Abstract
Chk1 is a protein kinase that is the effector molecule in the G2 DNA damage checkpoint. Chk1 homologues have an N-terminal kinase domain, and a C-terminal domain of ∼200 amino acids that contains activating phosphorylation sites for the ATM/R kinases, though the mechanism of activation remains unknown. Structural studies of the human Chk1 kinase domain show an open conformation; the activity of the kinase domain alone is substantially higher in vitro than full-length Chk1, and coimmunoprecipitation studies suggest the C-terminal domain may contain an autoinhibitory activity. However, we show that truncation of the C-terminal domain inactivates Chk1 in vivo. We identify additional mutations within the C-terminal domain that activate ectopically expressed Chk1 without the need for activating phosphorylation. When expressed from the endogenous locus, activated alleles show a temperature-sensitive loss of function, suggesting these mutations confer a semiactive state to the protein. Intragenic suppressors of these activated alleles cluster to regions in the catalytic domain on the face of the protein that interacts with substrate, suggesting these are the regions that interact with the C-terminal domain. Thus, rather than being an autoinhibitory domain, the C-terminus of Chk1 also contains domains critical for adopting an active configuration.
INTRODUCTION
DNA damage checkpoints halt cell cycle progression to allow time for the repair machinery to correct the lesion. The ultimate targets of these checkpoints are the cyclin-dependent kinases that promote the transition from G1 into S-phase, and from G2 in to mitosis (O'Connell et al., 2000; O'Connell and Cimprich, 2005). Two major effectors of the DNA damage checkpoints are the tumor suppressor and transcription factor p53, and the protein serine/threonine kinase Chk1. The importance of checkpoints in genome integrity is highlighted by the frequent inactivation of p53 signaling in tumors, leading to G1/S checkpoint defects and a blockade to apoptosis (Giono and Manfredi, 2006). Conversely, Chk1 and its upstream regulators, which control the G2 DNA damage checkpoint, are rarely (if ever) mutated in tumors, suggesting this pathway may be essential for cancer cell viability. Indeed, inhibition or ablation of Chk1 greatly sensitizes p53 mutant tumor cells to genotoxic therapies (Suganuma et al., 1999; Jackson et al., 2000; Koniaras et al., 2001; Macip et al., 2006). Although Chk1 is essential for embryonic development in the mouse (Liu et al., 2000), this may not be the case for most cells in adult tissues. Therefore, there is intense interest in developing specific Chk1 inhibitors for cancer chemotherapy (Garber, 2005).
The G2 DNA damage checkpoint is highly conserved in evolution, and model systems, such as the fission yeast Schizosaccharomyces pombe, have been invaluable in dissecting this signaling pathway. On detection of DNA damage, several protein complexes cooperate to ensure Chk1 activation, leading to a 5–10-fold increase over its basal activity (Capasso et al., 2002; den Elzen and O'Connell, 2004; Harvey et al., 2004; Latif et al., 2004). This in turn ensures that Cdc2 is kept in an inactive state through regulation of the Wee1 kinases and Cdc25 phosphatases that regulate Cdc2 inhibitory tyrosine-15 (Y15) phosphorylation (Furnari et al., 1997, 1999; O'Connell et al., 1997; Raleigh and O'Connell, 2000; Lopez-Girona et al., 2001a). The magnitude of Chk1 activation is not dependent on the level of DNA damage, but rather the duration of Chk1 activity (Latif et al., 2004). That is, checkpoint arrest is achieved when Chk1 activity is above a threshold necessary to maintain Cdc2 Y15 phosphorylation. Moreover, inactivation of Chk1 during a checkpoint arrest is sufficient to cause immediate entry into lethal mitoses (Latif et al., 2004).
All Chk1 homologues have an N-terminal kinase domain, and a C-terminal domain of ∼200 amino acids that has only limited homology across species. Chk1 activation requires the phosphorylation of residues (S317 and S345 in humans, and S345 in S. pombe) in the C-terminal domain by the PI3-K–related protein kinases ATR (Rad3 in S. pombe) and ATM (Tel1 in S. pombe), hereafter referred to as ATM/R (Liu et al., 2000; Lopez-Girona et al., 2001b; Zhao and Piwnica-Worms, 2001; Capasso et al., 2002; Gatei et al., 2003). Recovery from the checkpoint arrest requires Chk1 dephosphorylation, which in S. pombe is catalyzed by the type 1 phosphatase Dis2 (den Elzen et al., 2004; den Elzen and O'Connell, 2004). This and additional phosphatases have been implicated in the inactivation of human Chk1, as have additional mechanisms including ubiquitin-dependent proteolysis (Calonge and O'Connell, 2008).
Molecular details of how the phosphorylation of the C-terminal domain activates the N-terminal kinase domain are poorly understood, though are clearly important to delineate. Structural studies of human Chk1 showed the kinase domain to be in an open conformation (Chen et al., 2000); that is, this domain does not require activating phosphorylation that is commonplace in many protein kinases. However, as part of this study, removal of the C-terminal domain led to a potent activation of the protein kinase activity of isolated N-terminal kinase domain, suggesting the C-terminal domain might be autoinhibitory (Chen et al., 2000). Indeed, studies in Xenopus cell-free systems uncovered an activating mutation (T377A) within one of the conserved motifs of the C-terminal domain (Wang and Dunphy, 2000), referred to in this article as “region 1,” suggesting this region to be involved in negative regulation of Chk1. Further, additional studies in Xenopus oocyte extracts have shown that ectopically expressed N- and C-terminal domains can physically interact. In this context, the C-terminal domain binding attenuates the activity of the kinase domain, and phospho-mimetic acidic substitutions at the ATM/R phosphorylation sites blocks binding and inhibition (Katsuragi and Sagata, 2004).
Based on these findings, the model has emerged that the C-terminal domain blocks the activity of the kinase domain, and when phosphorylated by ATM/R, this inhibition is relieved. However, the same acidic substitutions at the ATM/R phosphorylation sites in the context of full-length Chk1 are inactivating in both S. pombe (Capasso et al., 2002) and in Xenopus (Katsuragi and Sagata, 2004). Moreover, several other mutations in the C-terminal domain of S. pombe Chk1 are inactivating (Latif et al., 2004), including residues in the other conserved region of the C-terminal domain, referred to here as “region 2,” including a putative pseudosubstrate motif (Palermo et al., 2008). Thus, regulation of Chk1 by the C-terminal domain must include mechanisms other than a simple reversible interaction with the kinase domain. Other functions for the C-terminal domain, such as nuclear localization (Dunaway et al., 2005), activating interaction with mediator proteins, or substrate recognition may be essential for the function of endogenous Chk1, though not as important for the assays in the Xenopus oocyte extracts.
We have carried out an analysis of the C-terminal domain of S. pombe Chk1. By deletion analysis we find that, in contrast to the in vitro kinase assays, the C-terminal domain is essential for Chk1 function in vivo. Using regulated ectopic expression and mutagenesis of the two regions of the C-terminal domain that are most highly conserved between S. pombe and human Chk1, we identified both inactivating (nonfunctional) and activating (“Superactive”) mutations. Intragenic suppressors of one activating mutation (E472D), which recover wild-type function, identified mutations in the kinase domain that cluster into residues on the substrate-binding face. This suggests that these are the regions that interact with the C-terminal domain and that this interaction may occlude the active site. This screen also identified a three-amino acid deletion in the C-terminal domain (Δ359-61) that is itself an activating mutation in an otherwise wild-type Chk1. However, when expressed at endogenous levels, these Superactive Chk1 molecules have a temperature-sensitive loss of function. We propose these mutations render Chk1 in a partially active state that passes a threshold of activity for cell cycle arrest in the context of ectopic expression, but is intrinsically unstable and below the threshold of activity for G2 arrest. Thus, the C-terminal domain of Chk1 exerts inhibitory effects and yet is also essential for Chk1 to adopt a fully functional conformation in vivo.
MATERIALS AND METHODS
General S. pombe Methods
All strains are derivatives of 972h− and 975h+. Standard methods were used for strain construction, the propagation of cultures, and introduction of plasmids by transformation (Moreno et al., 1991). Survival assays on plates containing methyl methanesulfonate (MMS) used 10-fold serial dilutions of cultures starting at 1 × 106 cells/ml, and 5 μl of each dilution plated for 4 d (30 and 36°C) or 5 d (25°C). UV-C survival assays were used to confirm MMS survival data. Microscopy was performed on a Nikon E800 Microscope (Melville, NY) and images captured on a Spot XE camera (Diagnostic Instruments, Sterling Heights, MI). Cell length measurements were made using an eye-piece micrometer, using septated cells, or if cell cycle arrest, no length was recorded. For derepression of the nmt1 promoter (Basi et al., 1993; Maundrell, 1993), exponential cultures growing in minimal media supplemented with 10 μg/ml thiamine were washed three times in thiamine-free medium and then grown for the indicated times.
Mutagenesis of the C-Terminal Domain
For truncation mutagenesis, fragments of the Chk1 open reading frame (ORF) were amplified by PCR, and cloned into pREP41X (XhoI-BamHI). Fidelity of PCR was confirmed by sequencing. For site-specific mutagenesis, a Chk1 cDNA in pLitmus28 was mutagenized by the method of Kunkel et al. (1987) and confirmed by sequencing. Mutated cDNAs were then cloned into pREP41X (Basi et al., 1993).
Suppressors of Superactive Chk1
A pREP41X-Chk1E472D plasmid was transformed into chk1::ura4 cells, and transformants were selected on media lacking thiamine. Under these conditions, only plasmids containing suppressor mutations are viable, though the vast majority truncate or delete the chk1 ORF. Transformants were then replica-plated onto medium containing thiamine and 0.01% MMS. Colonies surviving on MMS plates contain a plasmid-borne functional Chk1. DNA was extracted from these colonies, and the plasmids were recovered into Escherichia coli. These plasmids were retested and sequenced. The resulting suppressors (Table 1) came from 11 independent transformations and plasmid preparations and as are thus likely to represent independent reisolation in the case of multiple isolates.
Table 1.
Characteristics and frequency of allele isolation for intragenic suppressors of nmt1-driven chk1-E472D
| Region | Allele | No. of isolates | Size (μm) |
|---|---|---|---|
| Controls | Vector | N/A | 14.2 ± 1.0 |
| WT | N/A | 30.1 ± 4.9 | |
| E472D | N/A | Arrested | |
| A | Δ 41–44 | 1 | 18.6 ± 2.6 |
| Δ 54–59 | 1 | 22.4 ± 2.9 | |
| Δ 57 | 1 | 18.4 ± 1.9 | |
| Δ 56–57 | 2 | 24.7 ± 1.9 | |
| B | Δ 190 | 1 | 17.7 ± 0.9 |
| C | Δ 217 | 1 | 28.8 ± 4.3 |
| Δ 218–219 | 2 | 16.6 ± 1.4 | |
| D | Δ 359–361 | 1 | 16.3 ± 1.0 |
| E | Frame shift 379–382 AKKA-VLFI* | 4 | 21.5 ± 3.2 |
Length at division is for cells after 16 h of growth in the absence of thiamine at 30°C. Values ares mean ± SD, n = 50.
Construction of Alleles at the Chk1 Locus
Mutations derived from the intragenic suppressor screen were introduced into a genomic clone of chk1 containing a C-terminal triple hemagglutinin (HA) tag and integrated back into the chk1 locus in a chk1::ura4 leu1-32 strain by cotransformation with pREP3X (LEU2). Leu+ transformants were selected and replica-plated to obtain Ura− (replacement of chk1::ura4) colonies now containing an intact (mutated) chk1 ORF. Integration events were confirmed by Southern blotting.
Chk1 Biochemistry
Chk1 expression was assayed by Western blotting, with either polyclonal anti-Chk1 antibodies (O'Connell et al., 1997), anti-glutathione S-transferase (GST; Santa Cruz Biotechnology, Santa Cruz, CA)) for GST-tagged Chk1, or 12CA5 (Roche, Indianapolis, IN) for detection of HA-tagged Chk1. For activity assays, GST-tagged Chk1 was collected on glutathione Sepharose, and activity was assayed on peptide substrate as described (Harvey et al., 2004; Kosoy et al., 2007).
RESULTS
The C-Terminus of Chk1 Is Essential for Function In Vivo
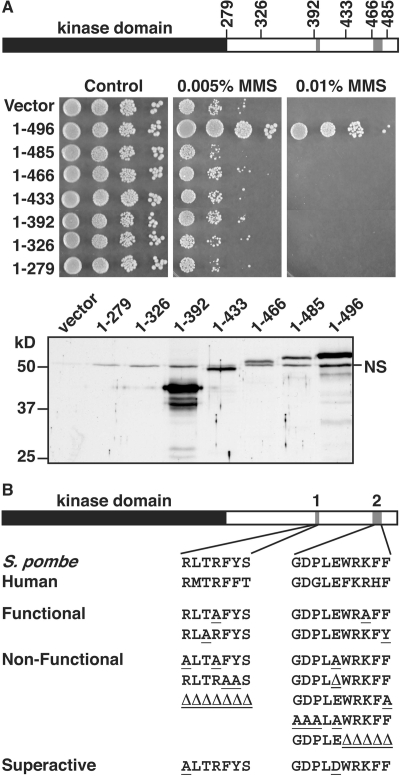
The kinase activity of human Chk1 lacking the C-terminal domain is significantly elevated over the full-length protein (Chen et al., 2000). This suggested the C-terminal domain has an autoinhibitory activity, and so we aimed to test this in S. pombe where mutant proteins can be expressed in cells lacking endogenous Chk1. Overexpression of Chk1 from the wild-type nmt1 promoter causes a lethal G2 cell cycle arrest (Walworth et al., 1993) that does not require activating phosphorylation (Lopez-Girona et al., 2001b). However, expression from an attenuated nmt1 promoter in pREP41X (Basi et al., 1993), which is ∼60-fold lower than that from wild-type nmt1 (Forsburg, 1993) is well tolerated and rescues a chk1Δ even when repressed (a further 25-fold reduced expression; Figure 1A). Using this system, we assayed the ability of a number of C-terminal truncations spanning the entire domain down to the last 11 amino acids to rescue the MMS sensitivity of chk1Δ. All truncations failed to rescue chk1Δ (Figure 1A). Therefore, these alleles encode nonfunctional proteins even though the truncation to 392 maintains sequences required for nuclear localization and 14-3-3 binding (Dunaway et al., 2005). The truncations to 392, 433, 466, and 485 were expressed to equivalent levels to the full-length protein, but our polyclonal antibody did not detect truncations to 279 or 326. Either these are unstable proteins or lack the epitopes recognized by this antibody. Nevertheless, had the truncations deleted an inhibitory motif, these alleles should minimally rescue and in the case of Chk1 activation, cause a cell cycle delay. Thus, the C-terminus of Chk1 is essential for function in vivo.
Figure 1.
The C-terminal domain of Chk1 is required for function in vivo. (A) Full-length Chk1 (1–496) and the indicated C-terminal truncations were expressed from an attenuated nmt1 promoter (pREP41) under repressing conditions (+ thiamine) in cells containing a null allele of chk1. Resistance to MMS was assayed by colony formation over 4 d at 30°C. The Western blot with polyclonal anti-Chk1 antibodies shows expression levels 24 h after promoter derepression. NS, nonspecific band. (B) Regions 1 and 2 of the C-terminal domain show high conservation across species. Alignment of S. pombe and human sequences is shown. Amino acid substitutions or deletions (Δ) are underlined, which were expressed from an attenuated nmt1 promoter. Functionality of these mutants was determined by the same assay shown in A and by cell length measurements when expressed under derepressing conditions (− thiamine) for 16 h at 30°C. Superactive mutants (R394A and E472D) fail to form colonies on media lacking thiamine.
Two Conserved Regions of the C-Terminal Domain Modulate Chk1 Function
In addition to the ATM/R phosphorylation sites, there are two regions within the C-terminal domain that are highly conserved from S. pombe to humans. Using pREP41X-driven Chk1, we mutated or deleted residues within these domains. Not surprisingly, deletion of both regions abolished Chk1 function as assayed by complementation of the MMS sensitivity of chk1Δ (Figure 1B). Several point mutations did not affect Chk1 function, including T396A, which is analogous to the activating T377A mutation in Xenopus Chk1 (Wang and Dunphy, 2000). Importantly, two mutations were activating, in that they caused a potent cell cycle arrest when expressed from this attenuated nmt1 promoter in the absence of thiamine (Figures 1B, 2, and 4), whereas wild-type Chk1 does not, and we have labeled these alleles as “Superactive.” These alleles show that these conserved regions contain residues that restrain Chk1 activity, which may be in keeping with the autoinhibitory model.
Figure 2.
chk1-E472D signals a potent G2 cell cycle arrest. (A) chk1Δ cells transformed with vector only (pREP41), pREP41 driven chk1 or chk1-E472D were grown in the presence and absence of thiamine for 4 d at 30°C. Cells expressing chk1-E472D fail to form colonies. (B) The same strains shown in A were grown in the absence of thiamine for 16 h at 30°C, and DIC images were acquired of live cells. Cells expressing chk1 (2) as modestly elongated (G2 delay), whereas cell division has ceased in cells expressing chk1-E472D (3). Bar, 10 μm. (C) Expression of chk1 or chk1-E472D from pREP1 or pREP41 was induced for 16 h at 30°C, whereupon thiamine was added to the culture to repress the nmt1 promoter, and a sample was collected for protein extraction. Additional samples were taken after an additional 2 and 4 h. Chk1 levels were assayed by Western blotting using polyclonal anti-Chk1 antibodies. (D) GST, GST-Chk1, GST-Chk1E472D, and the kinase dead GST-Chk1D155E were expressed from the nmt1 promoter and affinity-captured on glutathione Sepharose from a total of 2 mg of whole cell extract. Captured GST-Chk1 fusion proteins were detected by Western blotting using anti-GST antibodies from a one-tenth aliquot of the Sepharose, split into two samples (1 and 3) represented 25 and 75% of the aliquot. Kinase assays were performed in triplicate each using 25% of the captured proteins (corresponding to 500 μg of extract). Data shown are mean ± SD.
Figure 4.
Additional activating mutations in the C-terminal domain of Chk1. (A) chk1Δ cells transformed with vector only (pREP41), pREP41 driven chk1, chk1-R394A, or chk1-Δ359-361 were grown in the presence and absence of thiamine for 4 d at 30°C. Cells expressing chk1-R394A or chk1-Δ359-361 fail to form colonies. (B) The same strains shown in A were grown in the absence of thiamine for 16 h at 30°C, and DIC images were acquired of live cells. Cell division has ceased in cells expressing chk1-R394A or chk1-Δ359-361. Bar, 10 μm. (C) Expression of the three activated alleles of chk1 partially rescue the MMS sensitivity of chk1Δ. chk1Δ cells expressing the indicated alleles from pREP41 in the presence of thiamine were assayed for colony formation over 4 d at 30°C. (D) Cells were grown in the presence or absence of thiamine for 4 d at 30°C. The D155E kinase-dead mutation suppresses the lethality caused by superactive chk1 alleles.
Characterization of chk1-E472D
One of these activating mutations, R394A, is in region 1. The other, E472D in region 2, is iso-allelic to a temperature-sensitive allele of chk1 (chk1-ts1) in the context of expression from the endogenous chk1 locus (Latif et al., 2004). chk1-ts1 is functional at 25°C, but not at 36°C, where it fails to be activated by ATM/R-mediated phosphorylation. We therefore characterized this allele in more detail as it confers properties expected from an important region in controlling Chk1 regulation.
Expression of chk1-E472D from the attenuated nmt1 promoter blocks colony formation (Figure 2A). This is associated with dramatic cell elongation due to a G2 cell cycle arrest (Figure 2B), reminiscent of expression of wild-type Chk1 from the wild-type nmt1 promoter. Despite this gain of function, Chk1-E472D is a less stable protein than wild-type Chk1, as assayed by both steady-state protein levels, and the reduction in protein levels after nmt1 promoter repression by the addition of thiamine (Figure 2C). This effect on Chk1 stability is also observable with endogenous expression (Latif et al., 2004).
We next assayed the basal kinase activity of Chk1 and Chk1-E472D. As with endogenously expressed Chk1-E472D (encoded by chk1-ts1), the kinase activity of this activated mutant was substantially lower than wild type, even allowing for its lower level of expression (Figure 2D). Thus, the activating affect of the E472D mutation is not associated with increased activity assayed in vitro, suggesting an alternative activating mechanism within the cell. Despite this, the activating effect of this mutation and R394A does require Chk1 kinase activity, as combining these mutations with the catalytically inactivating D155E mutation results in a nonfunctional allele (see Figure 4D).
Isolation of Intragenic Suppressors of chk1-E472D
To further characterize the Superactive phenotype of expression of chk1-E472D from attenuated nmt1, we took advantage of the observation that spontaneous suppressors of its lethality are selected for on plates lacking thiamine. If Chk1 was activated by the E472D mutation through interfering with inhibitory intramolecular interactions, then we hypothesized that such suppressors may cluster to regions that mediate this interaction. Therefore, we used this spontaneous suppression to screen for second site mutations in Chk1 that reverse its activation. Most mutations that suppress the lethality are simple loss-of-function mutations that remove chk1 and/or its kinase activity. Hence, we applied a secondary screen: not only must the suppressors grow in the absence of thiamine, we also required that they retain the ability to rescue the MMS sensitivity of chk1Δ.
A total of 14 suppressors were isolated that fulfilled both criteria (Figure 3, Table 1). Interestingly, all suppressors were deletions of amino acids. Nine of the suppressors were present in three regions of the kinase domain. We compared the position of these mutations in S. pombe Chk1 to that of the analogous regions of the crystal structure of the Human Chk1 kinase domain (Chen et al., 2000). Each of these clusters mapped to loops either side of the catalytic cleft on the substrate interaction face of the kinase domain. However, because these double mutants complement chk1Δ, they do not abolish Chk1 kinase activity.
Figure 3.
Intragenic suppressors of nmt1-driven chk1-E472D. (A) Spontaneous suppressors of the lethality caused by derepression of chk1-E472D were selected over a chk1Δ background, and alleles that now grew in the absence of thiamine but still rescued the MMS hypersensitivity of chk1Δ were sequenced and fall into five regions (A–E) shown in the schematic of Chk1. (B) Representative isolates for each region are shown for complementation of the MMS sensitivity of chk1Δ and the ability to form colonies on plates lacking thiamine.
The remaining five suppressors were in the C-terminal domain (Figure 3, Table 1). One was an internal in-frame deletion of residues 359-361. The other four suppressors, isolated in independent experiments, contained an identical frame-shifting mutation leading to premature termination at amino acid 382 of 496 and thus lack the activating E472D mutation. This was an unexpected result, because the truncation series we made by PCR (Figure 1A) were all nonfunctional, including a termination at amino acid 392. These data highlight the complexity of regulatory effects conferred by the C-terminal domain.
Superactive Alleles of chk1 Show Expression-Level–dependent Phenotypes
We next separated the suppressor mutations from the E472D mutation in the context of pREP41X and assayed functionality using the assays described above. All suppressor mutations were wild type, with the exception of the deletion of residues 359-361, which in additional to E472D and R394A, is an additional Superactive allele of chk1 (Figure 4). The other two double mutant combinations (E472D/R394A and Δ359-61/R394A) retain the superactive phenotype (not shown), and thus E472D and Δ359-61 show a specific mutual suppression.
When expressed in the absence of thiamine from attenuated nmt1, each of these alleles confers a G2 cell cycle arrest (Figures 2, 4). However, when expressed at a lower level in the presence of thiamine, all three Superactive alleles poorly complemented the MMS sensitivity of chk1Δ (Figure 4C). Thus, these alleles have expression-level–dependent phenotypes. This suggests that the Superactive phenotypes, each dependent on kinase activity (Figure 4D), may be due to crossing a threshold of activity required for cell cycle arrest that is dependent on overexpression. That is, these mutations confer a partially active conformation on Chk1, and when expressed at sufficient levels, the threshold required for cell cycle arrest is passed. By contrast, for wild-type Chk1 to pass this threshold, either a substantially higher level of expression is required or activation by ATM/R-dependent phosphorylation. Notably, double mutants between these activated alleles and S345A, a nonphosphorylatable form of Chk1 (Capasso et al., 2002), retained their gain-of-function phenotype (data not shown).
Analysis of chk1 Alleles Expressed from the Endogenous Locus
All of the phenotypes described above were generated from chk1 alleles expressed from the attenuated nmt1 promoter. We were mindful that one of the Superactive alleles, chk1-E472D, is iso-allelic to our previously characterized temperature-sensitive loss-of-function chk1-ts1 allele (Latif et al., 2004). Therefore, we integrated the mutations in the C-terminal domain at the chk1 locus and included double mutant combinations of the Superactive alleles. These alleles were then characterized for functionality by MMS sensitivity (Figure 5) and for their activation by phosphorylation (Figure 6) at 25, 30, and 36°C.
Figure 5.
MMS sensitivity of chk1 mutations expressed from the chk1 locus. The indicated mutations were introduced into the chk1 locus and assayed for sensitivity to 0.0075% MMS at 25°C (5 d) and at 30 and 36°C (4 d).
Figure 6.
Activation of Chk1 mutants by phosphorylation. The indicated strains carrying chk1 mutations at the endogenous locus were treated with 0.01% MMS for 7 h at the indicated temperatures. Activating phosphorylation of Chk1 causes a mobility shift. Note that the most functional alleles (E472D R394A and R394A) show robust Chk1 activation; whereas the temperature-sensitive alleles show poor or no phosphorylation, which is reduced at 36°C.
Using MMS sensitivity as an assay for Chk1 function, two of three Superactive mutations, E472D and Δ359-61, showed a temperature-sensitive loss of function (Figure 5). These mutant proteins were poorly phosphorylated compared with wild-type Chk1, especially at 36°C (Figure 6). The third activated allele, R394A, was active at all temperatures (Figure 5), suggesting this mutation conferred a conformation closer to the wild-type state. Chk1R394A is phosphorylated by ATM/R, albeit to lesser extent than wild-type Chk1 (Figure 6). When combined with E472D, R394A rescued its temperature sensitivity (Figure 5) and activating phosphorylation (Figure 6). Thus, this double mutant protein must adopt a more wild-type conformation, though is still superactive when expressed from the nmt1 promoter. Conversely, neither E472D nor R394A rescued the temperature sensitivity or phosphorylation of the Δ359-61 mutation. The phosphorylation of Chk1Δ359-61 is significantly lower than wild type at all temperatures, suggesting this mutation cannot be converted to a fully active state by phosphorylation.
These data suggest that the functionality of these mutants under endogenous expression levels is, at least in part, determined by their ability to be phosphorylated by ATM/R, or for this phosphorylation to be protected from dephosphorylation by Dis2. Finally, the frame shifting mutation truncating Chk1 after 382 of 496 amino acids, which also removes the HA tag used to detect Chk1 phosphorylation, was barely functional at 25°C and was nonfunctional at 30 and 36°C. This is in keeping with the requirement for the C-terminus in Chk1 function (Figure 1). Thus, this truncation is only functional when overexpressed, suggesting it is lacking essential regions required for Chk1 function, which include both the conserved Regions 1 and 2.
Temperature-dependent Gain of Function for Superactive Chk1 Mutants
The different phenotypes depending on expression level suggested that mutational activation of Chk1 is related to, but not identical to activation by phosphorylation. That is, the mutations may affect the conformation of the protein such that they are active only at lower temperatures and that the gain of function may be due to unrestrained activity, which is not necessarily supraphysiologic.
We therefore tested whether expression of these alleles from nmt1 confers a G2 cell cycle arrest at the elevated temperature of 36°C (Figure 7). Under these conditions, the E472D mutation retained its gain of function, and the cells failed to form colonies. Therefore, when overexpressed, E472D is no longer temperature-sensitive and presumably crosses a threshold of activity required for cell cycle arrest. In keeping with the lack of temperature sensitivity with endogenous expression, the R394A mutation still caused a significant cell cycle delay, and hence the cells formed small colonies. Conversely, the Δ359-61 mutation did not cause a cell cycle arrest at 36°C. Therefore, this mutation is temperature-sensitive regardless of expression level. This highlights the positive and negative regulatory effects of the C-terminal domain on Chk1 function.
Figure 7.
Temperature-dependent effects of activating mutations. chk1Δ cells transformed with vector only (pREP41), pREP41 driven chk1, chk1-E472D, chk1-R394A, or chk1-Δ359-361 were grown in the presence and absence of thiamine for 4 d at 30°C or 36°C. Note that chk1-Δ 359-61–expressing cells form colonies in the absence of thiamine only at 36°C.
DISCUSSION
Regulation of Chk1 activity is crucial in the DNA damage response. Failure to activate Chk1 after DNA damage leads to lethal mitoses (Walworth et al., 1993; Capasso et al., 2002), whereas a failure to inactivate Chk1 upon completion of repair leads to a lethal G2 cell cycle arrest (den Elzen and O'Connell, 2004). The fulcrum that modulates Chk1 activity is its C-terminal phosphorylation by ATM/R, though how this influences the activity of the N-terminal kinase domain remains obscure.
The notion that the C-terminal domain may be autoinhibitory was consistent with the high in vitro kinase activity of human Chk1 lacking this domain (Chen et al., 2000). This was further supported by studies with the overexpression of Chk1 domains in Xenopus oocyte extracts, where the C-terminalf domain coimmunoprecipitated with the kinase domain, and could interfere with a block to germinal vesicle breakdown created by Chk1 kinase domain overexpression (Katsuragi and Sagata, 2004). These experiments suggested that the C-terminal domain somehow blocked the activity of the kinase domain when expressed in trans. However, by their nature, these experiments could not indicate how or if these domains might interact in the context of a single molecule in cis.
This autoinhibition model was not consistent with the isolation of loss-of-function mutations in the C-terminal domain of S. pombe Chk1 (Capasso et al., 2002; Latif et al., 2004; Palermo et al., 2008). We have assayed for intermolecular interactions between N- and C-termini of S. pombe Chk1 by coimmunoprecipitation and yeast two-hybrid analysis and have found no evidence for this (Kosoy et al., 2007). However, we do not rule out that this interaction could occur in vivo, particularly in the context of intramolecular interaction within full-length molecules. It is possible that, in these yeast systems, Chk1 fragments are not expressed to a level that is high enough to allow their association. Alternatively, an association may be disrupted by extraction buffers, although Chk1 can be extracted in its inactive state from unirradiated cells (den Elzen and O'Connell, 2004). We also cannot rule out that this is a difference between S. pombe and Xenopus Chk1. In this regard, we note that T377A is an activating mutation in Xenopus Chk1 (Wang and Dunphy, 2000), and yet the analogous mutation (T396A) has no effect on S. pombe Chk1 (Figure 1).
There is also the confounding finding that while acidic substitutions at the ATM/R phosphorylation sites block the intermolecular N- and C-terminal interactions in trans, as would be predicted if activation blocked this interaction, and yet these substitutions in the context of full-length Chk1 are inactivating (Capasso et al., 2002; Katsuragi and Sagata, 2004). Furthermore, the C-terminal domain has been shown to be responsible for the nuclear localization of Chk1 (Dunaway et al., 2005), and for interaction with 14-3-3 proteins (Chen et al., 1999; Jiang et al., 2003), suggesting it positively mediates Chk1 function.
Our data show that the C-terminal domain is critical for Chk1 function in vivo; although the kinase domain alone is highly active in vitro, it is functionally dead in vivo. Although some of these deletions cover the domains required for 14-3-3 binding (amino acids 291–303) and nuclear localization (amino acids 377–382; Dunaway et al., 2005), we found that truncation of Chk1 at amino acid 392 was nonfunctional. Interestingly, our screen did identify a partially functional truncation at amino acid 382. However, several of the point mutations in the conserved C-terminal regions, which are lost in the truncations at 382 and 392, were also nonfunctional. Thus, there are several regions in the C-terminal domain that are essential for Chk1 function.
The isolation of activating mutations in Chk1, at least when expressed from the attenuated nmt1 promoter, indicates that particular residues are required to restrain Chk1 activity in the absence of ATM/R-mediated activation. The fact that the activating effects are suppressed back to the wild-type state by clusters of small amino acid deletions on the same face of the kinase domain suggests these regions may either be the site of interaction between domains or indirectly affect such an interaction. However, the activating mutations cannot lead to full activation of Chk1, as achieved by phosphorylation, because persistent Chk1 phosphorylation leads to a lethal G2 arrest (den Elzen et al., 2004; den Elzen and O'Connell, 2004). Indeed, two of the three activating mutations (Δ359-61 and E472D) are temperature-sensitive loss of function when expressed at endogenous levels, and all including R394A are poorly phosphorylated by ATM/R, especially at 36°C (Figure 6). Therefore, we propose these mutations are in a semiactive state; when expressed at higher levels they behave as Chk1 activated by phosphorylation, whereas at endogenous levels of expression, they are poorly activated by DNA damage and cannot pass a threshold of activity at elevated temperatures that is required for G2 cell cycle arrest.
The data indicate that the Δ359-61, R394A, and E472D mutations disrupt the inactive conformation of Chk1, possibly through blocking interaction with the kinase domain. However, these mutations also attenuate Chk1 activation by ATM/R compared with wild type, though in the case of R394A, the level of activation is sufficient for MMS resistance. This highlights these regions of Chk1 as critical in its regulation by reversible phosphorylation. It is therefore clear that the model in which the C-terminal domain is simply autoinhibitory is incomplete and rather that these domains control the dynamic regulation of Chk1.
Since the discovery of Chk1 (Walworth et al., 1993), S. pombe has provided a paradigm of Chk1 function and regulation. With the interest in Chk1 as a target in anticancer therapy, knowledge of the molecular events in Chk1 activation will enable the development of small molecule inhibitors that do not need to be limited to ATP analogs. Although efficacious as protein kinase inhibitors, this class of molecules suffers from lack of specificity in some cases that may confound their usefulness. Because the regions of the C-terminal domain in S. pombe Chk1 that we have studied are highly conserved in human Chk1, they are likely to be important in the regulation of all Chk1 homologues, and structural studies will facilitate a complete understanding of the mechanism of Chk1 activation.
ACKNOWLEDGMENTS
We are grateful to J. Raleigh and J. Ross who were involved in early aspects of this work. This work was supported by National Institutes of Health, National Cancer Institute Grant CA100076 and Grant PDF0202498 from the Susan G. Komen Breast Cancer Research Foundation.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-04-0444) on August 20, 2008.
REFERENCES
- Basi G., Schmid E., Maundrell K. TATA box mutations in the Schizosaccharomyces pombe nmt1 promoter affect transcription efficiency but not the transcription start point or thiamine repressibility. Gene. 1993;123:131–136. doi: 10.1016/0378-1119(93)90552-e. [DOI] [PubMed] [Google Scholar]
- Calonge T. M., O'Connell M. J. Turning off the G2 DNA damage checkpoint. DNA Repair. 2008;7:136–140. doi: 10.1016/j.dnarep.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capasso H., Palermo C., Wan S., Rao H., John U. P., O'Connell M. J., Walworth N. C. Phosphorylation activates Chk1 and is required for checkpoint-mediated cell cycle arrest. J. Cell Sci. 2002;115:4555–4564. doi: 10.1242/jcs.00133. [DOI] [PubMed] [Google Scholar]
- Chen L., Liu T. H., Walworth N. C. Association of Chk1 with 14-3-3 proteins is stimulated by DNA damage. Genes Dev. 1999;13:675–685. doi: 10.1101/gad.13.6.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P., et al. The 1.7 A crystal structure of human cell cycle checkpoint kinase Chk1, implications for Chk1 regulation. Cell. 2000;100:681–692. doi: 10.1016/s0092-8674(00)80704-7. [DOI] [PubMed] [Google Scholar]
- den Elzen N., Kosoy A., Christopoulos H., O'Connell M. J. Resisting arrest: recovery from checkpoint arrest through dephosphorylation of Chk1 by PP1. Cell Cycle. 2004;3:529–533. [PubMed] [Google Scholar]
- den Elzen N. R., O'Connell M. J. Recovery from DNA damage checkpoint arrest by PP1-mediated inhibition of Chk1. EMBO J. 2004;23:908–918. doi: 10.1038/sj.emboj.7600105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunaway S., Liu H. Y., Walworth N. C. Interaction of 14-3-3 protein with Chk1 affects localization and checkpoint function. J. Cell Sci. 2005;118:39–50. doi: 10.1242/jcs.01570. [DOI] [PubMed] [Google Scholar]
- Forsburg S. L. Comparison of Schizosaccharomyces pombe expression systems. Nucleic Acids Res. 1993;21:2955–2956. doi: 10.1093/nar/21.12.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furnari B., Blasina A., Boddy M. N., McGowan C. H., Russell P. Cdc25 inhibited in vivo and in vitro by checkpoint kinases Cds1 and Chk1. Mol. Biol. Cell. 1999;10:833–845. doi: 10.1091/mbc.10.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furnari B., Rhind N., Russell P. Cdc25 mitotic inducer targeted by chk1 DNA damage checkpoint kinase. Science. 1997;277:1495–1497. doi: 10.1126/science.277.5331.1495. [DOI] [PubMed] [Google Scholar]
- Garber K. New checkpoint blockers begin human trials. J. Natl. Cancer Inst. 2005;97:1026–1028. doi: 10.1093/jnci/dji224. [DOI] [PubMed] [Google Scholar]
- Gatei M., et al. Ataxia-telangiectasia-mutated (ATM) and NBS1-dependent phosphorylation of Chk1 on Ser-317 in response to ionizing radiation. J. Biol. Chem. 2003;278:14806–14811. doi: 10.1074/jbc.M210862200. [DOI] [PubMed] [Google Scholar]
- Giono L. E., Manfredi J. J. The p53 tumor suppressor participates in multiple cell cycle checkpoints. J. Cell. Physiol. 2006;209:13–20. doi: 10.1002/jcp.20689. [DOI] [PubMed] [Google Scholar]
- Harvey S. H., Sheedy D. M., Cuddihy A. R., O'Connell M. J. Coordination of DNA damage responses via the Smc5/Smc6 complex. Mol. Cell. Biol. 2004;24:662–674. doi: 10.1128/MCB.24.2.662-674.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J. R., Gilmartin A., Imbrugia C., Winkler J. D., Marshall L. A., Roshak A. An indolocarbazole inhibitor of human chk1 abrogates cell cycle arrest caused by DNA damage. Cancer Res. 2000;60:566–572. [PubMed] [Google Scholar]
- Jiang K., Pereira E., Maxfield M., Russell B., Goudelock D. M., Sanchez Y. Regulation of Chk1 includes chromatin association and 14-3-3 binding following phosphorylation on Ser-345. J. Biol. Chem. 2003;278:25207–25217. doi: 10.1074/jbc.M300070200. [DOI] [PubMed] [Google Scholar]
- Katsuragi Y., Sagata N. Regulation of Chk1 kinase by autoinhibition and ATR-mediated phosphorylation. Mol. Biol. Cell. 2004;15:1680–1689. doi: 10.1091/mbc.E03-12-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koniaras K., Cuddihy A. R., Christopolous H., Hogg A., O'Connell M. J. Inhibition of Chk1-dependent G2 DNA damage checkpoint radiosensitises p53 mutant human cells. Oncogene. 2001;20:7453–7463. doi: 10.1038/sj.onc.1204942. [DOI] [PubMed] [Google Scholar]
- Kosoy A., Calonge T. M., Outwin E. A., O'Connell M. J. Fission yeast Rnf4 homologs are required for DNA repair. J. Biol. Chem. 2007;282:20388–20394. doi: 10.1074/jbc.M702652200. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Latif C., Elzen N. R., O'Connell M. J. DNA damage checkpoint maintenance through sustained Chk1 activity. J. Cell Sci. 2004;117:3489–3498. doi: 10.1242/jcs.01204. [DOI] [PubMed] [Google Scholar]
- Liu Q., et al. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 2000;14:1448–1459. [PMC free article] [PubMed] [Google Scholar]
- Lopez-Girona A., Kanoh J., Russell P. Nuclear exclusion of Cdc25 is not required for the DNA damage checkpoint in fission yeast. Curr. Biol. 2001a;11:50–54. doi: 10.1016/s0960-9822(00)00026-9. [DOI] [PubMed] [Google Scholar]
- Lopez-Girona A., Tanaka K., Chen X. B., Baber B. A., McGowan C. H., Russell P. Serine-345 is required for Rad3-dependent phosphorylation and function of checkpoint kinase Chk1 in fission yeast. Proc. Natl. Acad. Sci. USA. 2001b;98:11289–11294. doi: 10.1073/pnas.191557598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macip S., Kosoy A., Lee S. W., O'Connell M. J., Aaronson S. A. Oxidative stress induces a prolonged but reversible arrest in p53-null cancer cells, involving a Chk1-dependent G2 checkpoint. Oncogene. 2006;25:6037–6047. doi: 10.1038/sj.onc.1209629. [DOI] [PubMed] [Google Scholar]
- Maundrell K. Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene. 1993;123:127–130. doi: 10.1016/0378-1119(93)90551-d. [DOI] [PubMed] [Google Scholar]
- Moreno S., Klar A., Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Method. Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- O'Connell M. J., Cimprich K. A. G2 damage checkpoints: what is the turn-on? J. Cell Sci. 2005;118:1–6. doi: 10.1242/jcs.01626. [DOI] [PubMed] [Google Scholar]
- O'Connell M. J., Raleigh J. M., Verkade H. M., Nurse P. Chk1 is a wee1 kinase in the G2 DNA damage checkpoint inhibiting cdc2 by Y15 phosphorylation. EMBO J. 1997;16:545–554. doi: 10.1093/emboj/16.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell M. J., Walworth N. C., Carr A. M. The G2-phase DNA-damage checkpoint. Trends Cell Biol. 2000;10:296–303. doi: 10.1016/s0962-8924(00)01773-6. [DOI] [PubMed] [Google Scholar]
- Palermo C., Hope J. C., Freyer G. A., Rao H., Walworth N. C. Importance of a C-terminal conserved region of chk1 for checkpoint function. PLoS ONE. 2008;3:e1427. doi: 10.1371/journal.pone.0001427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh J. M., O'Connell M. J. The G(2) DNA damage checkpoint targets both Wee1 and Cdc25. J. Cell Sci. 2000;113:1727–1736. doi: 10.1242/jcs.113.10.1727. [DOI] [PubMed] [Google Scholar]
- Suganuma M., Kawabe T., Hori H., Funabiki T., Okamoto T. Sensitization of cancer cells to DNA damage-induced cell death by specific cell cycle G2 checkpoint abrogation. Cancer Res. 1999;59:5887–5891. [PubMed] [Google Scholar]
- Walworth N., Davey S., Beach D. Fission yeast chk1 protein kinase links the rad checkpoint pathway to cdc2. Nature. 1993;363:368–371. doi: 10.1038/363368a0. [DOI] [PubMed] [Google Scholar]
- Wang S. X., Dunphy W. G. Activation of Xenopus Chk1 by mutagenesis of threonine-377. FEBS Lett. 2000;487:277–281. doi: 10.1016/s0014-5793(00)02370-x. [DOI] [PubMed] [Google Scholar]
- Zhao H., Piwnica-Worms H. ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol. Cell. Biol. 2001;21:4129–4139. doi: 10.1128/MCB.21.13.4129-4139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]