Nussenzweig R. S., Roberts D. R. Identification of malaria-infected mosquitoes by a two-site enzyme-linked immunosorbent assay. Am J Trop Med Hyg. 1984 Mar;33(2):227–231. doi: 10.4269/ajtmh.1984.33.227. [DOI] [PubMed] [Google Scholar]
- Campbell C. K., Payne A. L., Teall A. J., Brownell A., Mackenzie D. W. Cryptococcal latex antigen test positive in patient with Trichosporon beigelii infection. Lancet. 1985 Jul 6;2(8445):43–44. doi: 10.1016/s0140-6736(85)90093-5. [DOI] [PubMed] [Google Scholar]
- Dismukes W. E. Cryptococcal meningitis in patients with AIDS. J Infect Dis. 1988 Apr;157(4):624–628. doi: 10.1093/infdis/157.4.624. [DOI] [PubMed] [Google Scholar]
- Dolan C. T. Specificity of the latex-cryptococcal antigen test. Am J Clin Pathol. 1972 Oct;58(4):358–364. doi: 10.1093/ajcp/58.5.358. [DOI] [PubMed] [Google Scholar]
- Dromer F., Salamero J., Contrepois A., Carbon C., Yeni P. Production, characterization, and antibody specificity of a mouse monoclonal antibody reactive with Cryptococcus neoformans capsular polysaccharide. Infect Immun. 1987 Mar;55(3):742–748. doi: 10.1128/iai.55.3.742-748.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert T. F., Kozel T. R. Production and characterization of monoclonal antibodies specific for Cryptococcus neoformans capsular polysaccharide. Infect Immun. 1987 Aug;55(8):1895–1899. doi: 10.1128/iai.55.8.1895-1899.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Figueroa J. I., Hamilton A. J., Bartholomew M. A., Harada T., Fenelon L., Hay R. J. Preparation of species-specific murine monoclonal antibodies against the yeast phase of Paracoccidioides brasiliensis. J Clin Microbiol. 1990 Aug;28(8):1766–1769. doi: 10.1128/jcm.28.8.1766-1769.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman J. S., Kaufman L., Koenig M. G. Diagnosis of cryptococcal meningitis. Value of immunologic detection of cryptococcal antigen. N Engl J Med. 1971 Aug 19;285(8):434–436. doi: 10.1056/NEJM197108192850804. [DOI] [PubMed] [Google Scholar]
- Hamilton A. J., Bartholomew M. A., Fenelon L. E., Figueroa J., Hay R. J. A murine monoclonal antibody exhibiting high species specificity for Histoplasma capsulatum var. capsulatum. J Gen Microbiol. 1990 Feb;136(2):331–335. doi: 10.1099/00221287-136-2-331. [DOI] [PubMed] [Google Scholar]
- Hamilton A. J., Bartholomew M. A., Figueroa J., Fenelon L. E., Hay R. J. Production of species-specific murine monoclonal antibodies against Cryptococcus neoformans which recognize a noncapsular exoantigen. J Clin Microbiol. 1991 May;29(5):980–984. doi: 10.1128/jcm.29.5.980-984.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker P. E., Hughes G. J., Wilson K. J. Peptide fragmentation suitable for solid-phase microsequencing. Use of N-bromosuccinimide and BNPS-skatole (3-bromo-3-methyl-2-[(2-nitrophenyl)thio]-3H-indole). Biochem J. 1980 May 1;187(2):515–519. doi: 10.1042/bj1870515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., Mosley R. L., Cherniak R., Reyes G. H., Kozel T. R., Reiss E. Serological, electrophoretic, and biological properties of Cryptococcus neoformans antigens. Infect Immun. 1988 Feb;56(2):424–431. doi: 10.1128/iai.56.2.424-431.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevost E., Newell R. Commercial cryptococcal latex kit: clinical evaluation in a medical center hospital. J Clin Microbiol. 1978 Nov;8(5):529–533. doi: 10.1128/jcm.8.5.529-533.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read S. M., Northcote D. H. Minimization of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Anal Biochem. 1981 Sep 1;116(1):53–64. doi: 10.1016/0003-2697(81)90321-3. [DOI] [PubMed] [Google Scholar]
- Reiss E., Cherniak R., Eby R., Kaufman L. Enzyme immunoassay detection of IgM to galactoxylomannan of Cryptococcus neoformans. Diagn Immunol. 1984;2(2):109–115. [PubMed] [Google Scholar]
- Spiropulu C., Eppard R. A., Otteson E., Kozel T. R. Antigenic variation within serotypes of Cryptococcus neoformans detected by monoclonal antibodies specific for the capsular polysaccharide. Infect Immun. 1989 Oct;57(10):3240–3242. doi: 10.1128/iai.57.10.3240-3242.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner S. H., Cherniak R., Reiss E. Fractionation and characterization of galactoxylomannan from Cryptococcus neoformans. Carbohydr Res. 1984 Feb 15;125(2):343–349. doi: 10.1016/0008-6215(84)85172-1. [DOI] [PubMed] [Google Scholar]
- Vartivarian S. E., Reyes G. H., Jacobson E. S., James P. G., Cherniak R., Mumaw V. R., Tingler M. J. Localization of mannoprotein in Cryptococcus neoformans. J Bacteriol. 1989 Dec;171(12):6850–6852. doi: 10.1128/jb.171.12.6850-6852.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Moer A., Salhi S. L., Cherniak R., Pau B., Garrigues M. L., Bastide J. M. An anti-Cryptococcus neoformans monoclonal antibody directed against galactoxylomannan. Res Immunol. 1990 Jan;141(1):33–42. doi: 10.1016/0923-2494(90)90099-k. [DOI] [PubMed] [Google Scholar]