Abstract
Targeted proteasomal degradation mediated by E3 ubiquitin ligases controls cell cycle progression, and alterations in their activities likely contribute to malignant cell proliferation. S phase kinase-associated protein 2 (Skp2) is the F-box component of an E3 ubiquitin ligase complex that targets p27Kip1 and cyclin E1 to the proteasome. In human melanoma, Skp2 is highly expressed, regulated by mutant B-RAF, and required for cell growth. We show that Skp2 depletion in melanoma cells resulted in a tetraploid cell cycle arrest. Surprisingly, co-knockdown of p27Kip1 or cyclin E1 failed to prevent the tetraploid arrest induced by Skp2 knockdown. Enhanced Aurora A phosphorylation and repression of G2/M regulators cyclin B1, cyclin-dependent kinase 1, and cyclin A indicated a G2/early M phase arrest in Skp2-depleted cells. Furthermore, expression of nuclear localized cyclin B1 prevented tetraploid accumulation after Skp2 knockdown. The p53 status is most frequently wild type in melanoma, and the tetraploid arrest and down-regulation of G2/M regulatory genes were strongly dependent on wild-type p53 expression. In mutant p53 melanoma lines, Skp2 depletion did not induce cell cycle arrest despite up-regulation of p27Kip1. These data indicate that elevated Skp2 expression may overcome p53-dependent cell cycle checkpoints in melanoma cells and highlight Skp2 actions that are independent of p27Kip1 degradation.
INTRODUCTION
Ubiquitin-mediated proteolysis of cell cycle regulators is critical for tight control of normal cell proliferation. The rate-limiting step in ubiquitin-dependent degradation is substrate recognition by E3 ubiquitin ligases. Altered expression and/or activity of E3 ubiquitin ligases in cancer cells leads to deregulated proteolysis and aberrant proliferation (Guardavaccaro and Pagano, 2004; Nakayama and Nakayama, 2006). It is critical to understand how E3 ubiquitin ligases contribute to malignant cell proliferation, because they represent a potential new class of therapeutic targets (Nalepa et al., 2006).
One major class of E3 ubiquitin ligases that regulates cell cycle progression contains Skp1/Cullin/F-box protein (SCF) complexes (Krek, 1998; Patton et al., 1998). Within these complexes, the cullin protein serves as a scaffold for the E2 ubiquitin-conjugating enzyme and Skp1/F-box protein complex. The F-box protein determines the substrate specificity of the SCF complex; ∼70 exist in the human genome. The F-box protein S phase kinase-associated protein 2 (Skp2) has received attention as a putative oncogene. Its expression correlates with tumor malignancy in several tumor types, and the SKP2 gene is amplified in small cell lung and biliary tract cancers (reviewed in Guardavaccaro and Pagano, 2004). High expression of Skp2 is sufficient to promote anchorage-independent growth (Carrano et al., 1999), and Skp2 cooperates with mutant Ras to transform rat fibroblasts (Gstaiger et al., 2001). Evidence from transgenic mice models shows that expression of Skp2 in the T-lymphoid lineage cooperates with activated N-Ras to induce T cell lymphomas in mice (Latres et al., 2001), whereas expression of Skp2 in the prostate glands initiates hyperplasia and low-grade carcinoma (Shim et al., 2003). Skp2 recognizes multiple targets including p27Kip1, cyclin E1, p21Cip1, p57Kip2 and origin recognition complex-1 (Orc1) (Carrano et al., 1999; Tsvetkov et al., 1999; Nakayama et al., 2000; Mendez et al., 2002; Bornstein et al., 2003; Kamura et al., 2003). Of these targets, the cyclin-dependent kinase inhibitor p27Kip1 has received most attention, because the phenotypes of the Skp2 knockout mouse are largely reversed by co-knockout of p27Kip1 (Nakayama et al., 2000; Nakayama et al., 2004).
Melanoma is the deadliest form of skin cancer. It originates from melanocytes, the pigment producing cells in the skin, and/or their progenitors. The mechanism underlying aberrant cell cycle progression in melanoma cells is poorly defined, although recent evidence indicates an important role for the mitogen-activated protein kinase kinase (MEK)-extracellular signal-regulated kinase (ERK)1/2 pathway, because approximately two thirds of melanomas have activating mutations in the serine-threonine kinase B-RAF (Davies et al., 2002). Skp2 is highly expressed in human melanoma (Li et al., 2004; Woenckhaus et al., 2005), is regulated by B-RAF (Bhatt et al., 2007), and is required for melanoma cell growth in vitro and in vivo (Katagiri et al., 2006; Sumimoto et al., 2006; Bhatt et al., 2007). By contrast, Skp2 expression is low in benign nevi (Li et al., 2004), which exhibit senescence-like characteristics (Michaloglou et al., 2005). Here, we demonstrate that Skp2 regulates cell cycle progression by causing a tetraploid arrest, decreased expression of mitotic regulators such as cyclin B1, and an increase in 8N cells likely due to endoreplication. These effects were independent of accumulated p27Kip1 the main studied Skp2 substrate, and only partially dependent on accumulated cyclin E1. Rather, the effects of Skp2 depletion on the cell cycle were prevented by expression of nuclear localized cyclin B1. Additionally, the tetraploid arrest and repression of mitotic regulators was dependent on expression of wild-type, but not mutant, p53. These findings show that Skp2 regulates cell cycle progression in melanoma cells in a manner independent of p27Kip1 accumulation and likely acts to override p53 surveillance mechanisms in melanomas.
MATERIALS AND METHODS
Cell Culture
Human melanoma cell lines WM115, WM278, and WM983A were obtained from Dr. Meenhard Herlyn (Wistar Institute, Philadelphia, PA). SK-MEL-28 cells were purchased from American Type Culture Collection (Manassas, VA). Cells were maintained in modified chemically defined medium (MCDB) 153 medium containing 20% Leibovitz L-15 medium, 2% (vol/vol) fetal bovine serum, 0.2% (wt/vol) sodium bicarbonate, and 5 μg/ml insulin.
RNA Interference
For short-interfering RNA (siRNA) transfections, 2 × 105 cells were transfected with final concentration of 25 nM siRNA (Dharmacon RNA Technologies, Lafayette, CO) by using Oligofectamine (Invitrogen, Carlsbad, CA). The sequences of the individual siRNAs are as follows: Skp2 #7, CUAAAGGUCUCUGGUGUUUUU; Skp2 #8, GAUGGUACCCUUCAACUGUUU; Skp2 #13, GGUAUCGCCUAGCGUCUGAUU; p27Kip1, GGAGCAAUGCGCAGGAAUAUU; Cyclin E1, GGAAAUCUAUCCUCCAAAGUU; and p53 #5, GAGGUUGGCUCUGACUGUAUU. A nontargeting siRNA from Dharmacon RNA Technologies was used as a control. For experiments involving simultaneous knockdowns, melanoma cells were transfected with 25 nM of each individual siRNA to give a final concentration of 50 nM. Cells were transfected for 4 h in serum-free medium after which culture medium was added. Cells were harvested after 72 h, unless otherwise indicated.
For short-hairpin RNA experiments, we used the BLOCK-iT lentiviral expression system (Invitrogen). SK-MEL-28 cells expressing the tetracycline repressor (SK-MEL-28-TR) were generated as described previously (Boisvert-Adamo and Aplin, 2008). The sequences for the Skp2 shRNA oligos were based on siRNA #13 by using the hairpin sequence 5′-TTCAAGAGA-3′. Annealed oligonucleotides (oligos) were ligated into the pENTR/H1/TO vector, and sequence-verified constructs were then recombined into the pLenti4/BLOCK-iT-DEST vector. The generated pLenti4/BLOCK-iT/Skp2 shRNA was transfected along with the packaging plasmids pLP1, pLP2, and pLP/VSVG into 293FT cells by using FuGENE HD (Roche Diagnostics, Indianapolis, IN). Harvested lentivirus-containing medium was used to infect SK-MEL-28-TR cells. Transduced cells were selected with zeocin over 2 wk. Expression of the shRNA was induced with doxycycline at a final concentration of 0.1 μg/ml.
Western Blotting
Melanoma cell lysates were analyzed for protein expression by Western blotting as described previously (Bhatt et al., 2007). The following primary antibodies were used: Skp2 (H-435), cyclin A (H-432), cyclin E1 (HE-12), p53 (DO-1), and ERK1/2 (K-23) were from Santa Cruz Biotechnology (Santa Cruz, CA); cyclin B (Ab-3), cyclin-dependent kinase 1 (CDK1; Ab-3), and p57Kip2 (Ab-5) were from Lab Vision-NeoMarkers (Fremont, CA); Skp2 (8D9) was from Zymed Laboratories (South San Francisco, CA); p21Cip1 (sx-118) and p27Kip1 (clone 57) were from BD Biosciences (San Jose CA); phospho T288 aurora-A (D13A11) and total aurora-A were from Cell Signaling Technology (Danvers, MA); and Orc1 (#209) was from Dr. C. Obuse (Nara Institute of Science and Technology, Nara, Japan; Tatsumi et al., 2003). Western blots were developed using SuperSignal chemiluminescent substrate (Pierce Chemical, Rockford) and quantitated with a Fluor-S MultiImager and Quantity-One software (Bio-Rad, Hercules, CA).
Flow Cytometry
Cells (1 × 106) were harvested, washed in phosphate-buffered saline (PBS), and fixed in ice-cold 70% ethanol. Cells were permeabilized with 0.1% Triton X-100 and 100 μg/ml RNaseA in PBS at 37°C for 2 h. DNA was stained with 50 μg/ml propidium iodide (Invitrogen, Carlsbad, CA) at 37°C for 15 min. Cells (1 × 105/sample) were analyzed in FACS Canto (BD Biosciences, San Jose, CA). For apoptosis studies, cells were analyzed by flow cytometry for cleaved caspase 3 staining, as described previously (Boisvert-Adamo and Aplin, 2006). As a positive control, cells were treated with 10 μM etoposide (Sigma-Aldrich, St. Louis, MO) for 48 h.
Immunofluorescence
Cells were fixed in 3.7% formaldehyde, permeabilized with 0.5% Triton X-100, and stained with antibodies to nucleoporin (N43620; BD Biosciences), and appropriate Alexa Fluor-conjugated secondary antibodies. Staining was viewed on Olympus BX61 upright microscope equipped for epifluorescence.
Real-Time Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted from melanoma cells by using Versagene RNA isolation kit (Gentra Systems, Minneapolis, MN). RNA (1 μg) was reverse transcribed, and 1/20 of the resulting cDNA (cDNA) was used to detect mRNA abundance with primers for actin (forward, TACCTCATGAAGATCCTCACC; reverse, TTTCGTGGATGCCACAGGAC), cyclin B1 (forward, AGAACCTGAGCCAGAACC; reverse, TTGCTCTTCCTCAAGTTGTC), CDK1 (forward, TACACATGAGGTAGTAACACTCTG; reverse, GATGCTAGGCTTCCTGGT), and cyclin A2 (forward, GAAGTACCAGACTACCATGAG; reverse, CTTCAAACTTTGAGGCTAACAG). All primers were designed to give <300-base pair products; primer specificity was indicated by melt curve analysis. Reactions were performed using SYBR Green mix and MyiQ real-time PCR detection system (Bio-Rad). Relative mRNA levels were calculated using the comparative Ct method (ΔCt) (Pfaffl, 2001).
Inducible Cyclin B1 Cell Lines
Wild-type and 3A-cyclin B1 cDNA were kind gifts from Dr. Eisuke Nishida (Kyoto University, Kyoto, Japan) (Toyoshima et al., 1998). cDNAs were cloned into pENTR/3C vector, and, after verification by DNA sequencing, were recombined with pLenti4/TO/DEST using the LR Clonase II kit (Invitrogen). The generated pLenti4/TO/WT-Cyclin B1 and pLenti4/TO/3A-Cyclin B1 were packaged in 293FT cells, and the resulting lentiviral supernatants were used to infect a WM115 cell line expressing the tetracycline repressor (WM115-TR). Infected cells were selected with zeocin for 2 wk. Doxycycline (0.1 μg/ml) was used to induce gene expression.
RESULTS
Skp2 Regulates Melanoma Cell Cycle Progression
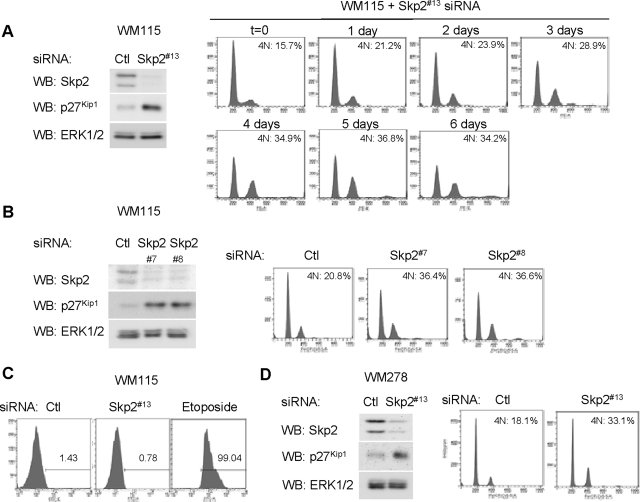
We recently demonstrated that Skp2 is required for melanoma cell growth (Bhatt et al., 2007); however, the mechanisms underlying these effects are unknown. Consistent with our previous study, transfection of vertical growth phase WM115 cells with siRNA targeting Skp2 efficiently reduced Skp2 expression and up-regulated levels of p27Kip1 (Figure 1A, left). DNA content profiles showed that the percentages of Skp2 knockdown cells in the G0/G1 and S stages were decreased compared with controls, but percentages with 4N were progressively increased over 6 d (Figure 1A, right). At later time points (5 and 6 d), a small 8N peak was also detected indicative of endoreplication, a cell cycle that consists of repeated rounds of DNA synthesis without concomitant cell division (Edgar and Orr-Weaver, 2001). To rule out effects of “off-targets,” we used two additional sequences to target Skp2 (Figure 1B, left). Accumulation of 4N was observed with both additional duplexes (Figure 1B, right), although the lower percentages of 4N and 8N cells correlated with decreased knockdown efficiency. Nevertheless, these data argue against off-target actions. Because the Skp2 siRNA duplex #13 was the most efficient, this individual sequence was used in subsequent experiments.
Figure 1.
Skp2 knockdown promotes 4N accumulation and endoreplication in melanoma cells. (A) B-RAFV600D-harboring WM115 cells were transfected with Skp2 #13 siRNA or a nontargeting control (Ctl) siRNA. Seventy-two hours after transfection, cells were lysed and Skp2, p27Kip1 and ERK1/2 protein levels determined by Western blot analysis. Cell cycle profiles were analyzed by propidium iodide staining and flow cytometry after the time period indicated. Representative profiles are shown, and percentage of G2/M cells from one of two independent experiments is shown. (B) WM115 cells were transfected with Skp2 siRNA duplexes #7 and #8 and analyzed, as described above. (C) Six days after transfection, control and Skp2 knockdown WM115 cells were analyzed by cleaved caspase 3 staining and flow cytometry. Etoposide (10 μM for 48 h) treated WM115 cells were used as positive control. (D) B-RAFV600E-harboring WM278 cells were transfected with Skp2 #13 or Ctl siRNA. Lysates were analyzed by Western blotting for Skp2 and ERK1/2 levels. Six days after transfection, the cell cycle profile of control and Skp2 knockdown WM278 cells was analyzed by propidium iodide staining and flow cytometry.
Down-regulation of Skp2 expression in small-cell lung cancer cells induces apoptosis (Yokoi et al., 2003); however, we detected neither enhanced cleaved caspase-3 staining (Figure 1C) nor increased annexin V staining (data not shown) after Skp2 knockdown in WM115 cells. To determine whether our effects on 4N accumulation could be extrapolated to other melanoma cell lines, we knocked down Skp2 in vertical growth phase melanoma WM278 cells. Consistent with data from WM115, Skp2 knockdown in WM278 cells led to an increase in the 4N peak, although the 8N accumulation was not readily detectable in these cells (Figure 1D). These data show that Skp2 depletion affects cell cycle progression, but it does not directly regulate apoptosis in melanoma cells.
Depletion of p27Kip1 Does Not Reverse Effects of Skp2 Knockdown on the Cell Cycle
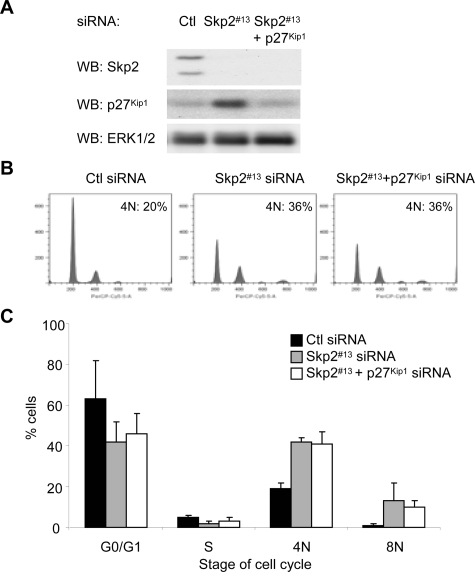
Mice studies have shown that combined knockout of p27Kip1 and Skp2 reverses effects of Skp2 knockout alone (Kossatz et al., 2004; Nakayama et al., 2004). To determine whether similar effects are observed in human melanoma cells, we performed double siRNA knockdown experiments. We optimized co-knockdown conditions to deplete p27Kip1 in Skp2 knockdown cells to levels comparable with control cells (Figure 2A). Surprisingly, Skp2 and p27Kip1 double knockdown melanoma cells still accumulated with 4N and 8N DNA contents (Figure 2B, quantitated in C). These results suggest that Skp2 effects on cell cycle progression in melanoma cells are independent of the accumulation of p27Kip1.
Figure 2.
p27Kip1 co-knockdown with Skp2 does not reverse cell cycle arrest. WM115 cells were transfected with control siRNA, Skp2 #13 siRNA alone, or Skp2 plus p27Kip1 siRNA for 6 d. (A) Cells lysates analyzed by Western blotting for Skp2, p27Kip1, and ERK1/2 (loading control). (B) Cells were stained with propidium iodide and analyzed by FACS. (C) Average percentage and SD of cells with G0/G1, S, 4N, and 8N DNA contents from three independent experiments are shown.
Skp2 Also Targets Orc1 and Cyclin E1 in Human Melanoma Cells
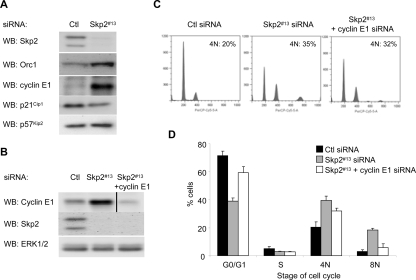
Multiple Skp2 targets, in addition to p27Kip1, have been described. We examined the effect of Skp2 knockdown on additional targets, including p21Cip1, p57Kip2, cyclin E1, and Orc1 (Carrano et al., 1999; Tsvetkov et al., 1999; Nakayama et al., 2000; Mendez et al., 2002; Bornstein et al., 2003; Kamura et al., 2003). Western blot analysis showed that cyclin E1 and Orc1 were both Skp2-regulated in melanoma cells; p57Kip2 was slightly up-regulated, whereas levels of p21Cip1 were decreased (Figure 3A). We determined the requirement of Orc1 and cyclin E1 in Skp2 effects on the cell cycle in double knockdown experiments. Orc1 is a key determinant of genomic sites in which prereplication complexes are to be assembled (DePamphilis, 2003). Despite efficient co-knockdown of Orc1 with Skp2, an accumulation of 4N cells was still detected (Supplemental Figure 1), although a small reduction in 8N accumulation was observed. Similarly, we efficiently co-knocked down cyclin E1 and Skp2 (Figure 3B). Reduction of cyclin E1 efficiently reduced the size of the 8N DNA peak that is induced after Skp2 depletion but only slightly reduced 4N accumulation (Figure 3C; quantitated in D). These data indicate that up-regulation of cyclin E1 and possibly Orc1 after Skp2 depletion may promote endoreplicative cycles in melanoma cells.
Figure 3.
Depletion of cyclin E1 reduces the Skp2 knockdown effect on endoreplication. (A) WM115 cells were transfected with control or Skp2 #13 siRNA. Cell lysates were analyzed for protein expression of Skp2, Orc1, cyclin E1, p21Cip1, and p57Kip2. (B–D) WM115 cells were transfected with control siRNA, Skp2 #13 siRNA alone, or Skp2 plus cyclin E1 siRNA for 6 d. (B) Cell lysates analyzed by Western blotting for Skp2, cyclin E1, and ERK1/2 (loading control). (C) Cells were stained with propidium iodide and analyzed by FACS. (D) Average percentage and SD of cells with G0/G1, S, 4N, and 8N DNA contents from three independent experiments are shown.
Skp2 Knockdown Is Associated with Decreased Expression of Mitotic Genes
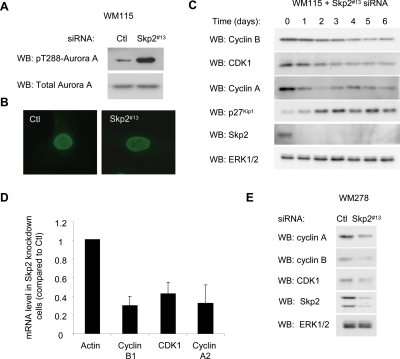
We next characterized the cell cycle arrest in Skp2 knockdown cells. The mitotic kinase Aurora-A is activated in late G2 (Marumoto et al., 2005). Skp2 knockdown cells displayed enhanced activation loop phosphorylation of Aurora-A (Figure 4A). Nuclear envelope breakdown occurs at the onset of anaphase. More than 95% of Skp2 knockdown cells displayed an intact nuclear envelope by nucleoporin staining (Figure 4B), but they stained negatively for phospho-histone H3 (data not shown). These data indicate that Skp2 knockdown cells arrest within G2/early M before the formation of mitotic chromosomes. We next examined the expression of regulators of the G2/M transition, specifically CDK1, cyclin B1, and cyclin A2 during a Skp2 time-course knockdown. Cyclin A was rapidly down-regulated followed by decreases in the expression of cyclin B1 and CDK1 in response to Skp2 knockdown compared with controls (Figure 4C). Down-regulation of cyclin B1, CDK1, and cyclin A occurred at the mRNA level, as determined by qRT-PCR (Figure 4C). Notably, a similar down-regulation of cyclin B1, CDK1, and cyclin A was observed after Skp2 knockdown in WM278 cells (Figure 4D). Together, these results are consistent with down-regulation of multiple mitotic regulators contributing to the cell cycle arrest in Skp2 knockdown cells.
Figure 4.
Effect of Skp2 knockdown on mitotic regulators. (A) Lysates from control and Skp2 knockdown WM115 cells were analyzed by Western blotting for levels of phosphoThr288 and total Aurora-A. (B) Control and Skp2 knockdown cells were analyzed by immunofluorescence for nucleoporin and costained with Hoechst reagent to visualize the nuclei. (C) Skp2 knockdown cells were lysed at the times indicated after transfection, and lysates analyzed for the levels of cyclin B, CDK1, cyclin A2, p27Kip1, Skp2, and ERK1/2 by Western blotting. (D) qRT-PCR analysis of the indicated genes in Skp2 depleted or control WM115 cells 3 d after transfection. The graph represents the average and SD for the percentage of change in mRNA level relative to actin from three independent experiments. (E) WM278 cell lysates were analyzed for the levels of cyclin B, CDK1, cyclin A2, Skp2, and ERK1/2 by Western blotting.
Expression of Nuclear-localized Cyclin B1 Rescued Cell Cycle Defects in Skp2 Knockdown Cells
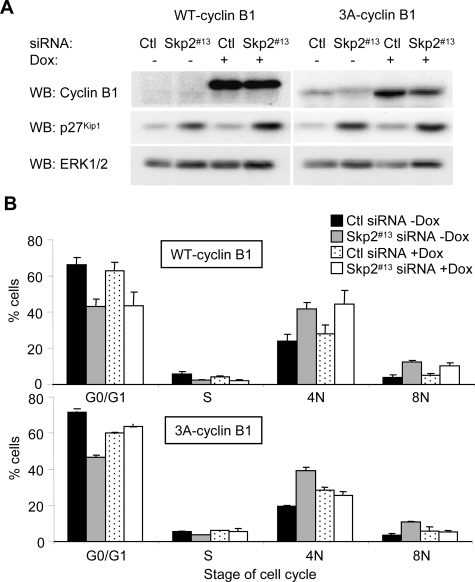
Because cyclin B1 is the major cyclin that drives the G2/M transition, we tested whether cyclin B1 expression was sufficient to rescue the Skp2 knockdown-induced arrest. Doxycycline-inducible expression of wild-type cyclin B1 in WM115 was not sufficient to overcome Skp2-induced G2/M arrest (Figure 5, A and B; representative profiles are provided in Supplemental Figure 2). It has been demonstrated that cyclin B/CDK1 complexes need to be activated and localized in the nucleus to promote the G2/M transition and that cyclin B1 is excluded from the nucleus during G2 (Toyoshima et al., 1998; Taylor et al., 1999). We therefore engineered WM115 cells to inducibly express a mutant cyclin B1 (3A-cyclin B1) that resides in the nucleus due to lack of intact nuclear export signal (Figure 5A). Notably, expression of 3A-cyclin B1 was able to reverse the G2 arrest induced after Skp2 knockdown (Figure 5B). p27Kip1 levels remained high in Skp2 knockdown cells expressing 3A-cyclin B1, uncoupling regulation of p27Kip1 levels from G2/M progression. Thus, nuclear expression of cyclin B1 prevents the effects of Skp2 knockdown.
Figure 5.
Expression of 3A-cyclin B1 prevents the Skp2 knockdown-induced 4N arrest. (A) WM115-TR-WT-cyclin B1 and WM115-TR-3A-cyclin B1 cells were transfected with control or Skp2 #13 siRNA for 6 d in the presence or absence of 0.1 μg/ml doxycycline. Cell lysates analyzed by Western blotting for cyclin B1, p27Kip1, and ERK1/2 (loading control). (B) Cells were stained with propidium iodide and analyzed by FACS. Average percentage and SD of cells with G0/G1, S, 4N, and 8N DNA contents from three independent experiments are shown.
Cell Cycle Arrest after Skp2 Depletion Is Dependent on p53
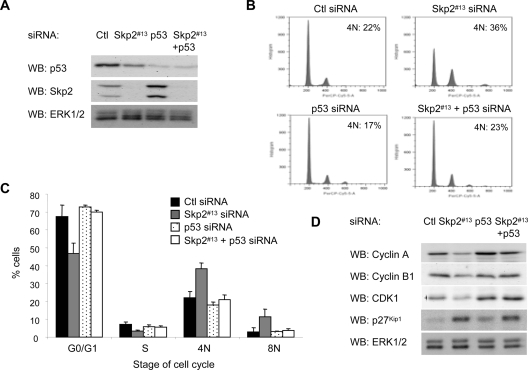
As shown in Figure 4, Skp2 depletion is associated with decreased mRNA levels of G2/M regulators. The tumor suppressor p53 is known to repress transcription of CDK1, cyclin B1, and cyclin A2, at least in part through the binding of a p53–NF-Y complex to multiple CCAAT boxes within the promoter regions of these genes (Bolognese et al., 1999; Farina et al., 1999; Manni et al., 2001). Because the p53 status is wild type in WM115 and many other melanoma cell lines (Smalley et al., 2007), we determined the requirement of p53 in G2/M arrest and repression of mitotic regulators after Skp2 knockdown. We individually or jointly depleted cells of Skp2 and p53 (Figure 6A). p53 knockdown alone caused a reproducible increase in the Skp2 levels, an effect that may be mediated by p53 down-regulation of Cks1, a cofactor that stabilizes Skp2 expression (Rother et al., 2007). Notably, the Skp2 knockdown-induced G2/M arrest was efficiently reduced when p53 was co-knocked down in these cells (Figure 6B; quantitated in C). Similar effects were obtained with a second distinct p53 siRNA duplex (Supplemental Figure 3). Furthermore, cyclin B1, cyclin A, and CDK1 protein levels were enhanced in Skp2/p53 knockdown cells compared with Skp2 knockdown alone cells, and they were similar to levels in control cells (Figure 6D). The levels of p27Kip1, however, remained elevated in Skp2/p53 co-knockdown cells, providing further evidence that increased p27Kip1 levels could be separated from Skp2 effects on the cell cycle. Together, these data suggest that the cell cycle arrest and down-regulation of mitotic genes induced by Skp2 knockdown requires p53.
Figure 6.
G2/M accumulation after Skp2 depletion is dependent on p53. WM115 cells were transfected with control, Skp2 #13 siRNA alone, p53 #5 siRNA alone, or Skp2 plus p53 siRNA for 6 d. (A) Cells were lysed, and lysates analyzed by Western blotting for Skp2, p53, and ERK1/2 (loading control). (B) Cells were stained with propidium iodide and analyzed by FACS. (C) The average percentage and SD of cells in G0/G1, S, 4N, and 8N DNA contents from three independent experiments are shown. (D) Cell lysates analyzed by Western blotting for cyclin A, cyclin B1, CDK1, p27Kip1, and ERK1/2.
Lack of Tetraploidy after Skp2 Knockdown in Mutant p53 Cell Lines
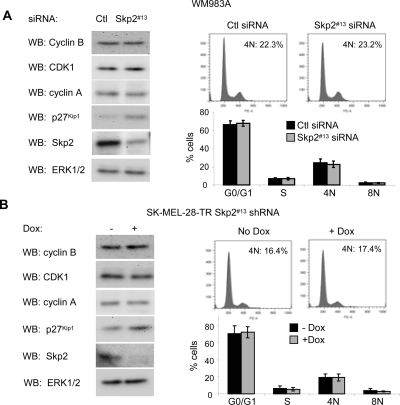
Although most melanomas are wild type for p53, a few harbor p53 mutations. We next determined whether Skp2 knockdown induced a cell cycle arrest in two mutant p53 cell lines. WM983A and SK-MEL-28 cells harbor p53 mutants, P278F and L145R, respectively (Weiss et al., 1993; Ikediobi et al., 2006; Smalley et al., 2007). siRNA-mediated knockdown of Skp2 in WM983A up-regulated p27Kip1 levels, but it did not alter the expression of cyclin B1, CDK1, and cyclin A (Figure 7A, left). Additionally, in propidium iodide experiments, we did not observe accumulation of tetraploidy after Skp2 knockdown (Figure 7A, right). Similar experiments were performed in SK-MEL-28 cells, except that we generated doxycycline-inducible Skp2 shRNA cells due to resistance of these cells to transfection. Inducible knockdown of Skp2 in SK-MEL-28 cells enhanced p27Kip1 levels but did not elicit effects on G2/M regulators or 4N accumulation (Figure 7B). Together, these data indicate that Skp2 actions on the cell cycle are dependent on wild-type p53.
Figure 7.
Lack of tetraploid accumulation after Skp2 depletion in mutant p53 cells. (A) WM983A cells were transfected with Skp2 #13 siRNA or a nontargeting control (Ctl) siRNA. Six days after transfection cell lysates were analyzed for cyclin B, CDK1, cyclin A, p27Kip1, Skp2, and ERK1/2 levels by Western blotting. Cell cycle profiles were analyzed by propidium iodide staining and flow cytometry. Representative profiles are shown and percentages of 4N cells are shown. Average percentage and SD of cells in G0/G1, S, 4N, and 8N DNA content from three independent experiments are shown. (B) SK-MEL-28-TR/Skp2 shRNA were induced with 0.1 μg/ml doxycycline for 6 d. Cells were analyzed by Western blotting and propidium iodide staining, as in described in A.
DISCUSSION
The importance of proteasomal degradation in tumor growth is underscored by the recent successful treatment of multiple myeloma patients with the general proteasomal inhibitor, Bortezomib. The F-box protein, Skp2 may regulate cell cycle progression in a variety of cancers. We have previously shown that Skp2 expression is enhanced in human melanoma cells compared with melanocytes (Bhatt et al., 2007) corroborating in situ data from human tumor samples (Li et al., 2004; Woenckhaus et al., 2005). Furthermore, we and others have shown that Skp2 is required for melanoma cell growth in vitro and in vivo (Katagiri et al., 2006; Sumimoto et al., 2006; Bhatt et al., 2007). This current study provides important new insight underlying Skp2 effects on the cell cycle progression in melanoma cells.
Skp2 knockdown cells display two main alterations in their cell cycle profile: accumulation of cells with 4N and 8N DNA content. The 4N accumulation likely reflects cellular arrest in G2/early M phase. Although G1 tetraploid arrest is also possible (Andreassen et al., 2001), we believe this is less likely given the high phosphorylation of the mitotic kinase Aurora-A and the reversal by expression of nuclear-localized cyclin B1. The 8N peak signifies endoreplication that consists of rounds of DNA synthesis without concomitant cell division (Edgar and Orr-Weaver, 2001). Similar 4N and 8N DNA phenotypes were observed in fibroblasts and hepatocytes derived from Skp2 knockout mice (Nakayama et al., 2000). A major difference in our findings to those in knockout mice is the role of accumulated p27Kip1. It is clear that p27Kip1 is targeted by Skp2 in G2 and M phases and that concomitant loss of p27Kip1 expression is sufficient to reverse the effects of Skp2 knockout in several cell types (Kossatz et al., 2004; Nakayama et al., 2004). However, our results in human melanoma cells demonstrate that reducing p27Kip1 to basal levels is not sufficient to reverse the effect of Skp2 depletion. These results may highlight important differences between Skp2 targets in normal and tumor cells and are consistent with findings from an activated K-Ras mouse model of lung carcinoma. In this model, expression of the p27Kip1 mutant T187A, which is resistant to Skp2-mediated degradation, did not improve tumor-free survival compared with wild-type mice (Timmerbeul et al., 2006). Cyclin E1 is another target of Skp2 (Nakayama et al., 2000). Our data indicate that the failure to degrade cyclin E1 as cells proceed through S phase likely promotes the endocycling phenotype. A role for cyclin E in endoreplication was previously highlighted in E-type cyclin knockout mice, which are embryonic lethal due to a failure of trophoblast cells to undergo normal endoreplicative cycles (Geng et al., 2003).
After Skp2 knockdown, we also observed repression of several G2/M regulators that contain multiple CCAAT boxes within their promoter region. CCAAT boxes in these promoters are bound by the coactivator NF-Y in complex with p53 (Sciortino et al., 2001; Caretti et al., 2003; Imbriano et al., 2005). This prompted us to analyze the dependence of Skp2 effects on p53, which is typically wild type in melanoma (Volkenandt et al., 1991; Weiss et al., 1993). We showed that the cell cycle arrest and decreased expression of G2/M regulators in Skp2 knockdown cells was dependent on p53 expression. Although p53 regulation of p21Cip1 mediates many of the actions of p53, we did not detect an increase of p21Cip1 after Skp2 knockdown (Figure 3), and co-knockdown of p21Cip1 with Skp2 did not reverse the G2/M accumulation (data not shown).
The mechanism underlying altered p53 function after Skp2 knockdown in melanoma warrants further investigation. This is not a trivial issue because p53 can be posttranslationally modified by several distinct mechanisms at multiple sites and recruit coactivators and corepressors (Kruse and Gu, 2008). A recent study showed that Skp2 sequesters the acetyltransferase p300, from binding p53 (Kitagawa et al., 2008). This action inhibited p53 acetylation and transactivation of p21Cip1, PUMA, and Bax after DNA damage, and it enhanced the sensitivity of cancer cells to apoptosis. To date, we have not detected alterations in the acetylation of p53 at C-terminal sites after Skp2 knockdown (data not shown), although notably DNA-damage–inducing agents are not present in our experimental conditions. Although we cannot rule out a similar mechanism because low levels of acetylation may be undetectable in basal conditions, our data indicate that Skp2 blocks the repressive functions of p53 on expression G2/M regulators and that it elicits effects on cell cycle progression. Ongoing experiments are designed to elucidate the mechanism of Skp2 effects on the repressor functions of p53. An additional feature of the Kitagawa study was that Skp2 actions were not dependent of its F-box function and down-regulation of p27Kip1. This notion is consistent with our findings and those of others (Kim et al., 2003) that Skp2 elicits actions on the cell cycle in addition to down-regulation of p27Kip1.
Skp2 depletion in melanoma cells leads to inhibition of cell proliferation despite the expression of mutant B-RAF. It is known that expression of oncogenes early in tumorigenesis leads to a senescent-like cell cycle arrest. Mutant B-RAF expression in human melanocytes initially leads to several rounds of proliferation, but subsequently it leads to a senescent-like arrest (Michaloglou et al., 2005), consistent with the observation that benign nevus cells harbor B-RAF mutations (Dong et al., 2003; Pollock et al., 2003). Activation of p53 is associated with the DNA-damage response, which inhibits G2/M progression. A DNA-damage response has been detected in preneoplastic lesions (Bartkova et al., 2005; Gorgoulis et al., 2005) and occurs during oncogene-induced cell cycle arrest (Bartkova et al., 2006; Di Micco et al., 2006), including arrested mutant B-RAF–expressing melanocytes (Denoyelle et al., 2006). Because we observe a G2 arrest after depletion of Skp2, up-regulation of Skp2 may represent one mechanism, in addition to loss of p16Ink4a, used by melanoma cells to overcome tumorigenesis barriers imposed during mutant B-RAF–mediated senescent-like arrest.
Supplementary Material
ACKNOWLEDGMENTS
We thank Ethan Abel and Whitney Longmate for help with lentiviral transduction and apoptosis assays, respectively. We are grateful to Dr. Meenhard Herlyn for the melanoma cell lines and Dr. Kevin Pumiglia (Albany Medical College, Albany, NY) for comments on the manuscript. This work was supported by a National Institutes of Health grant GM-067893 (to A.E.A.) and a National Cancer Center predoctoral fellowship (to R. H.).
Abbreviations used:
- CDK1
cyclin-dependent kinase 1
- Orc1
origin recognition complex-1
- qRT-PCR
real-time quantitative reverse transcription-polymerase chain reaction
- SCF
Skp1/Cullin/F-box protein
- shRNA
short hairpin RNA
- siRNA
short-interfering RNA
- Skp2
S phase kinase-associated protein 2.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-11-1137) on August 20, 2008.
REFERENCES
- Andreassen P. R., Lohez O. D., Lacroix F. B., Margolis R. L. Tetraploid state induces p53-dependent arrest of nontransformed mammalian cells in G1. Mol. Biol. Cell. 2001;12:1315–1328. doi: 10.1091/mbc.12.5.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartkova J., et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- Bartkova J., et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–637. doi: 10.1038/nature05268. [DOI] [PubMed] [Google Scholar]
- Bhatt K. V., Hu R., Spofford L. S., Aplin A. E. Mutant B-RAF signaling and cyclin D1 regulate Cks1/S-phase kinase-associated protein 2-mediated degradation of p27(Kip1) in human melanoma cells. Oncogene. 2007;26:1056–1066. doi: 10.1038/sj.onc.1209861. [DOI] [PubMed] [Google Scholar]
- Boisvert-Adamo K., Aplin A. E. B-RAF and PI-3 kinase signaling protect melanoma cells from anoikis. Oncogene. 2006;25:4848–4856. doi: 10.1038/sj.onc.1209493. [DOI] [PubMed] [Google Scholar]
- Boisvert-Adamo K., Aplin A. E. Mutant B-RAF mediates resistance to anoikis via Bad and Bim. Oncogene. 2008;27:3301–3312. doi: 10.1038/sj.onc.1211003. [DOI] [PubMed] [Google Scholar]
- Bolognese F., et al. The cyclin B2 promoter depends on NF-Y, a trimer whose CCAAT-binding activity is cell-cycle regulated. Oncogene. 1999;18:1845–1853. doi: 10.1038/sj.onc.1202494. [DOI] [PubMed] [Google Scholar]
- Bornstein G., Bloom J., Sitry-Shevah D., Nakayama K., Pagano M., Hershko A. Role of the SCFSkp2 ubiquitin ligase in the degradation of p21Cip1 in S phase. J. Biol. Chem. 2003;278:25752–25757. doi: 10.1074/jbc.M301774200. [DOI] [PubMed] [Google Scholar]
- Caretti G., Salsi V., Vecchi C., Imbriano C., Mantovani R. Dynamic recruitment of NF-Y and histone acetyltransferases on cell-cycle promoters. J. Biol. Chem. 2003;278:30435–30440. doi: 10.1074/jbc.M304606200. [DOI] [PubMed] [Google Scholar]
- Carrano A. C., Eytan E., Hershko A., Pagano M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat. Cell Biol. 1999;1:193–199. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- Davies H., et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- Denoyelle C., et al. Anti-oncogenic role of the endoplasmic reticulum differentially activated by mutations in the MAPK pathway. Nat. Cell Biol. 2006;8:1053–1063. doi: 10.1038/ncb1471. [DOI] [PubMed] [Google Scholar]
- DePamphilis M. L. The ‘ORC cycle’: a novel pathway for regulating eukaryotic DNA replication. Gene. 2003;310:1–15. doi: 10.1016/s0378-1119(03)00546-8. [DOI] [PubMed] [Google Scholar]
- Di Micco R., et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444:638–642. doi: 10.1038/nature05327. [DOI] [PubMed] [Google Scholar]
- Dong J., Phelps R. G., Qiao R., Yao S., Benard O., Ronai Z., Aaronson S. A. BRAF oncogenic mutations correlate with progression rather than initiation of human melanoma. Cancer Res. 2003;63:3883–3885. [PubMed] [Google Scholar]
- Edgar B. A., Orr-Weaver T. L. Endoreplication cell cycles: more for less. Cell. 2001;105:297–306. doi: 10.1016/s0092-8674(01)00334-8. [DOI] [PubMed] [Google Scholar]
- Farina A., Manni I., Fontemaggi G., Tiainen M., Cenciarelli C., Bellorini M., Mantovani R., Sacchi A., Piaggio G. Down-regulation of cyclin B1 gene transcription in terminally differentiated skeletal muscle cells is associated with loss of functional CCAAT-binding NF-Y complex. Oncogene. 1999;18:2818–2827. doi: 10.1038/sj.onc.1202472. [DOI] [PubMed] [Google Scholar]
- Geng Y., Yu Q., Sicinska E., Das M., Schneider J. E., Bhattacharya S., Rideout W. M., Bronson R. T., Gardner H., Sicinski P. Cyclin E ablation in the mouse. Cell. 2003;114:431–443. doi: 10.1016/s0092-8674(03)00645-7. [DOI] [PubMed] [Google Scholar]
- Gorgoulis V. G., et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434:907–913. doi: 10.1038/nature03485. [DOI] [PubMed] [Google Scholar]
- Gstaiger M., Jordan R., Lim M., Catzavelos C., Mestan J., Slingerland J., Krek W. Skp2 is oncogenic and overexpressed in human cancers. Proc. Natl. Acad. Sci. USA. 2001;98:5043–5048. doi: 10.1073/pnas.081474898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardavaccaro D., Pagano M. Oncogenic aberrations of cullin-dependent ubiquitin ligases. Oncogene. 2004;23:2037–2049. doi: 10.1038/sj.onc.1207413. [DOI] [PubMed] [Google Scholar]
- Ikediobi O. N., et al. Mutation analysis of 24 known cancer genes in the NCI-60 cell line set. Mol. Cancer Ther. 2006;5:2606–2612. doi: 10.1158/1535-7163.MCT-06-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbriano C., Gurtner A., Cocchiarella F., Di Agostino S., Basile V., Gostissa M., Dobbelstein M., Del Sal G., Piaggio G., Mantovani R. Direct p53 transcriptional repression: in vivo analysis of CCAAT-containing G2/M promoters. Mol. Cell. Biol. 2005;25:3737–3751. doi: 10.1128/MCB.25.9.3737-3751.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamura T., Hara T., Kotoshiba S., Yada M., Ishida N., Imaki H., Hatakeyama S., Nakayama K., Nakayama K. I. Degradation of p57Kip2 mediated by SCFSkp2-dependent ubiquitylation. Proc. Natl. Acad. Sci. USA. 2003;100:10231–10236. doi: 10.1073/pnas.1831009100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri Y., Hozumi Y., Kondo S. Knockdown of Skp2 by siRNA inhibits melanoma cell growth in vitro and in vivo. J. Dermatol. Sci. 2006;42:215–224. doi: 10.1016/j.jdermsci.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Kim S. Y., Herbst A., Tworkowski K. A., Salghetti S. E., Tansey W. P. Skp2 regulates Myc protein stability and activity. Mol. Cell. 2003;11:1177–1188. doi: 10.1016/s1097-2765(03)00173-4. [DOI] [PubMed] [Google Scholar]
- Kitagawa M., Lee S. H., McCormick F. Skp2 suppresses p53-dependent apoptosis by inhibiting p300. Mol. Cell. 2008;29:217–231. doi: 10.1016/j.molcel.2007.11.036. [DOI] [PubMed] [Google Scholar]
- Kossatz U., Dietrich N., Zender L., Buer J., Manns M. P., Malek N. P. Skp2-dependent degradation of p27kip1 is essential for cell cycle progression. Genes Dev. 2004;18:2602–2607. doi: 10.1101/gad.321004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krek W. Proteolysis and the G1-S transition: the SCF connection. Curr. Opin. Genet. Dev. 1998;8:36–42. doi: 10.1016/s0959-437x(98)80059-2. [DOI] [PubMed] [Google Scholar]
- Kruse J. P., Gu W. SnapShot: p53 posttranslational modifications. Cell. 2008;133:930. doi: 10.1016/j.cell.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latres E., Chiarle R., Schulman B. A., Pavletich N. P., Pellicer A., Inghirami G., Pagano M. Role of the F-box protein Skp2 in lymphomagenesis. Proc. Natl. Acad. Sci. USA. 2001;98:2515–2520. doi: 10.1073/pnas.041475098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Murphy M., Ross J., Sheehan C., Carlson J. A. Skp2 and p27kip1 expression in melanocytic nevi and melanoma: an inverse relationship. J. Cutan. Pathol. 2004;31:633–642. doi: 10.1111/j.0303-6987.2004.00243.x. [DOI] [PubMed] [Google Scholar]
- Manni I., Mazzaro G., Gurtner A., Mantovani R., Haugwitz U., Krause K., Engeland K., Sacchi A., Soddu S., Piaggio G. NF-Y mediates the transcriptional inhibition of the cyclin B1, cyclin B2, and cdc25C promoters upon induced G2 arrest. J. Biol. Chem. 2001;276:5570–5576. doi: 10.1074/jbc.M006052200. [DOI] [PubMed] [Google Scholar]
- Marumoto T., Zhang D., Saya H. Aurora-A–a guardian of poles. Nat. Rev. Cancer. 2005;5:42–50. doi: 10.1038/nrc1526. [DOI] [PubMed] [Google Scholar]
- Mendez J., Zou-Yang X. H., Kim S. Y., Hidaka M., Tansey W. P., Stillman B. Human origin recognition complex large subunit is degraded by ubiquitin-mediated proteolysis after initiation of DNA replication. Mol. Cell. 2002;9:481–491. doi: 10.1016/s1097-2765(02)00467-7. [DOI] [PubMed] [Google Scholar]
- Michaloglou C., Vredeveld L. C., Soengas M. S., Denoyelle C., Kuilman T., van der Horst C. M., Majoor D. M., Shay J. W., Mooi W. J., Peeper D. S. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- Nakayama K., et al. Targeted disruption of Skp2 results in accumulation of cyclin E and p27(Kip1), polyploidy and centrosome overduplication. EMBO J. 2000;19:2069–2081. doi: 10.1093/emboj/19.9.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K., Nagahama H., Minamishima Y. A., Miyake S., Ishida N., Hatakeyama S., Kitagawa M., Iemura S.-I., Natsume T., Nakayama K. I. Skp2-mediated degradation of p27 regulates progression into mitosis. Dev. Cell. 2004;6:661–672. doi: 10.1016/s1534-5807(04)00131-5. [DOI] [PubMed] [Google Scholar]
- Nakayama K. I., Nakayama K. Ubiquitin ligases: cell-cycle control and cancer. Nat. Rev. Cancer. 2006;6:369–381. doi: 10.1038/nrc1881. [DOI] [PubMed] [Google Scholar]
- Nalepa G., Rolfe M., Harper J. W. Drug discovery in the ubiquitin-proteasome system. Nat. Rev. Drug Discov. 2006;5:596–613. doi: 10.1038/nrd2056. [DOI] [PubMed] [Google Scholar]
- Patton E. E., Willems A. R., Tyers M. Combinatorial control in ubiquitin-dependent proteolysis: don't Skp the F-box hypothesis. Trends Genet. 1998;14:236–243. doi: 10.1016/s0168-9525(98)01473-5. [DOI] [PubMed] [Google Scholar]
- Pfaffl M. W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock P. M., et al. High frequency of BRAF mutations in nevi. Nat. Genet. 2003;33:19–20. doi: 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- Rother K., Li Y. Y., Tschop K., Kirschner R., Muller G. A., Mossner J., Engeland K. Expression of cyclin-dependent kinase subunit 1 (Cks1) is regulated during the cell cycle by a CDE/CHR tandem element and is downregulated by p53 but not by p63 or p73. Cell Cycle. 2007;6:853–862. doi: 10.4161/cc.6.7.4017. [DOI] [PubMed] [Google Scholar]
- Sciortino S., Gurtner A., Manni I., Fontemaggi G., Dey A., Sacchi A., Ozato K., Piaggio G. The cyclin B1 gene is actively transcribed during mitosis in HeLa cells. EMBO Rep. 2001;2:1018–1023. doi: 10.1093/embo-reports/kve223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim E. H., Johnson L., Noh H. L., Kim Y. J., Sun H., Zeiss C., Zhang H. Expression of the F-box protein SKP2 induces hyperplasia, dysplasia, and low-grade carcinoma in the mouse prostate. Cancer Res. 2003;63:1583–1588. [PubMed] [Google Scholar]
- Smalley K. S., et al. An Organometallic Protein Kinase Inhibitor Pharmacologically activates p53 and induces apoptosis in human melanoma cells. Cancer Res. 2007;67:1–9. doi: 10.1158/0008-5472.CAN-06-1538. [DOI] [PubMed] [Google Scholar]
- Sumimoto H., Hirata K., Yamagata S., Miyoshi H., Miyagishi M., Taira K., Kawakami Y. Effective inhibition of cell growth and invasion of melanoma by combined suppression of BRAF (V599E) and Skp2 with lentiviral RNAi. Int. J. Cancer. 2006;118:472–476. doi: 10.1002/ijc.21286. [DOI] [PubMed] [Google Scholar]
- Tatsumi Y., Ohta S., Kimura H., Tsurimoto T., Obuse C. The ORC1 cycle in human cells: I. cell cycle-regulated oscillation of human ORC1. J. Biol. Chem. 2003;278:41528–41534. doi: 10.1074/jbc.M307534200. [DOI] [PubMed] [Google Scholar]
- Taylor W. R., DePrimo S. E., Agarwal A., Agarwal M. L., Schonthal A. H., Katula K. S., Stark G. R. Mechanisms of G2 arrest in response to overexpression of p53. Mol. Biol. Cell. 1999;10:3607–3622. doi: 10.1091/mbc.10.11.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerbeul I., et al. Testing the importance of p27 degradation by the SCFskp2 pathway in murine models of lung and colon cancer. Proc. Natl. Acad. Sci. USA. 2006;103:14009–14014. doi: 10.1073/pnas.0606316103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima F., Moriguchi T., Wada A., Fukuda M., Nishida E. Nuclear export of cyclin B1 and its possible role in the DNA damage-induced G2 checkpoint. EMBO J. 1998;17:2728–2735. doi: 10.1093/emboj/17.10.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetkov L. M., Yeh K. H., Lee S. J., Sun H., Zhang H. p27(Kip1) ubiquitination and degradation is regulated by the SCF(Skp2) complex through phosphorylated Thr187 in p27. Curr. Biol. 1999;9:661–664. doi: 10.1016/s0960-9822(99)80290-5. [DOI] [PubMed] [Google Scholar]
- Volkenandt M., Schlegel U., Nanus D. M., Albino A. P. Mutational analysis of the human p53 gene in malignant melanoma. Pigment Cell Res. 1991;4:35–40. doi: 10.1111/j.1600-0749.1991.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Weiss J., Schwechheimer K., Cavenee W. K., Herlyn M., Arden K. C. Mutation and expression of the p53 gene in malignant melanoma cell lines. Int. J. Cancer. 1993;54:693–699. doi: 10.1002/ijc.2910540427. [DOI] [PubMed] [Google Scholar]
- Woenckhaus C., Maile S., Uffmann S., Bansemir M., Dittberner T., Poetsch M., Giebel J. Expression of Skp2 and p27KIP1 in naevi and malignant melanoma of the skin and its relation to clinical outcome. Histol. Histopathol. 2005;20:501–508. doi: 10.14670/HH-20.501. [DOI] [PubMed] [Google Scholar]
- Yokoi S., Yasui K., Iizasa T., Takahashi T., Fujisawa T., Inazawa J. Down-regulation of SKP2 induces apoptosis in lung-cancer cells. Cancer Sci. 2003;94:344–349. doi: 10.1111/j.1349-7006.2003.tb01444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.