Abstract
Mitosis in Saccharomyces cerevisiae depends on IPL1 kinase, which genetically interacts with GLC8. The metazoan homologue of GLC8 is inhibitor-2 (I-2), but its function is not understood. We found endogenous and ectopic I-2 localized to the spindle, midzone, and midbody of mitotic human epithelial ARPE-19 cells. Knockdown of I-2 by RNA interference produced multinucleated cells, with supernumerary centrosomes, multipolar spindles and lagging chromosomes during anaphase. These defects did not involve changes in levels of protein phosphatase-1 (PP1), and the multinuclear phenotype was rescued by overexpression of I-2. Appearance of multiple nuclei and supernumerary centrosomes required progression through the cell cycle and I-2 knockdown cells failed cytokinesis, as observed by time-lapse microscopy. Inhibition of Aurora B by hesperadin produced multinucleated cells and reduced H3S10 phosphorylation. I-2 knockdown enhanced this latter effect. Partial knockdown of PP1Cα prevented multiple nuclei caused by either knockdown of I-2 or treatment with hesperadin. Expression of enhanced green fluorescent protein-I-2 or hemagglutinin-I-2 made cells resistant to hesperadin. We propose that I-2 acts to enhance Aurora B by inhibiting specific PP1 holoenzymes that dephosphorylate Aurora B substrates necessary for chromosome segregation and cytokinesis. Conserved together throughout eukaryotic evolution, I-2, PP1 and Aurora B function interdependently during mitosis.
INTRODUCTION
Protein phosphatase-1 (PP1) is a protein serine/threonine phosphatase extraordinarily conserved among eukaryotes as an essential gene. Its critical function occurs in mitosis because various eukaryotic cells undergo metaphase arrest due to PP1 mutations or inhibition (Booher and Beach, 1989; Doonan and Morris, 1989; Ohkura et al., 1989; Doonan et al., 1991; Fernandez et al., 1992). PP1 reacts with many phosphoprotein substrates and thereby has a variety of cellular functions (Bollen and Stalmans, 1992; Bollen, 2001; Cohen, 2002). Researchers now rationalize that PP1 exists in cells as a set of distinctive multisubunit holoenzymes (Aggen et al., 2000; Cohen, 2002), which are made up of one of the three major isoforms of PP1 catalytic subunit paired with a regulatory subunit containing a RVxF motif for recognition by PP1C. Based on screening for PP1 binding to recombinant proteins, there are nearly 200 regulatory subunits with RVxF motifs. These subunits control PP1 holoenzyme subcellular localization, catalytic activity, and impact substrate specificity, in part by physical association with substrates.
Several lines of evidence suggest that PP1 acts in opposition to Aurora B to control the phosphorylation of substrates required for proper mitosis (Hsu et al., 2000; Tang et al., 2004; Pinsky et al., 2006). Aurora B kinase functions in checkpoint signaling, chromosome alignment, release of syntelic and merotelic attachments, and completion of cytokinesis (Kallio et al., 2002; Ditchfield et al., 2003; Hauf et al., 2003; Guse et al., 2005; Knowlton et al., 2006). In budding yeast, the ipl1 (Aurora kinase) mutant suffered severe chromosome missegregation, which was suppressed by mutant alleles of the phosphatase GLC7 (Francisco et al., 1994). Phosphorylation of S10 on Histone H3, an indicator of Aurora B activity during mitosis, is governed by the balance of Ipl1/Aurora B kinase and Glc7/PP1 phosphatase in budding yeast and nematodes (Hsu et al., 2000; Pinsky et al., 2006). Aurora B activity is regulated by complex mechanisms, involving association with different proteins and autophosphorylation of T232 in its activation loop (Emanuele et al., 2008; Rosasco-Nitcher et al., 2008). This results in precise spatiotemporal activation of Aurora B and generation of intracellular gradients of phosphorylation that determine spatial orientation of events during mitosis (Fuller et al., 2008). In addition, during mitosis in yeast and mammalian cells PP1 becomes phosphorylated at Thr320 by CDK2 with reduction in phosphatase activity (Dohadwala et al., 1994; Yamano et al., 1994; Li et al., 2006). Further details on what holoenzyme forms of PP1 are critical for mitosis, and how PP1 holoenzymes are regulated during mitosis, remain unknown. The evidence indicates Aurora B and PP1 balance one another in controlling mitosis.
Inhibitor-2 (I-2) is the most ancient of more than eight different PP1-specific inhibitor proteins, and I-2 is conserved among all eukaryotes, including yeast, Caenorhabditis elegans, Drosophila, Xenopus, and humans (Gruppuso et al., 1985; Roach et al., 1985; Tung et al., 1995; Li et al., 2007). Evidence indicates involvement of I-2 in regulation of the cell cycle, especially mitosis. The expression level of I-2 fluctuates during the cell cycle and peaks during mitosis (Brautigan et al., 1990), when I-2 becomes phosphorylated at a conserved PXTP site by CDK2:cyclin B1 (Leach et al., 2003; Li et al., 2006). I-2 is localized to centrosomes during interphase, and it associates with PP1 bound to the centrosomal kinase Nek2A and activates this kinase, involving separate regions of the I-2 (Eto et al., 2002). I-2 directly associates with Aurora A without involvement of PP1 and increases the kinase activity (Satinover et al., 2004). Recently, we discovered that I-2 directly binds and regulates the specificity of Pin1, a prolyl isomerase reactive with various mitotic phosphoproteins (Li et al., 2008). Nevertheless, there is no loss-of-function evidence for the function of I-2 in the cell cycle of mammalian cells. One clue for possible function comes from study of Glc8, the yeast homologue of I-2, which compensates for mutations in Ipl1 (Aurora) kinase to correct severe missegregation of chromosomes during mitosis (Tung et al., 1995).
Here, we report that knockdown of I-2 by RNA interference (RNAi) leads to lagging anaphase chromosomes and failure of cytokinesis, with subsequent formation of multinucleated cells with supernumerary centrosomes and multipolar spindles. Knockdown of I-2 and Aurora B chemical inhibition are similar, and both are rescued by partial knockdown of PP1, suggesting that I-2 is required for proper chromosome segregation and cytokinesis by regulating the balance of PP1-Aurora B activities during mitosis, similar to IPL1, GLC7, and GLC8 in yeast. Our results show functional conservation of this trio of proteins and suggest that aneuploidy in human disease may in part be due to alteration of I-2 levels.
MATERIALS AND METHODS
Cell Culture and Transfection
Human adult retinal epithelial cells (ARPE-19; ATCC CRL-2302) were grown according to American Type Culture Collection recommendations. Cells were not used past P4-P5 to ensure a consistent phenotype. Cells were plated into either six-well plates (50,000 cells/well) or 60-mm dishes (100,000 cells), and 24 h later they were transfected with small interfering RNA (siRNA) (80 nM) using Oligofectamine (Invitrogen, Carlsbad, CA) following the manufacturer's instructions. Transfection was repeated 48 h later. For plasmid transfections, 2 × 106 cells were nucleofected (Amaxa Biosystems, Gaithersburg, MD) with plasmids (5 μg in 0.1 ml) by using program T-28 and seeded at 100,000 cells per well in six-well plates (transfection efficiency, >90%). Cells were analyzed after 72 h. Taxol and nocodazole were purchased from Sigma-Aldrich (St. Louis, MO).
siRNA, Short Hairpin RNA (shRNA), and Plasmid Preparation
Preliminary experiments (Supplemental Figure S1) compared knockdown of I-2 by using two different siRNAs from Dharmacon RNA Technologies (Lafayette, CO) and two different shRNAs, and the more efficient sequences were used in this study. The siRNA for I-2 was designed using the Dharmacon program (http://www.dharmacon.com/). The shRNA targeting the coding region different from siRNA was designed using Oligoengine (http://www.oligoengine.com/). The sense and antisense oligonucleotides were annealed and cloned into the BglII and HindIII sites of the pSUPER vector (Brummelkamp et al., 2002), named pSUPER-I-2. The sequence targeted by siRNA is 5′-GTGGGATGAAATGAACATC-3′, and the sequence targeted by shRNA is 5′-GCGGGAATGTCGACGAGGA-3′. PP1Cα was knocked down by a SMARTpool siRNA (Dharmacon RNA Technologies) transfected once. Human I-2 was cloned into pRK7-HA3 vector (Eto et al., 2002) for expression of I-2 in mammalian cells with a N-terminal triple-hemagglutinin (HA) epitope tag. The pRK7-I-2-HA3 was mutated by polymerase chain reaction (PCR) to generate expression plasmids that contain two silent mutations in the 19 base pair I-2 shRNA target sequence. The mutations were confirmed by DNA sequencing. Human full length I-2 was cloned into pEGFP-C3 vector for expression in mammalian cells with N-terminal enhanced green fluorescent protein (EGFP) tag.
Immunoblotting
Western blotting was done as described previously (Stefansson and Brautigan, 2006) and used the following primary antibodies: sheep polyclonal anti-I-2 (1:500); chicken anti-pan PP1 (1:20,000); mouse anti-actin (1:1000) (Sigma-Aldrich); rabbit anti-PHI-1 (1:500); mouse anti-Nek2A antibody (1:1000) (BD Biosciences, San Jose, CA); goat anti-PP1Cα (1:5000) (Santa Cruz Biotechnology, Santa Cruz, CA). Goat anti-rabbit Alexa Fluor 680 and donkey anti-sheep Alexa Fluor 680 were purchased from Invitrogen and used at a 1:3000 dilution. Goat anti-mouse IRDye 800 and anti-chicken IRDye 800 antibodies were purchased from Rockland Immunochemicals (Gilbertsville, PA) and used at a 1:3000 dilution.
Cell Cycle Analysis
Cells transfected with siRNAs or pSUPER plasmids were resuspended in 0.5 ml of phosphate-buffered saline (PBS), fixed by adding 4.5 ml of ice-cold 70% ethanol, and stored at 4°C. Before analysis, fixed cells were washed in PBS and incubated with propidium iodide staining solution (0.1% Triton X-100 in PBS, 0.04 mg/ml propidium iodide; 0.2 mg/ml RNase A) for 30 min at room temperature. Analysis was on a FACSCalibur cytometer (BD Biosciences, San Jose, CA). Ten thousand cells were analyzed per sample.
ARPE-19 cells transfected with EGFP-I-2 were subjected to bivariate assay as described previously (Robinson and International Society for Analytical Cytology, 1997).
Microscopy
Immunofluorescent microscopy was done as described previously (Eto et al., 2002), with sheep polyclonal anti-I-2 (1:100), rabbit anti-phospho(Ser10) histone H3 (1:100) (Millipore, Billerica, MA); human anti-centromere (1:200) (Antibodies, Davis, CA); mouse anti-γ-tubulin (1:1000) (Sigma-Aldrich); mouse anti-Cep57; and rabbit anti-centrin (1:500) (Sigma-Aldrich). Rhodamine Red-X–conjugated goat anti-mouse, cyanin (Cy)3-conjugated goat anti-human, and Oregon Green-conjugated goat anti-rabbit or goat anti-sheep secondary antibodies were used at 1:1000 (Invitrogen). Fluorescein isothiocyanate-conjugated monoclonal anti-α-tubulin antibody (Sigma-Aldrich) was used at 1:200.
Intensities of pH3S10 staining were analyzed by the image analysis software Openlab (Improvision, Coventry, United Kingdom). Comparison of H3pS10 staining was performed by creating a mask (region of interest) around Hoechst 33342 stained material. The mean pixel intensity of H3pS10 staining in the masked region was corrected by subtracting background pixel intensity, and quantified relative to DNA staining in the same region.
RESULTS
I-2 Expression and Localization in Mitotic ARPE-19 Cells
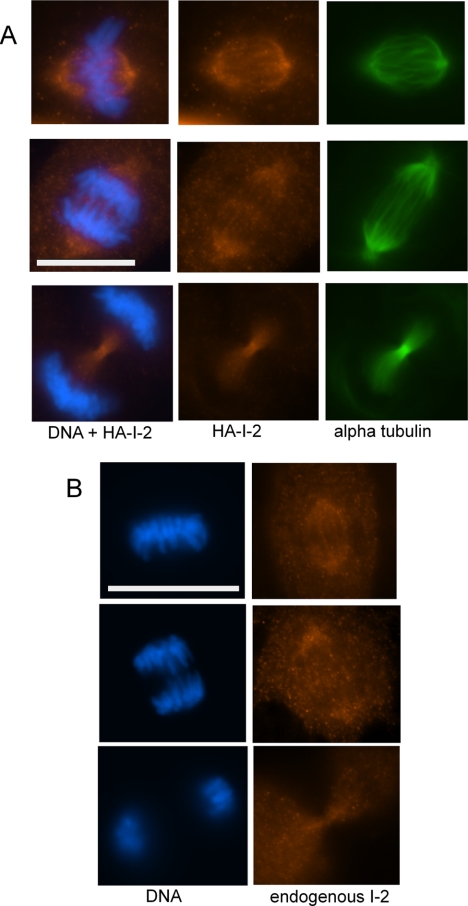
We screened various tissue culture cell lines by immunoblotting for endogenous I-2 (relative to actin), and we found the lowest I-2 levels in diploid human retinal epithelial ARPE-19 cells, compared with >15-fold higher levels expressed in HeLa cells (data not shown). Immunofluorescent microscopy of ARPE-19 cells during interphase showed prominent colocalization of I-2 and γ-tubulin at centrosomes (Eto et al., 2002), in addition to diffuse cytoplasmic staining. During mitosis we observed that levels of I-2 in ARPE-19 cells increased, as reported previously for fibroblasts (Brautigan et al., 1990). Endogenous I-2 stained with affinity-purified sheep anti-I-2 antibody showed changes in localization during mitosis that were faithfully duplicated by HA-tagged I-2 protein expressed at low levels and stained with anti-HA antibody (Figure 1, A and B). At metaphase, I-2 localized at spindle poles and along spindle fibers (top rows) and during anaphase I-2 remained on the spindles but partially relocalized into the midzone between the separating chromosomes (middle rows). Aurora B localizes in the midzone during anaphase. Then, during telophase I-2 became highly concentrated in the midbody (bottom rows). The relocalization during mitosis suggests I-2 may have multiple actions, at different stages of mitosis and regulate processes at specific times and at specific intracellular sites.
Figure 1.
Localization of I-2 during mitosis in ARPE-19 cells. (A) ARPE-19 cells expressing HA-I-2 at low levels were fixed and stained with anti-HA (orange), anti-α-tubulin (green) antibody, and Hoechst 33342 (blue). Cells at metaphase (top), early anaphase (middle), and late anaphase (bottom) are shown. (B) Localization of endogenous I-2 in ARPE-19 cells at metaphase (top), early anaphase (middle), and late anaphase (bottom) that were fixed and stained with Hoechst 33342 (blue) and sheep anti-I-2 antibody (orange). Bar, 20 μm.
Knockdown of I-2 by RNAi Results in Multinucleated Cells
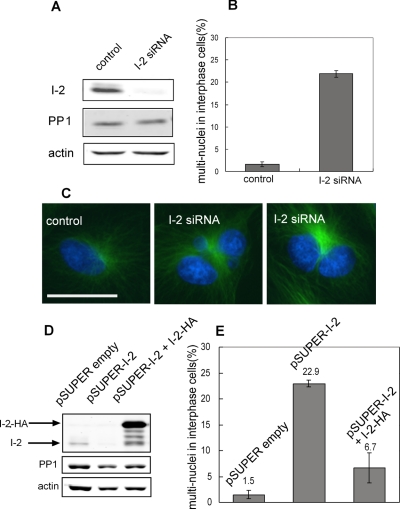
Knockdown of I-2 in human epithelial ARPE-19 cells by RNAi (either siRNA or shRNA) produced multinucleated cells, due to defects in mitosis. Quantitative immunoblotting using fluorescent antibodies was used to analyze the extent of knockdown by siRNA and shRNA against different target sequences (Supplemental Figure S1). The more effective siRNA sequence knocked down I-2 protein >85%, relative to actin as loading control (Figure 2A). Fluorescent microscopy of interphase and mitotic cells stained with anti-I-2 antibodies confirmed the extensive knockdown of the endogenous I-2 protein and the specificity of the immunostaining (Supplemental Figure S2). Microscopy revealed multiple nuclei in 23% of I-2 knockdown cells, a significant increase compared with 2% of cells transfected with control siRNA (Figure 2B). The mitotic index of I-2 knockdown cells and cells transfected with control siRNA was the same, ∼3%. The doubled DNA content in cells was confirmed by flow cytometry (see below). Staining of DNA and α-tubulin (Figure 2C) showed a single nucleus and one microtubule array in control cells, compared with multiple nuclei and a single microtubule array in knockdown cells. We suspected that knockdown of I-2 yielded cells with multiple nuclei due to a failure of mitosis.
Figure 2.
Knockdown of I-2 yields multinucleated cells. (A) ARPE-19 cells were transfected with siRNA against I-2, or luciferase, as control. Cells were collected 72 h after transfection, and extracts were prepared and immunoblotted for I-2, with actin as a loading control, and anti-pan-PP1 as an additional control. (B) Control and I-2 knockdown cells were fixed and stained to quantitate the percentage of cells with multiple nuclei (500 cells in each group). Data are plotted as mean ± SD of results from three independent experiments. (C) Cells were fixed 72 h after siRNA transfection and stained with anti-α-tubulin (green) and Hoechst 33342 (blue) to show multiple nuclei. Bar, 25 μm. (D) ARPE-19 cells were nucleofected with pSUPER empty vector, pSUPER-I-2 shRNA, and pSUPER-I-2 shRNA + pK7-HA-I-2. The shRNA targeted a different sequence in I-2 than that used for siRNA. The pK7-HA-I-2 encodes I-2 with silent mutations to preserve the amino acid sequence but changes the nucleotide sequence to avoid knockdown by RNAi. Cell extracts were analyzed by immunoblotting with affinity-purified sheep anti-I-2, mouse anti-actin, and chicken anti-PP1. The migration of endogenous I-2 and ectopic HA-tagged I-2 are indicated with arrows. (E) Cells with multiple nuclei were scored (500 cells/group), and data are plotted as mean ± SD of values from three independent experiments.
Control experiments demonstrated the multinucleated phenotype was specific for knockdown of I-2. First, either siRNA or shRNA that target different coding sequences within I-2 gave comparable protein knockdown (80–85%; Figure 2) and produced the same fraction of multinucleated cells in the population. This makes it unlikely the phenotype is due to the transfection or off-target effects of the RNAi. Second, knockdown of I-2 did not cause a corresponding depletion of the levels of PP1 catalytic subunits, which were unchanged relative to cells transfected with a control siRNA (Figure 2A). Therefore, PP1 levels were not dependent on endogenous I-2, and changes in levels of PP1 did not account for the phenotypes seen in I-2 knockdown cells. Furthermore, knockdown of I-2 did not seem to produce a general increase in cellular PP1 activity, to account for the phenotypes. By digital immunofluorescent microscopy there was no difference in the phosphorylation of S19 in myosin light chains or in T232 of Aurora B (both PP1 substrates) in I-2 knockdown versus control knockdown cells (data not shown). As another additional control, we used siRNA to knockdown a different PP1-specific inhibitor protein, called PHI-1. Knockdown of PHI-1 did not result in multinucleated cells and also did not alter either PP1 or I-2 levels in ARPE-19 cells (data not shown). The results demonstrate that these different PP1 inhibitor proteins have separate functions. Knockdown of these PP1 inhibitor proteins did not change the levels of PP1 in cells or cause major changes in PP1 activity in cells.
The ultimate proof of specificity of RNAi is to reverse the phenotype by coexpression of a plasmid mutated to preserve the protein amino acid sequence but to avoid the knockdown process. Such a plasmid encoding I-2 with an N-terminal HA tag was cotransfected with the pSUPER shRNA plasmid. The knockdown of the endogenous I-2 by pSUPER shRNA was >80% based on immunoblotting (Figure 2D). Expression of the HA-I-2 rescued the shRNA induction of multinucleated cells, reducing the rate from 23% of the I-2 knockdown cells to <7% in the cells coexpressing HA-I-2, compared with <2% in control cells nucleofected with empty pSUPER plasmid (Figure 2E). The level of HA-I-2 expression was clearly much higher than endogenous I-2 in ARPE-19 cells (Figure 2D). We note that overexpression of HA-I-2 or EGFP-I-2 in ARPE-19 cells caused little change in the fluorescence-activated cell sorting (FACS) profile (Supplemental Figure S3) and no change in mitotic index (∼3%), indicating that the rescue of the multinucleated phenotype was not due to cell cycle arrest. The results of the controls and rescue demonstrated that formation of multinucleated ARPE-19 cells was due to RNAi reduction of levels of I-2 protein.
Multiple Centrosomes and Multipolar Spindles Arise from Cytokinesis Failure in I-2 Knockdown Cells
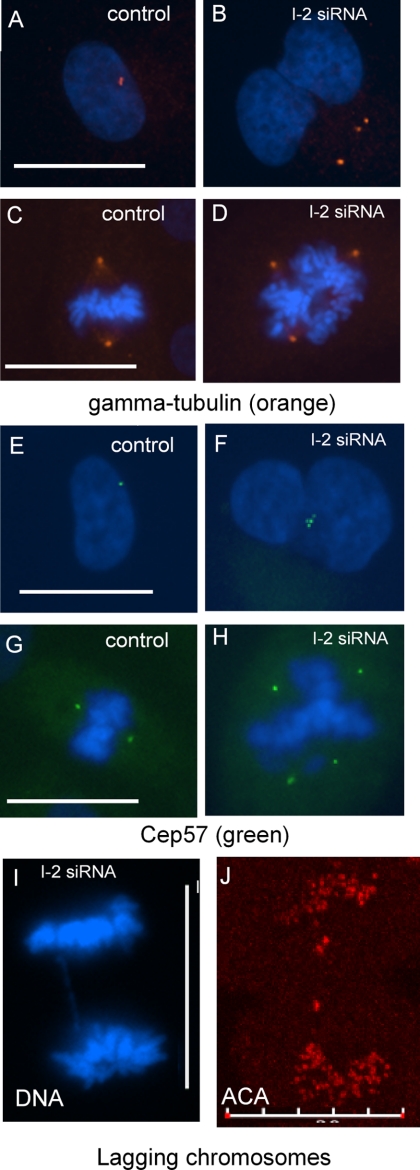
Multiple centrosomes and multipolar mitotic spindles occurred in cells with I-2 knocked down by siRNA. Control cells had one, or two closely spaced, centrosomes stained by anti-γ-tubulin (Figure 3A) that became the spindle poles during mitosis (Figure 3C). By comparison I-2 knockdown cells had multiple centrosomes stained for γ-tubulin during interphase (Figure 3B) that led to multipolar spindles during mitosis (Figure 3D). We confirmed that these γ-tubulin–containing foci were indeed centrosomes by staining the centrosome matrix with anti-Cep57 antibodies (Figure 3, E–H). In addition, multiple centrosomes were stained using anti-centrin antibody in I-2 knockdown cells (data not shown). The γ-tubulin staining showed multiple centrosomes in 15% of I-2 knockdown cells, compared with <1% in ARPE-19 cells transfected with control siRNA. The α-tubulin staining showed 13% of mitotic I-2 knockdown cells had multipolar or tripolar spindles, compared with 2% of control cells (data not shown). Even in I-2 knockdown cells with bipolar spindles, lagging chromosomes were visualized in the midzone of cells during anaphase by anti-centromere antigen (ACA) staining (Figure 3, I and J). We concluded that knockdown of I-2 compromised proper chromosome segregation.
Figure 3.
Centrosome amplification and mitotic defects due to I-2 knockdown in ARPE-19 cells. (A–D) Centrosome amplification was found in both interphase and mitotic cells. ARPE-19 cells were fixed 72 h after transfection with siRNA for I-2, or a control siRNA, and stained with antibody against γ-tubulin to identify centrosomes (orange). Centrosome amplification was evident in both interphase (B) and mitotic (D) I-2 knockdown cells, compared with corresponding control cells (A and C). Centrosome amplification was found in 18% of I-2 knockdown cells and 2% in control cells. (E–NH). ARPE-19 cells transfected with siRNA were fixed and stained with Hoechst 33342 plus antibody against Cep57 (green) to independently identify centrosomes. The Cep57 staining reveals centrosome amplification in both interphase (F) and mitotic (H) I-2 knockdown cells, compared with control (E and G). (I and J) Lagging chromosomes were detected in I-2 knockdown cells based on wide-field microscopic image of Hoechst 33342-stained DNA in anaphase cells (I) and a confocal image of an anaphase cell stained with ACA (J). In 100 I-2 knockdown anaphase cells, 7.5 ± 1.5% of the cells had lagging chromosomes, compared with 1.5 ± 1.5% in control cells. Bars, 25 μm (A, C, E and G) and 20 μm (I and J).
Did the extra centrosomes seen in I-2 knockdown cells form by reduplication in one cell cycle or were duplicated centrosomes and chromosomes retained in a single cell due to failure of cytokinesis? (Nigg, 2002). Arrest of control ARPE-19 cells at S phase by hydroxyurea treatment prevented centrosome duplication, indicating the process in ARPE-19 cells is limited to once per cell cycle. Knockdown of I-2 did not interfere with S phase arrest by hydroxyurea treatment (Figure 4A) and hydroxyurea had a minimal effect on knockdown of I-2, slightly reducing the extent of protein depletion (Figure 4B). Arrest of cell cycle progression with hydroxyurea yielded <4% of I-2 knockdown cells with multiple centrosomes, a significant reduction compared with 18% of I-2 knockdown cells that were allowed to proceed through the cell cycle (Figure 4C). I-2 knockdown cells arrested at S phase did not accumulate multiple nuclei based on microscopic examination (data not shown). We concluded that appearance of multiple centrosomes in I-2 knockdown cells was not due to extra centrosome duplication within the same cell cycle. Instead, the results support a hypothesis that I-2 knockdown cells accumulate multiple nuclei and supernumerary centrosomes due to failure of cytokinesis.
Figure 4.
I-2 knockdown phenotypes are prevented by cell cycle arrest at S phase. ARPE-19 cells were transfected with siRNA for I-2 or luciferase as control, and after 24 h were treated with 1.5 mM hydroxyurea in the presence of the siRNA for an additional 48 h. Cells were fixed for flow cytometry analysis, or for γ-tubulin staining or for Western blot. (A) DNA content profiles of cells were determined by flow cytometry, with DNA content measured by propidium iodide staining. (B) Cells transfected with siRNA in the presence of hydroxyurea were collected and immunoblotted for I-2, with actin as loading control. (C) After transfection with I-2 siRNA or control siRNA for 72 h, the percentage of cells (of 100 total) with abnormal centrosomes was counted. Cultures with or without hydroxyurea treatment were compared. Data are plotted as mean ± SD from three independent experiments.
This hypothesis was supported by direct microscopic observation of mitotic I-2 knockdown cells. We collected phase contrast images in time-lapse to make movies of cells during mitosis (Supplemental Figure S4 and Supplemental Movies). Control cells rounded up at metaphase and then formed a cleavage furrow during anaphase and separated in two daughter cells (Supplemental Figure S4A). In 21 of 100 mitotic I-2 knockdown cells, the rounded metaphase cells began to elongate but did not complete cleavage furrows and retracted back to a single cell, without undergoing cytokinesis (Supplemental Figure S4, B–D). In contrast, no cytokinesis defect was found in 100 control cells. The rate of cytokinesis failure matches the fraction of multinucleated cells formed in response to I-2 knockdown. We concluded that reversion of cytokinesis resulted in the observed phenotypes in I-2 knockdown cells.
Phosphorylation of Histone H3 Ser10 in Response to Hesperadin and I-2 Knockdown
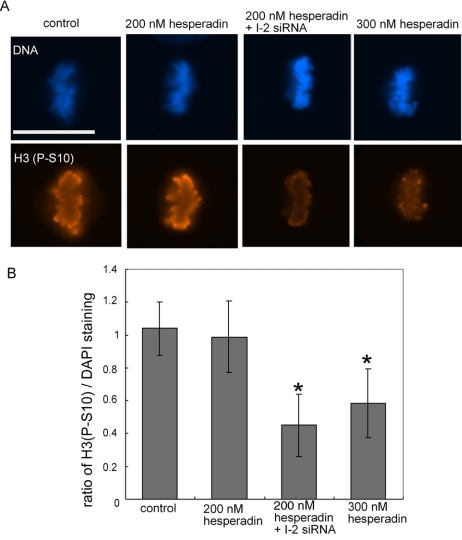
During mitosis chromosome segregation and cytokinesis depend on Aurora B kinase; therefore, we asked whether I-2 was involved in regulation of Aurora B. An abundant substrate of Aurora B is Ser10 of histone H3 (H3S10), which can be detected in mitotic cells by using phosphosite-specific antibody (Figure 5). Phosphorylation of H3S10 during metaphase was the same in control cells and in cells treated with hesperadin at 200 nM. Thus, Aurora B could be partially inhibited without a net decrease in the steady-state phosphorylation of H3S10. However, inhibition of Aurora B kinase with a higher concentration of 300 nM hesperadin significantly reduced H3S10 phosphorylation (Figure 5). Knockdown of I-2 by itself did not reduce phosphorylation of H3S10; however, in combination with 200 nM hesperadin knockdown of I-2 significantly reduced H3S10 phosphorylation, matching the inhibition produced by 300 nM hesperadin (Figure 5). This suggested to us that knockdown of I-2 was equivalent to a partial reduction in Aurora B activity. Because I-2 knockdown could reduce phosphorylation of H3S10, we reasoned that either Aurora B or H3S10 is under control of a PP1 holoenzyme that is sensitive to I-2 inhibition.
Figure 5.
Effects of I-2 knockdown and hesperadin on phosphorylation of Histone H3(S10). (A) Cells were transfected with I-2 siRNA or control siRNA for 48 h, treated with 200 or 300 nM hesperadin, respectively, for 2 h, and then they were fixed and stained with Hoechst 33342 (blue) and anti-phosphosite antibody for S10 in histone H3 (H3S10; orange). Bar, 25 μm. (B) The integrated intensity of H3S10 staining was quantified relative to Hoechst 33342 staining for individual mitotic cells. The graph shows the mean ± SD of the integrated intensity of H3S10 staining of 30 mitotic cells from three independent experiments (10 cells each experiment) of each treatment. *, p < 0.001.
Mitotic Defects in I-2 Knockdown Cells Are Mimicked by Aurora Kinase Inhibition and Rescued by Partial PP1 Knockdown
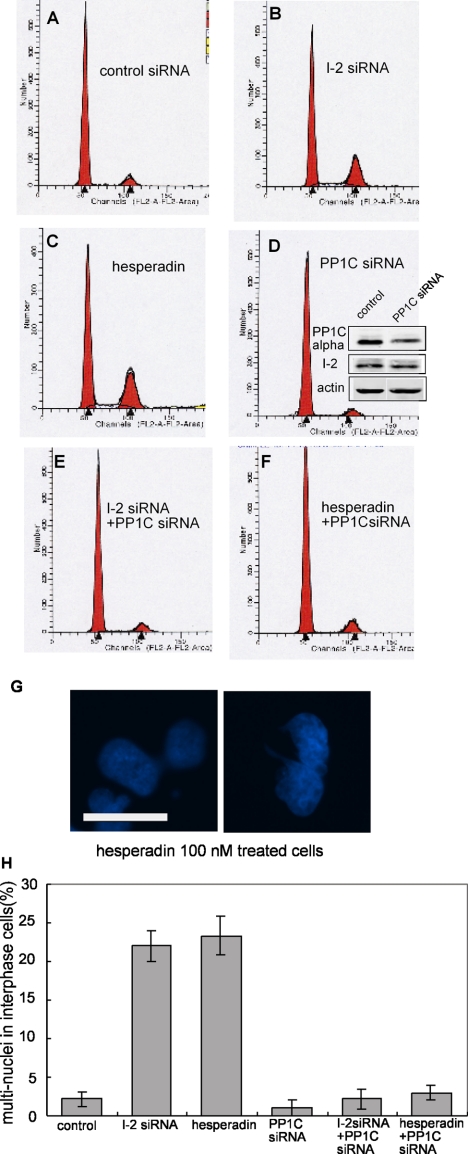
Aurora B inhibition by the drug hesperadin produced multinucleated ARPE-19 cells, resembling the phenotype seen with I-2 knockdown (Figure 6). I-2 knockdown produced multinucleated cells, reflected in a significant increase in the fraction of cells with 4N DNA content by FACS analysis, relative to controls (Figure 6, A and B). The fraction of cells with 4N DNA content matched the fraction of cells with multiple nuclei seen by fluorescent microscopy by using double staining with Hoechst 33342 and anti-α-tubulin (Figure 6H). Partial inhibition of Aurora B by low dose hesperadin (100 nM) produced a significant increase in cells with 4N DNA content (Figure 6C), equal to the increase seen by I-2 knockdown. Hesperadin at 100 nM produced 23% of cells with multiple nuclei (Figure 6G), and this fraction of multinucleated cells was the same as for I-2 knockdown (Figure 6H). Cells treated with 100 nM hesperadin exhibited multiple centrosomes stained for γ-tubulin and centrin (data not shown). Note that this dose of hesperadin was much lower than the dose required to alter H3S10. Together, the results indicated that knockdown of I-2 was equivalent to partial inhibition of Aurora B by hesperadin.
Figure 6.
Mitotic defects produced by I-2 knockdown are mimicked by Aurora B inhibition and rescued by partial PP1C knockdown. (A–F) ARPE-19 cells were transfected for 72 h with siRNA against luciferase, as control (A), I-2 (B), PP1Cα (D), or both I-2 and PP1Cα (E). For hesperadin treatment, 100 nM hesperadin was added to the medium 42 h after transfection with control siRNA (C) or siRNA against PP1Cα (F), and cells were incubated for another 30 h. The DNA content of cells was measured by flow cytometry, with propidium iodide staining. Profiles are representative of three independent experiments. Knockdown of PP1Cα was measured by blotting with anti-PP1Cα antibody, by using actin as loading control (inset in D). (G) Hoechst 33342 staining showed cells with multiple nuclei after 100 nM hesperadin treatment. Bar, 25 μm. (H) Percentage of cells with multiple nuclei after treatment with siRNA or hesperadin (500 total/group), plotted as mean ± SD from three independent experiments.
Testing a range of concentrations of siRNA, we found conditions that partially knocked down PP1Cα (to 40% of endogenous; see inset in Figure 6D). These conditions gave no increase in cells with 4N DNA, and the flow cytometry profile matched that for cells treated with control siRNA (Figure 6, D vs. A). However, partial knockdown of PP1α catalytic subunit by siRNA prevented the increase in cells with 4N DNA produced by either Aurora B inhibition (Figure 6, E vs. B) or I-2 knockdown (Figure 6, F vs. C). Immunoblotting confirmed the same efficient knockdown of I-2 either alone or in conjunction with partial PP1C knockdown (Supplemental Figure S5). Separate assays confirmed that cells with 4N DNA content by flow cytometry reflected the fraction of multinucleated cells generated by either knockdown of I-2 or inhibition of Aurora B by hesperadin (Figure 6H). Similar results were obtained with partial knockdown of any one of the three isoforms of PP1 catalytic subunit (data not shown). The data showed that reduction in PP1C levels could rescue cells and compensate for knockdown of I-2 or inhibition of Aurora B with hesperadin.
Equivalence of I-2 Knockdown and Aurora B Inhibition in Relief of Taxol-induced Mitotic Arrest
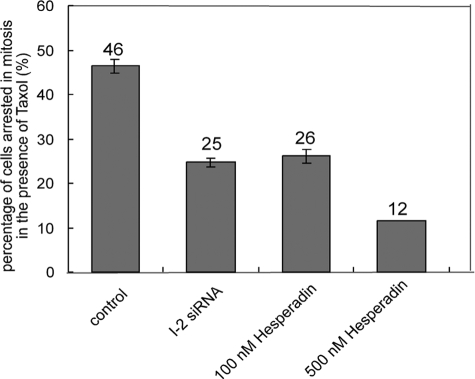
Taxol induces mitotic arrest, which is relieved by hesperadin inhibition of Aurora B (Hauf et al., 2003) that provides a bypass of the spindle checkpoint. We found taxol treatment arrested 40–50% of ARPE-19 cells in mitosis, and knockdown of I-2 significantly reduced the mitotic index, to the same extent as inhibition of Aurora B with 100 nM hesperadin (Figure 7). Again, knockdown of I-2 was equivalent to partial inhibition of Aurora B. A higher concentration of hesperadin (500 vs. 100 nM) allowed more cells to proceed through mitosis in the presence of taxol, showing the range of the assay (Figure 7). Knockdown of I-2 or inhibition of Aurora B released cells from taxol-induced mitotic arrest, presumably by a reduction in phosphorylation of a critical protein by the action of PP1.
Figure 7.
Override of taxol-induced mitotic arrest by I-2 knockdown or hesperadin treatment. ARPE-19 cells were transfected for 48 h with siRNA against I-2 or luciferase as control, and then they were incubated with 5 μM taxol in the presence of siRNA for another 12 h, before fixation and staining with Hoechst 33342. For hesperadin treatment, cells were treated with 100 or 500 nM hesperadin for 2 h, incubated with 5 μM taxol in the presence of hesperadin for another 12 h, and then fixed and stained with Hoechst 33342 to identify the percentage of mitotic cells (500 cells/group). Data are means ± SD of values from three independent experiments.
Hesperadin-induced Multinucleation Is Rescued by Overexpression of I-2
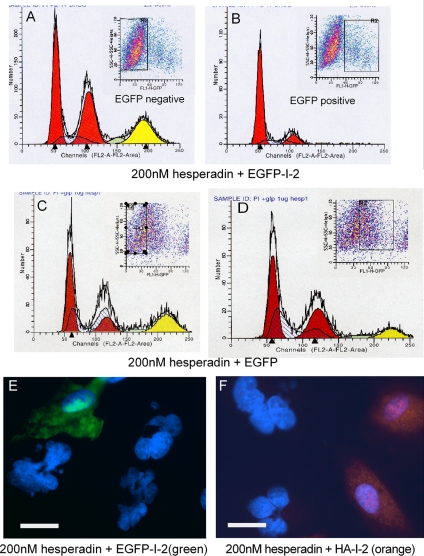
Because I-2 knockdown seemed equivalent to Aurora B inhibition, we tested whether I-2 overexpression would have the opposite effect and rescue or reverse Aurora B inhibition. We transfected ARPE-19 cells with a plasmid encoding EGFP-I-2, treated the cells with 200 nM hesperadin for 24 h, and applied bivariate flow cytometry to divide the population into EGFP-positive and EGFP-negative subsets and analyze for DNA content (Figure 8). Hesperadin treatment caused significant accumulation of cells with 8N and 4N DNA content in the EGFP-I-2–negative population (Figure 8A). Parallel analysis by immunofluorescent microscopy confirmed the flow cytometry results, showing the fraction of cells with higher DNA content corresponded to the fraction of multinucleated cells. In contrast, cells expressing EGFP-I-2 in the same experiment were relatively resistant to hesperadin treatment, with a flow cytometry profile that was not much different from untransfected ARPE-19 cells (Figure 8B; ∼70% of the cells in G1 with 2N DNA). Expression of EGFP plus hesperadin treatment of ARPE-19 cells was analyzed as another control, and bivariate flow cytometry separated EGFP-positive and EGFP-negative cells and showed both populations of cells had nearly the same increase in cells with 4N and 8N DNA content (Figure 8, C and D). Thus, expression of EGFP-I-2 produced a resistance to hesperadin that was not due to the EGFP portion of the fusion protein. Hesperadin treatment produced a high proportion of multinucleated cells, but those cells expressing either EGFP-I-2 (Figure 8E) or HA-I-2 (Figure 8F) had single, normal-sized nuclei. This showed that different tags on the I-2 did not interfere with its function in cells. We concluded that I-2 overexpression rendered cells resistant to hesperadin-induced mitotic defects that lead to formation of multinucleated cells.
Figure 8.
Ectopic expression of I-2 prevents mitotic defects generated by hesperadin inhibition of Aurora B. (A–D) ARPE-19 cells were transfected to express EGFP-I-2 (A and B) or EGFP, as a control (C and D), and incubated with 200 nM hesperadin for 24 h. Finally, cells were fixed and subjected to fluorescent activated cell sorting. The cell population was sorted into EGFP-negative (A and C) and EGFP-positive (B and D) populations, marked by the rectangle in each FACS profile. The DNA content of each subset was determined by flow cytometry based on propidium iodide staining, in three independent experiments. Representative profiles are shown. Red peaks mark cell populations with 2N or 4N DNA content; yellow peaks designate cell populations with 8N DNA content. (E and F) Cells expressing either EGFP-I-2 or HA-I-2 were treated with 200 nM hesperadin for 24 h, fixed, and examined by immunofluorescent microscopy. (E) The EGFP (green)-positive cells had single, normal-sized nuclei compared with neighboring EGFP-negative cells that had multiple nuclei or abnormally large nuclei. (F) HA (orange)-positive cells with single, normal-sized nuclei were compared with neighboring HA-negative cells with abnormal nuclei containing extra DNA. Bars, 25 μm (E and F).
DISCUSSION
This study shows that phosphatase inhibitor 2 (I-2) is required for the successful completion of mitosis in human epithelial cells. Knockdown of I-2 by RNAi reduced the I-2 protein levels and resulted in accumulation of cells with multiple nuclei and supernumerary centrosomes. We demonstrate the effects are dependent on progression through the cell cycle, and we show by time-lapse microscopy that the phenotypes arise from failed cytokinesis and subsequent retention of duplicated chromosomes and centrosomes in single cells. Cytokinesis failure and accumulation of multi-nucleated cells have been associated with lagging chromosomes during anaphase and telophase (Shi and King, 2005). We observed lagging chromosomes in I-2 knockdown cells and faulty chromosome segregation could have caused or contributed to the failure of cytokinesis. Asymmetric kinetochore–microtubule attachments can cause chromosomes to not congress to the metaphase plate in a timely manner. Aurora B kinase is required for correction of asymmetric kinetochore–microtubule attachments and maintenance of the spindle checkpoint (Kallio et al., 2002; Hauf et al., 2003). Aurora B is thought to promote resolution of microtubule attachments by phosphorylation of MCAK and Hec1 proteins at kinetochores (Walczak and Heald, 2008). Aurora B and PP1 form a phosphorylation gradient across the midzone during mitosis to determine the location of the cleavage furrow for cytokinesis (Fuller et al., 2008). These considerations suggest the mitotic function of I-2 is linked to the regulation and actions of Aurora B.
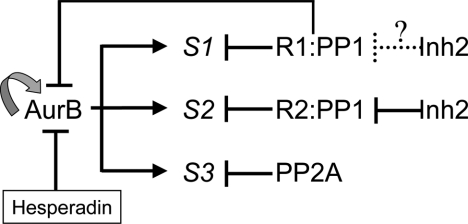
Consistent with a relationship between I-2 and Aurora B, we found knockdown of I-2 was equivalent to hesperadin partial inhibition of Aurora B. This was in terms of formation of multinucleated cells, override of the spindle checkpoint imposed by taxol, and phosphorylation of histone H3S10. Conversely, I-2 overexpression prevented the formation of multinucleated cells in response to hesperadin without causing cell cycle arrest. We suspect that Aurora B-dependent phosphorylation events were enhanced by I-2 overexpression probably by inhibition of certain PP1 holoenzymes. Knockdown of I-2 could have generated lagging chromosomes by increasing PP1 activity, thereby indirectly reducing phosphorylation of Aurora B substrates at kinetochores. In addition, both I-2 and Aurora B are localized to the midzone in anaphase and to the midbody during cytokinesis. Previous studies have shown that Aurora B regulates cytokinesis by phosphorylation of certain substrates in the midzone and midbody (Delaval et al., 2004; Guse et al., 2005). Therefore, we hypothesize that in addition to lagging chromosomes, the failure of cytokinesis in I-2 knockdown cells was due at least partially to reduced Aurora B activity and/or reduced phosphorylation of its substrates in the midzone and midbody, because of enhanced local PP1 activity. I-2 might not regulate the PP1 holoenzyme reactive with Aurora B because we did not observe a change in anti-phospho-T232 staining of Aurora B in the midzone or midbody of I-2 knockdown cells, relative to cells treated with control siRNA. However, there could be other I-2–dependent mechanisms for Aurora B activation in addition to T232 phosphorylation (e.g., other phosphosites in Aurora B) that would not be detected by pT232 immunostaining. Alternatively, I-2 may primarily be regulating the activity of PP1 holoenzymes that dephosphorylate Aurora B substrates. We propose there are one or more I-2 sensitive holoenzyme forms of PP1 that inactivate Aurora B and/or dephosphorylate Aurora B substrates during mitosis to account for the phenotypes (Figure 9).
Figure 9.
Model for regulation of Aurora B by PP1 and I-2 during mitosis. Aurora B is activated by autophosphorylation of T232 in its activation loop, and this reaction can be enhanced by specific binding partners and is reversed by PP1 that inactivates the kinase. Distinct PP1 holoenzymes consist of a catalytic subunit and different regulatory subunits (PP1 R1 and PP1 R2). Different PP1 holoenzymes or PP2A dephosphorylate Aurora B substrates (S1, S2, and S3) to keep the balance of phosphorylation events during different stages of mitosis. Knockdown of I-2 seems equivalent to partial inhibition of Aurora B by indirectly releasing PP1 activity against Aurora B substrates. Conversely, overexpression of I-2 makes cells resistant to partial Aurora B inhibition by hesparadin presumably by inhibiting the counteracting PP1 activity against Aurora B substrates. I-2 binds and inhibits a subset of PP1 holoenzymes (marked by solid inhibition arrow) to regulate phosphorylation of Aurora B substrates. I-2 may also regulate another subset PP1 holoenzymes (marked by dotted inhibition arrow) to regulate Aurora B autophosphorylation.
Besides Aurora kinases, another Ser/Thr kinase, Nek2A, has been claimed to regulate kinetochore–microtubule attachments and spindle checkpoint signaling (Lou et al., 2004; Du et al., 2008). Our previous studies have shown that I-2 binds to Nek2A–PP1 complexes to activate the Nek2A (Eto et al., 2002; Li et al., 2007). The Nek2A–PP1 complex involves reciprocal negative feedback and autoactivation, predicted to respond as a bistable switch (Ferrell, 2002). Therefore, Nek2A is another candidate that might mediate the actions of I-2 and be affected by RNAi knockdown. However, we found knockdown of Nek2A did not mimic the effects of knockdown of I-2. Furthermore, coincident knockdown of Nek2A in I-2 knockdown cells did not enhance or reverse the appearance of abnormal or multiple nuclei (Supplemental Figure S6). This implies that knockdown of I-2 produces phenotypes independent of Nek2A.
More than 30 y ago, I-2 was discovered as a heat-stable protein inhibitor of PP1 (Huang and Glinsmann, 1976) and has been used over the years as a selective inhibitor to implicate PP1 in various biological processes, and to differentiate between PP2A, PP2C, and other phosphatases (Ingebritsen and Cohen, 1983; Cohen, 1991). The approach depended on the assumption that I-2 inhibited all the PP1 present. More recent comparison of I-2 with another PP1-specific inhibitor protein, CPI-17, used affinity chromatography on immobilized inhibitor protein to separate from cell extracts-specific pools of PP1 holoenzymes with different regulatory subunits (Eto et al., 2004). Thus, one needs to consider that actions of I-2 are mediated through selective inhibition of only a fraction of PP1 in cells. The specificity of PP1 inhibitor proteins for different PP1 holoenzymes accounts for the inability of PHI-1 knockdown to mimic knockdown of I-2. Knockdown of PHI-1 reduces the rates of cell spreading and migration (Tountas and Brautigan, 2004; Tountas et al., 2004), probably through deregulation of a juxtamembrane form of PP1 that is responsible for PHI-1 localization in cells and tissues. Our view is that different PP1 inhibitor proteins regulate specific PP1 holoenzymes to regulate discrete cellular processes. To fully understand the basis for I-2 function in mitosis will require identification of the mitotic PP1 complex(es) inhibited by I-2, a future goal of investigations.
Although thought to be a simple protein inhibitor for PP1, I-2 functions in a network of biochemical reactions during mitosis and has several possible actions in cells that might be compromised by knockdown. I-2 is specifically phosphorylated at a conserved PXTP site during early mitosis by the kinase cdc2-cyclinB (Leach et al., 2003; Li et al., 2006). Biochemical studies of PP1::I-2 heterodimer showed years ago that phosphorylation-dephosphorylation of this PXTP site in I-2 causes conformational activation of bound PP1 (Ballou et al., 1985). Therefore, mitotic phosphorylation of I-2 might conceivably involve activation of specific PP1 holoenzymes. In yeast, either deletion or overexpression of the I-2 homologue GLC8 suppresses mutations in Ipl1 (Aurora kinase), suggesting possible activation or inhibition of GLC7 (PP1). Treatment of mitotic cells with high-dose hesperadin (500 nM) did not interfere with mitotic phosphorylation of I-2 (data not shown), indicating that Aurora B was not required. Phosphorylation of the PXTP site in I-2 also effectively eliminates its binding to the Pin1 prolyl isomerase (Li et al., 2008), an enzyme that reacts with a host of mitotic phosphoproteins (Shen et al., 1998). Binding of I-2 to Pin1 drastically alters substrate specificity of Pin1 (Li et al., 2008), providing another possible mechanism for I-2 to control events during mitosis. Thus, during mitosis I-2 regulates kinases, phosphatases and Pin1 prolyl isomerase to coordinate events in a complex network of regulatory reactions.
Control of chromosome segregation is a conserved function of I-2. In Drosophila, I-2 is a maternal function gene and the I-2 protein is loaded into the oocyte for early rounds of mitosis within the syncytial embryo (Wang et al., 2008). Embryos of mutant mothers with reduced levels of I-2 exhibit defects in chromosome segregation, with DNA bridges linking adjacent nuclei, and they show loss of synchrony of mitosis across the embryo (Wang et al., 2008). Reduction of maternal I-2 levels results in a profound loss of viability in terms of hatching rate and survival of the larvae, effects that are partially rescued by dose-dependent transgenic expression of Drosophila I-2. These in vivo results provide strong support for our conclusions based on experiments in ARPE-19 cells. Furthermore, we now have evidence from two organisms in addition to yeast that I-2 plays a critical role in mitosis.
Aurora B kinase is over expressed in a variety of human tumors (Bischoff et al., 1998; Vischioni et al., 2006), and is implicated in tumorigenesis (Sorrentino et al., 2005). Aurora B kinase has been highlighted as a target for anticancer drug development, small molecule inhibitors have been generated, and these already show promising antitumor activity (Keen and Taylor, 2004). Our discovery that I-2 is highly expressed in several cancer cell lines, and ectopic I-2 overexpression makes cells resistant to hesperadin, suggest that I-2 levels may at least influence the sensitivity of tumors to Aurora B inhibition. Further, I-2 itself might be a target for anti-tumor drug development, and blocking its action or suppressing its levels may increase the sensitivity of tumor cells to drug inhibition of Aurora B kinase.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from U.S. Public Health Service, National Institutes of Health grants GM-56362 (to D.L.B.) and GM-63045 (to P.T.S.).
Abbreviations used:
- PP1
protein phosphatase 1
- I-2
phosphatase inhibitor-2.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-05-0460) on August 20, 2008.
REFERENCES
- Aggen J. B., Nairn A. C., Chamberlin R. Regulation of protein phosphatase-1. Chem. Biol. 2000;7:R13–R23. doi: 10.1016/s1074-5521(00)00069-7. [DOI] [PubMed] [Google Scholar]
- Ballou L. M., Villa-Moruzzi E., Fischer E. H. Subunit structure and regulation of phosphorylase phosphatase. Curr. Top. Cell. Regul. 1985;27:183–192. doi: 10.1016/b978-0-12-152827-0.50022-0. [DOI] [PubMed] [Google Scholar]
- Bischoff J. R., et al. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J. 1998;17:3052–3065. doi: 10.1093/emboj/17.11.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen M. Combinatorial control of protein phosphatase-1. Trends Biochem. Sci. 2001;26:426–431. doi: 10.1016/s0968-0004(01)01836-9. [DOI] [PubMed] [Google Scholar]
- Bollen M., Stalmans W. The structure, role, and regulation of type 1 protein phosphatases. Crit. Rev. Biochem. Mol. Biol. 1992;27:227–281. doi: 10.3109/10409239209082564. [DOI] [PubMed] [Google Scholar]
- Booher R., Beach D. Involvement of a type 1 protein phosphatase encoded by bws1+ in fission yeast mitotic control. Cell. 1989;57:1009–1016. doi: 10.1016/0092-8674(89)90339-5. [DOI] [PubMed] [Google Scholar]
- Brautigan D. L., Sunwoo J., Labbe J. C., Fernandez A., Lamb N. J. Cell cycle oscillation of phosphatase inhibitor-2 in rat fibroblasts coincident with p34cdc2 restriction. Nature. 1990;344:74–78. doi: 10.1038/344074a0. [DOI] [PubMed] [Google Scholar]
- Brummelkamp T. R., Bernards R., Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- Cohen P. Classification of protein-serine/threonine phosphatases: identification and quantitation in cell extracts. Methods Enzymol. 1991;201:389–398. doi: 10.1016/0076-6879(91)01035-z. [DOI] [PubMed] [Google Scholar]
- Cohen P. T. Protein phosphatase 1-targeted in many directions. J. Cell Sci. 2002;115:241–256. doi: 10.1242/jcs.115.2.241. [DOI] [PubMed] [Google Scholar]
- Delaval B., Ferrand A., Conte N., Larroque C., Hernandez-Verdun D., Prigent C., Birnbaum D. Aurora B-TACC1 protein complex in cytokinesis. Oncogene. 2004;23:4516–4522. doi: 10.1038/sj.onc.1207593. [DOI] [PubMed] [Google Scholar]
- Ditchfield C., Johnson V. L., Tighe A., Ellston R., Haworth C., Johnson T., Mortlock A., Keen N., Taylor S. S. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J. Cell Biol. 2003;161:267–280. doi: 10.1083/jcb.200208091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohadwala M., da Cruz e Silva E. F., Hall F. L., Williams R. T., Carbonaro-Hall D. A., Nairn A. C., Greengard P., Berndt N. Phosphorylation and inactivation of protein phosphatase 1 by cyclin-dependent kinases. Proc. Natl. Acad. Sci. USA. 1994;91:6408–6412. doi: 10.1073/pnas.91.14.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doonan J. H., MacKintosh C., Osmani S., Cohen P., Bai G., Lee E. Y., Morris N. R. A cDNA encoding rabbit muscle protein phosphatase 1α complements the Aspergillus cell cycle mutation, bimG11. J. Biol. Chem. 1991;266:18889–18894. [PubMed] [Google Scholar]
- Doonan J. H., Morris N. R. The bimG gene of Aspergillus nidulans, which is required for completion of anaphase, encodes a homologue of mammalian phosphoprotein phosphatase. Cell. 1989;57:987–996. doi: 10.1016/0092-8674(89)90337-1. [DOI] [PubMed] [Google Scholar]
- Du J., et al. The mitotic checkpoint kinase NEK2A regulates kinetochore microtubule attachment stability. Oncogene. 2008;27:4107–4114. doi: 10.1038/onc.2008.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuele M. J., Lan W., Jwa M., Miller S. A., Chan C. S., Stukenberg P. T. Aurora B kinase and protein phosphatase 1 have opposing roles in modulating kinetochore assembly. J. Cell Biol. 2008;181:241–254. doi: 10.1083/jcb.200710019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eto M., Elliott E., Prickett T. D., Brautigan D. L. Inhibitor-2 regulates protein phosphatase-1 complexed with NimA-related kinase to induce centrosome separation. J. Biol. Chem. 2002;277:44013–44020. doi: 10.1074/jbc.M208035200. [DOI] [PubMed] [Google Scholar]
- Eto M., Kitazawa T., Brautigan D. L. Phosphoprotein inhibitor CPI-17 specificity depends on allosteric regulation of protein phosphatase-1 by regulatory subunits. Proc. Natl. Acad. Sci. USA. 2004;101:8888–8893. doi: 10.1073/pnas.0307812101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez A., Brautigan D. L., Lamb N. J. Protein phosphatase type 1 in mammalian cell mitosis: chromosomal localization and involvement in mitotic exit. J. Cell Biol. 1992;116:1421–1430. doi: 10.1083/jcb.116.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell J. E., Jr Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Curr. Opin. Cell Biol. 2002;14:140–148. doi: 10.1016/s0955-0674(02)00314-9. [DOI] [PubMed] [Google Scholar]
- Francisco L., Wang W., Chan C. S. Type 1 protein phosphatase acts in opposition to IpL1 protein kinase in regulating yeast chromosome segregation. Mol. Cell. Biol. 1994;14:4731–4740. doi: 10.1128/mcb.14.7.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller B. G., Lampson M. A., Foley E. A., Rosasco-Nitcher S., Le K. V., Tobelmann P., Brautigan D. L., Stukenberg P. T., Kapoor T. M. Midzone activation of aurora B in anaphase produces an intracellular phosphorylation gradient. Nature. 2008;453:1132–1136. doi: 10.1038/nature06923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruppuso P. A., Johnson G. L., Constantinides M., Brautigan D. L. Phosphorylase phosphatase regulatory subunit. J. Biol. Chem. 1985;260:4288–4294. [PubMed] [Google Scholar]
- Guse A., Mishima M., Glotzer M. Phosphorylation of ZEN-4/MKLP1 by aurora B regulates completion of cytokinesis. Curr. Biol. 2005;15:778–786. doi: 10.1016/j.cub.2005.03.041. [DOI] [PubMed] [Google Scholar]
- Hauf S., Cole R. W., LaTerra S., Zimmer C., Schnapp G., Walter R., Heckel A., van Meel J., Rieder C. L., Peters J. M. The small molecule hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J. Cell Biol. 2003;161:281–294. doi: 10.1083/jcb.200208092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu J. Y., et al. Mitotic phosphorylation of histone H3 is governed by Ipl1/aurora kinase and Glc7/PP1 phosphatase in budding yeast and nematodes. Cell. 2000;102:279–291. doi: 10.1016/s0092-8674(00)00034-9. [DOI] [PubMed] [Google Scholar]
- Huang F. L., Glinsmann W. H. Separation and characterization of two phosphorylase phosphatase inhibitors from rabbit skeletal muscle. Eur. J. Biochem. 1976;70:419–426. doi: 10.1111/j.1432-1033.1976.tb11032.x. [DOI] [PubMed] [Google Scholar]
- Ingebritsen T. S., Cohen P. The protein phosphatases involved in cellular regulation. 1. Classification and substrate specificities. Eur. J. Biochem. 1983;132:255–261. doi: 10.1111/j.1432-1033.1983.tb07357.x. [DOI] [PubMed] [Google Scholar]
- Kallio M. J., McCleland M. L., Stukenberg P. T., Gorbsky G. J. Inhibition of aurora B kinase blocks chromosome segregation, overrides the spindle checkpoint, and perturbs microtubule dynamics in mitosis. Curr. Biol. 2002;12:900–905. doi: 10.1016/s0960-9822(02)00887-4. [DOI] [PubMed] [Google Scholar]
- Keen N., Taylor S. Aurora-kinase inhibitors as anticancer agents. Nat. Rev. Cancer. 2004;4:927–936. doi: 10.1038/nrc1502. [DOI] [PubMed] [Google Scholar]
- Knowlton A. L., Lan W., Stukenberg P. T. Aurora B is enriched at merotelic attachment sites, where it regulates MCAK. Curr. Biol. 2006;16:1705–1710. doi: 10.1016/j.cub.2006.07.057. [DOI] [PubMed] [Google Scholar]
- Leach C., Shenolikar S., Brautigan D. L. Phosphorylation of phosphatase inhibitor-2 at centrosomes during mitosis. J. Biol. Chem. 2003;278:26015–26020. doi: 10.1074/jbc.M300782200. [DOI] [PubMed] [Google Scholar]
- Li M., Satinover D. L., Brautigan D. L. Phosphorylation and functions of inhibitor-2 family of proteins. Biochemistry. 2007;46:2380–2389. doi: 10.1021/bi602369m. [DOI] [PubMed] [Google Scholar]
- Li M., Stefansson B., Wang W., Schaefer E. M., Brautigan D. L. Phosphorylation of the Pro-X-Thr-Pro site in phosphatase inhibitor-2 by cyclin-dependent protein kinase during M-phase of the cell cycle. Cell. Signal. 2006;18:1318–1326. doi: 10.1016/j.cellsig.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Li M., Stukenberg P. T., Brautigan D. L. Binding of phosphatase inhibitor-2 to prolyl isomerase Pin1 modifies specificity for mitotic phosphoproteins. Biochemistry. 2008;47:292–300. doi: 10.1021/bi701819k. [DOI] [PubMed] [Google Scholar]
- Lou Y., Yao J., Zereshki A., Dou Z., Ahmed K., Wang H., Hu J., Wang Y., Yao X. NEK2A interacts with MAD1 and possibly functions as a novel integrator of the spindle checkpoint signaling. J. Biol. Chem. 2004;279:20049–20057. doi: 10.1074/jbc.M314205200. [DOI] [PubMed] [Google Scholar]
- Nigg E. A. Centrosome aberrations: cause or consequence of cancer progression? Nat. Rev. Cancer. 2002;2:815–825. doi: 10.1038/nrc924. [DOI] [PubMed] [Google Scholar]
- Ohkura H., Kinoshita N., Miyatani S., Toda T., Yanagida M. The fission yeast dis2+ gene required for chromosome disjoining encodes one of two putative type 1 protein phosphatases. Cell. 1989;57:997–1007. doi: 10.1016/0092-8674(89)90338-3. [DOI] [PubMed] [Google Scholar]
- Pinsky B. A., Kotwaliwale C. V., Tatsutani S. Y., Breed C. A., Biggins S. Glc7/protein phosphatase 1 regulatory subunits can oppose the Ipl1/aurora protein kinase by redistributing Glc7. Mol. Cell. Biol. 2006;26:2648–2660. doi: 10.1128/MCB.26.7.2648-2660.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach P., Roach P. J., DePaoli-Roach A. A. Phosphoprotein phosphatase inhibitor-2. Identification as a species of molecular weight 31,000 in rabbit muscle, liver, and other tissues. J. Biol. Chem. 1985;260:6314–6317. [PubMed] [Google Scholar]
- Robinson J. P. International Society for Analytical Cytology. Current Protocols in Cytometry. New York: Wiley; 1997. [Google Scholar]
- Rosasco-Nitcher S. E., Lan W., Khorasanizadeh S., Stukenberg P. T. Centromeric Aurora-B activation requires TD-60, microtubules, and substrate priming phosphorylation. Science. 2008;319:469–472. doi: 10.1126/science.1148980. [DOI] [PubMed] [Google Scholar]
- Satinover D. L., Leach C. A., Stukenberg P. T., Brautigan D. L. Activation of Aurora-A kinase by protein phosphatase inhibitor-2, a bifunctional signaling protein. Proc. Natl. Acad. Sci. USA. 2004;101:8625–8630. doi: 10.1073/pnas.0402966101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M., Stukenberg P. T., Kirschner M. W., Lu K. P. The essential mitotic peptidyl-prolyl isomerase Pin1 binds and regulates mitosis-specific phosphoproteins. Genes Dev. 1998;12:706–720. doi: 10.1101/gad.12.5.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q., King R. W. Chromosome nondisjunction yields tetraploid rather than aneuploid cells in human cell lines. Nature. 2005;437:1038–1042. doi: 10.1038/nature03958. [DOI] [PubMed] [Google Scholar]
- Sorrentino R., et al. Aurora B overexpression associates with the thyroid carcinoma undifferentiated phenotype and is required for thyroid carcinoma cell proliferation. J. Clin. Endocrinol. Metab. 2005;90:928–935. doi: 10.1210/jc.2004-1518. [DOI] [PubMed] [Google Scholar]
- Stefansson B., Brautigan D. L. Protein phosphatase 6 subunits with conserved saps domain target Ikappa Bepsilon. J. Biol. Chem. 2006;281:39891–39896. doi: 10.1074/jbc.M608155200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X., Szeto A., Xu B. The role of protein phosphatase one (PP1) in regulation of Histone H3 phosphorylation. Mol. Biol. Cell. 2004;15:255a–255a. [Google Scholar]
- Tountas N. A., Brautigan D. L. Migration and retraction of endothelial and epithelial cells require PHI-1, a specific protein-phosphatase-1 inhibitor protein. J. Cell Sci. 2004;117:5905–5912. doi: 10.1242/jcs.01506. [DOI] [PubMed] [Google Scholar]
- Tountas N. A., Mandell J. W., Everett A. D., Brautigan D. L. Juxtamembrane localization of the protein phosphatase-1 inhibitor protein PHI-1 in smooth muscle cells. Histochem. Cell Biol. 2004;121:343–350. doi: 10.1007/s00418-004-0642-8. [DOI] [PubMed] [Google Scholar]
- Tung H. Y., Wang W., Chan C. S. Regulation of chromosome segregation by Glc8p, a structural homolog of mammalian inhibitor 2 that functions as both an activator and an inhibitor of yeast protein phosphatase 1. Mol. Cell. Biol. 1995;15:6064–6074. doi: 10.1128/mcb.15.11.6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vischioni B., Oudejans J. J., Vos W., Rodriguez J. A., Giaccone G. Frequent overexpression of aurora B kinase, a novel drug target, in non-small cell lung carcinoma patients. Mol. Cancer Ther. 2006;5:2905–2913. doi: 10.1158/1535-7163.MCT-06-0301. [DOI] [PubMed] [Google Scholar]
- Walczak C. E., Heald R. Mechanisms of mitotic spindle assembly and function. Int. Rev. Cytol. 2008;265:111–158. doi: 10.1016/S0074-7696(07)65003-7. [DOI] [PubMed] [Google Scholar]
- Wang W., Cronmiller C., Brautigan D. L. Maternal phosphatase inhibitor-2 is required for proper chromosome segregation and mitotic synchrony during Drosophila embryogenesis. Genetics. 2008;179:1823–1833. doi: 10.1534/genetics.108.091959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano H., Ishii K., Yanagida M. Phosphorylation of dis2 protein phosphatase at the C-terminal cdc2 consensus and its potential role in cell cycle regulation. EMBO J. 1994;13:5310–5318. doi: 10.1002/j.1460-2075.1994.tb06865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.