Abstract
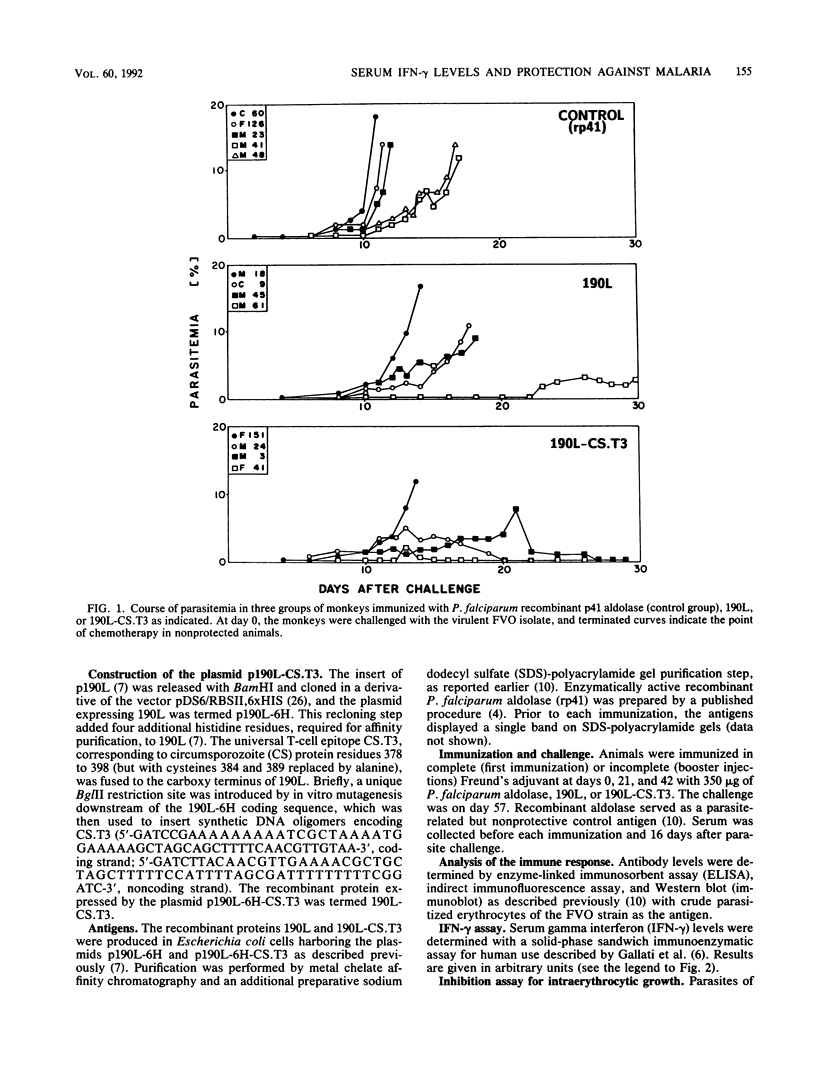
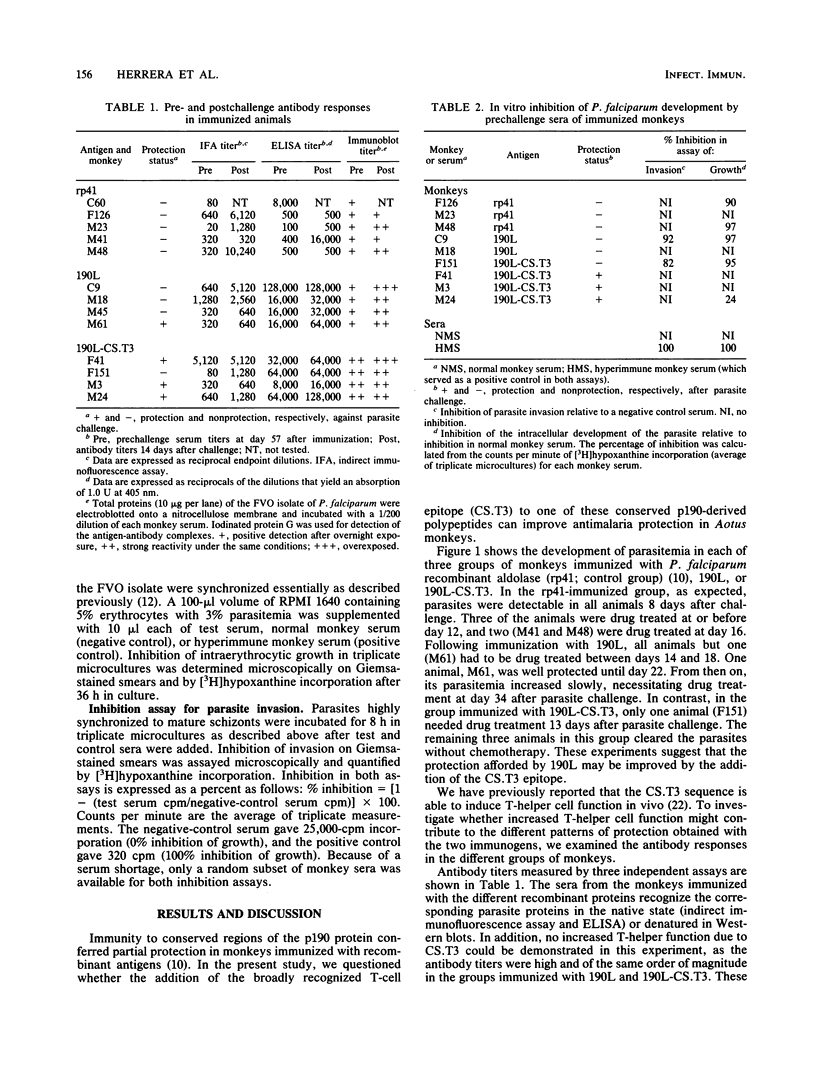
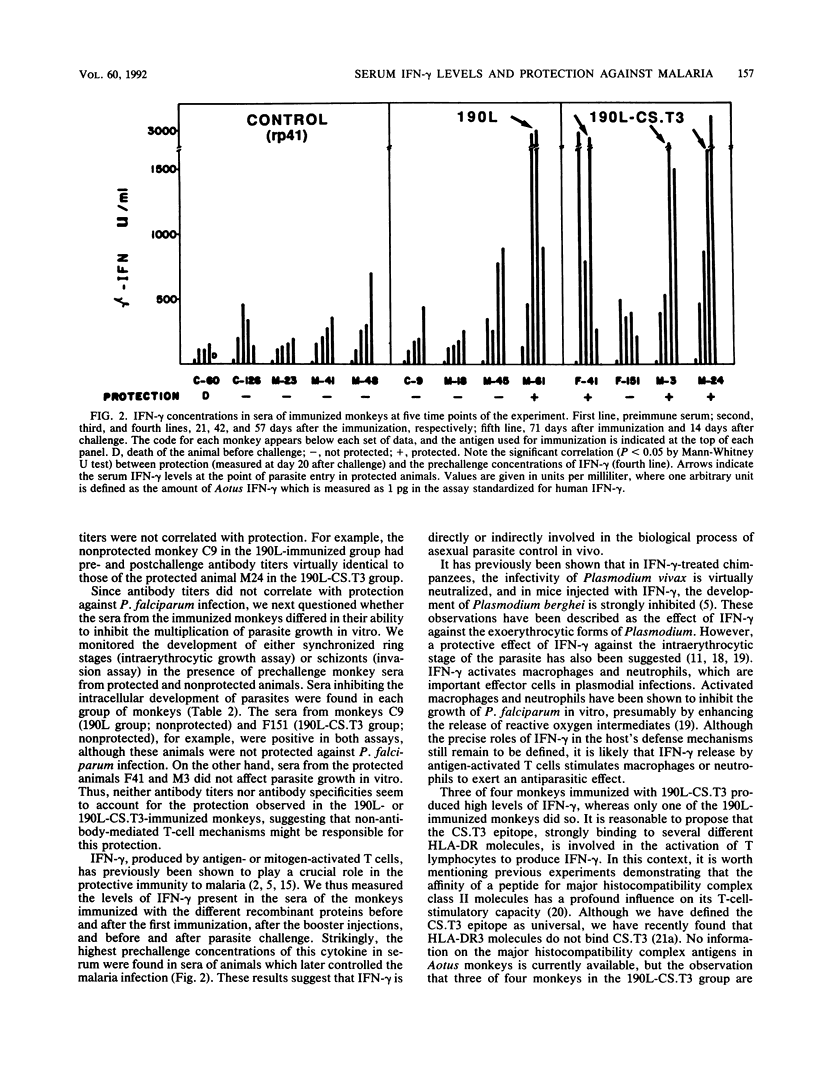
The major surface antigen p190 of the human malaria parasite Plasmodium falciparum contains nonpolymorphic, immunogenic stretches of amino acids which are attractive components for a subunit vaccine against malaria. One such polypeptide, termed 190L, is contained in the 80-kDa processing product of p190, which constitutes the major coat component of mature merozoites. We report here that immunization of Aotus monkeys with 190L gives only poor protection against P. falciparum challenge. However, addition by genetic engineering of a universal T-cell epitope (CS.T3) to 190L improved immunity, and as a result three of four monkeys were protected following challenge infection with blood-stage parasites. Neither antibody against the immunizing antigens or against blood-stage parasites nor the capacity of the monkeys' sera to inhibit in vitro parasite invasion correlated with protection. However, in contrast to sera from nonprotected monkeys, sera from protected animals contained elevated levels of gamma interferon. These results suggest that gamma interferon is directly or indirectly involved in the process of asexual parasite control in vivo.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheung A., Leban J., Shaw A. R., Merkli B., Stocker J., Chizzolini C., Sander C., Perrin L. H. Immunization with synthetic peptides of a Plasmodium falciparum surface antigen induces antimerozoite antibodies. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8328–8332. doi: 10.1073/pnas.83.21.8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I. A., Hunt N. H., Butcher G. A., Cowden W. B. Inhibition of murine malaria (Plasmodium chabaudi) in vivo by recombinant interferon-gamma or tumor necrosis factor, and its enhancement by butylated hydroxyanisole. J Immunol. 1987 Nov 15;139(10):3493–3496. [PubMed] [Google Scholar]
- Crisanti A., Müller H. M., Hilbich C., Sinigaglia F., Matile H., McKay M., Scaife J., Beyreuther K., Bujard H. Epitopes recognized by human T cells map within the conserved part of the GP190 of P. falciparum. Science. 1988 Jun 3;240(4857):1324–1326. doi: 10.1126/science.2453924. [DOI] [PubMed] [Google Scholar]
- Döbeli H., Trzeciak A., Gillessen D., Matile H., Srivastava I. K., Perrin L. H., Jakob P. E., Certa U. Expression, purification, biochemical characterization and inhibition of recombinant Plasmodium falciparum aldolase. Mol Biochem Parasitol. 1990 Jun;41(2):259–268. doi: 10.1016/0166-6851(90)90189-s. [DOI] [PubMed] [Google Scholar]
- Ferreira A., Schofield L., Enea V., Schellekens H., van der Meide P., Collins W. E., Nussenzweig R. S., Nussenzweig V. Inhibition of development of exoerythrocytic forms of malaria parasites by gamma-interferon. Science. 1986 May 16;232(4752):881–884. doi: 10.1126/science.3085218. [DOI] [PubMed] [Google Scholar]
- Gallati H., Pracht I., Schmidt J., Häring P., Garotta G. A simple, rapid and large capacity ELISA for biologically active native and recombinant human IFN gamma. J Biol Regul Homeost Agents. 1987 Jul-Sep;1(3):109–118. [PubMed] [Google Scholar]
- Gentz R., Certa U., Takacs B., Matile H., Döbeli H., Pink R., Mackay M., Bone N., Scaife J. G. Major surface antigen p190 of Plasmodium falciparum: detection of common epitopes present in a variety of plasmodia isolates. EMBO J. 1988 Jan;7(1):225–230. doi: 10.1002/j.1460-2075.1988.tb02803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttinger M., Romagnoli P., Vandel L., Meloen R., Takacs B., Pink J. R., Sinigaglia F. HLA polymorphism and T cell recognition of a conserved region of p190, a malaria vaccine candidate. Int Immunol. 1991 Sep;3(9):899–906. doi: 10.1093/intimm/3.9.899. [DOI] [PubMed] [Google Scholar]
- Guttinger M., Romagnoli P., Vandel L., Meloen R., Takacs B., Pink J. R., Sinigaglia F. HLA polymorphism and T cell recognition of a conserved region of p190, a malaria vaccine candidate. Int Immunol. 1991 Sep;3(9):899–906. doi: 10.1093/intimm/3.9.899. [DOI] [PubMed] [Google Scholar]
- Hall R., Hyde J. E., Goman M., Simmons D. L., Hope I. A., Mackay M., Scaife J., Merkli B., Richle R., Stocker J. Major surface antigen gene of a human malaria parasite cloned and expressed in bacteria. 1984 Sep 27-Oct 3Nature. 311(5984):379–382. doi: 10.1038/311379a0. [DOI] [PubMed] [Google Scholar]
- Herrera S., Herrera M. A., Perlaza B. L., Burki Y., Caspers P., Döbeli H., Rotmann D., Certa U. Immunization of Aotus monkeys with Plasmodium falciparum blood-stage recombinant proteins. Proc Natl Acad Sci U S A. 1990 May;87(10):4017–4021. doi: 10.1073/pnas.87.10.4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaratilake L. M., Ferrante A., Rzepczyk C. The role of T lymphocytes in immunity to Plasmodium falciparum. Enhancement of neutrophil-mediated parasite killing by lymphotoxin and IFN-gamma: comparisons with tumor necrosis factor effects. J Immunol. 1991 Jan 15;146(2):762–767. [PubMed] [Google Scholar]
- Lambros C., Vanderberg J. P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979 Jun;65(3):418–420. [PubMed] [Google Scholar]
- Lyon J. A., Geller R. H., Haynes J. D., Chulay J. D., Weber J. L. Epitope map and processing scheme for the 195,000-dalton surface glycoprotein of Plasmodium falciparum merozoites deduced from cloned overlapping segments of the gene. Proc Natl Acad Sci U S A. 1986 May;83(9):2989–2993. doi: 10.1073/pnas.83.9.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay M., Goman M., Bone N., Hyde J. E., Scaife J., Certa U., Stunnenberg H., Bujard H. Polymorphism of the precursor for the major surface antigens of Plasmodium falciparum merozoites: studies at the genetic level. EMBO J. 1985 Dec 30;4(13B):3823–3829. doi: 10.1002/j.1460-2075.1985.tb04154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheshwari R. K., Czarniecki C. W., Dutta G. P., Puri S. K., Dhawan B. N., Friedman R. M. Recombinant human gamma interferon inhibits simian malaria. Infect Immun. 1986 Sep;53(3):628–630. doi: 10.1128/iai.53.3.628-630.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride J. S., Newbold C. I., Anand R. Polymorphism of a high molecular weight schizont antigen of the human malaria parasite Plasmodium falciparum. J Exp Med. 1985 Jan 1;161(1):160–180. doi: 10.1084/jem.161.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller H. M., Früh K., von Brunn A., Esposito F., Lombardi S., Crisanti A., Bujard H. Development of the human immune response against the major surface protein (gp190) of Plasmodium falciparum. Infect Immun. 1989 Dec;57(12):3765–3769. doi: 10.1128/iai.57.12.3765-3769.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockenhouse C. F., Schulman S., Shear H. L. Induction of crisis forms in the human malaria parasite Plasmodium falciparum by gamma-interferon-activated, monocyte-derived macrophages. J Immunol. 1984 Sep;133(3):1601–1608. [PubMed] [Google Scholar]
- Playfair J. H., Dockrell H., Taverne J. Macrophages as effector cells in immunity to malaria. Immunol Lett. 1985;11(3-4):233–237. doi: 10.1016/0165-2478(85)90173-7. [DOI] [PubMed] [Google Scholar]
- Schaeffer E. B., Sette A., Johnson D. L., Bekoff M. C., Smith J. A., Grey H. M., Buus S. Relative contribution of "determinant selection" and "holes in the T-cell repertoire" to T-cell responses. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4649–4653. doi: 10.1073/pnas.86.12.4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui W. A., Tam L. Q., Kramer K. J., Hui G. S., Case S. E., Yamaga K. M., Chang S. P., Chan E. B., Kan S. C. Merozoite surface coat precursor protein completely protects Aotus monkeys against Plasmodium falciparum malaria. Proc Natl Acad Sci U S A. 1987 May;84(9):3014–3018. doi: 10.1073/pnas.84.9.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinigaglia F., Guttinger M., Kilgus J., Doran D. M., Matile H., Etlinger H., Trzeciak A., Gillessen D., Pink J. R. A malaria T-cell epitope recognized in association with most mouse and human MHC class II molecules. Nature. 1988 Dec 22;336(6201):778–780. doi: 10.1038/336778a0. [DOI] [PubMed] [Google Scholar]
- Sinigaglia F., Guttinger M., Romagnoli P., Takacs B. Malaria antigens and MHC restriction. Immunol Lett. 1990 Aug;25(1-3):265–270. doi: 10.1016/0165-2478(90)90125-a. [DOI] [PubMed] [Google Scholar]
- Sinigaglia F., Takacs B., Jacot H., Matile H., Pink J. R., Crisanti A., Bujard H. Nonpolymorphic regions of p190, a protein of the Plasmodium falciparum erythrocytic stage, contain both T and B cell epitopes. J Immunol. 1988 May 15;140(10):3568–3572. [PubMed] [Google Scholar]
- Strych W., Miettinen-Baumann A., Lottspeich F., Heidrich H. G. Isolation and characterization of the 80,000 dalton Plasmodium falciparum merozoite surface antigen. Parasitol Res. 1987;73(5):435–441. doi: 10.1007/BF00538201. [DOI] [PubMed] [Google Scholar]
- Stueber D., Ibrahimi I., Cutler D., Dobberstein B., Bujard H. A novel in vitro transcription-translation system: accurate and efficient synthesis of single proteins from cloned DNA sequences. EMBO J. 1984 Dec 20;3(13):3143–3148. doi: 10.1002/j.1460-2075.1984.tb02271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe K., Mackay M., Goman M., Scaife J. G. Allelic dimorphism in a surface antigen gene of the malaria parasite Plasmodium falciparum. J Mol Biol. 1987 May 20;195(2):273–287. doi: 10.1016/0022-2836(87)90649-8. [DOI] [PubMed] [Google Scholar]


