Abstract
We have previously reported that Nodal, a member of the TGF-β superfamily, acts through activin receptor-like kinase 7 (ALK7) to inhibit ovarian cancer cell proliferation. To determine the mechanism underlying their effects, a cell cycle gene array was performed and cyclin G2 mRNA was found to be strongly up-regulated by Nodal and ALK7. To study the function and regulation of cyclin G2 in ovarian cancer cells, expression constructs were generated. We found that cyclin G2 protein level decreased rapidly after transfection, and this decrease was prevented by 26S proteasome inhibitors. Immunoprecipitation and pull-down studies showed that ubiquitin, Skp1, and Skp2 formed complexes with cyclin G2. Knockdown of Skp2 by siRNA increased, whereas overexpression of Skp2 decreased cyclin G2 levels. Nodal and ALK7 decreased the expression of Skp1 and Skp2 and increased cyclin G2 levels. Overexpression of cyclin G2 inhibited cell proliferation whereas cyclin G2-siRNA reduced the antiproliferative effect of Nodal and ALK7. Taken together, these findings provide strong evidence that cyclin G2 is degraded by the ubiquitin–proteasome pathway and that Skp2 plays a role in regulating cyclin G2 levels. Furthermore, our results also demonstrate that the antiproliferative effect of Nodal/ALK7 on ovarian cancer cells is in part mediated by cyclin G2.
INTRODUCTION
Cyclins are critical regulators of the cell cycle (Morgan, 1997). Classical cyclins activate specific cyclin-dependent kinases (Cdks) to promote cell cycle progression (Pines, 1995; Morgan, 1997). Cyclin G2 (CCNG2), first cloned in human in 1996 (Bates et al., 1996; Horne et al., 1996), belongs to a group of unconventional cyclins that include cyclin G1 and cyclin I. Increasing evidence suggests that cyclin G2 exerts important inhibitory effects on cell proliferation. First, cyclin G2 is up-regulated by growth inhibitory signals (Horne et al., 1997; Martinez-Gac et al., 2004). Second, overexpression of cyclin G2 results in inhibition of cell proliferation in several human cell lines (Bennin et al., 2002; Kim et al., 2004; Liu et al., 2004). Finally, expression of cyclin G2 is significantly decreased in thyroid neoplasms (Ito et al., 2003) and oral cancer (Kim et al., 2004). Although the mechanisms by which cyclin G2 induces cell growth arrest are not entirely clear, it has been reported that it interacts with active protein phosphatase 2A (PP2A) and blocks transition of G1 to S phase (Bennin et al., 2002). More recently, it was found that cyclin G2 is associated with the centrosome to regulate microtubule stability and that its growth inhibitory effect is p53-dependent (Arachchige Don et al., 2006).
The synthesis and execution of cyclins are tightly regulated during cell cycle. The ubiquitin-proteasome system (UPS) plays an important role in the degradation of cyclic proteins (Ciechanover, 1994; Pines and Lindon, 2005). The UPS consists of two steps: the covalent attachment of multiple ubiquitin molecules to the target protein and the degradation of the polyubiquitinated protein by the 26S proteasome complex (Pines and Lindon, 2005). Ubiquitination involves an enzyme cascade comprising an activating enzyme (E1), a conjugating enzyme (E2), and a ubiquitin ligase (E3; Pines and Lindon, 2005). Two major types of E3 ligases, the Skp1-Cullin1-F-box-protein (SCF) complex and the anaphase-promoting complex/cyclosome (APC/C), are thought to control the timely entry into S phase and the onset of anaphase, respectively (Jackson and Eldridge, 2002; Nakayama and Nakayama, 2006). The SCF complex contains four components: Skp1, Cul1, Rbx-1 and an F-box protein. The F-box proteins are responsible for target protein recognition and recruitment (Nakayama and Nakayama, 2006). S-phase kinase–associated protein 2 (Skp2), originally identified as an interacting protein of cyclin A/Cdk2 (Zhang et al., 1995), is one of the F-box proteins of the SCF E3 ligase complexes. Skp2 has been shown to control the stability of a number of cell cycle regulators, such as p27 (Carrano et al., 1999; Sutterluty et al., 1999; Tsvetkov et al., 1999; Nakayama et al., 2000; Bloom and Pagano, 2003), p57 (Kamura et al., 2003), p21 (Bornstein et al., 2003), p130 (Tedesco et al., 2002), Smad 4 (Liang et al., 2004), and Tob1 (Hiramatsu et al., 2006).
The transforming growth factor (TGF)-β superfamily consists of a large group of structurally related molecules that have important functions in embryonic development as well as in many physiological processes throughout adult life (Massague and Chen, 2000; Chang et al., 2002). Members of the TGF-β superfamily elicit diverse biological responses by regulating many cellular activities, such as proliferation, differentiation, adhesion, migration, and apoptosis and their abnormal signaling is associated with diseases, including cancer (Massague and Chen, 2000; Bierie and Moses, 2006). Signaling of TGF-β superfamily is mediated through type I and type II serine/threonine kinase receptors and intracellular Smad proteins (Wrana et al., 1994; Zimmerman and Padgett, 2000). Seven type I receptors, referred to as activin receptor-like kinase (ALK) 1-7, have been characterized in mammals (Graham and Peng, 2006). Our laboratory has cloned several human ALK7 isoforms derived from alternative splicing of the ALK7 gene (Roberts et al., 2003). Nodal, one of ligands for ALK7, plays an important role in mesoderm formation and left-right axis patterning during embryonic development (Reissmann et al., 2001; Brennan et al., 2002; Eimon and Harland, 2002; Nonaka et al., 2002). We have previously reported that overexpression of Nodal and activation of ALK7 resulted in inhibition of proliferation and induction of apoptosis in ovarian cancer cells (Xu et al., 2004; Xu et al., 2006), suggesting that the Nodal-ALK7 pathway plays a role in ovarian cancer development.
To study the mechanisms by which Nodal-ALK7 inhibits ovarian cancer cell proliferation, we performed a gene array and found that the cyclin G2 mRNA level was increased by Nodal and ALK7. Here, we report that cyclin G2 is partly involved in the Nodal-ALK7–induced cell growth arrest. In addition, we have provided the first evidence that cyclin G2 has a short life span and is degraded through the ubiquitin–proteasome pathway. Furthermore, we have found that cyclin G2 levels are regulated by Skp2. Finally, we have demonstrated that Nodal and ALK7 down-regulate Skp1 and Skp2 expression and increase endogenous and exogenous cyclin G2 levels.
MATERIALS AND METHODS
Cell Culture, Expression Constructs, Transient Transfection, and Adenoviral Infection
Two “immortalized” ovarian surface epithelial cell lines, IOSE-397 and IOSE-398 (provided by Dr. Nelly Auersperg, University of British Columbia, Vancouver; Maines-Bandiera et al., 1992) and an ovarian cancer cell line, OV2008, were cultured as described previously (Xu et al., 2006; He et al., 2007). Human Nodal, wild-type ALK7 (ALK7-wt), constitutively active ALK7 (ALK7-ca), dominant negative (DN)-Smad2, and DN-Smad3 constructs have been described previously (Xu et al., 2004, 2006). Cyclin G2 plasmids were generated by PCR using cDNA from IOSE-397 cells as the template and a pair of primers (a forward primer with a KpnI linker: 5′-GGGGGTACCCAGATGAAGGATTTGGGGGCA-3′ and a reverse primer with an XbaI linker: 5′-GTGCTCTAGAGCAGATGGAAAGCACAGTG TT-3′). Several vectors (pcDNA3.1, pcDNA4, pEGFP-N1, and p3XFLAG-CMV-7.1) were used for CCNG2 cloning as shown in Supplemental Figure S2. Two cyclin G2 mutants, with full and partial deletion of the PEST region, were generated using the same forward primer and a reverse primer 5′-GGCGGATCCCTACCGCGGGAGGTTCTGGGCTGTGCGCCTTGA-3′ (for ΔPEST) or 5′-GGCGGATCCCTACCGCGGACTTTCACTTTCATCAAAACAAC-3′ (for PEST24). Adenoviral infection and transient transfection using 25-kDa polyethylenimine (PEI, Sigma, Oakville, ON, Canada) were carried out as previously reported (Xu et al., 2004, 2006).
RNA Extraction and Quantitative Real-Time RT-PCR
Total RNA was extracted from cells using TRIZOL reagent (Invitrogen Canada, Burlington, ON, Canada) as described previously (Xu et al., 2004). Real-time RT-PCR was performed using TaqMan Gene Expression Assay system from Applied Biosystems (Streetsville, ON, Canada). TaqMan MGB probes (FAM dye-labeled) for human cyclin G2 and eukaryotic 18S rRNA were used to amplify cyclin G2 and 18S rRNA, respectively, according to the manufacturer's instruction. Assays were conducted in triplicate and repeated three times.
Immunoprecipitation and Western Blot Analysis
Cell lysates were prepared and Western blot was performed as reported previously (Xu et al., 2004, 2006). Immunoprecipitation was carried out using a Flag-tagged protein immunoprecipitation (IP) kit (Sigma). Briefly, cells were washed twice with 1× PBS and lysed with lysis buffer (50 mM Tris/HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100) on ice for 30 min. Cell lysates were transferred to a microtube and centrifuged at 12,000× g for 15 min. The supernatant was then transferred to a new tube and incubated with 20 μl of packed anti-flag M2 affinity gel at 4°C for 16 h. Samples were then centrifuged at 5,000× g for 30 s, and the supernatant was discarded. After three washes with TBS, the sample was eluted with 2× SDS sample buffer by boiling and loaded into SDS-PAGE gel. Western blot analyses were performed using specific antibodies.
Proteasome Inhibitor Treatment and Protein Stability Assay
OV2008 cells were treated with proteasome inhibitors, MG-132 or lactacystin (Calbiochem, Mississauga, ON, Canada), for different periods of time after transient transfection with cyclin G2 plasmid vectors. DMSO was used as a vehicle control. For protein stability assay, OV2008 cells were transiently transfected with plasmid DNA of full-length cyclin G2 or PEST deletion mutants in six-well plate for 16 h and recovered in the presence or absence of cycloheximide (5 μg/ml, Sigma). Total proteins were extracted, and an equal amount of protein was subjected to Western blotting.
Pulldown Assay
OV2008 cells were transiently transfected with cyclin G2-V5, Flag-Skp2 (provided by Dr. Poon, Department of Biochemistry, Hong Kong University of Science and Technology; Fung et al., 2002), and hemagglutinin (HA)-ubiquitin (obtained from Dr. Benchimol, Department of Biology, York University; Leng et al., 2003), either alone or in combination, for 16 h using the PEI method. After recovery for 2 h, cells were lysed in a lysis buffer containing 50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole, and 0.05% Tween 20 (pH 8.0). After sonication, cell lysates were centrifuged at 10,000× g for 15 min to pellet cellular debris and DNA. The supernatant was then transferred to a new tube. Fifteen microliters of Ni-NTA magnetic agarose beads were added to whole cell extracts and incubated at 4°C for 16 h. After three washes with a washing buffer containing 50 mM NaH2PO4, 300 mM NaCl, 20 mM imidazole, and 0.05% Tween 20 (pH 8.0), beads were boiled in 2× SDS sample buffer for 3 min. Eluted proteins were separated by SDS-PAGE gel and analyzed by Western blotting.
Skp2-siRNA Treatment
OV2008 cells were cultured into six-well plate at a density of 1.5×105 per well for 24 h before transfection. After washing with OPTI-MEM I medium (Invitrogen), cells was transiently transfected with control-siRNA or Skp2-siRNA (Santa Cruz Biotechnology, Santa Cruz, CA) at a final concentration of 200 nM using 0.28 μl of 0.18 mM 25-kDa PEI. The desired amount of siRNA and the required PEI were diluted separately into 10 μl of 150 mM NaCl and incubated for 5 min. These solutions were then mixed and further incubated at room temperature for 12 min. The PEI/siRNA mixture was then diluted into OPTI-MEM I medium and added to cells. After incubation for 8 h, cells were transfected with 3 μg of empty vector or cyclin G2 plasmid DNA for 16 h using the PEI method as indicated above and recovered for 2 h. The total protein was extracted and subjected to Western blotting.
Fluorescence Microscopy
Immunofluorescence was performed in OV2008 cells cotransfected with cyclin G2 and either empty vector, Flag-Skp2, or ALK7-ca. For the Skp2-siRNA experiment, OV2008 cells were first transfected with control-siRNA or Skp2-siRNA for 8 h and then transfected with cyclin G2. After transfection of plasmid DNAs for 16 h and recovery for additional 6 h, cells were fixed with cold methanol/acetone (1:1 volume) and permeabilized with 0.2% Triton X-100. Cells were incubated sequentially using the following antibodies in PBS containing 0.5% BSA: anti-Flag (1:100 dilution, Sigma, for Flag-Skp2), anti c-myc (1:100 dilution, Sigma, for ALK7-ca-c-myc), or anti-Skp2 (1:50 dilution, Cell Signaling Technology, Beverly, MA, for endogenous Skp2), Alexa Fluor 594 anti-rabbit (1:100 dilution, Molecular Probes, Eugene, OR, for Skp2 and ALK7-ca) or anti-V5 (1:100 dilution, Invitrogen, for cyclin G2), and finally Alexa Fluor 488 anti-mouse (1:100, Molecular Probes, for cyclin G2-V5). DAPI was included in last antibody solution to label nuclei. Fluorescent microscopy was performed using Nikon Eclipse TE2000-U microscope (Melville, NY) at 30× magnification.
Regulation of Endogenous Cyclin G2 by Nodal, ALK7, and PI3K Inhibitor
To study the effect of ALK7-ca on endogenous cyclin G2 expression, OV2008 cells were plated in six-well plate for 16 h and then transiently transfected with increasing amount of ALK7-ca using Lipofectamine 2000 (Invitrogen), according to the manufacturer's instruction. After transfection for 6 h, cells were recovered for 24 h, and cell lysates were then prepared. To determine if Nodal regulates cyclin G2 expression, OV2008 cells were incubated for 24 h with 500 ng/ml recombinant mouse Nodal (rmNodal, R&D Systems, Hornby, ON, Canada). LY294002 (10 μM, Calbiochem), an inhibitor of the phosphoinositol-3-kanase (PI3K) pathway that has been shown to regulate cyclin G2 expression (Le et al., 2007), was used as a positive control. The regulation of cyclin G2 expression by Nodal and proteasome inhibitor, as well as the interaction between cyclin G2 and Skp2 were also examined in IOSE-398 cells. Cells were incubated with 500 ng/ml rmNodal or 5 μM MG-132 for 24 h and the endogenous cyclin G2 expression was analyzed by Western blotting probed with an anti-cyclin G2 antibody (1:300, Santa Cruz Biotechnology). For IP of endogenous cyclin G2 complex, IOSE-398 cells were seeded in 10-cm dishes and cultured for 16 h. Cells were then treated with 500 ng/ml rmNodal or 5 μM MG-132 for 24 h. Cell lysate was prepared in IP buffer (20 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin, and protease inhibitor cocktail). IP reactions were performed by incubating the same amount of cell lysate (300 μg) with anti-Skp2 antibody (1:100, Santa Cruz Biotechnology) or anti-rabbit IgG (1:100, Santa Cruz Biotechnology) at 4°C for 16 h. After further incubation with protein A agarose beads (GE Healthcare, Waukesha, WI) at 4°C for 3 h, the immune complexes were collected by centrifugation (10,000× g) at 4°C for 1 min and eluted into 30 μl 3× SDS sample buffer.
Cell Proliferation Assay and Flow Cytometry
Cell proliferation was determined using a ELISA bromodeoxyuridine (BrdU) kit (Roche Diagnostics, Alameda, CA) as reported earlier (Xu et al., 2004). For flow cytometry, cells were plated into 6-cm dishes at 2×105 cells per dish for 24 h and transfected with an empty vector, ALK7-ca, or cyclin G2 for 16 h. Cells were then incubated in a complete medium for 30 or 54 h before fixation and stained with propidium iodide (PI). The cell cycle profile was determined using a FACScan (Becton Dickinson, San Diego, CA) by measuring fluorescence from cells stained with PI according to the protocol provided by the manufacturer.
Generation of Cyclin G2-siRNA Construct and OV2008 Cell Lines Stably Transfected with Cyclin G2-siRNA
Cyclin G2 siRNA (tattccatccactcatgat) was designed based on human cyclin G2 cDNA sequence (GenBank accession no. L49506). It was ligated into a mammalian expression vector (pBluGFP) that simultaneously express a small fragment of RNA and green fluorescent protein (GFP) as we have previously described (Lee et al., 2007). Stable cell lines were generated using a single-cell cloning technique. Briefly, OV2008 cells were plated into 6-cm dishes at 2×105 cells per dish for 24 h. Cells were cultured in a serum-free OPTI-MEM I medium for 1 h and then transfected with either pBluGFP or pBluGFP-CCNG2siRNA construct for 16 h. After recovery for 24 h in a complete medium, cells were trypsinized, washed, and passaged into six 10-cm dishes at very low density. After incubation for 7 d, a dozen GFP-expressing colonies were selected and isolated using a small-cylinder isolation method. These cells were then passaged into 96-well plates by serial dilution. A single, GFP-positive cell was selected and cultured for another 14 d. GFP-expressing cells were passaged onto a 24-well plate and were grown thereafter. Knockdown efficiency was determined by RT-PCR using cyclin G2 specific primers. A control cell line, OV-GFP, and a cyclin G2-siRNA cell line, OV-siCCNG2, were transfected with different amount of cyclin G2, Nodal, or ALK7-ca plasmid DNA (0.1–0.4 μg) for 16 h and then recovered for 48 h. Cell proliferation was determined by BrdU assays.
Statistical Analysis
Data were represented as mean value±SEM of replicated samples in one experiment or replicated experiments as indicated in figure legends. Statistical analysis was done using one-way ANOVA, followed by a Tukey-Kramer multiple comparisons test (Graph Pad InStat Software, Graph Pad, San Diego, CA). Student's t test was used for the comparison between two groups. Differences were considered significant at values of p<0.05.
RESULTS
Activin Receptor-like Kinase 7 Up-Regulates Cyclin G2 mRNA Expression
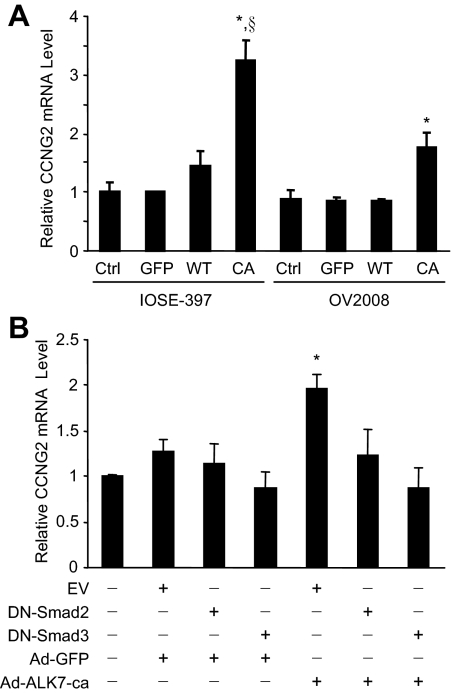
We have previously shown that Nodal acts through ALK7 to inhibit proliferation and induce apoptosis in ovarian cancer cells (Xu et al., 2004, 2006). To explore the potential mechanisms by which the Nodal-ALK7 pathway regulates cell proliferation, a cell cycle gene array was performed. Cyclin G2 was one of the genes strongly up-regulated by the overexpression of Nodal or ALK7-ca (Supplemental Figure S1). To confirm this, IOSE-397 and OV2008 cells were infected with adenovirus carrying wild-type or constructively active ALK7 (ALK7-ca). Quantitative real-time PCR performed at 48 h after infection showed that the level of cyclin G2 mRNA was significantly increased by ALK7-ca (Figure 1A). Because Nodal and ALK7 activate Smad2/3 to regulate IOSE and EOC cell proliferation (Xu et al., 2004, 2006), we determined whether Smad2/3 is also involved in ALK7-regulated cyclin G2 mRNA expression. When dominant negative Smad2 or Smad3 was cotransfected with ALK7-ca, the effect of ALK7-ca was abolished (Figure 1B), suggesting that ALK7-ca induces cyclin G2 mRNA expression via Smad2/3.
Figure 1.
ALK7 up-regulates cyclin G2 mRNA expression. (A) IOSE-397 and OV2008 cells were infected without or with Ad-GFP (GFP), Ad-ALK7-wt (WT), or Ad-ALK7-ca (CA) for 4 h and recovered for 16 h. Total RNA was extracted at 48 h after infection and subjected to real-time PCR. ALK7-ca increased cyclin G2 mRNA in both cell lines as compared with ALK7-wt, GFP control, and noninfection control (Ctrl). *p<0.05 versus other groups in the same cell line; § p<0.05 versus same infection in OV2008 cells. (B) OV2008 cells were transiently transfected with an empty vector (EV), dominant negative Smad2 (DN-Smad2), or DN-Smad3 for 16 h and then infected with Ad-GFP or Ad-ALK7-ca. Overexpression of DN-Smad2 or DN-Smad3 abolished the effect of ALK7-ca on cyclin G2 expression. *p<0.05 versus EV control. Data were expressed as fold of the control and represent mean±SEM of three experiments.
Cyclin G2 Is Degraded through the 26S Proteasome
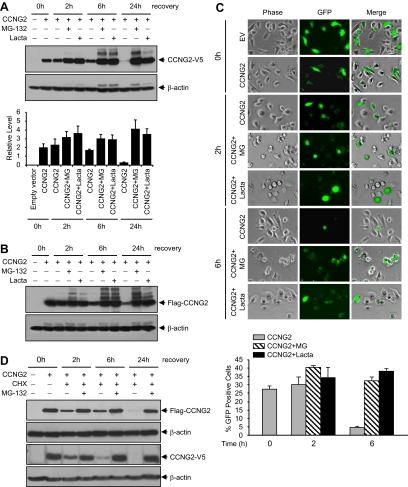
To determine if cyclin G2 mediates the antiproliferative action of Nodal/ALK7, we generated six human cyclin G2-expressing constructs (Supplemental Figure S2A). After transfection of a V5-tagged CCNG2 plasmid into OV2008 or IOSE-397 cells, the expression of exogenous cyclin G2 was detected by a V5 antibody (Invitrogen, Supplemental Figure S2B). We observed that cyclin G2 levels started to decline as early as 6 h after transfection (Figure 2A). To determine whether the short life span of cyclin G2 is due to a rapid degradation via the proteasome pathway, OV2008 cells were transfected with CCNG2-V5 or Flag-CCNG2 plasmids for 16 h and recovered for different periods of time in the presence or absence of 26S proteasome inhibitors, MG-132 and lactacystin, respectively. In the presence of these proteasome inhibitors, cyclin G2 protein levels increased, especially at 24 h after transfection (Figure 2, A and B). Similar results were obtained with the pCCNG2-GFP plasmid. The number of GFP-positive cells after transfection with pCCNG2-GFP decreased at 6 h after transfection; however, this decrease was prevented by the proteasome inhibitors (Figure 2C). Finally, cells were transfected with either the CCNG2-V5 or Flag-CCNG2 plasmid and treated with cycloheximide (CHX), which blocks de novo protein synthesis, alone or in combination with MG-132. As shown in Figure 2D, MG-132 prevented the degradation of cyclin G2 at all time points tested.
Figure 2.
Proteasome inhibitors increase cyclin G2 levels. (A) Expression of CCNG2-V5 in the absence or presence of proteasome inhibitors. OV2008 cells were transiently transfected with 3 μg/ml/well of CCNG2-V5 plasmid in six-well plate for 16 h and recovered in the presence or absence of 50 μM MG-132 or lactacystin for 0–24 h. Cyclin G2 was detected using an antibody against V5. A representative Western blot and a graph with normalized densitometry data (mean±SEM of three experiments) are shown. (B) Expression of Flag-CCNG2 at the same conditions as in A. Cyclin G2 was detected using an antibody against Flag. (C) OV2008 cells were transiently transfected with pCCNG2-GFP or empty vector (EV) for 16 h. GFP-positive cells were determined by fluorescence microscopy at 0, 2, or 6 h after transfection in the presence or absence of MG-132 or lactacystin. A histogram represents the percentage of CCNG2-GFP–positive cells in total cells. At least 800 cells were counted from each individual experiment, and data represent mean±SEM of three experiments. (D) OV2008 cells were transfected with either the Flag-CCNG2 pr CCNG2-V5 plasmid for 16 h and then treated with cycloheximide (CHX, 5 μg/ml) alone or together with MG-132 (10 μM) for 2–24 h. MG-132 prevented the degradation of cyclin G2.
Cyclin G2 Is Physically Associated with Ubiquitin, Skp1, and Skp2
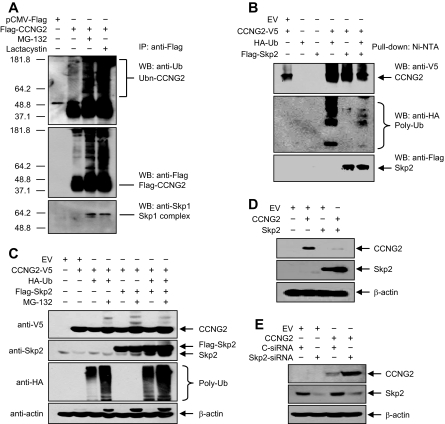
To determine if cyclin G2 is ubiquitinated, OV2008 cells were transfected with p3xFLAG-CCNG2 plasmid and treated with or without MG-132 or lactacystin for 2 h. The Flag-tagged cyclin G2 was immunoprecipitated, and polyubiquitin was detected using an antibody against ubiquitin (Figure 3A). An increase in ubiquitination of cyclin G2 was observed in the presence of proteasome inhibitors (Figure 3, A and B). It has been shown that SCF E3 ligases are responsible for the ubiquitination of several proteins involved in inhibiting G1/S transition (Nakayama and Nakayama, 2006). Because cyclin G2 has been reported to induce a G1/S cell cycle arrest (Bennin et al., 2002) and because our gene array showed a decrease in Skp1 expression by Nodal and ALK7-ca in IOSE-397 cells (Supplemental Figure S1), we determined whether cyclin G2 may ubiquitinated by SCF, by reprobing the blot with an anti-Skp1 antibody. As shown in Figure 3A, Skp1 was detected in the cyclin G2 complex in the presence of proteasome inhibitors, suggesting that SCF may be involved in cyclin G2 ubiquitination. Skp2 is one of the F-box proteins of SCF E3 ligase complexes implicated in the degradation of key cell cycle regulators, including p27 (Nakayama and Nakayama, 2006). To determine if Skp2 can form a complex with cyclin G2 and ubiquitin, cells were cotransfected with cyclin G2, ubiquitin, and Skp2 plasmids, and Ni-NTA beads were used to pull down cyclin G2. Immunoblotting revealed that Skp2 and ubiquitin were pulled down with cyclin G2 (Figure 3B).
Figure 3.
Cyclin G2 forms complexes with ubiquitin, Skp1 and Skp2 and is regulated by Skp2. (A) OV2008 cells were transfected with p3xFLAG-CCNG2 plasmid and recovered in the presence of 50 μM MG-132 or lactacystin for 2 h. The Flag-tagged CCNG2 was immunoprecipitated, and Western blots were performed using antibodies against ubiquitin, Flag, and Skp1. (B) Detection of cyclin G2/ubiquitin/Skp2 complex in pulldown samples. Cells in 10-cm dish were transfected with 5 μg of CCNG2-V5, HA-ubiquitin (Ub), and Flag-Skp2 plasmids, either alone or in combination. Ni-NTA beads were used to pulldown CCNG2-V5. CCNG2, ubiquitin, and Skp2 were detected using antibodies against V5, HA, and Flag, respectively. (C) Effect of Skp2 and MG-132 on cyclin G2 levels. Cells in 10-cm dish were cotransfected with 5 μg of CCNG2-V5, HA-Ub, and Flag-Skp2 plasmids in the presence or absence of MG-132. Western blots were performed using anti-V5, anti-HA, and anti-Skp2 antibodies. (D) Overexpression of Skp2 decreased cyclin G2. Cells in six-well plate were transfected with 3 μg of CCNG2-V5 and Flag-Skp2 plasmid alone or in combination. Western blots were performed using antibodies against V5 and Flag for CCNG2 and Skp2, respectively. (E) Knockdown of Skp2 increased cyclin G2 level. Cells were cotransfected with CCNG2-V5 and Skp2-siRNA or control-siRNA (C-siRNA), and Western blots were performed using anti-V5 and anti-Skp2 antibodies. Skp2-siRNA decreased endogenous Skp2 levels and enhanced CCNG2-V5 levels.
Skp2 Down-Regulates Cyclin G2 Levels
To determine if Skp2 regulates cyclin G2 levels, several experiments were performed. First, OV2008 cells were cotransfected with cyclin G2 and Skp2 or ubiquitin in the absence or presence of MG-132. Overexpression of Skp2 or ubiquitin decreased cyclin G2 levels (Figure 3, C and D), but their effects were blocked by MG-132 (Figure 3C). Second, Skp2-siRNA was cotransfected with cyclin G2 plasmid DNA. Skp2-siRNA decreased Skp2 expression and increased cyclin G2 levels when compared with the control siRNA (Figure 3E). Finally, we used immunofluorescent microscopy to confirm the effect of Skp2 on cyclin G2 levels. Compared with the cells cotransfected with cyclin G2 and control pcDNA3.1 vector, cotransfection of cyclin G2 with Skp2 plasmids resulted in very weak cyclin G2 signals (Supplemental Figure S3A). On the other hand, when cells were transfected with Skp2-siRNA, Skp2 was weakly detected, whereas strong cyclin G2 signals were observed (Supplemental Figure S3B). In contrast to the cotransfection of cyclin G2 and Skp2, strong signals for both cyclin G2 and ALK7 were detected in cells cotransfected with cyclin G2 and ALK7-ca (Supplemental Figure S3A).
The PEST Motif Is Involved in Cyclin G2 Stability
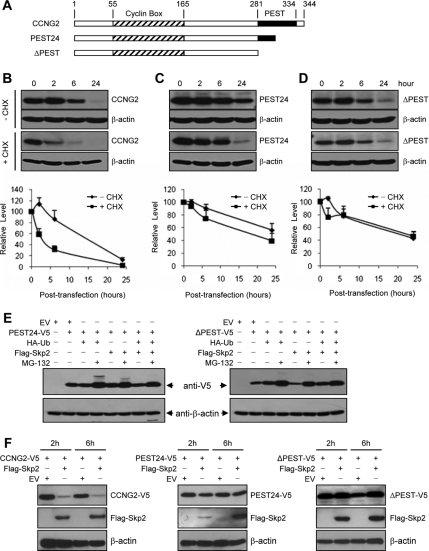
Human cyclin G2 contains a C-terminal PEST motif known as a destabilization domain (Horne et al., 1996). To investigate the involvement of PEST on cyclin G2 stability, we generated PEST deletion mutants in which PEST domain is partially (PEST24) or completely (ΔPEST) removed (Figure 4A). OV2008 cells were transiently transfected with CCNG2, PEST24, or ΔPEST and recovered for different periods of time in the presence or absence of CHX. We observed that the expression of cyclin G2 peaked at 2 h and declined thereafter. When de novo synthesis of protein was blocked by CHX, the level of cyclin G2 was decreased at 2 h (Figure 4B), once again indicating that the synthesized cyclin G2 has a short life span. Compared with the full-length cyclin G2, the PEST deletion mutants were more stable (Figure 4, C and D). To determine if the PEST motif is involved in cyclin G2 degradation via the proteasome pathway, cells were cotransfected with the partial or full PEST deletion mutant, ubiquitin, and Skp2, in the absence or presence of MG-132. The level of PEST24 and ΔPEST was still higher in the MG-132–treated groups (Figure 4E). To test if deletion of the PEST motif alters the effect of Skp2, OV2008 cells were cotransfected with full-length cyclin G2, PEST24, or ΔPEST, and Skp2 or its control vector, for 16 h, followed by 2 or 6 h of recovery. Again, overexpression of Skp2 strongly decreased the level of full-length cyclin G2. Skp2 slightly decreased the expression level of PEST24; however, it did not affect the level of ΔPEST (Figure 4F).
Figure 4.
PEST motif is involved in cyclin G2 stability. (A) Schematic presentation of cyclin G2 structure. Full-length cyclin G2 contains 344 amino acids. PEST24 is a half-PEST mutant which contains the first 24 amino acids of PEST. Mutant ΔPEST contains no PEST. (B–D) Stability of cyclin G2 and its PEST deletion mutants. OV2008 cells were transiently transfected with the full-length, partial or full PEST deletion of CCNG2 plasmids and recovered for 2–24 h in the presence or absence of cycloheximide (CHX). Representative blots are shown. Graphs represent densitometry data of four experiments. The expression of cyclin G2 peaked at 2 h and declined thereafter. Compared with the full-length cyclin G2, the PEST deletion mutants were relatively more stable. (E) MG-132 increased the levels of PEST deletion mutants. Cells were transfected with PEST24 (left panels) or ΔPEST (right panels), ubiquitin, and Skp2 for 16 h and then incubated with or without MG-132 for 6 h. (F) Effect of Skp2 on cyclin G2 and its PEST deletion mutants. Cells were cotransfected with Skp2 and cyclin G2 or its PEST mutants for 16 h and recovered for 2 or 6 h. Expression levels of cyclin G2 or its mutants were assessed by Western blotting probed with an anti-V5 antibody. Expression of Skp2 was detected using an anti-Flag antibody.
Nodal and ALK7-ca Up-Regulate Cyclin G2 and Down-Regulate Skp1 and Skp2 Levels
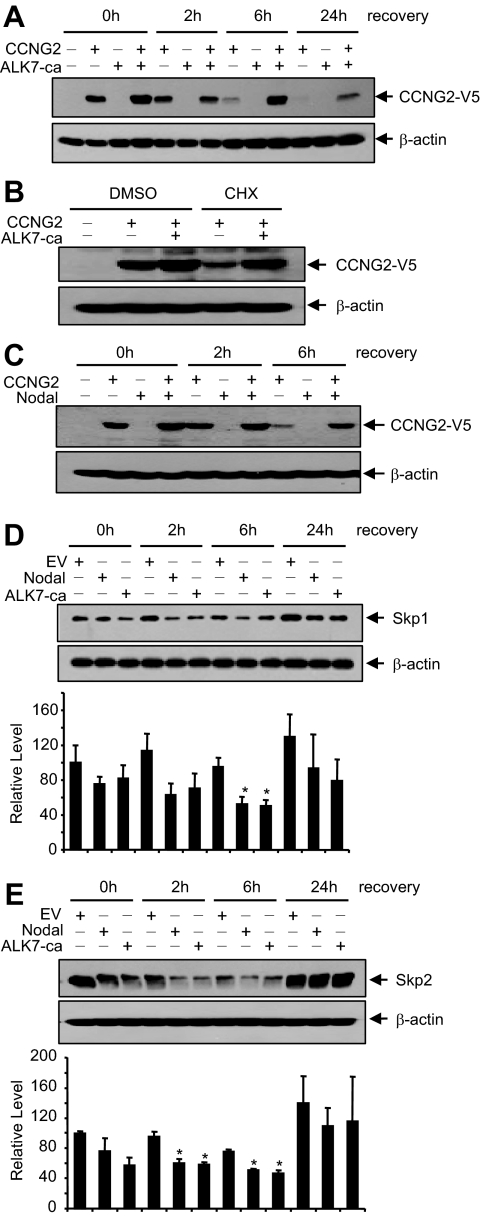
Because we found that Nodal/ALK7 down-regulated Skp1 mRNA in the gene array experiment (Supplemental Figure S2), we examined whether Nodal and ALK7 regulate cyclin G2, Skp1, and Skp2 levels. OV2008 cells were cotransfected with cyclin G2 and ALK7-ca, alone or in combination. As expected, cyclin G2 protein level was decreased at 6 and 24 h after transfection; however, this decrease was prevented by cotransfection with ALK7-ca (Figure 5A). Furthermore, in cells treated with CHX, ALK7-ca also increased cyclin G2 protein levels (Figure 5B), suggesting that ALK7 prevents the degradation of cyclin G2. Similarly, cotransfection of Nodal also prevented the decrease of cyclin G2 at 6 h after transfection (Figure 5C). On the other hand, when OV2008 cells were transfected with Nodal or ALK7-ca and Skp1 and Skp2 levels were determined at various time points after transfection, we observed that overexpression of Nodal or ALK7-ca decreased Skp1 (Figure 5D) and Skp2 (Figure 5E) expression.
Figure 5.
Nodal and ALK7-ca up-regulate cyclin G2 and down-regulate Skp1/2 expression. (A) ALK7-ca increased cyclin G2 level. OV2008 cells were transiently transfected with CCNG2-V5 and ALK7-ca, alone or in combination, for 16 h and then recovered for 0–24 h. Cotransfection with ALK7-ca resulted in an increase in cyclin G2 level. (B) ALK7 prevented cyclin G2 degradation. OV2008 cells were transfected with CCNG2-V5 and ALK7-ca and then recovered in the presence of cycloheximide (CHX, 5 μg/ml) or DMSO (control) for 6 h. (C) Cotransfection of Nodal and CCNG2-V5 resulted in an increase in cyclin G2 level at 6 h after overnight transfection. (D and E) Effects of Nodal and ALK7-ca on Skp1 (D) and Skp2 (E) expression. OV2008 cells were transiently transfected with Nodal or ALK7-ca for 16 h. Total protein was extracted at different time points after transfection, subjected to SDS-PAGE and Western blotting, and probed by anti-Skp1 and -Skp2 antibodies. Histograms show normalized densitometry data (mean±SEM) from three experiments. *p<0.05 versus EV control at the same time point.
Regulation of Endogenous Cyclin G2 Levels
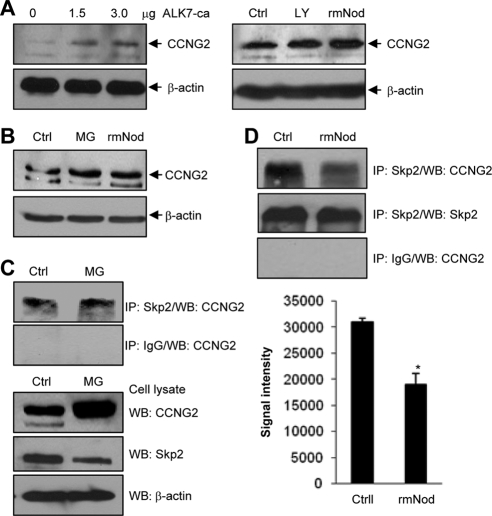
To further investigate the regulation of cyclin G2, we examined the effect of Nodal/ALK7 and proteasome inhibitors on endogenous cyclin G2 levels. Transfection of OV2008 cells with different amounts of ALK7-ca plasmid DNA resulted in an increase in cyclin G2 levels (Figure 6A, left panel). OV2008 cells were also treated with rmNodal and LY294002, a PI3K inhibitor previously shown to increase cyclin G2 levels in breast cancer cells (Le et al., 2007). As shown in Figure 6A (right panel), treatment with Nodal and LY294002 both increased cyclin G2 levels. Similarly, in IOSE-398 cells, incubation with rmNodal or MG-132 increased cyclin G2 levels (Figure 6B). To detect the endogenous cyclin G2/Skp2 complex, IOSE-398 cells were treated with MG-132 or its vehicle control for 24 h, followed by IP using an anti-Skp2 antibody or IgG as a negative control. Cyclin G2 was detected in samples precipitated by the anti-Skp2 antibody, but not in the IgG control samples (Figure 6C). Western blots of cell lysates revealed that cyclin G2 level was higher in the MG-132–treated sample. In a separate experiment, IOSE-398 cells were incubated with rmNodal, and cell lysates were subjected to IP. Again, cyclin G2 was detected in the anti-Skp2–precipitated samples. Interestingly, treatment with rmNodal resulted in a significant decrease in the level of cyclin G2/Skp2 complex (Figure 6D).
Figure 6.
Regulation of endogenous cyclin G2 level and cyclin G2/Skp2 association. (A) Cyclin G2 expression was induced by ALK7-ca, LY294002 (LY) and rmNodal (rmNod) in OV2008 cells. Cells were transfected with ALK7-ca (0–3 μg) for 6 h followed by 24-h recovery (left panel) or treated with10 μM LY294002 or 500 ng/ml rmNodal (right panel) for 24 h. Ctrl, negative control. (B) MG-132 and rmNodal increased cyclin G2 expression in IOSE-398 cells. Cells were incubated with 500 ng/ml rmNodal or 5 μM MG-132 for 24 h. (C) Detection of endogenous cyclin G2/Skp2 complex. IOSE-398 cells were treated with 5 μM MG-132 or its vehicle control for 24 h, followed by IP using an anti-Skp2 antibody or IgG as a negative control. Cyclin G2 was detected in samples precipitated by the anti-Skp2 antibody, but not in the IgG control samples. Western blots of cell lysates were also performed using anti-cyclin G2, anti-Skp2, and anti-β-actin antibodies. (D) Nodal decreased cyclin G2/Skp2 association. IOSE-397 ells were treated with 500 ng/ml rmNodal or its vehicle control for 24 h, followed by IP using the anti-Skp2 antibody. Cyclin G2 was detected in the anti-Skp2–precipitated samples and the level of cyclin G2/Skp2 complex was lower in the presence of rmNodal (top panel). Skp2 was detected using anti-Skp2 antibody (middle panel). Rabbit IgG was used as a negative control (bottom panel). Graph represents densitometry data of cyclin G2 in anti-Skp2 immunoprecipitated samples (mean±SEM of three experiments). *p<0.05 versus control.
Cyclin G2 Exerts an Antiproliferative Effect on Ovarian Cancer Cells
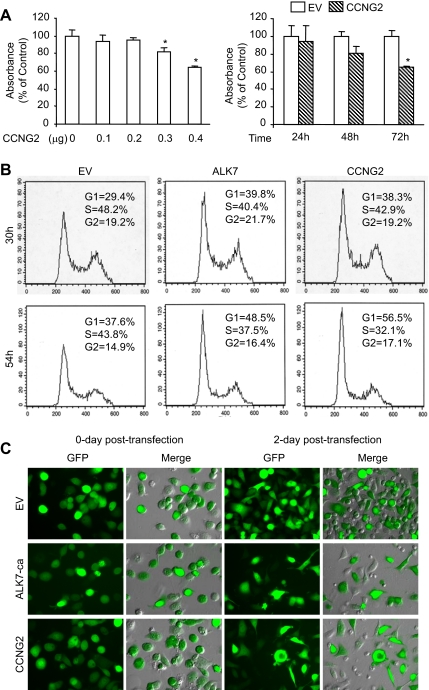
Next, we analyzed whether cyclin G2 has an effect on ovarian cancer cell proliferation and if it is involved in Nodal/ALK7-induced cell growth arrest. First, BrdU assays were performed and it was found that overexpression of cyclin G2 resulted in a decrease in cell proliferation in a dose- and time-dependent manner (Figure 7A). Second, flow cytometry was used to determine the effect of cyclin G2, as well as ALK7-ca, on cell cycle progression. Overexpression of cyclin G2 and ALK7-ca resulted in an increase in the number of cells in G1-phase and a decrease in the number of cells in S-phase (Figure 7B). The transfection efficiency was determined by cotransfection with pEGFP-N1 and either an empty vector (EV), ALK7-ca, or cyclin G2 constructs. GFP expression was monitored by fluorescent microscopy. We observed that more than 60% cells were GFP-positive after transfection with these plasmids. ALK7-ca and cyclin G2 decreased cell density at 48 h after transfection when compared with the EV control group (Figure 7C).
Figure 7.
Cyclin G2 inhibits cell proliferation. (A) Measurement of cell proliferation by BrdU assay. OV2008 cells were transiently transfected with CCNG2-V5 plasmid. Overexpression of CCNG2 resulted in a decrease in cell proliferation in a dose- and time-dependent manner. (B) Analysis of cell cycle profile by flow cytometry. OV2008 cells were transfected with an empty vector (EV), ALK7-ca, or CCNG2-V5. Both ALK7-ca and cyclin G2 increased the number of cells in G1 phase and decreased the number of cells in S phase. (C) Transfection efficiency. OV2008 cells were cotransfected with pEGFP and an empty vector (EV), ALK7-ca, or CCNG2-V5 constructs. More than 60% of GFP-positive cells were observed after transfection with these plasmids. At 2 d after transfection, cell density was lower in cyclin G2- and ALK7-ca–transfected cells.
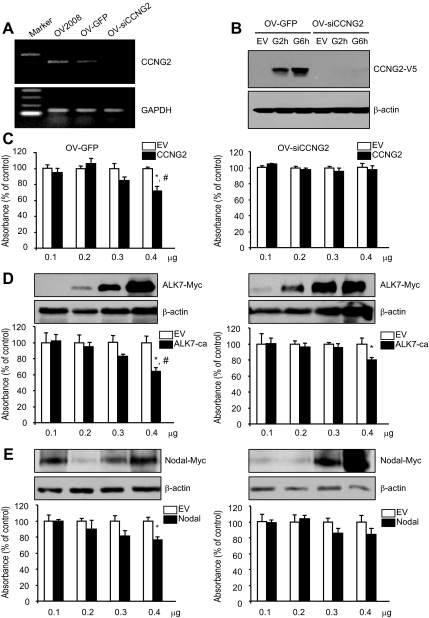
To investigate whether cyclin G2 mediates Nodal- and ALK7-ca–regulated cell proliferation, we generated two stable cell lines, an empty vector control cell line (OV-GFP) and a cyclin G2-siRNA cell line (OV-siCCNG2), from a parental cell line, OV2008. Stable cell lines express GFP as monitored by fluorescent microscopy (Supplemental Figure S4). Knockdown of cyclin G2 in the OV-siCCNG2 cell line was confirmed by RT-PCR (Figure 8A) and Western blot (Figure 8B). BrdU assays revealed a significant decrease in cell proliferation after transfection with cyclin G2 (Figure 8C), ALK7-ca (Figure 8D), or Nodal (Figure 8E) as compared with their vector controls in OV-GFP cells. The inhibitory effect of cyclin G2 on cell proliferation was blocked in OV-siCCNG2 cell line (Figure 8C, right panel). The action of Nodal and ALK7-ca was reduced, but not completely reversed, in the OV-siCCNG2 cell line (Figure 8, D and E, right panel). Western blot showed that the expression level of Nodal and ALK7-ca after transfection was similar between the two cell lines (Figure 8, D and E).
Figure 8.
Cyclin G2-siRNA reduced the effect of Nodal and ALK7-ca on cell proliferation. (A) Expression of cyclin G2 mRNA in control (OV-GFP) and cyclin G2-siRNA expressing (OV-siCCNG2) stable cell lines. Total RNA was extracted, reverse transcribed, and subjected to 30 and 18 cycles of PCR for CCNG2 and GAPDH, respectively. (B) Cyclin G2-siRNA knocked down the expression of exogenous cyclin G2. OV-GFP and OV-siCCNG2 cells were transfected with control vector (EV) or CCNG2-V5 plasmid for 16 h and then recovered for 2 (G2h) or 6 (G6h) h. Cyclin G2 level was detected by Western blot using an antibody against V5. The cyclin G2-siRNA inhibited the expression of exogenous cyclin G2 at 2 and 6 h after transfection. (C–E) Comparison of the effect of cyclin G2, ALK7-ca, and Nodal on proliferation between control (OV-GFP, left panel) and cyclin G2-siRNA–expressing cells (siCCNG2, right panel). Both cell lines were transfected with different amount of control vectors (EV), cyclin G2 (C), ALK7-ca (D), or Nodal (E). At 72 h after transfection, BrdU assays were performed. Cyclin G2-siRNA blocked the effect of cyclin G2 and reduced the effect of Nodal and ALK7-ca on cell proliferation. The overexpression of Nodal and ALK-ca was detected by Western blot analysis using an antibody against c-myc. *p<0.05 versus EV control in the same cell line. # p<0.05 versus same transfection in OV-siCCNG2 cell line.
DISCUSSION
Previously, we reported that Nodal and ALK7 induced cell growth arrest (Xu et al., 2004). However, the mechanism underlying the inhibition of proliferation by Nodal-ALK7 was unclear. In the present study, we demonstrated that cyclin G2, an unconventional cyclin, inhibits OV2008 cell proliferation and partially mediates the antiproliferative effect of Nodal and ALK7. Moreover, we demonstrated that cyclin G2 has a short life span and is degraded by the ubiquitin–proteasome pathway.
We observed that cyclin G2 levels decreased rapidly in OV2008 cells after transfection with cyclin G2 plasmid DNA. However, in the presence of proteasome inhibitors MG-132 and lactacystin, cyclin G2 protein level was increased significantly. When cycloheximide was used to block de novo protein synthesis, MG-132 also increased cyclin G2 levels, indicating that proteasome inhibitors increased cyclin G2 levels by inhibiting its degradation. In addition, IP studies revealed the presence of ubiquitin in the cyclin G2 complex. These findings strongly suggest that cyclin G2 is degraded via the ubiquitin–proteasome pathway. It has been shown that the UPS plays an essential role in a number of cellular processes including cell cycle progression (Nakayama and Nakayama, 2006). It also plays critical roles in cancer development by regulating the tumor suppressors or tumor-promoting proteins (Fuchs, 2002). SCF E3 ligase complexes are known to play a major role in the regulation of cell cycle modulators (Nakayama and Nakayama, 2006). In this study, we found that Skp1 and Skp2 are physically associated with cyclin G2. Also, overexpression of Skp2 decreased, whereas knockdown of Skp2 enhanced, cyclin G2 levels. Furthermore, the effect of Skp2 on cyclin G2 level was inhibited by MG-132. These results suggest that SCFskp2 may be involved, either directly or indirectly, in cyclin G2 ubiquitination. More studies are required to confirm that SCFskp2 is a E3 ligase responsible for cyclin G2 degradation.
A polypeptide sequence enriched in proline, glutamic acid, serine, and threonine (PEST motif) is responsible for rapid destruction of many unstable proteins (Rogers et al., 1986). A PEST motif has been found in the C-terminal region of human cyclin G2 (Horne et al., 1996). In the present study, we found that partial or full deletion of the PEST motif retained cyclin G2 expression, indicating that the PEST region is indeed involved in the stability of cyclin G2. The PEST motif has been shown to serve as a proteolytic signal and has been implicated as a recognition site for F-box proteins (Rechsteiner and Rogers, 1996; Kiernan et al., 2001). In this study, we observed that removal of the PEST motif greatly increased cyclin G2 stability. We also found that Skp2, while strongly decreasing the wild-type cyclin G2 level, had little or no effect on the levels of the PEST deletion mutants. These results suggest that the PEST motif is important for Skp2 to regulate cyclin G2 levels. It has been documented that recognition of substrates by SCF complex is dependent upon phosphorylation of the substrate proteins (Harper, 2002). Examination of the cyclin G2 sequence using bioinformatics tools revealed that the PEST motif contains multiple potential phosphorylation sites. Further studies will identify whether or not these sites are involved in cyclin G2 degradation.
Interestingly, although removal of the PEST motif greatly enhanced cyclin G2 stability, the proteasome inhibitor was still able to enhance their expression levels. Thus, it is possible that PEST is not entirely responsible for cyclin G2 degradation through the proteasome pathway and that there are additional mechanisms, including proteasome-independent proteolytic pathways, for cyclin G2 degradation. Because the deletion of PEST motif resulted in Skp2 resistance, it is also possible that additional E3 ligase(s) is involved in cyclin G2 degradation through the proteasome pathway. Consistent with these hypotheses, p27 has been reported to be degraded through multiple mechanisms. In addition to SCFSkp2 (Nakayama et al., 2000; Bloom and Pagano, 2003), p27 can also be degraded by KPC1 complex via ubiquitination (Kamura et al., 2004). Furthermore, calpain has also been shown to induce p27 degradation (Patel and Lane, 2000; Akashiba et al., 2006). Several studies have reported that PEST sequence can be targeted by calpain for degradation (Shumway et al., 1999; Sandoval et al., 2006). It will be interesting to investigate if calpain is also involved in cyclin G2 degradation via the PEST motif.
The involvement of TGF-β in tumorigenesis has been well established. Both tumor-suppressing and oncogenic activities of TGF-β have been reported (Wakefield and Roberts, 2002; Bierie and Moses, 2006). Several studies have suggested that suppression of Skp2 may be an important mechanism by which TGF-β induces cell cycle arrest (Wang et al., 2004; Liu et al., 2007). In the present study, we found that Nodal and ALK7 down-regulated Skp1 and Skp2 expression and up-regulated cyclin G2. We also observed that Nodal and ALK7-ca increased endogenous cyclin G2 levels. In addition, Nodal inhibits the association of endogenous cyclin G2 with Skp2. These findings suggest that the Nodal/ALK7 pathway increases cyclin G2 level in part by inhibiting cyclin G2 degradation. Because Nodal and ALK7 also increased cyclin G2 mRNA, it is very likely that they exert regulatory effects on cyclin G2 expression, either at transcriptional or posttranscriptional levels. Both cyclin G2 and ALK7-ca inhibited G1-S transition. Furthermore, cyclin G2-siRNA reduced the effect of Nodal and ALK7 on cell proliferation. These findings further support the role of Nodal and ALK7 in regulating cell proliferation and suggest that cyclin G2 partially mediates the antiproliferative effect of Nodal/ALK7 in ovarian cancer cells. Additional molecules, such as p27 and p21, which have been found to be up-regulated by Nodal/ALK7 in trophoblast cells (Munir et al., 2004) and in ovarian cancer cells (unpublished observations), may also play a role in mediating the effect of Nodal and ALK7 on cell proliferation. The decrease in Skp1/2 expression would likely increase the stability of cell cycle inhibitors, which in turn, would contribute to the antiproliferative action of Nodal and ALK7.
In summary, this study provides initial evidence that cyclin G2 is degraded through the ubiquitin–proteasome pathway and that SFCSkp2 may play a role in this process. We have also indentified that the PEST motif at the C-terminal of cyclin G2 is important for the rapid degradation of cyclin G2. Finally, we have demonstrated that cyclin G2 inhibits ovarian cancer cell proliferation and in part mediates the antiproliferative effects of Nodal and ALK7.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Canadian Institutes of Health Research (CIHR) Grants MOP-74622 and MOP-81370 to C.P. G.X. was a recipient of an Ontario Women's Health Council (OWHC) Scholars Award; C.P. is a recipient of a MidCareer Award from OWHC/CIHR, and B.Y. is a recipient of a Career Investigator Award (CI 5958) from the Heart and Stroke Foundation of Ontario.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-03-0259) on September 10, 2008.
REFERENCES
- Akashiba H., Matsuki N., Nishiyama N. Calpain activation is required for glutamate-induced p27 down-regulation in cultured cortical neurons. J. Neurochem. 2006;99:733–744. doi: 10.1111/j.1471-4159.2006.04100.x. [DOI] [PubMed] [Google Scholar]
- Arachchige Don A. S., Dallapiazza R. F., Bennin D. A., Brake T., Cowan C. E., Horne M. C. Cyclin G2 is a centrosome-associated nucleocytoplasmic shuttling protein that influences microtubule stability and induces a p53-dependent cell cycle arrest. Exp. Cell Res. 2006;312:4181–4204. doi: 10.1016/j.yexcr.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates S., Rowan S., Vousden K. H. Characterisation of human cyclin G1 and G2, DNA damage inducible genes. Oncogene. 1996;13:1103–1109. [PubMed] [Google Scholar]
- Bennin D. A., Don A. S., Brake T., McKenzie J. L., Rosenbaum H., Ortiz L., DePaoli-Roach A. A., Horne M. C. Cyclin G2 associates with protein phosphatase 2A catalytic and regulatory B′ subunits in active complexes and induces nuclear aberrations and a G1/S phase cell cycle arrest. J. Biol. Chem. 2002;277:27449–27467. doi: 10.1074/jbc.M111693200. [DOI] [PubMed] [Google Scholar]
- Bierie B., Moses H. L. TGF-beta and cancer. Cytokine Growth Factor Rev. 2006;17:29–40. doi: 10.1016/j.cytogfr.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Bloom J., Pagano M. Deregulated degradation of the cdk inhibitor p27 and malignant transformation. Semin. Cancer Biol. 2003;13:41–47. doi: 10.1016/s1044-579x(02)00098-6. [DOI] [PubMed] [Google Scholar]
- Bornstein G., Bloom J., Sitry-Shevah D., Nakayama K., Pagano M., Hershko A. Role of the SCFSkp2 ubiquitin ligase in the degradation of p21Cip1 in S phase. J. Biol. Chem. 2003;278:25752–25757. doi: 10.1074/jbc.M301774200. [DOI] [PubMed] [Google Scholar]
- Brennan J., Norris D. P., Robertson E. J. Nodal activity in the node governs left-right asymmetry. Genes Dev. 2002;16:2339–2344. doi: 10.1101/gad.1016202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrano A. C., Eytan E., Hershko A., Pagano M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat. Cell Biol. 1999;1:193–199. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- Chang H., Brown C. W., Matzuk M. M. Genetic analysis of the mammalian transforming growth factor-beta superfamily. Endocr. Rev. 2002;23:787–823. doi: 10.1210/er.2002-0003. [DOI] [PubMed] [Google Scholar]
- Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell. 1994;79:13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- Eimon P. M., Harland R. M. Effects of heterodimerization and proteolytic processing on Derriere and Nodal activity: implications for mesoderm induction in Xenopus. Development. 2002;129:3089–3103. doi: 10.1242/dev.129.13.3089. [DOI] [PubMed] [Google Scholar]
- Fuchs S. Y. The role of ubiquitin-proteasome pathway in oncogenic signaling. Cancer Biol. Ther. 2002;1:337–341. [PubMed] [Google Scholar]
- Fung T. K., Siu W. Y., Yam C. H., Lau A., Poon R. Y. Cyclin F is degraded during G2-M by mechanisms fundamentally different from other cyclins. J. Biol. Chem. 2002;277:35140–35149. doi: 10.1074/jbc.M205503200. [DOI] [PubMed] [Google Scholar]
- Graham H., Peng C. Activin receptor-like kinases: structure, function and clinical implications. Endocr. Metab. Immune Disord. Drug Targets. 2006;6:45–58. doi: 10.2174/187153006776056585. [DOI] [PubMed] [Google Scholar]
- Harper J. W. A phosphorylation-driven ubiquitination switch for cell-cycle control. Trends Cell Biol. 2002;12:104–107. doi: 10.1016/s0962-8924(01)02238-3. [DOI] [PubMed] [Google Scholar]
- He X., Pool M., Darcy K. M., Lim S. B., Auersperg N., Coon J. S., Beck W. T. Knockdown of polypyrimidine tract-binding protein suppresses ovarian tumor cell growth and invasiveness in vitro. Oncogene. 2007;26:4961–4968. doi: 10.1038/sj.onc.1210307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramatsu Y., et al. Degradation of Tob1 mediated by SCFSkp2-dependent ubiquitination. Cancer Res. 2006;66:8477–8483. doi: 10.1158/0008-5472.CAN-06-1603. [DOI] [PubMed] [Google Scholar]
- Horne M. C., Donaldson K. L., Goolsby G. L., Tran D., Mulheisen M., Hell J. W., Wahl A. F. Cyclin G2 is up-regulated during growth inhibition and B cell antigen receptor-mediated cell cycle arrest. J. Biol. Chem. 1997;272:12650–12661. doi: 10.1074/jbc.272.19.12650. [DOI] [PubMed] [Google Scholar]
- Horne M. C., Goolsby G. L., Donaldson K. L., Tran D., Neubauer M., Wahl A. F. Cyclin G1 and cyclin G2 comprise a new family of cyclins with contrasting tissue-specific and cell cycle-regulated expression. J. Biol. Chem. 1996;271:6050–6061. doi: 10.1074/jbc.271.11.6050. [DOI] [PubMed] [Google Scholar]
- Ito Y., et al. Decreased expression of cyclin G2 is significantly linked to the malignant transformation of papillary carcinoma of the thyroid. Anticancer Res. 2003;23:2335–2338. [PubMed] [Google Scholar]
- Jackson P. K., Eldridge A. G. The SCF ubiquitin ligase: an extended look. Mol. Cell. 2002;9:923–925. doi: 10.1016/s1097-2765(02)00538-5. [DOI] [PubMed] [Google Scholar]
- Kamura T., Hara T., Kotoshiba S., Yada M., Ishida N., Imaki H., Hatakeyama S., Nakayama K., Nakayama K. I. Degradation of p57Kip2 mediated by SCFSkp2-dependent ubiquitylation. Proc. Natl. Acad. Sci. USA. 2003;100:10231–10236. doi: 10.1073/pnas.1831009100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamura T., Hara T., Matsumoto M., Ishida N., Okumura F., Hatakeyama S., Yoshida M., Nakayama K., Nakayama K. I. Cytoplasmic ubiquitin ligase KPC regulates proteolysis of p27(Kip1) at G1 phase. Nat. Cell Biol. 2004;6:1229–1235. doi: 10.1038/ncb1194. [DOI] [PubMed] [Google Scholar]
- Kiernan R. E., Emiliani S., Nakayama K., Castro A., Labbe J. C., Lorca T., Nakayama Ki K., Benkirane M. Interaction between cyclin T1 and SCF(SKP2) targets CDK9 for ubiquitination and degradation by the proteasome. Mol. Cell. Biol. 2001;21:7956–7970. doi: 10.1128/MCB.21.23.7956-7970.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Shintani S., Kohno Y., Zhang R., Wong D. T. Cyclin G2 dysregulation in human oral cancer. Cancer Res. 2004;64:8980–8986. doi: 10.1158/0008-5472.CAN-04-1926. [DOI] [PubMed] [Google Scholar]
- Le X. F., Arachchige-Don A. S., Mao W., Horne M. C., Bast R. C., Jr Roles of human epidermal growth factor receptor 2, c-jun NH2-terminal kinase, phosphoinositide 3-kinase, and p70 S6 kinase pathways in regulation of cyclin G2 expression in human breast cancer cells. Mol. Cancer Ther. 2007;6:2843–2857. doi: 10.1158/1535-7163.MCT-07-0109. [DOI] [PubMed] [Google Scholar]
- Lee D. Y., Deng Z., Wang C. H., Yang B. B. MicroRNA-378 promotes cell survival, tumor growth, and angiogenesis by targeting SuFu and Fus-1 expression. Proc. Natl. Acad. Sci. USA. 2007;104:20350–20355. doi: 10.1073/pnas.0706901104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng R. P., Lin Y., Ma W., Wu H., Lemmers B., Chung S., Parant J. M., Lozano G., Hakem R., Benchimol S. Pirh2, a p53-induced ubiquitin-protein ligase, promotes p53 degradation. Cell. 2003;112:779–791. doi: 10.1016/s0092-8674(03)00193-4. [DOI] [PubMed] [Google Scholar]
- Liang M., Liang Y. Y., Wrighton K., Ungermannova D., Wang X. P., Brunicardi F. C., Liu X., Feng X. H., Lin X. Ubiquitination and proteolysis of cancer-derived Smad4 mutants by SCFSkp2. Mol. Cell. Biol. 2004;24:7524–7537. doi: 10.1128/MCB.24.17.7524-7537.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Cui Z. S., Luo Y., Jiang L., Man X. H., Zhang X. Effect of cyclin G2 on proliferative ability of SGC-7901 cell. World J. Gastroenterol. 2004;10:1357–1360. doi: 10.3748/wjg.v10.i9.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Wu G., Li W., Lobur D., Wan Y. Cdh1-anaphase-promoting complex targets Skp2 for destruction in transforming growth factor beta-induced growth inhibition. Mol. Cell. Biol. 2007;27:2967–2979. doi: 10.1128/MCB.01830-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines-Bandiera S. L., Kruk P. A., Auersperg N. Simian virus 40-transformed human ovarian surface epithelial cells escape normal growth controls but retain morphogenetic responses to extracellular matrix. Am. J. Obstet. Gynecol. 1992;167:729–735. doi: 10.1016/s0002-9378(11)91579-8. [DOI] [PubMed] [Google Scholar]
- Martinez-Gac L., Marques M., Garcia Z., Campanero M. R., Carrera A. C. Control of cyclin G2 mRNA expression by forkhead transcription factors: novel mechanism for cell cycle control by phosphoinositide 3-kinase and forkhead. Mol. Cell. Biol. 2004;24:2181–2189. doi: 10.1128/MCB.24.5.2181-2189.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J., Chen Y. G. Controlling TGF-beta signaling. Genes Dev. 2000;14:627–644. [PubMed] [Google Scholar]
- Morgan D. O. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu. Rev. Cell Dev. Biol. 1997;13:261–291. doi: 10.1146/annurev.cellbio.13.1.261. [DOI] [PubMed] [Google Scholar]
- Munir S., Xu G., Wu Y., Yang B., Lala P. K., Peng C. Nodal and ALK7 inhibit proliferation and induce apoptosis in human trophoblast cells. J. Biol. Chem. 2004;279:31277–31286. doi: 10.1074/jbc.M400641200. [DOI] [PubMed] [Google Scholar]
- Nakayama K., et al. Targeted disruption of Skp2 results in accumulation of cyclin E and p27(Kip1), polyploidy and centrosome overduplication. EMBO J. 2000;19:2069–2081. doi: 10.1093/emboj/19.9.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K. I., Nakayama K. Ubiquitin ligases: cell-cycle control and cancer. Nat. Rev. Cancer. 2006;6:369–381. doi: 10.1038/nrc1881. [DOI] [PubMed] [Google Scholar]
- Nonaka S., Shiratori H., Saijoh Y., Hamada H. Determination of left-right patterning of the mouse embryo by artificial nodal flow. Nature. 2002;418:96–99. doi: 10.1038/nature00849. [DOI] [PubMed] [Google Scholar]
- Patel Y. M., Lane M. D. Mitotic clonal expansion during preadipocyte differentiation: calpain-mediated turnover of p27. J. Biol. Chem. 2000;275:17653–17660. doi: 10.1074/jbc.M910445199. [DOI] [PubMed] [Google Scholar]
- Pines J. Cyclins and cyclin-dependent kinases: theme and variations. Adv. Cancer Res. 1995;66:181–212. doi: 10.1016/s0065-230x(08)60254-7. [DOI] [PubMed] [Google Scholar]
- Pines J., Lindon C. Proteolysis: anytime, any place, anywhere? Nat. Cell Biol. 2005;7:731–735. doi: 10.1038/ncb0805-731. [DOI] [PubMed] [Google Scholar]
- Rechsteiner M., Rogers S. W. PEST sequences and regulation by proteolysis. Trends Biochem. Sci. 1996;21:267–271. [PubMed] [Google Scholar]
- Reissmann E., Jornvall H., Blokzijl A., Andersson O., Chang C., Minchiotti G., Persico M. G., Ibanez C. F., Brivanlou A. H. The orphan receptor ALK7 and the Activin receptor ALK4 mediate signaling by Nodal proteins during vertebrate development. Genes Dev. 2001;15:2010–2022. doi: 10.1101/gad.201801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts H. J., Hu S., Qiu Q., Leung P. C., Caniggia I., Gruslin A., Tsang B., Peng C. Identification of novel isoforms of activin receptor-like kinase 7 (ALK7) generated by alternative splicing and expression of ALK7 and its ligand, Nodal, in human placenta. Biol. Reprod. 2003;68:1719–1726. doi: 10.1095/biolreprod.102.013045. [DOI] [PubMed] [Google Scholar]
- Rogers S., Wells R., Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986;234:364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- Sandoval A., Oviedo N., Tadmouri A., Avila T., De Waard M., Felix R. Two PEST-like motifs regulate Ca2+/calpain-mediated cleavage of the CaVbeta3 subunit and provide important determinants for neuronal Ca2+ channel activity. Eur J. Neurosci. 2006;23:2311–2320. doi: 10.1111/j.1460-9568.2006.04749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumway S. D., Maki M., Miyamoto S. The PEST domain of IkappaBalpha is necessary and sufficient for in vitro degradation by mu-calpain. J. Biol. Chem. 1999;274:30874–30881. doi: 10.1074/jbc.274.43.30874. [DOI] [PubMed] [Google Scholar]
- Sutterluty H., Chatelain E., Marti A., Wirbelauer C., Senften M., Muller U., Krek W. p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nat. Cell Biol. 1999;1:207–214. doi: 10.1038/12027. [DOI] [PubMed] [Google Scholar]
- Tedesco D., Lukas J., Reed S. I. The pRb-related protein p130 is regulated by phosphorylation-dependent proteolysis via the protein-ubiquitin ligase SCF(Skp2) Genes Dev. 2002;16:2946–2957. doi: 10.1101/gad.1011202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetkov L. M., Yeh K. H., Lee S. J., Sun H., Zhang H. p27(Kip1) ubiquitination and degradation is regulated by the SCF(Skp2) complex through phosphorylated Thr187 in p27. Curr. Biol. 1999;9:661–664. doi: 10.1016/s0960-9822(99)80290-5. [DOI] [PubMed] [Google Scholar]
- Wakefield L. M., Roberts A. B. TGF-beta signaling: positive and negative effects on tumorigenesis. Curr. Opin. Genet. Dev. 2002;12:22–29. doi: 10.1016/s0959-437x(01)00259-3. [DOI] [PubMed] [Google Scholar]
- Wang W., Ungermannova D., Jin J., Harper J. W., Liu X. Negative regulation of SCFSkp2 ubiquitin ligase by TGF-beta signaling. Oncogene. 2004;23:1064–1075. doi: 10.1038/sj.onc.1207204. [DOI] [PubMed] [Google Scholar]
- Wrana J. L., Attisano L., Wieser R., Ventura F., Massague J. Mechanism of activation of the TGF-beta receptor. Nature. 1994;370:341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- Xu G., Zhong Y., Munir S., Yang B. B., Tsang B. K., Peng C. Nodal induces apoptosis and inhibits proliferation in human epithelial ovarian cancer cells via activin receptor-like kinase 7. J. Clin. Endocrinol. Metab. 2004;89:5523–5534. doi: 10.1210/jc.2004-0893. [DOI] [PubMed] [Google Scholar]
- Xu G., Zhou H., Wang Q., Auersperg N., Peng C. Activin receptor-like kinase 7 induces apoptosis through up-regulation of Bax and down-regulation of Xiap in normal and malignant ovarian epithelial cell lines. Mol. Cancer Res. 2006;4:235–246. doi: 10.1158/1541-7786.MCR-05-0174. [DOI] [PubMed] [Google Scholar]
- Zhang H., Kobayashi R., Galaktionov K., Beach D. p19Skp1 and p45Skp2 are essential elements of the cyclin A-CDK2 S phase kinase. Cell. 1995;82:915–925. doi: 10.1016/0092-8674(95)90271-6. [DOI] [PubMed] [Google Scholar]
- Zimmerman C. M., Padgett R. W. Transforming growth factor beta signaling mediators and modulators. Gene. 2000;249:17–30. doi: 10.1016/s0378-1119(00)00162-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.