Abstract
In budding yeast, as in other eukaryotes, the Cdc7 protein kinase is important for initiation of DNA synthesis in vegetative cells. In addition, Cdc7 has crucial meiotic functions: it facilitates premeiotic DNA replication, and it is essential for the initiation of recombination. This work uses a chemical genetic approach to demonstrate that Cdc7 kinase has additional roles in meiosis. First, Cdc7 allows expression of NDT80, a meiosis-specific transcriptional activator required for the induction of genes involved in exit from pachytene, meiotic progression, and spore formation. Second, Cdc7 is necessary for recruitment of monopolin to sister kinetochores, and it is necessary for the reductional segregation occurring at meiosis I. The use of the same kinase to regulate several distinct meiosis-specific processes may be important for the coordination of these processes during meiosis.
INTRODUCTION
Sexually reproducing, eukaryotic cells use two types of cell division: mitosis and meiosis. In mitosis, chromosomes are replicated and the resulting sister chromatids are then segregated to produce two genetically identical daughter cells. In contrast, meiosis generates haploid gametes from diploid cells by having one round of DNA replication followed by two rounds of chromosome segregation. A unique feature of meiosis is that pairs of homologous sister chromatids are pulled to opposite poles (reductional segregation) at the first meiotic division (meiosis I). The second meiotic division (meiosis II) resembles mitosis in that sister chromatids are segregated to opposite poles (called equational segregation). In budding yeast, meiotic chromosome behavior arises from the interplay between meiosis-specific proteins and proteins involved in mitosis (Marston and Amon 2004; Wan et al., 2008).
Progression through meiosis relies on transcriptional cascades regulated in part by protein kinases (Vershon and Pierce 2000). Microarray analysis has identified several temporal waves of transcription. The first wave includes the early meiotic genes, which function specifically in premeiotic S phase and in meiotic prophase (Chu et al., 1998; Primig et al., 2000). Expression of early meiotic genes depends upon the transcriptional activator Ime1 (Kassir et al., 1988; Smith et al., 1990). The transition from meiotic prophase to the first meiotic division relies on a meiosis-specific transcriptional activator, Ndt80, as well as Cdc28-Clb1 kinase activity (Xu et al., 1995; Benjamin et al., 2003; Carlile and Amon 2008). Ndt80 binds to a sequence motif called the middle sporulation element, or MSE, upstream of genes in the second wave of transcription. These genes are called middle sporulation genes, and they are necessary for exit from pachytene, meiotic progression, and spore morphogenesis (Hepworth et al., 1995, 1998; Chu and Herskowitz 1998; Allers and Lichten 2001). Cells lacking NDT80 arrest at pachytene with duplicated, but unseparated, spindle pole bodies (Xu et al., 1995). One of the genes regulated by Ndt80 is CLB1, which, together with Cdc28 kinase, promotes execution of the first meiotic division (Chu and Herskowitz 1998; Pak and Segall 2002, Carlile and Amon 2008).
Faithful segregation of chromosomes requires correct attachment of spindle microtubules to kinetochores (Petronczki et al., 2003). In mitosis, cohesin complexes containing Mcd1/Scc1 are loaded onto chromosomes during DNA replication to hold sister chromatids together; these complexes persist until the onset of anaphase (Uhlmann and Nasmyth 1998). Kinetochores of sister chromatids (sister kinetochores) attach to microtubules emanating from opposite poles, a process called biorientation. The pulling force by spindle microtubules in combination with sister chromatid cohesion generates tension that stabilizes kinetochore–microtubule attachments. At the onset of anaphase, separase cleaves cohesin complexes along the chromosomes, and the sister chromatids segregate to opposite poles (Uhlmann et al., 1999).
Meiosis I is unique in that sister chromatids segregate to the same pole instead of opposite poles. This unique chromosome behavior requires several meiosis-specific processes (Petronczki et al., 2003). First, reciprocal recombination between nonsister chromatids of homologous chromosomes generates crossovers. Crossovers, in combination with sister chromatid cohesion, create physical connections between homologues that allow proper orientation at metaphase I. Second, sister kinetochores behave as a single unit (called mono-orientation) and attach to microtubules from the same pole. Third, cohesin complexes are removed from chromosomes in a stepwise manner. At the onset of anaphase I, arm cohesins are cleaved by separase (Buonomo et al., 2000). Centromeric cohesin complexes are retained until anaphase of meiosis II. This two step loss of cohesion is made possible by the substitution of the Mcd1/Scc1 subunit of the cohesin complex with a meiosis-specific protein, Rec8, which, when present at kinetochores, is resistant to cleavage at anaphase I (Klein et al., 1999; Watanabe and Nurse 1999). Failure in any of these processes in yeast leads to chromosome missegregation and inviable spores.
In budding yeast, mono-orientation of sister kinetochores at meiosis I requires that the monopolin complex, consisting of Mam1, Lrs4, and Csm1, be localized and maintained on kinetochores during metaphase I (Toth et al., 2000; Rabitsch et al., 2003). Mam1 is a meiosis-specific protein, whereas Lrs4 and Csm1 are constitutively present in mitotic and meiotic cells. In mitotic cells, Lrs4 and Csm1 form a complex and reside in the nucleolus. They are released from the nucleolus before meiosis I and localize to kinetochores together with Mam1. If a monopolin subunit is deleted, sister kinetochores biorient at meiosis I (Toth et al., 2000; Rabitsch et al., 2003). The association of the monopolin complex to kinetochores is regulated by several proteins, such as the polo-like kinase, Cdc5, and the meiosis-specific Spo13 protein (Clyne et al., 2003; Lee and Amon 2003). Spo13 is needed to maintain the kinetochore localization of monopolin during metaphase I (Lee et al., 2004; Katis et al., 2004).
Protein kinases required for mitotic cell division have been found to be important for the execution of meiosis-specific processes. For example, Cdc28-Clb5 is essential for premeiotic S phase and double-strand break (DSB) formation, Cdc28-Clb1 is important for entry into the meiotic divisions and Cdc5 is necessary for Holliday junction resolution and mono-orientation of sister kineotochores (Stuart and Wittenberg 1998; Smith et al., 2001; Clyne et al., 2003; Henderson et al., 2006; Carlile and Amon 2008). Another highly conserved cell cycle kinase with important roles in meiosis is Cdc7-Dbf4. Like the cyclin-dependent kinases, Cdc7-Dbf4 is essential for growth, and it is comprised of a catalytic subunit (Cdc7) and a regulatory subunit (Dbf4) (Johnston et al., 1999; Sclafani 2000). For simplicity this complex will hereinafter be referred to as Cdc7. Inactivation of Cdc7 in meiosis results in a delay in DNA replication and a prophase arrest with no recombination (Schild and Byers 1978; Valentin et al., 2005; Wan et al., 2006). The failure to recombine is because initiation of meiotic recombination is regulated by Cdc7 together with Cdc28-Clb5 via phosphorylation of the DSB protein Mer2 (Sasanuma et al., 2008; Wan et al., 2008). To elucidate additional roles of Cdc7 in meiosis, we have developed a chemical genetic approach to specifically inactivate Cdc7 kinase activity during meiosis (Bishop et al., 2001; Wan et al., 2006). This approach involves enlarging the ATP binding pocket of Cdc7 to create an analog-sensitive (cdc7-as) kinase that can be inhibited by the addition of purine analogs to the sporulation medium. Using cdc7-as, we have shown that Cdc7 kinase activity promotes meiotic progression by enabling transcription of NDT80. Furthermore, Cdc7 is required for mono-orientation of sister kinetochores by allowing recruitment of the monopolin subunit, Mam1, onto kinetochores.
MATERIALS AND METHODS
Plasmids
pLW55-P83L.
A 1.7-kb XhoI/XbaI fragment containing 230 base pairs upstream and 1543 base pairs downstream of the MCM5 start codon was amplified from SK1 genomic DNA and cloned into XhoI/XbaI-digested pRS306 to make pLW55. Site-directed mutagenesis using the QuikChange kit (Stratagene, La Jolla, CA) was performed to change codon 83 from CCT (proline) to CTA (leucine). Digestion of pLW55 with NruI cuts 119 base pairs upstream of the MCM5 ATG and targets the integration of the plasmid to the MCM5 locus. PCUP1-NDT80-3HA (YIplac211-CUP1-NDT80-3HA) was generously provided by David Stuart (University of Alberta, Edmonton, AB, Canada; Sopko et al., 2002). The plasmid carrying an untagged version of NDT80, PCUP1-NDT80, was created by digestion of YIplac211-CUP1-NDT80-3HA with NotI, followed by self-ligation. The removal of the 3HA tag from NDT80 was confirmed by DNA sequencing. PNDT80-NDT80 (pNKY1212), a CEN plasmid carrying NDT80 under the control of the NDT80 promoter, was provided by Nancy Kleckner (Harvard University, Cambridge, MA; Xu et al., 1995).
Strains
All strains are derived from the SK1 background. Genotypes can be found in Table 1. cdc7-as strains carry the as3 allele, which contains the L120A V181A mutations (Wan et al., 2006). The cdc7-as mcm5-bob1 diploid NH661 was constructed first by introducing mcm5-bob1 into the cdc7-as haploids NH144-32aF and NH144-33bF by two-step gene replacement (Rothstein 1991). The URA3 mcm5-bob1 plasmid pLW55-P83L was targeted to integrate immediately upstream of the MCM5 locus by digestion with NruI. The transformants were grown nonselectively in YEPD, and then plasmid popouts were selected on synthetic complete medium containing 5-fluoro-orotic acid (5-FOA). FOAR colonies were then screened for the presence of the mcm5-bob1 by assaying for suppression of the vegetative growth defect of cdc7-as plus 30 μM 4-amino-1-tert-butyl-3-(p-methylyphenyl)pyrazolo [3,4-d]pyrimidine (PP1) (see below). The cdc7-as mcm5-bob1 diploid was generated immediately before time courses. The appropriate haploids were mated on YPD and replica plated to SD-his-arg medium to select for diploids. After patching the diploids once onto SD-his-arg, the cells were inoculated into YPD medium for sporulation.
Table 1.
S. cerevisiae strains
| Name | Genotype | Source |
|---|---|---|
| NH144-32aF | MATa leu2Δ::hisG his4-x lys2 hoΔ::LYS2 cdc7-as3-9myc ura3 | Wan et al. (2006) |
| NH144-33bF | MATα leu2-K arg4-Nsp lys2 hoΔ::LYS2 cdc7-as3-9myc ura3 | Wan et al. (2006) |
| NH452F | MATa leu2Δ::hisG his4-xARG4lys2hoΔ::LYS2cdc7-as3-9mycura3 | Wan et al. (2006) |
| MATα leu2-k HIS4 arg4-Nsp lys2 hoΔ::LYS2 cdc7-as3-9myc ura3 | ||
| NH588 | Same as NH452F only red1::LEU2 | This work |
| NH589 | Same as NH452F only rec104Δ::LEU2 | This work |
| NH590 | Same as NH452F only mek1Δ::LEU2 | This work |
| NH661 | Same as NH452F only mcm5-P83L | This work |
| NH709 | Same as NH452F only rad9Δ::kanMX4 | This work |
| NH452F::CUP1-NDT80 | Same as NH452F only ura3::PCUP1-NDT80-3HA::URA3 | This work |
| NH379 | MATa leu2ΔhisG his4-xhoΔ::LYS2lys2ura3ARG4ndt80Δ::kanMX4 | This work |
| MATα leu2k HIS4 hoΔ::LYS2 lys2 ura3 arg4-Nsp ndt80Δ::kanMX6 | ||
| NH656 | MATa leu2 his4-xARG4lys2 hoΔ::LYS2cdc7-as3-9mycsml1Δ::3HA-URA3-3HA | This work |
| MATa leu2 HIS4 arg4-Nsp lys2 hoΔ::LYS2 cdc7-as3-9myc sml1Δ::3HA-URA3-3HA | ||
| NH660 | Same as NH656 only mec1Δ::kanMX4 | This work |
| NH875 | MATa leu2::tetR-GFP-LEU2hoΔ::LYS2ura3::tetOx240::URA3 lys2his3::hisGARG4 | This work |
| MATα leu2-K hoΔ::LYS2 ura3 lys2 HIS3 arg4-Nsp | ||
| CDC7 trp1::hisG::GAL-NDT80-TRP1 | ||
| cdc7-as3-9myc TRP1 | ||
| NH875::CUP1-NDT80-3HA | Same as NH875 only ura3::tetOx240::URA3 | This work |
| ura3::PCUP1-NDT80-3HA::URA3 | ||
| NH883 | Same as NH875::CUP1-NDT80-3HA only spo11Δ::natMX4 | This work |
| spo11Δ::natMX4 | ||
| NH874 | MATa leu2::tetR-GFP-LEU2 hoΔ::LYS2ura3::tetOx240::URA3lys2 his3::hisGarg4 | This work |
| MATα leu2-K hoΔ::LYS2 ura3 lys2 HIS3 arg4-Nsp | ||
| cdc7-as3trp1::hisG::GAL-NDT80-TRP1 | ||
| cdc7-as3-9myc TRP1 | ||
| NH874::CUP1-NDT80-3HA | same as NH874 only ura3::tetOx240::URA3 | This work |
| ura3::PCUP1-NDT80-3HA::URA3 | ||
| NH870::CUP1-NDT80-3HA | MATα leu2his3::hisGura3::PCUP1-NDT80-3HA::URA3trp1::hisGcdc7-as3lys2 | This work |
| MATa leu2 his3::hisG ura3 trp1::hisG cdc7-as3 lys2 | ||
| hoΔ::LYS2NDC10-6HA::HIS3MX6MAM1-9myc::TRP1 | ||
| hoΔ::LYS2 NDC10-6HA::HIS3MX6 MAM1-9myc::TRP1 |
RED1 and REC104 were deleted using pNH119 and pNH131, respectively (Hollingsworth and Johnson 1993). MEK1 was deleted with pTS1-1 (de los Santos and Hollingsworth 1999). RAD9 and NDT80 were deleted with kanMX4 using the polymerase chain reaction (PCR) method of Longtine et al. (1998). The mec1Δ sml1Δ diploid was made in two steps. First, a PCR fragment was generated using pMPY-3HA and used to substitute 3HA-URA3-3HA for the SML1 open reading frame (Schneider et al., 1995). The sml1Δ::3HA-URA3-3HA deletion was confirmed by Southern blot and then crossed to NH144-32aF, sporulated, and tetrads were dissected. Ura+ spore colonies containing sml1Δ-3HA-URA3-3HA were screened for cdc7-as by looking for lack of growth on YEPD plates containing 30 μM PP1. MEC1 was deleted from MATa and MATα cdc7-as sml1Δ::3HA-URA3-3HA spore colonies from this cross by using kanMX4, and the resulting haploids were mated to generate NH660. NH452F::CUP1-NDT80-3HA was generated by transforming NH452F with YIplac211-CUP1-NDT80-3HA digested with ApaI to target integration to ura3. The ndt80Δ::PCUP1-NDT80-3HA and ndt80Δ/NDT80 diploids were made by transformation of NH379 with YIplac211-CUP1-NDT80-3HA or pNKY1212, respectively.
A CDC7 diploid heterozygous for a tandem array of tet operators integrated at ura3 on chromosome V was created by selecting a MATa segregant (NH843-17-2) from a cross between 14154 (Benjamin et al., 2003) and 9375 that contains leu2::tetR-GFP:LEU2 and ura3::tetOX240:URA3 (Michaelis et al., 1997) (strains provided by A. Amon, Massachusetts Institute of Technology, Boston, MA). NH843-17-2 was then mated to NH144-33bF to make NH875. To make an isogenic diploid ectopically expressing PCUP1-NDT80-3HA (NH875::CUP1-NDT80-3HA) NH843-17-2 was crossed to NH144-33bF::CUP1-NDT80-3HA. A corresponding cdc7-as diploid (NH874) was made by crossing NH843-17-2 to SKY371 cdc7-as, selecting a MATa segregant (NH844-2-9-2) containing cdc7-as, leu2::tetR-GFP:LEU2, and ura3::tetOX240:URA3, and crossing it to NH144-33bF. In all cases, the presence of the Tet repressor-green fluorescent protein (GFP) and tet operators was confirmed by observation of a “green dot” by using fluorescence microscopy. The cdc7-as mutant was verified by failure to grow in YPD liquid medium containing 20 μM PP1. To make an isogenic diploid ectopically expressing PCUP1-NDT80-3HA (NH874::CUP1-NDT80-3HA), NH844-2-9-2 was crossed to NH144-33bF::CUP1-NDT80-3HA. To make NH870, the cdc7-as allele was first introduced into 7152, a MATa MAM1-9myc NDC10-6HA haploid, by two-step gene replacement using pNH256 (Wan et al., 2008). 7152 cdc7-as was then crossed to the MATα MAM1-9myc NDC10-6HA haploid 7153, and the spore colonies were screened for a MATα cdc7-as segregant that was backcrossed to 7152 cdc7-as. NH870::CUP1-NDT80 was generated by targeted integration of NcoI digested YIplac211-CUP1-NDT80 into the ura3 locus.
Plate Assay for cdc7-as mcm5-bob1
Single colonies were grown in YPD overnight at 30°C, diluted 1:1000, and 3 μl was spotted onto YPDcom and YPDcom +30 μM PP1. The plates were then incubated at 30°C for 1–2 d.
Microarray Experiments
Two independent isolates were sporulated in the absence or presence of 15 μM PP1 added at the time of transfer to Spo medium. Total RNA was prepared from snap-frozen cells as directed (RiboPure-Yeast; Ambion, Austin, TX), and labeled cDNA was prepared as described previously (Oliva et al., 2005). Two-color hybridization of 10-h samples and their 0-h controls was performed on custom arrays of spotted long PCR probes. Hybridizations were performed in technical duplicate. Detailed information regarding array composition, manufacture, and processing are available through ArrayExpress (www.ebi.ac.uk/ArrayExpress/) under accession A-MEXP-1379.
Data were normalized within the Bioconductor package (Gentleman et al., 2004) by fitting to a normal+exponential convolution model (Smyth 2005), and intensity-dependent bias was removed by loess correction. All spots except those manually flagged at time of scanning were included in further analysis. A linear model was fit to each pair of technical replicate arrays; individual isolates were analyzed separately. Pearson correlations of log2-fold change values from this fit were clustered as described previously (Eisen et al., 1998). A complete data set including raw and processed data is available through ArrayExpress (accession number E-MEXP-1765).
Time Courses
Two sporulation protocols were used. For the experiments shown in Figures 1–4, diploid cells were sporulated in 2% potassium acetate at a density of 3 × 107 cells/ml and shaken at 30°C as described in de los Santos and Hollingsworth (1999). For the experiment shown in Figure 5, cells were grown in YPD for 24 h, diluted in 1% YPA (1% yeast extract, 2% bactopeptone, and 1% potassium acetate) at OD660 = 0.2, and grown for 13–14 h to OD660 = 1.3–1.4. Cells were washed once with water and sporulated in the same volume of 2% potassium acetate (Spo medium). PP1 was added at a final concentration of 15 μM when cells were transferred to Spo medium in both sporulation protocols. Flow cytometry analysis was performed by fixing 3 ml of sporulating cells with 70% ethanol overnight at 4°C as described in Stuart and Wittenberg (1998). This analysis was performed at the Stony Brook University Hospital Research Flow Cytometry Facility (Stony Brook, NY). DSBs were examined at the naturally occurring YCR048w hot spot as described in Woltering et al. (2000).
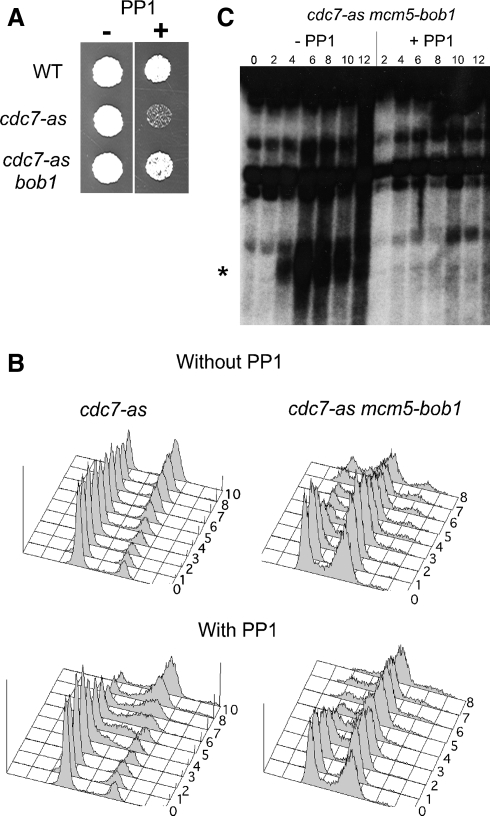
Figure 1.
Effect of PP1 on cdc7-as mcm5-bob1 strains. (A) Vegetative growth on YPD plates with or without 30 μM PP1. (B) NH452F (cdc7-as) and NH661 (cdc7-as mcm5-bob1) were analyzed by flow cytometry at 1-h intervals (indicated by numbers on the plots) after transfer to Spo medium in a time course with and without 15 μM PP1. The cdc7-as data have been published previously (Wan et al., 2006). (C) DSBs at the YCR048w hot spot (Wu and Lichten 1994). The asterisk indicates the DSB bands.
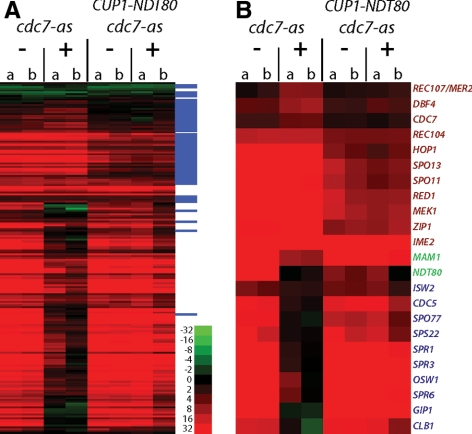
Figure 2.
Microarray analysis of RNAs from meiotic cells under various conditions. (A) Expression profile of ∼200 previously identified sporulation-specific genes at 10 h in cdc7-as (NH452F) and cdc7-asNDT80 (NH452F:CUP1-NDT80) with and without inhibitor. Relative RNA abundance in the indicated strains with (+) or without (−) 15 μM PP1 was determined by competitive hybridization to a T0 control. Early genes are tagged with a blue line in the far right column. Red indicates induction, green repression. Although the data exhibited a dynamic range of 216, only a range of 26 is shown here. Two independent colonies (a and b) were assayed. (B) Subset of specific genes from the data set in A. Early genes are indicated in red, delayed early genes in green, and middle sporulation genes in blue.
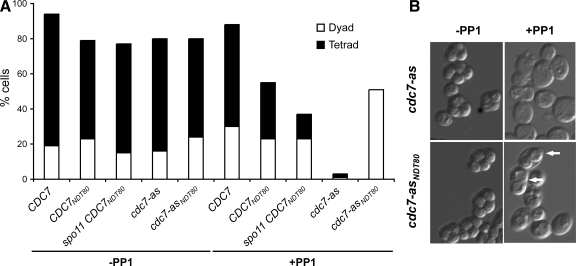
Figure 3.
Dyad and tetrad formation in cdc7-as and cdc7-asNDT80 diploids with or without PP1. (A) CDC7 (NH875), CDC7NDT80 (NH875::CUP1-NDT80-3HA), spo11 CDC7NDT80 (NH883), cdc7-as (NH874), and cdc7-asNDT80 (NH874::CUP1-NDT80-3HA) diploids were sporulated in the absence or presence (+PP1) of 15 μM PP1. The frequency of dyad and tetrad formation was determined by counting 200 cells after 24 h in Spo medium. The number is the average of two or three independent experiments. (B) cdc7-as (NH452F) and cdc7-asNDT80(NH452F::CUP1-NDT80) cells after >24 h in sporulation medium with and without 15 μM PP1 as indicated. White arrows point to dyads.
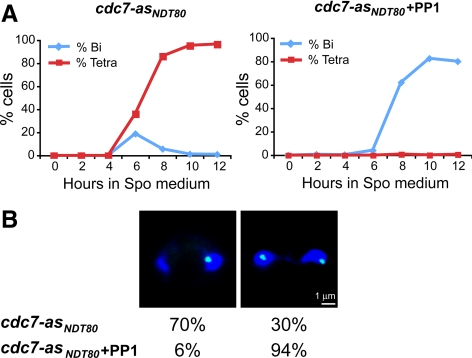
Figure 4.
Meiotic progression and chromosome V segregation in cdc7-asNDT80 cells with or without PP1. cdc7-asNDT80 (NH874::CUP1- NDT80) carrying one copy of chromosome V marked by tetR-GFP was sporulated in the absence or presence of 15 μM PP1. (A) Meiotic progression: cells were stained with DAPI, and 200 cells were counted at the indicated time points after transfer to sporulation medium to determine the percentage of binucleate and tetranucleate cells. The number shown is the average of two independent experiments. (B) Chromosome V segregation: binucleate cells were analyzed for the number of green dots/nucleus. For cdc7-asNDT80 − PP1, the 6-h time point was used and 40 cells were examined. For cdc7-asNDT80 + PP1, the 8-h time point was used and 376 cells were examined. Left, representative picture of reductional segregation taken from cdc7-asNDT80. Right, representative picture of equational segregation taken from cdc7-asNDT80 + PP1. DAPI is stained with blue, tetR-GFP is green.
Figure 5.
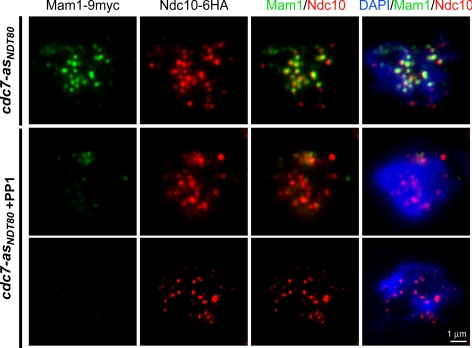
Localization of Mam1-9myc and Ndc10-6HA in cdc7-asNDT80 meiotic cells with or without PP1. cdc7-asNDT80 MAM1-9myc NDC10-6HA (NH870::CUP1-NDT80) was sporulated in the absence or presence (+PP1) of 15 μM PP1 for 6 or 8 h, respectively. Chromosome spreads were stained with anti-myc and anti-HA antibodies as well as with DAPI. Mam1-9myc staining is green; Ndc10-6HA is red and DAPI is blue.
Chromosome Spreads and Indirect Immunofluorescence Staining
Chromosome spreads were prepared as described in Loidl et al. (1991). The primary antibodies mouse anti-myc 9E10 for Mam1-9myc and rabbit anti-HA-11 for Ndc10-6HA were diluted (1:100 and 1:250, respectively) in 60 μl of blocking buffer and incubated with the spread chromosomes on a slide under a coverslip at 4°C overnight in a moist chamber. The spreads were washed with phosphate-buffered saline (PBS) and then incubated in PBS in a plastic chamber for 5 min. Residual PBS was drained off on a paper towel. The secondary antibodies goat anti-mouse coupled with fluorescein isothiocyanate (FITC) and goat anti-rabbit coupled to rhodamine were both used at 1:250 dilution in 60 μl of blocking buffer and incubated under a coverslip for 2.5 h in a dark moist chamber. The coverslip was washed off using PBS and placed to drain on a paper towel. One or two drops of VECTASHIELD mounting medium (Vector Laboratories, Burlingame, CA) with diamidino-2-phenylindole (DAPI) were added onto a coverslip that was then placed carefully on the slide with the spreads to avoid bubbles. All antibodies were provided by A. Amon.
Quantification of Immunofluorescence Staining
Chromosome spreads were examined for staining with DAPI, anti-hemagglutinin (HA) (Ndc10-6HA, rhodamine) and anti-myc (Mam1-9myc, FITC) antibodies using an Axioplan 2 microscope (Carl Zeiss, Thornwood, NY) with 100× objective. Images were collected and merged using Axiovision software (Carl Zeiss). Chromosome spreads (100) containing Ndc10-6HA foci were first selected and then scored for the presence of Mam1 foci. Only discrete dots with significant intensity were counted as foci. Colocalization of Ndc10-6HA and Mam1-9myc was observed by the creation of a yellow color in the merged images. The percentage of Mam1-9myc foci that colocalize with Ndc10-6HA foci per spread was determined.
Green Fluorescent Dots
To examine the segregation of a single pair of sister chromatids, strains heterozygous for a tandem array of 240 tet operator sites integrated 35 kb from ura3 that express tetR-GFP were used (Lee et al., 2004). Such strains exhibit a single green dot at G1 and after DNA replication when sister chromatids are held together by cohesion. For the green dot analysis, 1 ml of meiotic cells was collected and fixed in 3.7% formaldehyde (diluted in potassium phosphate, pH 6.4) for 10 min followed by two washes with 1 ml of potassium phosphate, pH 6.6. Cells were pelleted and resuspended in 1 ml of potassium phosphate, pH 7.4. DAPI staining was used to select binucleate cells. The number of green dots per nucleus was then determined.
RESULTS
The cdc7-as Arrest Is Not Suppressed by Mutations in the intra-S, DNA Damage, or Meiotic Recombination Checkpoints, or by Mutations That Affect Meiotic Chromosome Structure
Inhibition of Cdc7-as kinase activity during meiosis by addition of PP1 to cdc7-as cells causes a prophase arrest, no DSBs and no recombination (Wan et al., 2006). The reason for the prophase arrest is unclear: given that DSB-defective mutants are proficient in meiotic progression (Malone et al., 2004), the cdc7-as prophase arrest cannot be explained simply by a lack of DSBs. One possibility is that the absence of Cdc7 kinase activity creates a situation that triggers a checkpoint. However, mutations in genes that inactivate the intra-S phase, DNA damage, and meiotic recombination checkpoints fail to suppress the meiotic progression and sporulation defects of cdc7-as diploids in the presence of the inhibitor PP1 (Table 2). Consistent with the fact that cdc7-as mutants are unable to recombine, preventing DSB formation by mutation of REC104 also has no effect (Table 2).
Table 2.
Sporulation and spore viability of various mutants in the presence or absence of Cdc7 kinase activity
| Relevant genotype | Function | % sporulationa |
% spore viability (no. asci)b−PP1 | |
|---|---|---|---|---|
| −PP1 | +PP1c | |||
| cdc7-as | 83.5 | 0.0 | 91.3 | |
| sml1Δ cdc7-asd | Inhibitor of ribonucleotide reductase | 94.0 | 0.0 | 97.1 |
| mec1Δ smlΔ cdc7-as | Intra-S checkpoint | 85.5 | 0.0 | 37.1 |
| rad9Δ cdc7-as | DNA damage checkpoint | 84.5 | 0.0 | 43.2 |
| mek1Δ cdc7-as | Meiotic recombination checkpoint | 64.0 | 0.0 | <1.0 |
| rec104 cdc7-as | DSB formation | 51.0 | 0.0 | <1.0 |
| red1 cdc7-as | Formation of axial elements | 56.8 | 0.0 | <1.0 |
a Cells (200) were assayed by light microscopy.
b A minimum of 26 asci was dissected for each diploid.
c PP1 (15 μM) was added to liquid sporulation cultures.
d sml1Δ is needed to suppress the lethality of mec1Δ.
Another possibility is that CDC7 is required to prevent aberrant chromosome structures that cause cells to arrest. Axial elements are meiosis-specific structures created by condensation of sister chromatids along protein cores. Preventing axial element formation by mutation of RED1 had no effect on the cdc7-as arrest, however, indicating that aberrant axial elements are not responsible (Rockmill and Roeder 1990) (Table 2). To determine whether any of these double mutant combinations allow meiotic progression even though they do not produce asci, the strains were transferred to Spo medium and nuclei were stained with DAPI. In all of the double mutant diploids described above, 100% of the cells remained mono-nucleate in the presence of PP1, consistent with a prophase arrest. In contrast, >50% of the cells from the same cultures entered meiosis I in the absence of inhibitor (data not shown).
Bypassing the Requirement for CDC7 for DNA Replication Does Not Restore DSBs or Sporulation
The requirement for Cdc7 for DNA replication in mitotic cells can be bypassed by a point mutation in MCM5 (mcm5-bob1) (Hardy et al., 1997). If the cdc7-as meiotic arrest is due to problems in replication that are not subject to the MEC1 checkpoint, this phenotype might be suppressed by mcm5-bob1. mcm5-bob1 partially suppresses the vegetative growth defect of cdc7-as in the presence of PP1 in our SK1 strain background (Figure 1A). After several generations of vegetative growth, cdc7-as mcm5-bob1 diploids accumulate recessive lethal mutations, resulting in decreased spore viability. This phenotype was not observed with cdc7-as alone, indicating that there may be a synthetic effect between cdc7-as and mcm5-bob1, as has been observed previously for cdc7-as and tagged alleles of MER2 (Wan et al., 2008). Alternatively, mcm5-bob1 could produce a deleterious mutator phenotype on its own. This problem was circumvented by making the cdc7-as mcm5-bob1 diploid immediately before performing time courses. Under these conditions, cdc7-as mcm5-bob1 exhibited 90.5% sporulation and 95.2% spore viability in the absence of inhibitor.
The flow cytometry profiles of cdc7-as mcm5-bob1 with and without inhibitor were highly similar, in contrast to the cdc7-as diploid, where addition of PP1 induced an S phase delay (Figure 1B). Therefore mcm5-bob1 bypasses the role of CDC7 in premeiotic S phase, similar to its effect in vegetative cells. Meiotic progression and sporulation were blocked by PP1, however, and no DSBs were generated (Figure 1C; data not shown). The finding that mcm5-bob1 does not rescue the prophase arrest conferred by a deletion of CDC7 was observed previously by Sclafani (2000) and Sasanuma et al. (2008). This observation, coupled with the failure of mec1Δ to suppress the cdc7-as arrest, strongly argues against defects in premeiotic S phase being responsible for the failure of cells to enter meiosis I when Cdc7 is inactive.
CDC7 Is Required for Transcription of Middle Sporulation Genes
Given that the cdc7-as prophase arrest does not result from a checkpoint response, Cdc7 may actively promote entry into meiosis I. Meiotic progression requires Cdc28-Clb1 (CDK) activity as well as expression and activation of the transcription factor Ndt80 (Shuster and Byers 1989; Xu et al., 1995; Benjamin et al., 2003; Carlile and Amon 2008). CDC7 may therefore be required for CDK activity or for Ndt80-mediated transcription. Inactivation of Cdc7 has no effect on Cdc28-Clb5 activity during meiosis, however, making the former possibility unlikely (Wan et al., 2008). The possibility that Cdc7 activity is needed for Ndt80-mediated transcription can be tested by comparing the expression of middle sporulation genes in the presence or absence of Cdc7 kinase activity. Inactivation of CDK does not prevent middle gene transcription, whereas mutation of NDT80 does (Hepworth et al., 1998; Stuart and Wittenberg 1998; Benjamin et al., 2003). Cells derived from two independent cdc7-as colonies were transferred to Spo medium, and PP1 was added at time 0 to half of each culture. RNA was isolated after 10 h, and the RNA samples were compared using microarrays to RNA from the zero time cell cultures. To simplify the analysis, genes shown previously to be meiotically induced during meiosis were selected and clustered based on their expression pattern (Chu et al., 1998; Primig et al., 2000). The list of these genes can be found in Supplemental Table 1, and the data set for the entire genome can be found at ArrayExpress (www.ebi.ac.uk/ArrayExpress/; Accession number E-MEXP-1765). In the cdc7-as diploid without inhibitor, both early and middle gene transcripts were detected at the 10-h time point (Figure 2). Addition of inhibitor allowed early gene transcription but prevented transcription of many of the middle sporulation genes, including CLB1 (Figure 2). Therefore, Cdc7 seems to regulate NDT80-mediated transcription, as opposed to CDK activity. A similar result was recently published by Sasanuma et al. (2008).
NDT80 is a key meiotic regulator, and its regulation is complex. The NDT80 promoter contains two binding sites for the transcriptional repressor Ume6, and also contains two binding sites (the MSEs) for Ndt80 (i.e., NDT80 regulates its own transcription.) Furthermore, at least one of the Ndt80 binding sites is also a good binding site for a competing factor, the repressor Sum1 (Xie et al., 1999). Initial NDT80 expression is mediated by Ime1, which allows transcriptional activation from Ume6 sites, and occurs after early genes are transcribed (Rubin-Bejerano et al., 1996; Vershon and Pierce 2000). A small amount of Ndt80 from this “delayed early,” Ime1-dependent transcription competes with Sum1 for binding to the MSEs upstream of NDT80 (Pierce et al., 2003). Increasing expression of NDT80 from this positive feedback loop produces enough Ndt80, in conjunction with a weakening of Sum1 repression via Ime2, to allow Ndt80 to bind to MSEs upstream of middle sporulation genes, promoting the expression of these genes in mid-meiosis (Chu and Herskowitz 1998; Pak and Segall 2002).
No induction of NDT80 RNA was observed when Cdc7-as was inactivated, suggesting that CDC7 is required for some phase of the transcription of NDT80 (Figure 2). This idea is consistent with analysis of NDT80 RNA from meiotic time courses that showed reduced and greatly delayed NDT80 transcription in cdc7Δ mcm5-bob1 diploids (Sasanuma et al., 2008).
If lack of NDT80 transcription is the sole reason that cells are failing to enter meiosis I, then ectopic expression of NDT80 should bypass the cdc7-as arrest. NDT80 under control of the copper-inducible CUP1 promoter (PCUP1-NDT80-3HA) was introduced into the cdc7-as diploid (hereafter, referred to cdc7-asNDT80) (Sopko et al., 2002). Addition of copper reduced sporulation even in the absence of PP1, so this condition was not studied (data not shown). Fortunately, there is sufficient basal expression of NDT80 from the CUP1 promoter even in the absence of copper to bypass the cdc7-as + PP1 arrest: 51% asci were observed in cdc7-asNDT80 + PP1 compared with 3% for cdc7-as + PP1 (Figure 3A). This result was confirmed by microarray experiments demonstrating that NDT80 and other middle genes are expressed in the cdc7-asNDT80 + PP1 diploid (Figure 2; Supplemental Table 1).
The basal amount of NDT80 expressed from the CUP1 promoter is not sufficient on its own to provide sufficient Ndt80 protein to enter Meiosis I. PCUP1-NDT80-3HA fails to complement the sporulation defect of ndt80Δ (NH379::CUP1- NDT80-3HA), in contrast to NDT80 under control of its own promoter where 31% tetrads were observed. [In the latter strain (NH379/pNKY1212) NDT80 is present on a CEN plasmid, so the relatively low sporulation may be due to plasmid loss]. This experiment indicates that PCUP1-NDT80 is supplying a low level of NDT80 to cdc7-asNDT80 + PP1 cells, which can presumably then bind upstream of endogenous NDT80 to promote further NDT80 transcription, resulting in full induction of middle sporulation genes. This result is consistent with the idea that Cdc7 is important for the delayed early expression of NDT80.
Ectopic Expression of NDT80 in cdc7-as Plus PP1 Produces Exclusively Dyads
The suppression of the meiotic progression defect of cdc7-as + PP1 by ectopic expression of NDT80 revealed another phenotype for cdc7-as—all of the asci produced were dyads instead of tetrads (Figure 3). This phenotype is specific to the loss of Cdc7 kinase activity. Tetrads were predominantly formed in all of the various strains tested without inhibitor, as well as in diploids carrying a wild-type allele of CDC7 when PP1 was present (Figure 3A). Therefore, constitutive expression by PCUP1-NDT80 alone is not responsible for the dyad formation. One difference between CDC7NDT80 and cdc7-asNDT80 is a lack of DSBs in the cdc7-asNDT80 + PP1 strain (data not shown). To see whether a combination of no DSBs with constitutive NDT80 transcription results exclusively in dyads, the PCUP1-NDT80-3HA allele was integrated into a spo11Δ diploid to create spo11Δ CDC7NDT80. This diploid still makes tetrads, ruling out this idea (Figure 3A). Our results are consistent with observations made by Valentin et al. (2005) who showed that turning off transcription of DBF4 when cells were transferred to Spo medium results in an increased number of dyads.
Cdc7 Kinase Activity Is Required for Reductional Segregation
Dyad phenotypes have been observed in mutants defective in 1) mono-orientation of sister kinetochores, such as cdc5 and spo13Δ mutants (Klapholz and Esposito 1980; Schild and Byers 1980; Clyne et al., 2003; Lee and Amon 2003; Katis et al., 2004; Lee et al., 2004; Sharon and Simchen 1990); 2) coupling of the two meiotic divisions, such as spo12Δ (Klapholz and Esposito 1980; Buonomo et al., 2003; Marston et al., 2003); and 3) deposition of meiotic outer plaques on all four spindle pole bodies, such as heterozygous spo74Δ (Nickas et al., 2003). Class 1 and class 2 mutants produce diploid dyads in which chromosomes tend to segregate equationally or reductionally, respectively. Class 3 mutants result in haploid nonsister dyads (Davidow et al., 1980). To determine whether the dyad phenotype observed in cdc7-asNDT80 plus PP1 resembles any of these three classes, 249 dyads from cdc7-asNDT80 plus PP1 were dissected. Spore viability was high (86%). Of 178 two-viable spore dyads, all but two of the spore colonies were nonmaters and prototrophic for LEU2, suggesting that the spore colonies were diploid. This idea was confirmed by sporulating 40 randomly picked spore colonies in the absence of PP1 and dissecting the resulting tetrads. Spore viability in these asci was also high (>90%) and 2:2 segregation was observed for both MAT and LEU2. The heterozygosity for two chromosome III markers indicates that the cells are undergoing a single equational division, similar to cdc5 and spo11 spo13 mutants. No recombinants were observed between MAT and LEU2 in 176 two-viable spore dyads. Furthermore, no DSBs were observed at the YCR048w hotspot in the cdc7-asNDT80 diploid with PP1 (data not shown). Therefore, whereas ectopic expression of NDT80 can suppress the meiotic progression defect of cdc7-as + PP1, it does not relieve the requirement for CDC7 for DSB formation.
The genetic analysis of cdc7-asNDT80 plus PP1 dyads necessarily relies on those cells that sporulate and produce viable spores and thus may be subject to selection bias. A cytological analysis was therefore used to determine the segregation pattern of chromosome V by monitoring the distribution of a heterozygous green dot formed by binding of Tet repressor protein fused to GFP to an array of tet operator sites in binucleate cells (Toth et al., 2000). When chromosome V segregates reductionally, sister chromatids are connected to each other and a single green dot is seen in one nucleus of a binucleate cell. For equational segregation, sister chromatids disjoin to opposite poles, and both nuclei therefore exhibit a green dot. These predictions assume that there is no recombination between the tet operator sequences and the centromere. In cdc7-asNDT80, the peak of binucleate cells appeared 6 h after transfer to Spo medium and 70% of these cells exhibited reductional segregation of chromosome V (Figure 4). The 30% binucleate cells from cdc7-asNDT80 without PP1 that seem to exhibit equational segregation are most likely due to reductional segregation after recombination between the tet operator sequences and the centromere. The tetO tandem array is integrated at the ura3 locus that is on average 9.2 cM from the centromere (this value is based on six crosses from non-SK1 strains in the Saccharomyces Genome Database). Therefore, ∼18% of the meioses are predicted to exhibit recombination between the tet operators and the centromere and produce binucleate cells with a single green dot/nucleus. The fact that this number is less than the 30% observed for cdc7-asNDT80 − PP1 could be because the actual map distance in SK1 is higher than 9.2 cM or because there is some basal level of equational segregation in this diploid. The high spore viability of this strain makes the latter explanation unlikely. In cdc7-asNDT80 + PP1, binucleate cells were present at high frequency (∼81%) and <1% tetranucleate cells were observed (Figure 4A). Of the binucleate cells, 94% had one GFP dot in each nucleus, indicating that, under these conditions, chromosome V segregates equationally (Figure 4B). The cytological studies of chromosome V are consistent with the genetic analysis of chromosome III and support the conclusion that Cdc7 kinase activity is required for mono-orientation of sister kinetochores at meiosis I.
Cdc7 Kinase Activity Is Required for Mam1 Localization to Kinetochores
Mono-orientation of sister kinetochores at meiosis I requires that the monopolin complex consisting of Mam1, Lrs4, and Csm1 be recruited and maintained on kinetochores (Toth et al., 2000; Rabitsch et al., 2003). The equational segregation observed in the cdc7-asNDT80 + PP1 dyads suggests that monopolin function is disrupted in the absence of Cdc7 kinase activity. This idea was tested by examining the colocalization of the meiosis-specific monopolin subunit Mam1-9myc with the kinetochore component Ndc10-6HA on chromosome spreads in cdc7-asNDT80 without and with PP1.
In the absence of PP1, Mam1-9myc protein began to accumulate after 6 h in Spo medium, so this time point was used to analyze Mam1 localization to kinetochores (data not shown). Meiotic chromosome spreads (100) from the cdc7-asNDT80 − PP1 diploid were selected that exhibited Ndc10-6HA foci. Of these, 21% also exhibited Mam1-9myc foci. In the spreads where both proteins were present, 61% of the Ndc10 foci had associated Mam1-9myc staining (Figure 5; data not shown). In the cdc7-asNDT80 diploid + PP1, the 8-h time point was used for cytological analysis because this was the time at which the amount of Mam1-9myc protein was equivalent to the cdc7-asNDT80 − PP1 time point as determined by immunoblot analysis (data not shown). The 2-h delay in Mam1 production is likely due to the delay in DNA replication that occurs when Cdc7 is inactive (Wan et al., 2006). In this case, only four of 100 Ndc10-6HA–positive chromosome spreads exhibited Mam1-9myc foci, and the staining was weaker than without PP1 (Figure 5, middle); 96% of the spreads exhibited no Mam1-9myc foci (Figure 5, bottom). The number of Ndc10-6HA foci was the same, however, at both the 6- and 8-h time points in the presence or absence of PP1. The percentage of Ndc10-containing spreads with Mam1-9myc did not increase, even after 10 h in Spo medium (data not shown). In the four chromosome spreads containing both Mam1-9myc and Ndc10-6HA, only 20% of the kinetochores contained Mam1-9myc (Figure 5). These results indicate that Cdc7 kinase activity is required for reductional segregation by promoting Mam1 localization to kinetochores.
DISCUSSION
To study the function of Cdc7 in meiosis, we have used a chemical genetic approach to specifically inhibit the kinase activity of Cdc7 during meiosis while leaving the protein intact (Wan et al., 2006). Whereas our previous studies showed that Cdc7 phosphorylation of the DSB protein Mer2 is critical for recombination (Wan et al., 2008), this work demonstrates that the meiotic progression defect observed for cdc7 mutants is due to a requirement for Cdc7 kinase activity in the expression of NDT80. Furthermore, we show that phosphorylation by Cdc7 activity is critical for reductional segregation by recruiting the monopolin component Mam1 onto kinetochores.
Cdc7 Promotes Transcription of NDT80
When Cdc7-as is inactivated during meiosis with PP1, cells arrest before the first meiotic division with a single nucleus (Wan et al., 2006). The idea that lack of Cdc7 kinase activity indirectly causes an arrest by triggering a checkpoint is unlikely given that mutations in the intra-S, DNA damage, and meiotic recombination checkpoints do not relieve the arrest nor does suppressing premeiotic replication defects with mcm5-bob1. These results suggest that Cdc7 instead plays a positive role in meiotic progression. This hypothesis was confirmed by microarray analysis of cdc7-as cells that were arrested with PP1 for 10 h. Early meiotic genes were transcribed in these cells, consistent with the detection of early gene products such as Hop1 and Rec104 by immunoblot analysis of proteins from cdc7-as + PP1 cells (Wan et al., 2006) (data not shown). In contrast, NDT80, a key transcriptional regulator required for entry into meiosis I, as well as middle sporulation genes regulated by Ndt80, were not expressed when Cdc7-as was inactivated.
In addition to MSEs, the NDT80 promoter region contains a second regulatory element called upstream repression sequence 1 (URS1). URS1 is also found upstream of many early meiotic genes, such as HOP1, SPO13, and IME2 (Buckingham et al., 1990; Vershon et al., 1992). This sequence is bound in mitotic cells by Ume6 that in turn recruits the Sin3-Rpd3 histone deactylase complex (HDAC) as well as the Isw2 chromatin remodeling complex (Strich et al., 1994; Kadosh and Struhl 1998; Rundlett et al., 1998; Goldmark et al., 2000) (Figure 6). When cells are transferred to Spo medium, a combination of genetic and nutritional signals results in the expression of the Ime1 transcriptional activator (Vershon and Pierce 2000). Ime1 and Ume6 are phosphorylated by the kinase Rim11, and this phosphorylation promotes the formation and activity of a Ume6/Ime1 complex at URS1 (Rubin-Bejerano et al., 1996; Malathi et al., 1997).
Figure 6.
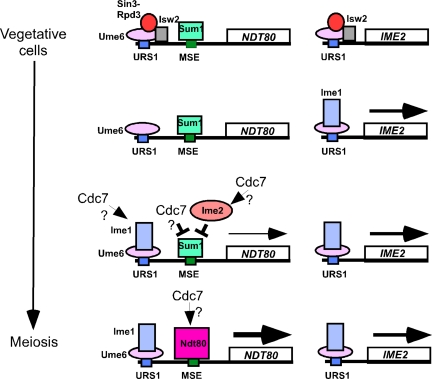
Model for Cdc7 regulation of NDT80 transcription. In vegetative cells, Ume6 is bound to URS1 elements and recruits the Sin3-Rpd3 HDAC as well as the Isw2 chromatin remodeling complex. Sum1 is bound to the MSE-1 element (only one URS1 and one MSE1 sequence are shown for simplicity). Transfer to Spo medium induces Ime1 that then displaces Sin3-Rpd3 and Isw2 and binds to Ume6 and promotes early gene transcription. The presence of Ime2 abrogates Sum1 repression of the NDT80 promoter, thereby resulting in the delayed early gene transcription of NDT80. Ndt80 then binds to MSEs in its own promoter to induce a second wave of NDT80 expression. Small arrows indicate a positive function by Cdc7. A cross-bar indicates negative regulation.
Although recruitment of Ime1 to URS1 sites allows induction of early gene transcription, it is not sufficient for NDT80 expression due to the presence of a repressor protein called Sum1 that is bound at MSE sites in the NDT80 promoter (Xie et al., 1999) (Figure 6). Sum1 repression is abrogated by the action of the Ime2 kinase, thereby allowing Ime1-driven transcription of NDT80 (Pak and Segall 2002). Because IME2 must be transcribed and the mRNA translated before the kinase can act, NDT80 transcription is delayed relative to other Ime1-dependent early genes. The resulting Ndt80 protein is activated by phosphorylation and then acts in a positive feedback loop, producing a large induction of NDT80 transcription (Pak and Segall 2002; Pierce et al., 2003; Chu and Herskowitz 1998) (Figure 6). Ndt80 then binds to MSEs upstream of middle sporulation genes, thereby activating their transcription.
This work shows that basal expression of NDT80 by ectopic expression off the CUP1 promoter allows cells to progress through meiosis and form spores even in the absence of Cdc7 kinase activity. Therefore, the meiotic progression defect of cdc7-as + PP1 can be explained simply by a failure to transcribe NDT80. Furthermore, Cdc7 must regulate NDT80 transcription through cis-acting sequences upstream of the NDT80 open reading frame. The level of NDT80 transcribed using the CUP1 promoter is insufficient for meiotic progression on its own, suggesting that PCUP1-NDT80 supplies a low level of Ndt80, which somehow engages the NDT80-positive feedback loop. Once this positive feedback loop has been engaged, Cdc7 must not be absolutely necessary for Ndt80 function because middle genes are expressed in cdc7-asNDT80 + PP1 even though Cdc7 kinase activity is inhibited. We cannot, however, at this time rule out the possibility that full Ndt80 function requires Cdc7 (Figure 6).
Because the Ndt80-positive feedback loop is normally “primed” by relatively low-level expression of Ndt80 as a delayed early gene, and because PCUP1-NDT80 probably makes only a low level of Ndt80 (because it is not sufficient for meiotic progression in an ndt80 null mutant), it is tempting to think that in the absence of Cdc7 activity, PCUP1-NDT80 is suppressing the defect in meiotic progression by providing a priming amount of Ndt80. In that case, the wild-type role of Cdc7 would be to assist in the delayed early phase of Ndt80 expression. For example, Cdc7 could directly or indirectly activate Ime1 specifically at the NDT80 promoter, thus activating delayed early expression, or it could directly or indirectly inactivate Sum1, thus derepressing delayed early expression, or it could act on some novel factor at the NDT80 promoter (Figure 6). Further work will be required to define the Cdc7 substrate that regulates NDT80 transcription.
Cdc7 Is Required for Reductional Segregation at Meiosis I
The idea that Cdc7 might play a role in reductional segregation was first suggested by Valentin et al. (2005) who noted that shutting off transcription of DBF4 at the time cells were transferred to sporulation medium results in an increase in dyads containing viable diploid spores. However, the long lag time of Dbf4 degradation in these experiments precluded a definitive conclusion. We show that when the meiotic progression defect of cdc7-as + PP1 is bypassed by PCUP1-NDT80, the resulting asci are exclusively dyads. Using both genetic and cytological analyses, the dyads were proven to result from a single equational division, confirming a role for Cdc7 kinase activity in reductional segregation.
Reductional segregation at meiosis I requires that sister chromatids be held together by centromeric cohesion and that mono-orientation of sister chromatids be created by binding of the monopolin complex at the kinetochores. Inactivation of Cdc7 results in a failure in both of these processes. This could be because Cdc7 is required for both processes, similar to SPO13 (Katis et al., 2004; Lee et al., 2004). In fact, the cdc7-asNDT80 + PP1 phenotype is identical to a spo11Δ spo13Δ mutant. Alternatively, cdc7-asNDT80 + PP1 could allow a continuation in meiotic progression such that chromosome segregation occurs under meiosis II-like conditions when centromeric cohesins are normally degraded, similar to what happens in spo12Δ mutants (Buonomo et al., 2003; Marston et al., 2003). In this regard, the cdc7-asNDT80 + PP1 resembles spo11Δ spo12Δ mam1Δ mutants (Rabitsch et al., 2003). Our data cannot distinguish between these two possibilities.
Whether Cdc7 is directly required for the retention of centromeric cohesins or not, Cdc7 kinase is needed to recruit the monopolin subunit Mam1 to kinetochores. Inactivation of cdc7-asNDT80 with PP1 reduces the amount of Mam1 present on chromosomes as well as the number of kinetochore-associated Mam1 foci. The kinetochore recruitment of monopolin components is interdependent, and the failure of Mam1 to localize to kinetochores could therefore be due to a defect in formation of the monopolin complex (Rabitsch et al., 2003). For example, Cdc7 could be important for release of Lrs4 and Csm1 from the nucleolus during meiosis. Alternatively, Cdc7 phosphorylation of one of the monopolin subunits could be a prerequisite for complex formation. It has been shown previously, for example, that Cdc28-Clb5 phosphorylation of serine 30 of Mer2 primes phosphorylation of S29 by Cdc7 and that these phosphorylation events are essential for Mer2 interaction with Rec114 and Mei4 and consequently, DSB formation (Henderson et al., 2006; Wan et al., 2008). The Cdc28-Clb5/Cdc7 combination site on Mer2 is TSSPFR. Intriguingly, both Mam1 and Lrs4 contain a putative Cdc28-Cdc7 combination site, SSSPNTKK in Mam1 and TSSPVK in Lrs4. Furthermore, Lrs4 is a substrate for CDK in vitro (Ubersax et al., 2003). However, mutation of the serines in the Lrs4 combination site to alanine fully complements the spore inviability of lrs4Δ (our unpublished data). Therefore, although Cdc7 may phosphorylate Lrs4, phosphorylation of the putative CDK/Cdc7 site in Lrs4 is not by itself essential for monopolin function as it is for Mer2 and meiotic recombination.
Finally, we note that the transcription of MAM1 is partially controlled by Ntd80, and therefore, indirectly controlled by Cdc7. The MAM1 gene coclusters with other members of the Ndt80 regulon in meiosis microarray time courses (data not shown), and it responds to the presence or absence of NDT80. The MAM1 promoter contains two core consensus sites for Ndt80 binding (CACAAAA). However, in the cdc7-asNDT80 + PP1 strain, MAM1 transcript levels at 10 h are as high as in cdc7-as without PP1 (Figure 2), and immunoblot analysis shows that wild-type levels of Mam1 protein are present in cdc7-asNDT80 + PP1 (data not shown). Thus, although MAM1 transcription is partially under Cdc7 control, this does not explain the failure of Mam1 to localize to kinetochores in the cdc7-asNDT80 strain. It is interesting, however, that in wild-type cells Cdc7 seems to control Mam1 at both transcriptional and posttranscriptional levels.
The polo-like kinase, Cdc5, is required for phosphorylation of Mam1 as well as monopolin recruitment to kinetochores (Clyne et al., 2003; Lee and Amon 2003). The fact that Cdc5 and Dbf4 physically interact with each other suggests that Cdc5 and Cdc7 may function together in localizing monopolin to kinetochores (Hardy and Pautz 1996). Cdc5 binds to target proteins via a “polo-box domain” that is a phospho-peptide binding module with a preference for sequences containing Ser-phospho-Ser-Pro (S-pS-P) (Elia et al., 2003). This sequence is contained within Cdc7/CDK combination sites and raises the possibility that Cdc7 may function with CDK to regulate Cdc5 binding to the substrates. However whether having two phosphorylated serines at these docking sites (pS-pS-P) would antagonize or enhance Cdc5 binding is not yet certain. In short, it is possible that Cdc7 combines with CDK to regulate the binding of Cdc5 to Lrs4 and/or Mam1 and that Cdc5 is the ultimate regulator.
Mono-orientation of sister kinetochores is an evolutionarily conserved feature of meiosis; yet, orthologues of the budding yeast monopolin subunits have not yet been discovered in metazoans. Cdc5 and Cdc7 are highly conserved protein kinases. The finding that they are required for mono-orientation in budding yeast suggests they may be involved in reductional segregation in mammalian cells as well and may ultimately pave the way for finding the functional equivalents of monopolin in multicellular organisms.
Cdc7: A Key Regulator of Meiosis
Meiosis consists of an ordered series of events beginning with premeiotic S phase when meiosis-specific cohesin complexes are established (Klein et al., 1999). DNA synthesis is then coordinated with DSB formation such that breaks occur only in places where replication has occurred (Borde et al., 2000). Exit from pachytene is induced by transcription of NDT80, which is coupled to double Holliday junction resolution and the formation of crossovers (Xu et al., 1995; Allers and Lichten 2001). Finally reductional segregation at the first meiotic division requires that mono-orientation of sister centromeres be established and that centromeric cohesion be protected. The finding that the highly conserved Cdc7 kinase plays a role in all of these processes suggests this kinase may be important for ensuring these steps occur in the proper order; however, how such coordination would occur is still unclear, however. It is intriguing to note for example, that thus far all of the proteins regulated by Cdc7 bind to chromosomes in some way—Mcm proteins for DNA replication, Mer2 for recombination, unknown proteins that affect regulation of the NDT80 promoter and monopolin recruitment to kinetochores (Sheu and Stillman 2006; Sasanuma et al., 2008; Wan et al., 2008; this work). Furthermore, the different functions of Cdc7 in meiosis are separable. For example, cells in which the cdc7-as + PP1 replication defect is suppressed with mcm5-bob1 still fail to recombine and arrest. Similarly, bypassing the Cdc7 requirement for NDT80 transcription does not restore recombination or promote monopolin recruitment. Understanding how Cdc7 works to coordinate these meiotic events awaits the discovery and molecular analysis of Cdc7's meiotic substrates, the elucidation of how Cdc7 is targeted to phosphorylate a diverse set of chromosome-associated proteins and an understanding of exactly how Cdc7 kinase activity is regulated during meiosis.
Supplementary Material
ACKNOWLEDGMENTS
A. Neiman provided helpful comments on the manuscript. We are grateful to A. Neiman and J. Segall for helpful discussions. We thank A. Amon, S. Keeney (Memorial Sloan-Kettering Cancer Center, New York, NY), N. Kleckner, and D. Stuart for plasmids and/or strains. We are especially grateful to A. Amon and G. Brar for teaching H.-C.L. how to do immufluorescence of meiotic chromosome spreads and for providing antibodies and reagents for these experiments. C. Zhang and K. Shokat generously provided the PP1 inhibitor. We thank A. Neiman for the use of a microscope, Y. Suda for help with the microscopy, and H. Wang for help with the microarrays. N.M.H. was supported by National Institutes of Health grant GM-50717 as well as the Office for the Vice President of Research at Stony Brook University. B. F. was supported by National Institutes of Health grant GM-064813.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-07-0755) on September 3, 2008.
REFERENCES
- Allers T., Lichten M. Differential timing and control of noncrossover and crossover recombination during meiosis. Cell. 2001;106:47–57. doi: 10.1016/s0092-8674(01)00416-0. [DOI] [PubMed] [Google Scholar]
- Benjamin K. R., Zhang C., Shokat K. M., Herskowitz I. Control of landmark events in meiosis by the CDK Cdc28 and the meiosis-specific kinase Ime2. Genes Dev. 2003;17:1524–1539. doi: 10.1101/gad.1101503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop A. C., Buzko O., Shokat K. M. Magic bullets for protein kinases. Trends Cell Biol. 2001;11:167–172. doi: 10.1016/s0962-8924(01)01928-6. [DOI] [PubMed] [Google Scholar]
- Borde V., Goldman A. S., Lichten M. Direct coupling between meiotic DNA replication and recombination initiation. Science. 2000;290:806–809. doi: 10.1126/science.290.5492.806. [DOI] [PubMed] [Google Scholar]
- Buckingham L. E., Wang H.-T., Elder R. T., McCarroll R. M., Slater M. R., Esposito R. E. Nucleotide sequence and promoter analysis of SPO13, a meiosis-specific gene of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA. 1990;87:9406–9410. doi: 10.1073/pnas.87.23.9406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonomo S. B., Clyne R. K., Fuchs J., Loidl J., Uhlmann F., Nasmyth K. Disjunction of homologous chromosomes in meiosis I depends on proteolytic cleavage of the meiotic cohesin Rec8 by separin. Cell. 2000;103:387–398. doi: 10.1016/s0092-8674(00)00131-8. [DOI] [PubMed] [Google Scholar]
- Buonomo S. B., Rabitsch K. P., Fuchs J., Gruber S., Sullivan M., Uhlmann F., Petronczki M., Toth A., Nasmyth K. Division of the nucleolus and its release of CDC14 during anaphase of meiosis I depends on separase, SPO12, and SLK19. Dev. Cell. 2003;4:727–739. doi: 10.1016/s1534-5807(03)00129-1. [DOI] [PubMed] [Google Scholar]
- Carlile T. M., Amon A. Meiosis I is established through division-specific translational control of a cyclin. Cell. 2008;133:280–291. doi: 10.1016/j.cell.2008.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu S., DeRisi J., Eisen M., Mulholland J., Botstein D., Brown P. O., Herskowitz I. The transcriptional program of sporulation in budding yeast [correction published in Science (1998). 282, 1421] Science. 1998;282:699–705. doi: 10.1126/science.282.5389.699. [DOI] [PubMed] [Google Scholar]
- Chu S., Herskowitz I. Gametogenesis in yeast is regulated by a transcriptional cascade dependent on Ndt80. Mol. Cell. 1998;1:685–696. doi: 10.1016/s1097-2765(00)80068-4. [DOI] [PubMed] [Google Scholar]
- Clyne R. K., Katis V. L., Jessop L., Benjamin K. R., Herskowitz I., Lichten M., Nasmyth K. Polo-like kinase Cdc5 promotes chiasmata formation and cosegregation of sister centromeres at meiosis I. Nat. Cell Biol. 2003;5:480–485. doi: 10.1038/ncb977. [DOI] [PubMed] [Google Scholar]
- Davidow L. S., Goetsch L., Byers B. Preferential occurrence of nonsister spores in two-spored asci of Saccharomyces cerevisiae: evidence for regulation of spore-wall formation by the spindle pole body. Genetics. 1980;94:581–595. doi: 10.1093/genetics/94.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de los Santos T., Hollingsworth N. M. Red1p: a MEK1-dependent phosphoprotein that physically interacts with Hop1p during meiosis in yeast. J. Biol. Chem. 1999;274:1783–1790. doi: 10.1074/jbc.274.3.1783. [DOI] [PubMed] [Google Scholar]
- Eisen M. B., Spellman P. T., Brown P. O., Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia A.E.H., Rellos P., Haire L. F., Chao J. W., Ivins F. J., Hoepker K., Mohammad D., Cantely L. C., Smerdon S. J., Yaffe M. B. The molecular basis for phosphodependent substrate targeting and regulation of Plks by the Polo-Box domain. Cell. 2003;115:83–95. doi: 10.1016/s0092-8674(03)00725-6. [DOI] [PubMed] [Google Scholar]
- Gentleman R. C., et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmark J. P., Fazzio T. G., Estep P. W., Church G. M., Tsukiyama T. The Isw2 chromatin remodeling complex represses early meiotic genes upon recruitment by Ume6p. Cell. 2000;103:423–433. doi: 10.1016/s0092-8674(00)00134-3. [DOI] [PubMed] [Google Scholar]
- Hardy C. F., Dryga O., Seematter S., Pahl P. M., Sclafani R. A. mcm5/cdc46-bob1 bypasses the requirement for the S phase activator Cdc7p. Proc. Natl. Acad. Sci. USA. 1997;94:3151–3155. doi: 10.1073/pnas.94.7.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy C.F.J., Pautz A. A novel role for Cdc5p in DNA replication. Mol. Cell. Biol. 1996;16:6775–6782. doi: 10.1128/mcb.16.12.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson K. A., Kee K., Maleki S., Santini P., Keeney S. Cyclin-dependent kinase directly regulates initiation of meiotic recombination. Cell. 2006;125:1321–1332. doi: 10.1016/j.cell.2006.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepworth S. R., Ebisuzaki L. K., Segall J. A 15-base-pair element activates the SPS4 gene midway through sporulation in Saccharomyces cerevisiae. Mol. Cell. Biol. 1995;15:3934–3944. doi: 10.1128/mcb.15.7.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepworth S. R., Friesen H., Segall J. NDT80 and the meiotic recombination checkpoint regulate expression of middle sporulation-specific genes in Saccharomyces cerevisiae. Mol. Cell. Biol. 1998;18:5750–5761. doi: 10.1128/mcb.18.10.5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth N. M., Johnson A. D. A conditional allele of the Saccharomyces cerevisiae HOP1 gene is suppressed by overexpression of two other meiosis-specific genes: RED1 and REC104. Genetics. 1993;133:785–797. doi: 10.1093/genetics/133.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston L. H., Masai H., Sugino A. First the CDKs, now the DDKs. Trends Cell Biol. 1999;9:249–252. doi: 10.1016/s0962-8924(99)01586-x. [DOI] [PubMed] [Google Scholar]
- Kadosh D., Struhl K. Targeted recruitment of the Sin3-Rpd3 histone deacetylase complex generates a highly localized domain of repressed chromatin in vivo. Mol. Cell. Biol. 1998;18:5121–5127. doi: 10.1128/mcb.18.9.5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassir Y., Granot D., Simchen G. IME1, a positive regulator of meiosis in Saccharomyces cerevisiae. Cell. 1988;52:853–862. doi: 10.1016/0092-8674(88)90427-8. [DOI] [PubMed] [Google Scholar]
- Katis V. L., Matos J., Mori S., Shirahige K., Zachariae W., Nasmyth K. Spo13 facilitates monopolin recruitment to kinetochores and regulates maintenance of centromeric cohesion during yeast meiosis. Curr. Biol. 2004;14:2183–2196. doi: 10.1016/j.cub.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Klapholz S., Esposito R. E. Isolation of SPO12-1 and SPO13-1 from a natural variant of yeast that undergoes a single meiotic division. Genetics. 1980;96:567–588. doi: 10.1093/genetics/96.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein F., Mahr P., Galova M., Buonomo S.B.C., Michaelis C., Nairz K., Nasmyth K. A central role for cohesins in sister chromatid cohesion, formation of axial elements and recombination during meiosis. Cell. 1999;98:91–103. doi: 10.1016/S0092-8674(00)80609-1. [DOI] [PubMed] [Google Scholar]
- Lee B. H., Amon A. Role of Polo-like kinase CDC5 in programming meiosis I chromosome segregation. Science. 2003;300:482–486. doi: 10.1126/science.1081846. [DOI] [PubMed] [Google Scholar]
- Lee B. H., Kiburz B. M., Amon A. Spo13 maintains centromeric cohesion and kinetochore coorientation during meiosis I. Curr. Biol. 2004;14:2168–2182. doi: 10.1016/j.cub.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Loidl J., K., N., Klein F. Meiotic chromosome synapsis in a haploid yeast. Chromosoma. 1991;100:221–228. doi: 10.1007/BF00344155. [DOI] [PubMed] [Google Scholar]
- Longtine M. S., McKenzie A., 3rd, Demarini D. J., Shah N. G., Wach A., Brachat A., Philippsen P., Pringle J. R. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Malathi K., Xiao Y., Mitchell A. P. Interaction of yeast repressor-activator protein Ume6 with glycogen synthase kinase 3 homology Rim11. Mol. Cell. Biol. 1997;17:7230–7236. doi: 10.1128/mcb.17.12.7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone R. E., Haring S. J., Foreman K. E., Pansegrau M. L., Smith S. M., Houdek D. R., Carpp L., Shah B., Lee K. E. The signal from the initiation of meiotic recombination to the first division of meiosis. Eukaryot. Cell. 2004;3:598–609. doi: 10.1128/EC.3.3.598-609.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston A. L., Amon A. Meiosis: cell-cycle controls shuffle and deal. Nat. Rev. 2004;5:983–997. doi: 10.1038/nrm1526. [DOI] [PubMed] [Google Scholar]
- Marston A. L., Lee B. H., Amon A. The Cdc14 phosphatase and the FEAR network control meiotic spindle disassembly and chromosome segregation. Dev. Cell. 2003;4:711–726. doi: 10.1016/s1534-5807(03)00130-8. [DOI] [PubMed] [Google Scholar]
- Michaelis C., Ciosk R., Nasmyth K. Cohesins: chromosomal proteins that prevent premature separation of sister chromatids. Cell. 1997;91:35–45. doi: 10.1016/s0092-8674(01)80007-6. [DOI] [PubMed] [Google Scholar]
- Nickas M. E., Schwartz C., Neiman A. M. Ady4p and Spo74p are components of the meiotic spindle pole body that promote growth of the prospore membrane in Saccharomyces cerevisiae. Eukaryotic Cell. 2003;2:431–445. doi: 10.1128/EC.2.3.431-445.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva A., Rosebrock A., Ferrezuelo F., Pyne S., Chen H., Skiena S., Futcher B., Leatherwood J. The cell cycle-regulated genes of Schizosaccharomyces pombe. PLoS Biol. 2005;3:e225. doi: 10.1371/journal.pbio.0030225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak J., Segall J. Regulation of the premiddle and middle phases of expression of the NDT80 gene during sporulation of Saccharomyces cerevisiae. Mol. Cell. Biol. 2002;22:6417–6429. doi: 10.1128/MCB.22.18.6417-6429.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petronczki M., Siomos M. F., Nasmyth K. Un menage a quatre: the molecular biology of chromosome segregation in meiosis. Cell. 2003;112:423–440. doi: 10.1016/s0092-8674(03)00083-7. [DOI] [PubMed] [Google Scholar]
- Pierce M., Benjamin K. R., Montano S. P., Georgiadis M. M., Winter E., Vershon A. K. Sum1 and Ndt80 proteins compete for binding to middle sporulation element sequences that control meiotic gene expression. Mol. Cell. Biol. 2003;23:4814–4825. doi: 10.1128/MCB.23.14.4814-4825.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primig M., Williams R. M., Winzeler E. A., Tevzadze G. G., Conway A. R., Hwang S. Y., Davis R. W., Esposito R. E. The core meiotic transcriptome in budding yeasts. Nat. Genet. 2000;26:415–423. doi: 10.1038/82539. [DOI] [PubMed] [Google Scholar]
- Rabitsch K. P., Petronczki M., Javerzat J. P., Genier S., Chwalla B., Schleiffer A., Tanaka T. U., Nasmyth K. Kinetochore recruitment of two nucleolar proteins is required for homolog segregation in meiosis I. Dev. Cell. 2003;4:535–548. doi: 10.1016/s1534-5807(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Rockmill B., Roeder G. S. Meiosis in asynaptic yeast. Genetics. 1990;126:563–574. doi: 10.1093/genetics/126.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R. Targeting, disruption, replacement and allele rescue: integrative DNA transformation in yeast. Methods Enzymol. 1991;194:281–301. doi: 10.1016/0076-6879(91)94022-5. [DOI] [PubMed] [Google Scholar]
- Rubin-Bejerano I., Mandel S., Robzyk K., Kassir Y. Induction of meiosis in Saccharomyces cerevisiae depends on conversion of the transcriptional repressor Ume6 to a positive regulator by its regulated association with the transcriptional activator Ime1. Mol. Cell. Biol. 1996;16:2518–2526. doi: 10.1128/mcb.16.5.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundlett S. E., Carmen A. A., Suka N., Turner B. M., Grunstein M. Transcriptional repression by UME6 involves deacetylation of lysine 5 of histone H4 by RPD3. Nature. 1998;392:831–835. doi: 10.1038/33952. [DOI] [PubMed] [Google Scholar]
- Sasanuma H., Hirota K., Fukuda T., Kakusho N., Kugou K., Kawasaki Y., Shibata T., Masai H., Ohta K. Cdc7-dependent phosphorylation of Mer2 facilitates initiation of yeast meiotic recombination. Genes Dev. 2008;22:398–410. doi: 10.1101/gad.1626608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild D., Byers B. Meiotic effects of DNA-defective cell division cycle mutations of Saccharomyces cerevisiae. Chromosoma. 1978;70:109–130. doi: 10.1007/BF00292220. [DOI] [PubMed] [Google Scholar]
- Schild D., Byers B. Diploid spore formation and other meiotic effects of two cell-division-cycle mutations of Saccharomyces cerevisiae. Genetics. 1980;96:859–876. doi: 10.1093/genetics/96.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider B. L., Seufert W., Steiner B., Yang Q. H., Futcher A. B. Use of polymerase chain reaction epitope tagging for protein tagging in Saccharomyces cerevisiae. Yeast. 1995;11:1265–1274. doi: 10.1002/yea.320111306. [DOI] [PubMed] [Google Scholar]
- Sclafani R. A. Cdc7p-Dbf4p becomes famous in the cell cycle. J. Cell Sci. 2000;113:2111–2117. doi: 10.1242/jcs.113.12.2111. [DOI] [PubMed] [Google Scholar]
- Sharon G., Simchen G. Mixed segregation of chromosomes during single-division meiosis of Saccharomyces cerevisiae. Genetics. 1990;125:475–485. doi: 10.1093/genetics/125.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu Y. J., Stillman B. Cdc7-Dbf4 phosphorylates MCM proteins via a docking site-mediated mechanism to promote S phase progression. Mol. Cell. 2006;24:101–113. doi: 10.1016/j.molcel.2006.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster E. O., Byers B. Pachytene arrest and other meiotic effects of the start mutations in Saccharomyces cerevisiae. Genetics. 1989;123:29–43. doi: 10.1093/genetics/123.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. E., Su Sophia S. Y., Neighborn L., Driscoll S. E., Mitchell A. P. Role of IME1 expression in regulation of meiosis in Saccharomyces cerevisiae. Mol. Cell. Biol. 1990;10:6103–6113. doi: 10.1128/mcb.10.12.6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. N., Penkner A., Ohta K., Klein F., Nicolas A. B-type cyclins CLB5 and CLB6 control the initiation of recombination and synaptonemal complex formation in yeast meiosis. Curr. Biol. 2001;11:88–97. doi: 10.1016/s0960-9822(01)00026-4. [DOI] [PubMed] [Google Scholar]
- Smyth G. K. Limma: linear models for microarray data. In: Gentleman R., Carey V., Dudoit S., Irizarry R., Huber W., editors. Bioinformatics and Computational Biology Solutions Using R and Bioconductor. New York: Springer; 2005. pp. 397–420. [Google Scholar]
- Sopko R., Raithatha S., Stuart D. Phosphorylation and maximal activity of Saccharomyces cerevisiae meiosis-specific transcription factor Ndt80 is dependent on Ime2. Mol. Cell. Biol. 2002;22:7024–7040. doi: 10.1128/MCB.22.20.7024-7040.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strich R., Surosky R. T., Steber C., Dubois E., Messenguy F., Esposito R. E. UME6 is a key regulator of nitrogen repression and meiotic development. Genes Dev. 1994;8:796–810. doi: 10.1101/gad.8.7.796. [DOI] [PubMed] [Google Scholar]
- Stuart D., Wittenberg C. CLB5 and CLB6 are required for premeiotic DNA replication and activation of the meiotic S/M checkpoint. Genes Dev. 1998;12:2698–2710. doi: 10.1101/gad.12.17.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth A., Rabitsch K. P., Galova M., Schleiffer A., Buonomo S. B., Nasmyth K. Functional genomics identifies monopolin: a kinetochore protein required for segregation of homologs during meiosis. Cell. 2000;103:1155–1168. doi: 10.1016/s0092-8674(00)00217-8. [DOI] [PubMed] [Google Scholar]
- Ubersax J. A., Woodbury E. L., Quang P. N., Paraz M., Blethrow J. D., Shah K., Shokat K. M., Morgan D. O. Targets of the cyclin-dependent kinase Cdk1. Nature. 2003;425:859–864. doi: 10.1038/nature02062. [DOI] [PubMed] [Google Scholar]
- Uhlmann F., Lottspeich F., Nasmyth K. Sister-chromatid separation at anaphase onset is promoted by cleavage of the cohesin subunit Scc1. Nature. 1999;400:37–42. doi: 10.1038/21831. [DOI] [PubMed] [Google Scholar]
- Uhlmann F., Nasmyth K. Cohesion between sister chromatids must be established during DNA replication. Curr. Biol. 1998;8:1095–1101. doi: 10.1016/s0960-9822(98)70463-4. [DOI] [PubMed] [Google Scholar]
- Valentin G., Schwob E., Della Seta F. Dual role of the CDC7-regulatory protein DBF4 during yeast meiosis. J. Biol. Chem. 2005;281:2828–2834. doi: 10.1074/jbc.M510626200. [DOI] [PubMed] [Google Scholar]
- Vershon A. K., Hollingsworth N. M., Johnson A. D. Meiotic induction of the yeast HOP1 gene is controlled by positive and negative regulatory elements. Mol. Cell. Biol. 1992;12:3706–3714. doi: 10.1128/mcb.12.9.3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vershon A. K., Pierce M. Transcriptional regulation of meiosis in yeast. Curr. Opin. Cell Bio. 2000;12:334–339. doi: 10.1016/s0955-0674(00)00104-6. [DOI] [PubMed] [Google Scholar]
- Wan L., Niu H., Futcher B., Zhang C., Shokat K. M., Boulton S. J., Hollingsworth N. M. Cdc28-Clb5 (CDK-S) and Cdc7-Dbf4 (DDK) collaborate to initiate meiotic recombination in yeast. Genes Dev. 2008;22:386–397. doi: 10.1101/gad.1626408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan L., Zhang C., Shokat K. M., Hollingsworth N. M. Chemical inactivation of Cdc7 kinase in budding yeast results in a reversible arrest that allows efficient cell synchronization prior to meiotic recombination. Genetics. 2006;174:1667–1774. doi: 10.1534/genetics.106.064303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Nurse P. Cohesin Rec8 is required for reductional chromosome segregation at meiosis. Nature. 1999;400:461–464. doi: 10.1038/22774. [DOI] [PubMed] [Google Scholar]
- Woltering D., Baumgartner B., Bagchi S., Larkin B., Loidl J., de los Santos T., Hollingsworth N. M. Meiotic segregation, synapsis, and recombination checkpoint functions require physical interaction between the chromosomal proteins Red1p and Hop1p. Mol. Cell. Biol. 2000;20:6646–6658. doi: 10.1128/mcb.20.18.6646-6658.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T.-C., Lichten M. Meiosis-induced double-strand break sites determined by yeast chromatin structure. Science. 1994;263:515–518. doi: 10.1126/science.8290959. [DOI] [PubMed] [Google Scholar]
- Xie J., Pierce M., Gailus-Durner V., Wagner M., Winter E., Vershon A. K. Sum1 and Hst1 repress middle sporulation-specific gene expression during mitosis in Saccharomyces cerevisiae. EMBO J. 1999;18:6448–6454. doi: 10.1093/emboj/18.22.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Ajimura M., Padmore R., Klein C., Kleckner N. NDT80, a meiosis-specific gene required for exit from pachytene in Saccharomyces cerevisiae. Mol. Cell. Biol. 1995;15:6572–6581. doi: 10.1128/mcb.15.12.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.