Abstract
Dendritic cells (DCs) are among the first immune cells to encounter HIV-1 at the initial infection. DCs efficiently transfer HIV-1 to CD4+ T cells via infectious or virological synapses formed between DCs and T cells. Retroviruses exploit the cytoskeletal network to facilitate viral infection and dissemination; however, the role of the cytoskeleton in DC-mediated HIV-1 transmission is unknown. Here, we report that intact cytoskeleton is essential for DC-mediated HIV-1 transmission to CD4+ T cells. We found that macropinocytosis of HIV-1 contributes to DC-mediated HIV-1 endocytosis and transmission. Blocking HIV-1 macropinocytosis and disrupting actin or microtubules in DCs with specific inhibitors significantly prevented DC-mediated HIV-1 trans-infection of CD4+ T cells. Altered HIV-1 trafficking and impaired formation of virological synapses primarily accounted for the inhibition of viral transmission by cytoskeletal inhibitors. Our results provide new insights into the mechanisms underlying DC-mediated HIV-1 transmission to CD4+ T cells via the cytoskeletal network.
Keywords: HIV-1, dendritic cells, transmission, cytoskeleton, macropinocytosis, CD4+ T cells
Introduction
Dendritic cells (DCs) are among the first immune cells to encounter HIV-1 (referred to subsequently as HIV) at the initial infection, they play an important role in viral pathogenesis (Cameron et al., 1992; Cameron et al., 2007; Hladik et al., 2007; Pope et al., 1994). Defining the mechanisms of DC-mediated HIV transmission can enhance our understanding of viral pathogenesis (Piguet and Steinman, 2007; Wu and KewalRamani, 2006). DCs efficiently transfer HIV to CD4+ T cells via infectious or virological synapses (VS) (Arrighi et al., 2004; Ganesh et al., 2004; Garcia, Nikolic, and Piguet, 2008; Garcia et al., 2005; Hladik et al., 2007; McDonald et al., 2003; Turville et al., 2004; Wang et al., 2007b), which are the synaptic junctions of DC-T-cells with concentrated HIV and viral receptors (McDonald et al., 2003). Immature DCs (iDCs) in submucosal tissues can capture HIV and migrate to lymphoid tissues, where they become mature DCs (mDCs) and effectively spread viral infection to CD4+ T cells [reviewed in (Wu, 2008; Wu and KewalRamani, 2006)]. Notably, mDCs significantly facilitate HIV endocytosis and efficiently concentrate HIV at VS (Garcia et al., 2005; Izquierdo-Useros et al., 2007; Turville et al., 2004; Wang et al., 2007b), which contributes to mDC-enhanced viral transmission, at least in part. However, the mechanisms underlying DC-mediated HIV transmission remain to be elucidated.
Retroviruses exploit the cytoskeletal network to facilitate viral infection and dissemination (Fackler and Krausslich, 2006; Naghavi and Goff, 2007). Understanding the role of cytoskeleton in cell-cell transmission of HIV can potentially aid in the development of interventions against HIV infection. Efficient HIV spread between CD4+ T cells occurs via actin-dependent VS (Chen et al., 2007; Jolly et al., 2004; Jolly, Mitar, and Sattentau, 2007). Recent studies suggest that actin-dependent filopodial bridges and intercellular membrane nanotubes mediate cell-to-cell transmission of retroviruses (Sherer et al., 2007; Sowinski et al., 2008). However, the role of the cytoskeleton in DC-mediated HIV transmission is unclear.
HIV trafficking in DCs contributes to viral transmission to CD4+ T cells (Fahrbach et al., 2007; Garcia et al., 2005; Izquierdo-Useros et al., 2007; McDonald et al., 2003; Trumpfheller et al., 2003; Turville et al., 2004; Wang et al., 2007b; Wiley and Gummuluru, 2006). DCs endocytose HIV or simian immunodeficiency virus (SIV) through various pathways, including clathrin-mediated endocytosis (Frank et al., 2002; Garcia et al., 2005; Wang et al., 2007b), receptor-mediated endocytosis (de Witte et al., 2007; Kwon et al., 2002), and likely macropinocytosis (Frank et al., 2002; Turville et al., 2004; Wang et al., 2007b). Macropinocytosis is an actin-dependent process by which DCs constitutively engulf large volumes of fluid and whole pathogens (Falcone et al., 2006; Marsh and Helenius, 2006; Sallusto et al., 1995; Wu and KewalRamani, 2006). Interestingly, mDCs are more efficient than iDCs in endocytosing HIV or SIV (Frank et al., 2002; Turville et al., 2004; Wang et al., 2007b). The large intracellular compartments that confine numerous HIV or SIV particles in mDCs (Frank et al., 2002; Turville et al., 2004; Wang et al., 2007b) appeared similar to macropinocytosis-mediated HIV entry into macrophages and brain microvascular endothelia (Liu et al., 2002; Maréchal et al., 2001). However, the role of macropinocytosis in DC-mediated HIV transmission is currently unknown.
In this study, we report that intact cytoskeleton is essential for DC-mediated HIV transmission to CD4+ T cells. Blocking actin-dependent macropinocytosis of HIV and disrupting actin or microtubules in DCs with specific inhibitors significantly inhibited DC-mediated HIV transmission. The inhibition of HIV transmission was mainly due to altered viral trafficking and impaired VS formation. These findings provide new insights into the mechanisms underlying DC-mediated HIV transmission to T cells, suggesting that reversible disruption of cytoskeleton by specific inhibitors could be considered as a potential therapeutic approach against HIV infection.
Results
F-actin and macropinocytosis contribute to DC endocytosis of HIV
HIV enters DCs primarily through endocytosis rather than the viral receptor-mediated fusion pathway (Cavrois et al., 2006; Dong et al., 2007; Janas et al., 2008). To measure HIV endocytosed in DCs, iDCs and lipopolysaccharide (LPS)-induced mDCs were pulsed with single-cycle, luciferase reporter HIV (HIV-Luc/JRFL) in the presence of specific inhibitors at various concentrations. These inhibitors included cytochalasin D (CytoD), an actin-depolymerizing drug (Fenteany and Zhu, 2003), nocodazole, an inhibitor of the polymerization of tubulin into microtubules (Fenteany and Zhu, 2003), and dimethyl amiloride (DMA), a macropinocytosis-specific inhibitor that does not affect receptor-mediated endocytosis (Maréchal et al., 2001; Sallusto et al., 1995). Dimethyl sulfoxide (DMSO) was used as a solvent control.
HIV-pulsed DCs were trypsinized after extensive washes to strip cell surface-bound HIV, and the Gag p24 in DCs lysates was quantified. The treatment with CytoD at a concentration of 0.5 μM decreased DC-associated p24 in iDCs and mDCs by 33% and 28% (P ≤ 0.004), respectively (Fig. 1A). In contrast, at higher concentrations (3 and 5 μM), CytoD treatment slightly increased p24 in iDCs by 2–13%, and in mDCs by 10–30%, suggesting different functions of CytoD at various concentrations. Nocodazole treatment of DCs did not reduce intracellular HIV p24 (Fig. 1B), indicating that HIV endocytosis in DCs is not decreased by disruption of microtubules. Interestingly, various concentrations of DMA treatment diminished HIV endocytosis in iDCs by 40–48% (P ≤ 0.001), and in mDCs by 32–48% (P ≤ 0.003) (Fig. 1C). Together, these data imply the involvement of F-actin and macropinocytosis in DC endocytosis of HIV, suggesting that F-actin-dependent macropinocytosis is not the only pathway that mediates HIV endocytosis in DCs. Indeed, other pathways such as clathrin-mediated HIV endocytosis in DCs have been previously reported (Frank et al., 2002; Wang et al., 2007b).
Fig. 1.

F-actin and macropinocytosis contribute to DC endocytosis of HIV. DCs (1×105) were pretreated separately (A) CytoD, (B) nocodazole, and (C) DMA at various concentrations as indicated, pulsed with HIV-Luc/JRFL (45 ng of p24) in the presence of DMSO (control in A-C) or the appropriate inhibitors. DCs were then washed, trypsinized and lysed for HIV Gag p24 quantification. Data shown are means ± SD (n = 3).
DC-mediated HIV transmission is dependent on cytoskeleton
To examine whether disruption of actin cytoskeleton prevents DC-mediated viral transmission, DCs were pulsed with small amounts of HIV-Luc/JRFL in the presence of CytoD, then washed and cocultured with CD4+ Hut/CCR5 T cells for measuring viral transmission. R5-tropic HIV was used in viral transmission assays given that DC-mediated infection and transmission of X4-tropic HIV is lower than that of R5-tropic HIV (Cameron et al., 2007; Granelli-Piperno et al., 1998; Pion et al., 2007; Smed-Sorensen et al., 2005; Wang et al., 2007a). Significantly, treatment of DCs with CytoD at various concentrations blocked mDC-mediated HIV transmission to CD4+ T cells by 67–82% (P ≤ 0.00003), while iDC-mediated viral transmission decreased by 28–48% (P ≤ 0.02) (Fig. 2A).
Fig. 2.

Cytoskeleton and HIV macropinocytosis contribute to DC-mediated HIV transmission. (A) CytoD, (B) nocodazole, and (C) DMA treatment of DCs inhibit DC-mediated HIV transmission to Hut/CCR5 cells. (D) Primary CD4+ T cells and (E) PBLs were used as target cells in the HIV transmission assays. DCs were pretreated with DMSO or various inhibitors, pulsed with HIV-Luc/JRFL (0.2 MOI) in the presence of DMSO or the appropriate inhibitors. DCs were washed, and then cocultured with target cells to measure viral transmission by detecting the luciferase activity in cell lysates. (F) Post-treatment of HIV-pulsed DCs with CytoD and nocodazole blocks DC-mediated HIV transmission to CD4+ T cells. DCs were pulsed with HIV-Luc/JRFL (0.2 MOI), and then treated with the inhibitors, washed, and cocultured with Hut/CCR5 cells in viral transmission assays. cps, counts per second. (G) and (H) Transient treatment of CytoD, DMA and nocodazole does not affect DC viability. DCs were treated separately with DMSO or inhibitors at 37°C for 2.5 h, washed and cultured for 3 days before the detection of viability. All data shown are means ± SD (n ≥ 3).
To examine the role of microtubules in DC-mediated HIV transmission, nocodazole was used to disrupt the microtubule network. Pretreatment of DCs with nocodazole dramatically blocks iDC- and mDC-mediated HIV transmission by 89–94% (P ≤ 0.000002) (Fig. 2B). As a negative control, no viral infection was detected in HIV-pulsed DCs without T cells (Fig. 2B, DC alone), indicating that the infection was from DC-mediated trans-infection of CD4+ T cells, rather than cis-infection of DCs. Thus, disrupting cytoskeleton inhibits DC-mediated HIV transmission.
Interestingly, blocking HIV macropinocytosis with DMA efficiently inhibited mDC-mediated HIV transmission by 46–88% (P ≤ 0.0008), whereas iDC-mediated viral transmission decreased by 34–70% (P ≤ 0.004) (Fig. 2C). The DMA inhibitory effect of iDC- and mDC-mediated HIV transmission was dose-dependent (Fig. 2C), suggesting that macropinocytosis-mediated HIV entry into DCs plays an important role in viral transmission, at least in part.
To confirm the results obtained using the allogeneic CD4+ T cell line Hut/CCR5, primary CD4+ T cells and peripheral blood lymphocytes (PBLs) were used as autologous target cells in DC-mediated HIV transmission assays. Separate treatment of DCs with CytoD and nocodazole blocked DC-mediated HIV transmission to activated primary CD4+ T cells by 71–97% (P ≤ 0.0001) (Fig. 2D), while the viral transmission to activated PBLs was reduced by 45–57% (P ≤ 0.002) (Fig. 2E). DMA treatment impaired DC-mediated HIV transmission to primary CD4+ T cells (Fig. 2D) and PBLs (Fig. 2E) by 28–47% (P ≤ 0.05) and 39–50% (P ≤ 0.01), respectively. Moreover, post-treatment of HIV-pulsed DCs with CytoD or nocodazole significantly impaired DC-mediated HIV transmission by 4–8-fold (P < 0.001) (Fig. 2F). As a control, the treatment of DCs with CytoD, nocodazole and DMA for 2.5 h did not affect DC viability after 3 days in culture (Fig. 2G, 2H). Together, these data suggest that intact cytoskeleton network is required for DC-mediated HIV transmission to CD4+ T cells.
Disruption of the cytoskeleton in DC-T-cell cocultures inhibits DC-mediated HIV transmission
To examine whether disruption of the cytoskeleton in DC-T-cell cocultures blocks HIV transmission to CD4+ T cells, DCs were pulsed with HIV-Luc/JRFL, washed and cocultured with Hut/CCR5 cells in the presence of CytoD or nocodazole for 5, 30, 60 and 120 min. Cells were then washed and cultured for 3 days before measuring HIV infection. CytoD treatment at the different time efficiently reduced DC-mediated HIV transmission 7–8-fold (P ≤ 0.0001) (Fig. 3A). No viral infection was detected in HIV-pulsed DCs without T cell coulture (not shown). As a parallel control, HIV infection of T cells decreased around 2-fold by the CytoD treatment, which is significantly lower than the inhibition of DC-mediated viral transmission (P < 0.0001) (Fig. 3B). Similar results and statistical analysis were obtained when replication-competent HIVNLAD8 was examined (Fig. 3C and 3D).
Fig. 3.
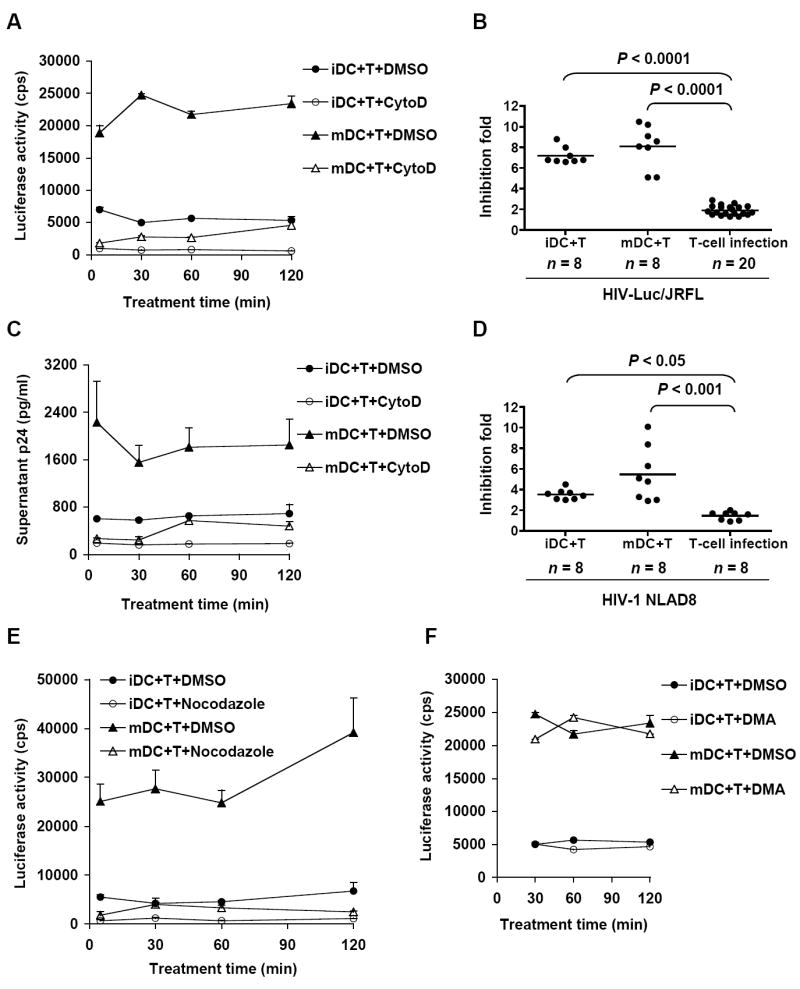
Disruption of the cytoskeleton in DC-T-cell cocultures inhibits DC-mediated HIV transmission. (A) and (C) CytoD treatment of DC-T-cell cocultures blocks HIV trans-infection of CD4+ T cells. DCs (3×105) were pulsed separately with HIV-Luc/JRFL (0.2 MOI) (A) or HIV-1NL-AD8 (40 ng of p24) (C), and cocultured with Hut/CCR5 cells in the presence or absence of CytoD for the indicated time. Cells were then washed and cocultured for an additional 3 days. HIV transmission was determined by measuring luciferase activity in cell lysates (A) or HIV p24 in supernatants (C). (B) and (D) Statistical analysis of the CytoD inhibition of DC-mediated HIV trans-infection compared with that of cis-infection of T cells. DC-mediated HIV transmission (iDC+T and mDC+T) was performed as described in A and C. In parallel, HIV cis-infection of Hut/CCR5 cells (T-cell infection) in the presence of CytoD was detected 3 days post-infection. Data shown are means of each time point from at least 3 independent experiments. Each dot represents one sample result from multiple independent experiments. (E) Nocodazole, but not (F) DMA in DC-T-cell cocultures blocks DC-mediated HIV transmission. HIV transmission assays were performed as described in (A). cps, counts per second. Data shown are means ± SD (n ≥ 3).
Moreover, significant inhibition of DC-mediated HIV transmission was observed when nocodazole was present in DC-T-cell cocultures (Fig. 3E). In contrast, the transient nocodazole treatment had no significant effect on HIV infection of CD4+ T cells (data not shown), consistent with a previous report indicating that HIV infection does not require microtubules (Bukrinskaya et al., 1998). As a control, DMA treatment in the T-cell coculture with HIV-pulsed DCs for 0.5~ 2 hr did not affect viral transmission (Fig. 3F), confirming that DMA inhibition of HIV transmission requires pretreatment of DCs to block macropinocytosis-mediated viral entry. Thus, disruption of cytoskeleton in DC-T-cell coculture efficiently inhibits DC-mediated HIV transmission to CD4+ T cells.
Cytoskeleton inhibitors alter HIV trafficking in mDCs
To investigate the mechanisms by which disruption of the cytoskeleton inhibits DC-mediated HIV transmission, viral trafficking were examined using Vpr-GFP-tagged replication-competent HIV (HIV-Vpr-GFP) (McDonald et al., 2002). Fewer HIV-Vpr-GFP were observed on the iDC surface or internalized in iDCs, while a large number of viral particles were internalized and concentrated in the cytosol of mDCs (Fig. 4A, left). These results confirm that mDCs significantly facilitate HIV endocytosis, consistent with the previous observations (Fahrbach et al., 2007; Frank et al., 2008; Garcia et al., 2005; McDonald et al., 2003; Turville et al., 2004; Wang et al., 2007b).
Fig. 4.
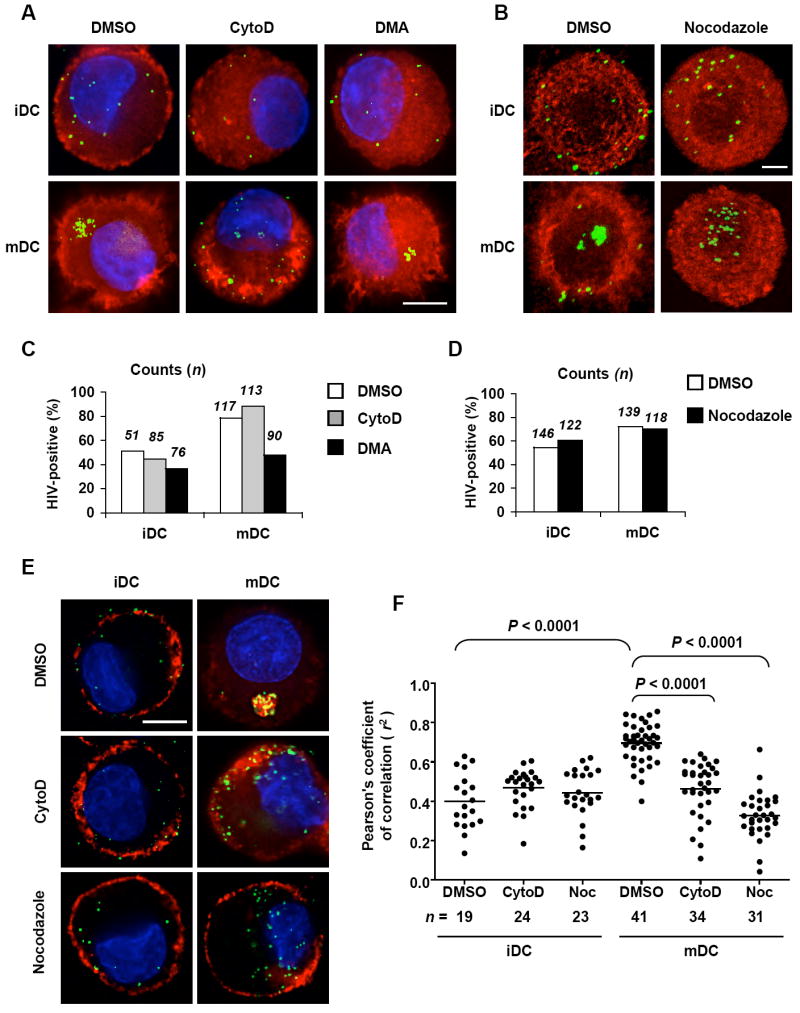
Cytoskeleton inhibitors alter HIV trafficking in mDCs. (A) CytoD treatment alters HIV trafficking in mDCs. DCs were pretreated with DMSO, CytoD, or DMA and pulsed with HIV-Vpr-GFP (green) (40 ng of p24) in the presence of the appropriate inhibitors. DCs were fixed and then stained for F-actin (red, phalloidin) and nucleus (blue, DAPI). (B) Nocodazole treatment alters HIV trafficking in mDCs. DCs were pulsed with HIV-Vpr-GFP (green) (40 ng of p24) in the presence of DMSO or nocodazole. DCs were fixed and stained for α-tubulin (red) and analyzed by confocal microscopy. Quantitative image analysis of HIV-positive DCs in the presence or absence of (C) CytoD, DMA, and (D) nocodazole. DCs were counted based on the experiments described in A and B. (E) Localization of CD81 and HIV in DCs treated with CytoD or nocodazole. DCs were pulsed separately with HIV-Vpr-GFP (green) (40 ng of p24) in the presence of DMSO or the inhibitors and stained for CD81 (red). (F) Pearson’s correlation analysis of HIV colocalization with CD81 in DCs. The analysis was based on the experiment described in E. The dots represent the r2 values of Pearson coefficient derived from individual DC images. Noc, nocodazole. Scale bars, 5 μm (A and E), and 3 μm (B).
Treatment of DCs with CytoD, but not DMA, resulted in depolarized F-actin in DCs and altered HIV trafficking (Fig. 4A, middle and right). The altered HIV trafficking was more profound in mDCs than that in iDCs, as viruses became diffused and localized more close to the plasma membrane of mDCs (Fig. 4A, middle). Furthermore, nocodazole treatment altered microtubule organization in DCs (Fig. 4B). The altered HIV trafficking in mDCs was also observed as HIV particles became diffusely localized closer to the plasma membrane of mDCs (Fig. 4B). Quantitative image analysis indicated that DMA treatment reduced HIV-positive iDCs and mDCs by 14% and 30% (P < 0.05), respectively, while the treatment with CytoD or nocodazole did not significantly alter the number of HIV-positive DCs (Fig. 4C and 4D). These data are consistent with the quantification results of DC-associated HIV p24 (Fig. 1).
HIV targets the CD81-rich compartment in mDCs and spreads to the DC-T-cell VS via a tetraspanin-sorting pathway (Fahrbach et al., 2007; Garcia, Nikolic, and Piguet, 2008; Garcia et al., 2005; Izquierdo-Useros et al., 2007). Notably, separate treatment with CytoD and nocodazole drastically disrupted HIV colocalization with CD81 in mDCs (Fig. 4E). Correlation analysis of the colocalization images indicated that the treatment of CytoD and nocodazole significantly impaired the colocalization of HIV with CD81 in mDCs (P < 0.0001) (Fig. 4F). The colocalization of CD81 and HIV in mDCs was significantly higher than that in iDCs (P < 0.0001) (Fig. 4F). Thus, disruption of the cytoskeleton alters HIV trafficking in mDCs, which likely contributes to the inhibition of viral transmission.
Examination of HIV trafficking in mDCs treated with various inhibitors by electron microscopy (EM)
To better visualize HIV trafficking within mDCs, transmission EM was used to examine mDCs pulsed with aldrithiol-2 (AT-2)-inactivated HIV in the presence of the macropinocytosis and cytoskeleton inhibitors. Previous studies have shown that AT-2-inactivated HIV is conformationally authentic and interacts with DCs similarly to infectious viruses (Frank et al., 2002; Frank et al., 2008; Turville et al., 2004; Wang et al., 2007b). Using ruthenium red labeling of plasma membranes, our recent EM studies demonstrated that significantly more HIV particles were internalized in the intracellular compartments in mDCs compared with those in iDCs (Wang et al., 2007b).
In addition to some surface-associated HIV, numerous intact HIV particles were observed in macropinosome-like compartments within mDCs in the presence of DMSO (Fig. 5A), which is consistent with our previous observations (Wang et al., 2007b). By contrast, DMA treatment significantly altered HIV trafficking, as fewer HIV particles were observed in endocytic compartments (Fig. 5B) that were generally much smaller. CytoD treatment appeared to result in diffused HIV distribution in numerous endocytic compartments (Fig. 5C). Nocodazole treated mDCs showed depolymerized microtubules and fewer HIV particles in HIV-containing compartments relative to the DMSO control (Fig. 5D).
Fig. 5.

Examination of HIV trafficking in mDCs treated with various inhibitors by EM. mDCs (6 × 105) were incubated with AT-2-inactivated HIV (2 μg of p24) at 37°C for 2 h in the presence of (A) DMSO, (B) DMA (C) CytoD, (D) nocodazole, DCs were then washed, fixed and processed for thin sections of conventional transmission electron microscopy. The black arrowheads point to HIV particles. Scale bars, 0.5 μm (A, B, and D), 1 μm (C). (E) Quantitative analysis of EM results. The numbers of mDCs with HIV compartments in 100 cells from each sample (left), and the numbers of cells that had compartments with greater than 5 virions per compartment (right) were labeled.
To better compare the effects of the inhibitor on HIV trafficking, quantitative analysis of EM images was performed. In each sample, 100 cells were imaged and scored for presence of virus within internal compartments. Additionally, the number of HIV-containing cells that had compartments with greater than 5 virions per compartment were counted. Compared with DMSO control, DMA treatment significantly reduced HIV entry into mDCs (P < 0.001) (Fig. 5E, left), and the inhibitor treatment largely altered HIV distribution in mDCs (Fig. 5E, right). These EM results suggest that, in mDCs, macropinocytosis of HIV is a major viral entry pathway, and that cytoskeletal inhibitors can alter HIV trafficking.
CytoD treatment of DCs transiently impairs the formation of DC-T-cell clustering
Our recent results indicate that DC-T-cell contact is required for DC-mediated HIV transmission (Wang et al., 2007b). To examine whether DC-T-cell clustering is impaired by the treatment of CytoD, nocodazole, or DMA, DCs were pretreated with these inhibitors, and pulsed with HIV-Luc/JRFL in the presence of the appropriate inhibitors. DCs were then cocultured with Hut/CCR5 cells and examined for DC-T-cell clustering. The transient treatment of HIV-pulsed DCs with CytoD, but not nocodazole and DMA, strongly blocked the formation of DC-T-cell clustering after 1 h in cocultures (Fig. 6). However, the impaired DC-T-cell clustering of CytoD-treated DCs re-established after 5-7 h in cocultures (Fig. 6), suggesting that CytoD treatment is reversible. Nevertheless, the impaired DC-T-cell clustering by CytoD treatment likely also contributed to the decreased HIV trans-infection.
Fig. 6.
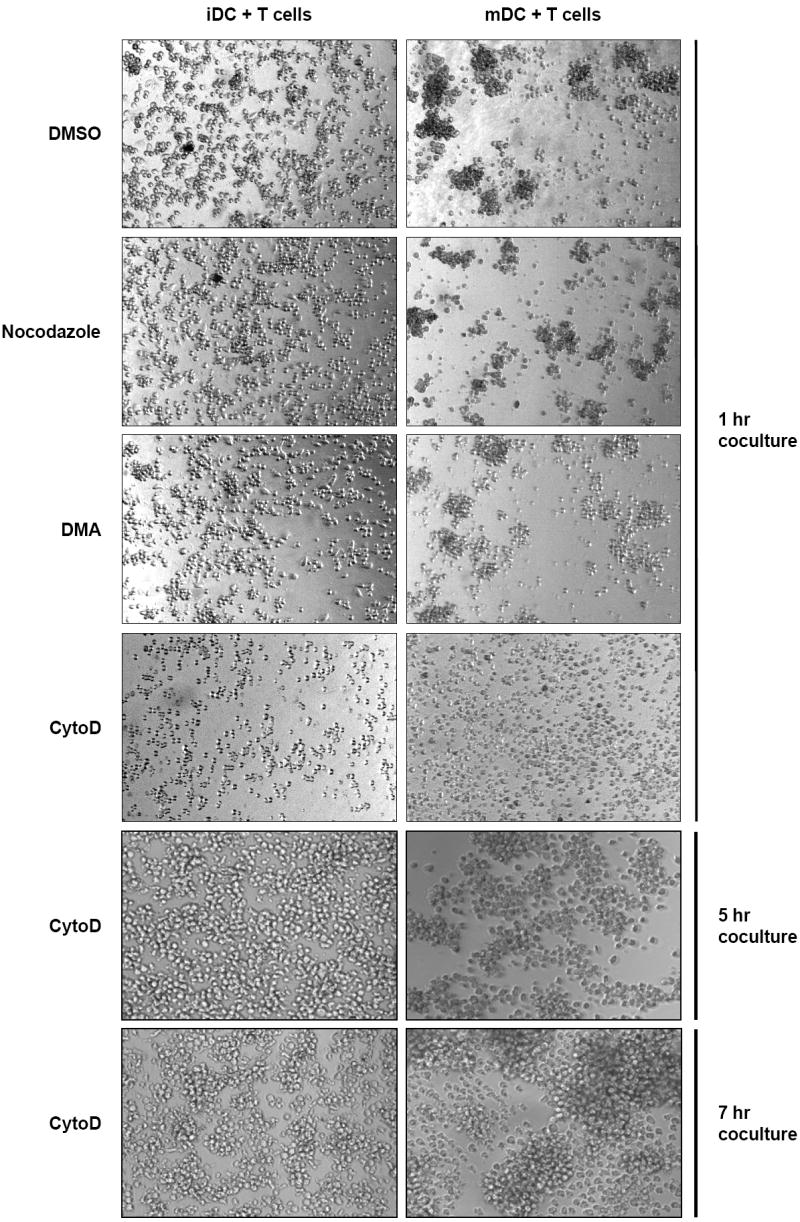
CytoD treatment of DCs transiently impairs the formation of DC-T-cell clustering. DCs were pretreated with DMSO, CytoD, nocodazole, or DMA, and pulsed with HIV-Luc/JRFL (0.2 MOI) in the presence of DMSO or the appropriate inhibitors. DCs were then cocultured with Hut/CCR5 cells for indicated time and examined by a light microscope. DC-T-cell clustering appears as dark aggregates of cells. Original magnifications, 40X.
Disrupting the cytoskeleton impairs the formation of VS
To examine if the disruption of the cytoskeleton impairs VS formation, HIV-Vpr-GFP-pulsed DCs were cocultured with CD4+ T cells in the presence or absence of the cytoskeleton inhibitors, and the VS were visualized by immunofluorescence microscopy. More HIV particles were recruited to mDC-T-cell junctions than those to iDC-T-cell junctions (Fig. 7A and 7B, upper). Intriguingly, treatment with CytoD (Fig. 7A) and nocodazole (Fig. 7B) strongly impaired VS formation between DCs and T cells. Quantitative image analysis of the DC-T-cell conjugates revealed that the CytoD and nocodazole treatment significantly impaired VS formation by 36–51% (P ≤ 0.001) (Fig. 7C and 7D, comparing the percentages of the “type a” conjugates in the presence or absence of the inhibitors). In addition, these observations were confirmed using autologous DCs and PBLs with specific staining of the T-cell marker CD3. Similar results were also obtained when DCs were treated with CytoD and nocodazole before or after HIV incubation (Wang and Wu, unpublished data). Thus, intact cytoskeleton is essential for forming the VS and enhancing DC-mediated HIV transmission.
Fig. 7.
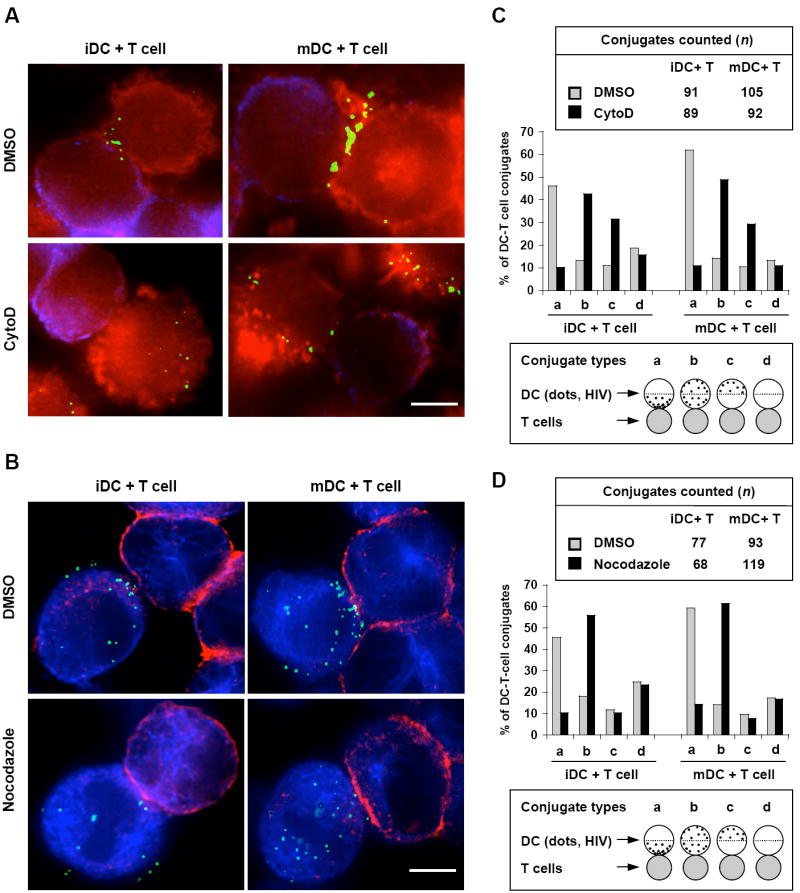
Disruption of the cytoskeleton impairs the formation of VS. (A) Actin disruption impairs VS formation between DCs and CD4+ T cells. DCs were pulsed with HIV-Vpr-GFP (green) (40 ng of p24), washed and cocultured with Hut/CCR5 cells in the presence of DMSO or CytoD. Cells were then fixed, stained for F-actin (red, phalloidin) and HA-tagged CCR5 (blue/purple) of Hut/CCR5 cells. (B) Nocodazole treatment of DCs impairs VS formation. DCs were pretreated with DMSO or nocodazole, and then pulsed separately with HIV-Vpr-GFP (green) (40 ng of p24) in the presence of DMSO or nocodazole. After washes, DCs were cocultured with Hut/CCR5 cells, fixed and stained for α-tubulin (blue) and HA-CCR5 (red) of Hut/CCR5 cells. Scale bars, 5 μm (A and B). (C) and (D) Quantitative image analysis of the DC-T-cell conjugates and the VS. DC-T-cell conjugates in C and D were counted and analyzed according to the experiments described in (A) and (B), respectively.
Discussion
Examining the role of the cytoskeleton in DC-mediated HIV transmission enriches our understanding of viral pathogenesis. The present study suggests that HIV exploits the cytoskeleton network of DCs to facilitate viral dissemination to CD4+ T cells through VS. We showed that disrupting actin or microtubules in DCs with specific inhibitors significantly inhibited DC-mediated HIV transmission. The inhibition of HIV transmission was mainly due to altered viral trafficking and impaired formation of VS. Our results define a new mechanism underlying DC-mediated HIV transmission to T cells through the cytoskeleton network.
In our experiments, transient treatment of cells with the cytoskeleton inhibitors appeared to disrupt the cytoskeleton network, rather than directly affecting HIV infectivity. It has been shown that the pretreatment of peripheral blood mononuclear cells, but not HIV, with CytoD, inhibited viral entry and infection (Iyengar, Hildreth, and Schwartz, 1998). In addition, Campbell et al. observed a modest increase of HIV infection when treating HeLa cell-derived HIV indicator cells with CytoD and performing viral infection in the presence of CytoD (Campbell, Nunez, and Hope, 2004). Although no significant cytotoxicity was detected with the transient treatment of the cytoskeleton inhibitors, these inhibitors may have broad or pleiotropic effects on intracellular trafficking and mechanical properties of cells. For example, the drug treatment may nonspecifically alter trafficking or function of some cellular proteins that are required for VS formation. Thus, further studies might provide the mechanism details underlying the role of cytoskeleton in DC-mediated HIV trans-infection.
Recent studies also suggest an important role of cytoskeleton in DC-mediated HIV transmission. Leukocyte-specific protein 1 (LSP1), an F-actin binding protein involved in leukocyte motility, binds to DC-SIGN (for “DC-specific intercellular adhesion molecule 3 [ICAM-3]-grabbing nonintegrin”) and directs internalized HIV to the proteasome in DCs for viral degradation (Smith et al., 2007). Silencing LSP1 expression in DCs promotes HIV transmission to CD4+ T cells, suggesting that HIV trafficking through the cytoskeleton is important for viral transmission (Smith et al., 2007). Moreover, HIV or DC-SIGN-specific antibodies can activate DC-SIGN signaling through the leukemia-associated Rho guanine nucleotide-exchange factor (LARG), which increases Rho-GTPase activity (Hodges et al., 2007). Activation of LARG in DCs facilitates HIV trans-infection of CD4+ T cells, likely by enhancing VS formation between DCs and T cells (Hodges et al., 2007).
Notably, different concentrations of CytoD treatment had distinct effects on DC uptake of HIV (Fig. 1A), which might result from the distinct inhibitory functions of CytoD at different concentrations. CytoD reversibly binds the barbed end of F-actin at low concentrations and prevents F-actin recycling. At higher concentrations, CytoD binds 2 actin monomers (G-actin) and promotes the hydrolysis of bound ATP, creating low affinity ADP-G actin forms (Fenteany and Zhu, 2003). In fact, CytoD treatment has concentration-dependent effects on the mechanical properties of the cells (Wakatsuki et al., 2001), which might influence HIV binding to DCs and viral trafficking in DCs. Moreover, higher concentrations of CytoD treatment of DCs might reduce or delay intracellular degradation of internalized HIV, resulting in intracellular retention of HIV and increased p24 detection.
We demonstrated that macropinocytosis partially contributed to HIV endocytosis in DCs, thereby affecting DC-mediated HIV transmission to CD4+ T cells. Gummuluru et al. have reported the existence of C-type lectin receptor-independent and heparan sulfate proteoglycan-independent mechanisms of DC-mediated HIV internalization and transmission (Gummuluru et al., 2003). We recently reported that mDCs are more efficient than iDCs to transfer HIV to various types of target cells independently of C-type lectins (Wang et al., 2007b). Previous studies indicated that mDCs significantly facilitate HIV endocytosis and efficiently concentrate HIV at the VS, which contributes to mDC-enhanced viral transmission, at least in part (Turville et al., 2004; Wang et al., 2007b). We have confirmed that HIV did not strongly colocalize with the markers of endolysosomal trafficking in DCs, such as transferrin and LysoTracker (Wang and Wu, unpublished results). These results are in accordance with previous observations (Fahrbach et al., 2007; Garcia, Nikolic, and Piguet, 2008; Garcia et al., 2005; Izquierdo-Useros et al., 2007; Trumpfheller et al., 2003; Turville et al., 2004), suggesting that HIV trafficking in DCs is not through the conventional endolysosomal pathway. Based on these published results and our present data, we propose that macropinocytosis-mediated HIV endocytosis in DCs, particularly in LPS-induced mDCs, may result in the formation and trafficking of macropinosomes that confine concentrated viral particles. Upon DC-T-cell contact, macropinosome-confined HIV in DCs might recycle to the cell surfaces and transfer to cocultured CD4+ T cells. Further studies of DC-mediated HIV transmission process using live cell images are carried out to examine this hypothesis.
Using dextran as a marker of endocytosis in DCs, previous studies reported that macropinocytosis in mDCs is significantly decreased relative to that in iDCs (Frank et al., 2002; Izquierdo-Useros et al., 2007; Sallusto et al., 1995). Indeed, we confirmed that uptake of fluorescein isothiocyanate-labeled dextran in iDCs was higher than that in mDCs (Wang and Wu, unpublished results). The differing efficiencies of HIV and dextran endocytosis in iDCs and mDCs might result from their distinct interactions. It has been shown that macropinocytosis and multilectin receptor-mediated endocytosis contribute to the uptake of dextran by DCs (Kato et al., 2000; Sallusto et al., 1995). DC maturation downregulates the cell-surface expression levels of some C-type lectin receptors, including mannose receptor and DC-SIGN (DC-specific intercellular adhesion molecule-3 grabbing nonintegrin) (Sallusto et al., 1995; Wang et al., 2007b). The downregulated receptors in mDCs might contribute to the decreased uptake of dextran relative to iDCs. Thus, it is important to note that dextran cannot be a marker for HIV endocytosis in DCs
Our previous study (Wang et al., 2007b) and the present results suggest that both cell surface-bound and internalized HIV contribute to DC-mediated viral transmission. Compared with iDC-mediated HIV transmission, viral trafficking in mDCs appears to play a more important role in trans-infection (Fahrbach et al., 2007; Garcia et al., 2005; Izquierdo-Useros et al., 2007; Turville et al., 2004; Wang et al., 2007b). By contrast, Cavrois et al. suggested that DC-mediated HIV trans-infection mainly derives from DC surface-bound virions (Cavrois, Neidleman, and Greene, 2008; Cavrois et al., 2007). Despite different approaches used in these studies, the dynamic trafficking and recycling of internalized HIV to DC surfaces could also mediate viral transmission, which should be an important consideration in DC-mediated HIV trans-infection. In addition, Cavrois et al. proposed that trypsin might be less potent than pronase at removing DC surface-bound HIV, but failed to present any results (Cavrois et al., 2007). By contrast, our comparison study indicated that pronase treatment (250 μg/ml) reduced iDC- and mDC-mediated HIV transmission by 48% and 24%, respectively (Wang et al., 2007b). These data were comparable to those of trypsin treatment at the same concentration (Wang et al., 2007b), suggesting that both trypsin and pronase may strip surface HIV from DCs with similar efficiencies. However, the proteolysis treatment may not completely remove DC surface-bound HIV due to the complex cell surface invaginations of DCs (Wang et al., 2007b).
Reversible or topical disruption of the cytoskeleton might be considered as a potential approach in the prevention and therapy of HIV infection. Indeed, there are many small molecules that either directly target the actin cytoskeleton, or inhibit actin-binding proteins and other immediate regulators of actin dynamics (Fenteany and Zhu, 2003). It would be interesting to explore whether some of these cytoskeleton inhibitors can be developed as antiviral agents against HIV transmission in vivo.
The actin cytoskeleton contributes to T cell activation by forming immunological synapses between antigen-presenting cells and T cells (Dustin and Cooper, 2000). Interestingly, the immunological synapses appear to share structural similarities with the VS and may play a role in HIV pathogenesis (Fackler, Alcover, and Schwartz, 2007; Piguet and Sattentau, 2004). While HIV facilitates cell-to-cell transmission by promoting VS formation, HIV infection impairs the formation of the immunological synapses (Thoulouze et al., 2006). HIV infection of DCs and T cells may result in a balance between the formation of the VS and the impairment of the immunological synapses, thereby enhancing cell-mediated HIV dissemination and impairing antiviral immune responses. Therefore, the influence of the structure and function of immunological synapses should be also considered in the development of potential anti-HIV interventions by targeting the cytoskeleton.
Materials and Methods
Cell culture
PBLs, CD4+ T cells and CD14+ monocytes were isolated from buffy coat units of healthy blood donors as previously described (Wang et al., 2007a). Monocyte-derived iDCs and LPS-induced mDCs were generated from purified CD14+ monocytes as previously described (Wang et al., 2007b). PBLs and primary CD4+ T cells were cultured in the presence of recombinant interleukin-2 (the NIH AIDS Research and Reference Reagent Program) and activated by phytohemagglutinin as described (Wang et al., 2007a). DCs and CD4+ T cells were more than 98.5% pure by flow cytometry analysis of surface markers as previously described (Dong et al., 2007; Wang et al., 2007b; Wu et al., 2002). HEK293T, Hut/CCR5, and GHOST/R5 cell lines have been previously described (Wang et al., 2007a).
HIV stocks
HIV-Luc/JRFL stocks were generated by cotransfections of HEK293T cells with the pLai3ΔenvLuc2 and an expression plasmid for HIV-1JRFL Env as previously described (Wang et al., 2007a). HIV-1NL-AD8 stocks were generated by transfections of HEK293T cells with the proviral construct pNLAD8 as previously described (Dong et al., 2007; Janas et al., 2008). The infectivity of the virus stocks was evaluated by limiting dilution on GHOST/R5 cells as previously described (Wang et al., 2007b). HIV-Vpr-GFP stocks were generated by cotransfections of HEK293T cells with pNLAD8 and a Vpr-GFP expression vector pGFP-Vpr (a gift from David McDonald, Case Western Reserve University) as previously described (McDonald et al., 2002). AT-2-inactivated R5-tropic HIV-1 (ADA/Supt1-CCR5 cl30) was a kind gift from Jeffery Lifson (AIDS Vaccine Program, SAIC, Frederick, MD).
HIV internalization, infection and transmission assays
For HIV internalization, DCs (1×105) were incubated separately with HIV-Luc/JRFL (45 ng of p24) at 37°C for 2 h. DCs were washed intensively, trypsinized and washed before lysis for HIV p24 quantification by ELISA as previously described (Wang et al., 2007b). HIV infection and transmission assays were performed as previously described (Dong et al., 2007; Wang et al., 2007a). For inhibitor treatment, DCs were preincubated separately with CytoD, DMA, or nocodazole (all inhibitors were purchased from Sigma-Aldrich) at the indicated concentrations at 37°C for 0.5 h, and then pulsed with infectious HIV (0.2 multiplicity of infection, MOI) at 37°C for 2 h in the presence of the appropriate inhibitors. For post-treatment of HIV-pulsed DCs with CytoD and nocodazole in HIV transmission assays, DCs were pulsed with HIV-Luc/JRFL (0.2 MOI), and then treated with the inhibitors for 30 min before wash and coculturing with CD4+ T cells. If not indicated in figures, the inhibitor concentrations used in HIV transmission assays were: CytoD (3 μM), nocodazole (20 μM), and DMA (100 μM). HIV-pulsed DCs were lysed for p24 detection or coculturing with the indicated target cells for viral transmission assays.
Examination of DC viability
DCs were treated separately with DMSO (control, 0.25%), CytoD (5 μM), DMA (100 μM), and nocodazole (20 and 35 μM) at 37°C for 2.5 h, washed and cultured for 3 days. DC viability was then determined by trypan blue (Invitrogen) exclusion according to the product instruction.
Examination of DC and T-cell clustering
DCs were pretreated separately with DMSO (0.25%), CytoD (3 μM), nocodazole (20 μM) and DMA (100 μM) at 37°C for 30 min, pulsed with HIV-Luc/JRFL at 37°C for 2 h in the presence of DMSO or the appropriate inhibitors. DCs were washed, cocultured with Hut/CCR5 cells at 37°C for indicated time, and then examined by the bright field of a microscope (Nikon Eclipse TE2000U).
Immunofluorescence, confocal microscopy and virological synapse assay
DCs were pretreated with DMSO (0.25%), CytoD (3 μM), or nocodazole (20 μM) at 37°C for 0.5 h, pulsed with HIV-Vpr-GFP in the presence or absence of the inhibitors at 37°C for 1.5 h. DCs were then washed, fixed and permeabilized. DCs were stained with DAPI, labeled with Alexa Fluor 546-conjugated phalloidin (Invitrogen), or stained separately with anti-α-tubulin (clone TU-01, Invitrogen) and anti-CD81 (clone JS-81, BD Biosciences), and then followed by Alexa Fluor 568-labeled secondary IgG (Invitrogen) as described (Wang et al., 2007a).
Virological synapse assays were performed as previously described (Garcia et al., 2005) with slight modifications. For the CytoD treatment in the cocultures, DCs (1× 105) were pulsed with HIV-Vpr-GFP (40 ng of p24) at 37°C for 2 h. After washes, HIV-pulsed DCs were cocultured with Hut/CCR5 cells (1× 105) for 0.5 h in presence of CytoD (3 μM) or DMSO (0.25%). Cells were then adhered on a poly-L-lysine-coated microscope slide at 37°C for 0.5 h, fixed with 4% paraformaldehyde for 1 h, and permeabilized with 0.1% Triton X-100 in PBS for 2 min at room temperature as described (Wang et al., 2007a). Cells were labeled with Alexa Fluor 546-conjugated phalloidin, and then stained for hemagglutinin (HA)-tagged CCR5 on Hut/CCR5 cells with anti-HA (Roche), and followed by Alexa Fluor 633-labeled secondary IgG (Invitrogen).
For the nocodazole treatment in the cocultures, DCs (1× 105) were pretreated with nocodazole (20 μM) at 37 °C for 0.5 h, and then incubated with HIV-Vpr-GFP (40 ng of p24) in the presence of nocodazole for 2 h. After washes, DCs were cocultured with Hut/CCR5 cells for 1 h, fixed and permeabilized as described above. Cells were first stained with anti-α-tubulin labeled with Alexa Fluor 647 using a Zenon One labeling kit (Invitrogen). Cells were washed, fixed, and then stained with anti-HA that has been labeled with Alexa Fluor 568 using a Zenon One labeling kit (Invitrogen). Stained cells were examined using a laser scanning confocal microscope (Leica TCS SP2) or a fluorescence microscope (Nikon Eclipse TE2000U) and analyzed with MetaMorph software (Version 7.0r4) as described (Wang et al., 2007a). Pearson correlation analysis for the quantification of colocalization was performed using ImageJ software with the JACoP plugin (Bolte and Cordelieres, 2006).
Electron microscopy
mDCs (6 × 105) were incubated with AT-2-inactivated HIVADA (2 μg of p24) at 37°C for 2 h in presence of DMA (100 μM), CytoD (3 μM), nocodazole (20 μM), or DMSO (0.25%). After a complete wash, DCs were fixed and processed for transmission electron microscopy as previously described (Wang et al., 2007a; Wang et al., 2007b). Thin sections were examined with a transmission electron microscope (JEOL 2100 LaB6).
Statistical analyses
Statistical analyses were performed using the ANOVA test, Wilcoxon’s paired, or Mann-Whitney’s unpaired t-test with Prism software.
Acknowledgments
We thank Eric Freed and Akira Ono for critical reading of the manuscript and members of the Wu laboratory for helpful discussions. We thank Alicia Janas for excellent assistance with confocal microscopy. We are grateful to Michael Emerman, Eric Freed, Vineet KewalRamani, Jeffery Lifson, and David McDonald for the kind gift of reagents. IL-2 was obtained from the NIH AIDS Research and Reference Reagent Program. This work was supported by grants to LW from the NIH (AI068493) and the Campbell Foundation. The Electron Microscopy Core Facility of the Medical College of Wisconsin (MCW) was supported by grants from the NIH (1S10RR022412-01) and Advancing a Healthier Wisconsin Program from the MCW.
References
- Arrighi JF, Pion M, Garcia E, Escola JM, van Kooyk Y, Geijtenbeek TB, Piguet V. DC-SIGN-mediated infectious synapse formation enhances X4 HIV-1 transmission from dendritic cells to T cells. J Exp Med. 2004;200(10):1279–88. doi: 10.1084/jem.20041356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolte S, Cordelieres FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc. 2006;224(Pt 3):213–32. doi: 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- Bukrinskaya A, Brichacek B, Mann A, Stevenson M. Establishment of a functional human immunodeficiency virus type 1 (HIV-1) reverse transcription complex involves the cytoskeleton. J Exp Med. 1998;188(11):2113–25. doi: 10.1084/jem.188.11.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron PU, Freudenthal PS, Barker JM, Gezelter S, Inaba K, Steinman RM. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science. 1992;257(5068):383–7. doi: 10.1126/science.1352913. [DOI] [PubMed] [Google Scholar]
- Cameron PU, Handley AJ, Baylis DC, Solomon AE, Bernard N, Purcell DF, Lewin SR. Preferential infection of dendritic cells during human immunodeficiency virus type 1 infection of blood leukocytes. J Virol. 2007;81(5):2297–306. doi: 10.1128/JVI.01795-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell EM, Nunez R, Hope TJ. Disruption of the actin cytoskeleton can complement the ability of Nef to enhance human immunodeficiency virus type 1 infectivity. J Virol. 2004;78(11):5745–55. doi: 10.1128/JVI.78.11.5745-5755.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavrois M, Neidleman J, Greene WC. The Achilles heel of the Trojan horse model of HIV-1 trans-infection. PLoS Pathogens. 2008;4(6):e1000051. doi: 10.1371/journal.ppat.1000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavrois M, Neidleman J, Kreisberg JF, Fenard D, Callebaut C, Greene WC. Human immunodeficiency virus fusion to dendritic cells declines as cells mature. J Virol. 2006;80(4):1992–9. doi: 10.1128/JVI.80.4.1992-1999.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavrois M, Neidleman J, Kreisberg JF, Greene WC. In vitro derived dendritic cells trans-infect CD4 T cells primarily with surface-bound HIV-1 virions. PLoS Pathog. 2007;3(1):e4. doi: 10.1371/journal.ppat.0030004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Hubner W, Spinelli MA, Chen BK. Predominant mode of human immunodeficiency virus transfer between T cells is mediated by sustained Env-dependent neutralization-resistant virological synapses. J Virol. 2007;81(22):12582–95. doi: 10.1128/JVI.00381-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Witte L, Nabatov A, Pion M, Fluitsma D, de Jong MA, de Gruijl T, Piguet V, van Kooyk Y, Geijtenbeek TB. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat Med. 2007;13(3):367–71. doi: 10.1038/nm1541. [DOI] [PubMed] [Google Scholar]
- Dong C, Janas AM, Wang J-H, Olson WJ, Wu L. Characterization of human immunodeficiency virus type 1 replication in immature and mature dendritic cells reveals dissociable cis- and trans-infection. J Virol. 2007;81(20):11352–11362. doi: 10.1128/JVI.01081-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin ML, Cooper JA. The immunological synapse and the actin cytoskeleton: molecular hardware for T cell signaling. Nat Immunol. 2000;1(1):23–9. doi: 10.1038/76877. [DOI] [PubMed] [Google Scholar]
- Fackler OT, Alcover A, Schwartz O. Modulation of the immunological synapse: a key to HIV-1 pathogenesis? Nat Rev Immunol. 2007;7(4):310–7. doi: 10.1038/nri2041. [DOI] [PubMed] [Google Scholar]
- Fackler OT, Krausslich HG. Interactions of human retroviruses with the host cell cytoskeleton. Curr Opin Microbiol. 2006;9(4):409–15. doi: 10.1016/j.mib.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Fahrbach KM, Barry SM, Ayehunie S, Lamore S, Klausner M, Hope TJ. Activated CD34-derived Langerhans cells mediate transinfection with human immunodeficiency virus. J Virol. 2007;81(13):6858–68. doi: 10.1128/JVI.02472-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone S, Cocucci E, Podini P, Kirchhausen T, Clementi E, Meldolesi J. Macropinocytosis: regulated coordination of endocytic and exocytic membrane traffic events. J Cell Sci. 2006;119(Pt 22):4758–69. doi: 10.1242/jcs.03238. [DOI] [PubMed] [Google Scholar]
- Fenteany G, Zhu S. Small-molecule inhibitors of actin dynamics and cell motility. Curr Top Med Chem. 2003;3(6):593–616. doi: 10.2174/1568026033452348. [DOI] [PubMed] [Google Scholar]
- Frank I, Piatak M, Jr, Stoessel H, Romani N, Bonnyay D, Lifson JD, Pope M. Infectious and whole inactivated simian immunodeficiency viruses interact similarly with primate dendritic cells (DCs): differential intracellular fate of virions in mature and immature DCs. J Virol. 2002;76(6):2936–51. doi: 10.1128/JVI.76.6.2936-2951.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank I, Stossel H, Getti A, Turville SG, Bess JW, Jr, Lifson JD, Sivin I, Romani N, Robbiani M. A fusion inhibitor prevents dendritic cell (DC) spread of immunodeficiency viruses but not DC activation of virus-specific T cells. J Virol. 2008;82(11):5329–5339. doi: 10.1128/JVI.01987-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesh L, Leung K, Lore K, Levin R, Panet A, Schwartz O, Koup RA, Nabel GJ. Infection of specific dendritic cells by CCR5-tropic human immunodeficiency virus type 1 promotes cell-mediated transmission of virus resistant to broadly neutralizing antibodies. J Virol. 2004;78(21):11980–7. doi: 10.1128/JVI.78.21.11980-11987.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia E, Nikolic DS, Piguet V. HIV-1 replication in dendritic cells occurs via a tetraspanin-containing compartment enriched in AP-3. Traffic. 2008;9(2):200–214. doi: 10.1111/j.1600-0854.2007.00678.x. [DOI] [PubMed] [Google Scholar]
- Garcia E, Pion M, Pelchen-Matthews A, Collinson L, Arrighi JF, Blot G, Leuba F, Escola JM, Demaurex N, Marsh M, Piguet V. HIV-1 trafficking to the dendritic cell-T-cell infectious synapse uses a pathway of tetraspanin sorting to the immunological synapse. Traffic. 2005;6(6):488–501. doi: 10.1111/j.1600-0854.2005.00293.x. [DOI] [PubMed] [Google Scholar]
- Granelli-Piperno A, Delgado E, Finkel V, Paxton W, Steinman RM. Immature dendritic cells selectively replicate macrophagetropic (M-tropic) human immunodeficiency virus type 1, while mature cells efficiently transmit both M- and T-tropic virus to T cells. J Virol. 1998;72(4):2733–7. doi: 10.1128/jvi.72.4.2733-2737.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gummuluru S, Rogel M, Stamatatos L, Emerman M. Binding of human immunodeficiency virus type 1 to immature dendritic cells can occur independently of DC-SIGN and mannose binding C-type lectin receptors via a cholesterol-dependent pathway. J Virol. 2003;77(23):12865–74. doi: 10.1128/JVI.77.23.12865-12874.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hladik F, Sakchalathorn P, Ballweber L, Lentz G, Fialkow M, Eschenbach D, McElrath MJ. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity. 2007;26(2):257–270. doi: 10.1016/j.immuni.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges A, Sharrocks K, Edelmann M, Baban D, Moris A, Schwartz O, Drakesmith H, Davies K, Kessler B, McMichael A, Simmons A. Activation of the lectin DC-SIGN induces an immature dendritic cell phenotype triggering Rho-GTPase activity required for HIV-1 replication. Nat Immunol. 2007;8(6):569–77. doi: 10.1038/ni1470. [DOI] [PubMed] [Google Scholar]
- Iyengar S, Hildreth JE, Schwartz DH. Actin-dependent receptor colocalization required for human immunodeficiency virus entry into host cells. J Virol. 1998;72(6):5251–5. doi: 10.1128/jvi.72.6.5251-5255.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo-Useros N, Blanco J, Erkizia I, Fernandez-Figueras MT, Borras FE, Naranjo-Gomez M, Bofill M, Ruiz L, Clotet B, Martinez-Picado J. Maturation of blood derived dendritic cells enhances HIV-1 capture and transmission. J Virol. 2007;81(14):7559–70. doi: 10.1128/JVI.02572-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janas AM, Dong C, Wang J-H, Wu L. Productive infection of human immunodeficiency virus type 1 in dendritic cells requires fusion-mediated viral entry. Virology. 2008;375(2):442–51. doi: 10.1016/j.virol.2008.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly C, Kashefi K, Hollinshead M, Sattentau QJ. HIV-1 cell to cell transfer across an Env-induced, actin-dependent synapse. J Exp Med. 2004;199(2):283–93. doi: 10.1084/jem.20030648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly C, Mitar I, Sattentau QJ. Requirement for an intact T-cell actin and tubulin cytoskeleton for efficient assembly and spread of human immunodeficiency virus type 1. J Virol. 2007;81(11):5547–60. doi: 10.1128/JVI.01469-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Neil TK, Fearnley DB, McLellan AD, Vuckovic S, Hart DN. Expression of multilectin receptors and comparative FITC-dextran uptake by human dendritic cells. Int Immunol. 2000;12(11):1511–9. doi: 10.1093/intimm/12.11.1511. [DOI] [PubMed] [Google Scholar]
- Kwon DS, Gregorio G, Bitton N, Hendrickson WA, Littman DR. DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity. 2002;16(1):135–44. doi: 10.1016/s1074-7613(02)00259-5. [DOI] [PubMed] [Google Scholar]
- Liu NQ, Lossinsky AS, Popik W, Li X, Gujuluva C, Kriederman B, Roberts J, Pushkarsky T, Bukrinsky M, Witte M, Weinand M, Fiala M. Human immunodeficiency virus type 1 enters brain microvascular endothelia by macropinocytosis dependent on lipid rafts and the mitogen-activated protein kinase signaling pathway. J Virol. 2002;76(13):6689–700. doi: 10.1128/JVI.76.13.6689-6700.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maréchal V, Prevost MC, Petit C, Perret E, Heard JM, Schwartz O. Human immunodeficiency virus type 1 entry into macrophages mediated by macropinocytosis. J Virol. 2001;75(22):11166–77. doi: 10.1128/JVI.75.22.11166-11177.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M, Helenius A. Virus entry: open sesame. Cell. 2006;124(4):729–40. doi: 10.1016/j.cell.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald D, Vodicka MA, Lucero G, Svitkina TM, Borisy GG, Emerman M, Hope TJ. Visualization of the intracellular behavior of HIV in living cells. J Cell Biol. 2002;159(3):441–52. doi: 10.1083/jcb.200203150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald D, Wu L, Bohks SM, KewalRamani VN, Unutmaz D, Hope TJ. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science. 2003;300(5623):1295–7. doi: 10.1126/science.1084238. [DOI] [PubMed] [Google Scholar]
- Naghavi MH, Goff SP. Retroviral proteins that interact with the host cell cytoskeleton. Curr Opin Immunol. 2007;19(4):402–7. doi: 10.1016/j.coi.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet V, Sattentau Q. Dangerous liaisons at the virological synapse. J Clin Invest. 2004;114(5):605–10. doi: 10.1172/JCI22812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet V, Steinman RM. The interaction of HIV with dendritic cells: outcomes and pathways. Trends Immunol. 2007;28(11):503–10. doi: 10.1016/j.it.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pion M, Arrighi JF, Jiang J, Lundquist CA, Hartley O, Aiken C, Piguet V. Analysis of HIV-1-X4 fusion with immature dendritic cells identifies a specific restriction that is independent of CXCR4 levels. J Invest Dermatol. 2007;127(2):319–23. doi: 10.1038/sj.jid.5700518. [DOI] [PubMed] [Google Scholar]
- Pope M, Betjes MG, Romani N, Hirmand H, Cameron PU, Hoffman L, Gezelter S, Schuler G, Steinman RM. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell. 1994;78(3):389–98. doi: 10.1016/0092-8674(94)90418-9. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182(2):389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer NM, Lehmann MJ, Jimenez-Soto LF, Horensavitz C, Pypaert M, Mothes W. Retroviruses can establish filopodial bridges for efficient cell-to-cell transmission. Nat Cell Biol. 2007;9(3):310–315. doi: 10.1038/ncb1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smed-Sorensen A, Lore K, Vasudevan J, Louder MK, Andersson J, Mascola JR, Spetz AL, Koup RA. Differential susceptibility to human immunodeficiency virus type 1 infection of myeloid and plasmacytoid dendritic cells. J Virol. 2005;79(14):8861–9. doi: 10.1128/JVI.79.14.8861-8869.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AL, Ganesh L, Leung K, Jongstra-Bilen J, Jongstra J, Nabel GJ. Leukocyte-specific protein 1 interacts with DC-SIGN and mediates transport of HIV to the proteasome in dendritic cells. J Exp Med. 2007;204(2):421–30. doi: 10.1084/jem.20061604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowinski S, Jolly C, Berninghausen O, Purbhoo MA, Chauveau A, Kohler K, Oddos S, Eissmann P, Brodsky FM, Hopkins C, Onfelt B, Sattentau Q, Davis DM. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat Cell Biol. 2008;10(2):211–219. doi: 10.1038/ncb1682. [DOI] [PubMed] [Google Scholar]
- Thoulouze MI, Sol-Foulon N, Blanchet F, Dautry-Varsat A, Schwartz O, Alcover A. Human immunodeficiency virus type-1 infection impairs the formation of the immunological synapse. Immunity. 2006;24(5):547–61. doi: 10.1016/j.immuni.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Trumpfheller C, Park CG, Finke J, Steinman RM, Granelli-Piperno A. Cell type-dependent retention and transmission of HIV-1 by DC-SIGN. Int Immunol. 2003;15(2):289–98. doi: 10.1093/intimm/dxg030. [DOI] [PubMed] [Google Scholar]
- Turville SG, Santos JJ, Frank I, Cameron PU, Wilkinson J, Miranda-Saksena M, Dable J, Stossel H, Romani N, Piatak M, Jr, Lifson JD, Pope M, Cunningham AL. Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood. 2004;103(6):2170–9. doi: 10.1182/blood-2003-09-3129. [DOI] [PubMed] [Google Scholar]
- Wakatsuki T, Schwab B, Thompson NC, Elson EL. Effects of cytochalasin D and latrunculin B on mechanical properties of cells. J Cell Sci. 2001;114(Pt 5):1025–36. doi: 10.1242/jcs.114.5.1025. [DOI] [PubMed] [Google Scholar]
- Wang JH, Janas AM, Olson WJ, KewalRamani VN, Wu L. CD4 coexpression regulates DC-SIGN-mediated transmission of human immunodeficiency virus type 1. J Virol. 2007a;81(5):2497–507. doi: 10.1128/JVI.01970-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JH, Janas AM, Olson WJ, Wu L. Functionally distinct transmission of human immunodeficiency virus type 1 mediated by immature and mature dendritic cells. J Virol. 2007b;81(17):8933–43. doi: 10.1128/JVI.00878-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley RD, Gummuluru S. Immature dendritic cell-derived exosomes can mediate HIV-1 trans infection. Proc Natl Acad Sci U S A. 2006;103(3):738–43. doi: 10.1073/pnas.0507995103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L. Biology of HIV mucosal transmission. Current Opinion in HIV and AIDS. 2008;3(5):534–540. doi: 10.1097/COH.0b013e32830634c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, KewalRamani VN. Dendritic-cell interactions with HIV: infection and viral dissemination. Nat Rev Immunol. 2006;6(11):859–68. doi: 10.1038/nri1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Martin TD, Vazeux R, Unutmaz D, KewalRamani VN. Functional evaluation of DC-SIGN monoclonal antibodies reveals DC-SIGN interactions with ICAM-3 do not promote human immunodeficiency virus type 1 transmission. J Virol. 2002;76(12):5905–14. doi: 10.1128/JVI.76.12.5905-5914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


