Abstract
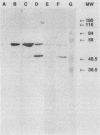
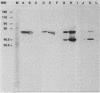
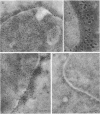
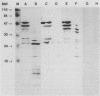
Monoclonal antibodies (MAbs) were generated against lysates of clinical Mycoplasma hominis isolates. Three of these, designated BG2, BA10, and FE6, recognized an integral membrane protein of M. hominis with an apparent molecular weight of 50,000 (p50). Electron microscopy studies demonstrated that this protein is distributed evenly over the cell surface. These anti-p50 MAbs were species specific for M. hominis; they reacted with 42% of 126 tested clinical M. hominis isolates and showed no reactivity to heterologous mycoplasma species. Immunoblot analysis after limited proteolysis of purified p50 demonstrated that the three MAbs reacted with different epitopes of the protein. Unlike BA10 and FE6, MAb BG2 induced a decrease in arginine metabolism and a reduction of CFU in metabolic inhibition tests. F(ab)2 fragments of MAb BG2 showed the same inhibitory effect as the intact MAb molecule, while Fab and Fc fragments had no influence on vital functions. Preincubation of the mycoplasmas with MAb BG2 followed by trypsin treatment yielded the same amount of CFU as the control without antibodies. In conclusion, the cell aggregates were resolved by the trypsin treatment. These experiments and tests with the antibody fragments led to the conclusion that only the intact MAb structure or the F(ab)2 structure had metabolic inhibition potential and that the observed metabolism inhibition as well as the apparent decrease in viability were a result of agglutination by MAb BG2.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander A. G., Lowes H. R., Kenny G. E. Identification of a mycoplasmal protein which binds immunoglobulins nonimmunologically. Infect Immun. 1991 Jun;59(6):2147–2151. doi: 10.1128/iai.59.6.2147-2151.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazek R., Schmitt K., Krafft U., Hadding U. Fast and simple procedure for the detection of cell culture mycoplasmas using a single monoclonal antibody. J Immunol Methods. 1990 Aug 7;131(2):203–212. doi: 10.1016/0022-1759(90)90191-w. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Kotani H., McGarrity G. J. Rapid and simple identification of mycoplasmas by immunobinding. J Immunol Methods. 1985 Dec 27;85(2):257–267. doi: 10.1016/0022-1759(85)90136-x. [DOI] [PubMed] [Google Scholar]
- Krause D. C., Baseman J. B. Inhibition of mycoplasma pneumoniae hemadsorption and adherence to respiratory epithelium by antibodies to a membrane protein. Infect Immun. 1983 Mar;39(3):1180–1186. doi: 10.1128/iai.39.3.1180-1186.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin J. S., Alpert S., Radnay K. M. Combined type-specific antisera in the identification of Mycoplasma hominis. J Infect Dis. 1975 Jun;131(6):727–730. doi: 10.1093/infdis/131.6.727. [DOI] [PubMed] [Google Scholar]
- Lin J. S., Kass E. H. Complement-dependent and complement-independent interactions between Mycoplasma hominis and antibodies in vitro. J Med Microbiol. 1975 Aug;8(3):397–404. doi: 10.1099/00222615-8-3-397. [DOI] [PubMed] [Google Scholar]
- Morrison-Plummer J., Leith D. K., Baseman J. B. Biological effects of anti-lipid and anti-protein monoclonal antibodies on Mycoplasma pneumoniae. Infect Immun. 1986 Aug;53(2):398–403. doi: 10.1128/iai.53.2.398-403.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårdh P. A. Mycoplasma hominis infection of the central nervous system in newborn infants. Sex Transm Dis. 1983 Oct-Dec;10(4 Suppl):331–334. [PubMed] [Google Scholar]
- Olson L. D., Shane S. W., Karpas A. A., Cunningham T. M., Probst P. S., Barile M. F. Monoclonal antibodies to surface antigens of a pathogenic Mycoplasma hominis strain. Infect Immun. 1991 May;59(5):1683–1689. doi: 10.1128/iai.59.5.1683-1689.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riethman H. C., Boyer M. J., Wise K. S. Triton X-114 phase fractionation of an integral membrane surface protein mediating monoclonal antibody killing of Mycoplasma hyorhinis. Infect Immun. 1987 May;55(5):1094–1100. doi: 10.1128/iai.55.5.1094-1100.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rurangirwa F. R., McGuire T. C., Musoke A. J., Kibor A. Differentiation of F38 mycoplasmas causing contagious caprine pleuropneumonia with a growth-inhibiting monoclonal antibody. Infect Immun. 1987 Dec;55(12):3219–3220. doi: 10.1128/iai.55.12.3219-3220.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt K., Däubener W., Bitter-Suermann D., Hadding U. A safe and efficient method for elimination of cell culture mycoplasmas using ciprofloxacin. J Immunol Methods. 1988 Apr 22;109(1):17–25. doi: 10.1016/0022-1759(88)90437-1. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn L. R., Ramsay J. R., Roberts L. K. Characterization of the metabolism inhibition antigen of Mycoplasma arthritidis. Infect Immun. 1985 Aug;49(2):357–364. doi: 10.1128/iai.49.2.357-364.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]