Abstract
Tumors derived from rat LA7 cancer stem cells (CSCs) contain a hierarchy of cells with different capacities to generate self-renewing spheres and tubules serially ex vivo and to evoke tumors in vivo. We isolated two morphologically distinct cell types with distinct tumorigenic potential from LA7-evoked tumors: cells with polygonal morphology that are characterized by expression of p21/WAF1 and p63 and display hallmarks of CSCs and elongated epithelial cells, which generate tumors with far less heterogeneity than LA7 CSCs. Serial transplantation of elongated epithelial cells results in progressive loss of tumorigenic potential; tumor heterogeneity; CD44, E-cadherin, and epithelial cytokeratin expression and increased α-smooth muscle actin I and vimentin expression. In contrast, serial transplantation of LA7 CSCs can be performed indefinitely and results in tumors that maintain their heterogeneity, consistent with self-renewal and multilineage differentiation potential. Collectively, our data show that polygonal cells are CSCs, whereas epithelial elongated cells are lineage-committed progenitors with tumorigenic potential, and suggest that tumor progenitors, although lacking indefinite self-renewal potential, nevertheless may make a substantial contribution to tumor development. Because LA7 cells can switch between conditions that favor maintenance of pure CSCs vs. differentiation into other tumor cell types, this cell system provides the opportunity to study factors that influence CSC self-renewal and differentiation. One factor, p63, was identified as a key gene regulating the transition between CSCs and early progenitor cells.
Keywords: CD133, CD44, mammospheres, tumor-initiating progenitors, p21/WAF1
The cancer stem cell (CSC) hypothesis posits that tumors are derived from mutated stem cells that have retained, or progenitors that have regained, the stem cell property of cell self-renewal (1). Only stem cells possess the capacity for indefinite self-renewal, whereas lineage-committed progenitor cells have lost the capacity for self-renewal but still possess extensive proliferation and differentiation potential (2). The ability to form and regenerate spheres in culture has been correlated with self-renewing stem cells and CSCs (3, 4). In addition, it has been shown that CD24−/CD44+/LIN− cells within human breast tumors have the capacity to initiate breast tumor growth in nonobese diabetic severe combined immunodeficient (NOD SCID) immunocompromised mice (5). The CD44+ phenotype has also been correlated with colon, prostate, and pancreatic cancer–initiating cells (6–8). However, these studies were unable to demonstrate tumor outgrowth from a single cell. Because CSCs could not be isolated and characterized in detail at the single-cell level, these studies could not resolve whether more than one type of cell within tumors might retain substantial, although not limitless, tumorigenicity.
We have used LA7AA10 cells, isolated by Dulbecco from a mammary adenocarcinoma induced in rat by dimethylbenzanthracene to study mammary gland differentiation (9–16) and, more recently, as a potential CSC model (17). A subclone of LA7AA10 cells, LA73F12ms (which we designate as stem LA7 [sLA7]) cells have the ability, at the single-cell level, to generate branched ductal-alveolar–like structures in three-dimensional (3D) cultures that morphologically and functionally recapitulate the tubuloalveolar architecture of the mammary tree. Exposure of sLA7 cells to lactogenic hormones, lipids, or differentiating agents (e.g., DMSO) results in the formation of dome-shaped structures representing the cellular changes that occur in the mammary gland at pregnancy, when alveoli are formed (9–16). sLA7 cells express CD29 and CD49f, which mark a mammary stem cell–enriched population in mice (17–19), as well as CD44 and CD133, which are markers for breast, colon, prostate, and pancreatic CSCs and brainstem cells (5–8, 20–23), and p21/WAF1, which is a gene involved in the maintenance of the quiescence of mammary, hematopoietic, and brain stem cells (24–26).
Most importantly, sLA7 cells, at the single-cell level, can initiate tumors in NOD-SCID mice (17). Such tumors have a heterogeneous morphology and contain cells with different capacities to generate self-renewing spheres and 3D organotypic growth (17). Because most cancers, like sLA7-generated tumors, are composed of a heterogeneous population of stem and nonstem cells and tumorigenic and nontumorigenic cells with marked differences in their capacity to proliferate and differentiate (27), we isolated different cell types from sLA7-generated tumors based on morphology and determined their ability to sustain sphere formation, their capacity for 3D organotypic growth, and their tumor-formation potential by transplanting them into NOD-SCID mice.
Here, we report that sLA7-generated tumors contain at least three distinct cell types: polygonal, epithelial elongated, and mesenchyme-like cells, distinguishable by their morphology and marker expression. The polygonal and epithelial elongated cells have different properties in terms of the ability to sustain sphere formation serially and capacity for 3D organotypic growth and in terms of tumor-development and tumor-sustaining capacity. The mesenchyme-like cells were not extensively characterized because these cells exhibited limited expansion capacity ex vivo and no capacity to generate spheres and 3D organotypic growth. Collectively, our data show that polygonal cells are self-renewing CSCs and epithelial elongated cells are lineage-committed progenitor cells and that the switch from CSCs to progenitors is associated with loss of p63.
Results
sLA7 Cells Give Rise to Heterogeneous Tumors.
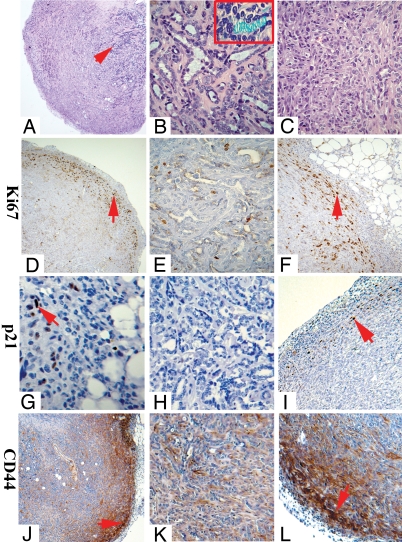
We generated tumors by injecting 105 sLA7 cells into NOD-SCID mice. Similar to tumors evoked by a single sLA7, these tumors had a morphologically complex core (Fig. 1 A and B) containing differentiated structures and functional secretory tubules, as shown by Alcian blue staining (Fig. 1B). Secretion capacity is indicative of the presence of terminally differentiated cells (15). Immunological analysis showed that the tumor core almost completely lacks cells staining for Ki67, a marker for cell proliferation (Fig. 1E) or CD44 (Fig. 1K), and has a large number of cytokeratin K14+ and K18+ cells (not shown), markers for mammary cell lineage–specific differentiation (19, 28). The paucity of Ki67+ cells confirms that the core consists predominantly of terminally differentiated cells. In addition, a complete lack of staining for p21/WAF1 was observed at the tumor core (Fig. 1H). In contrast, the peripheral area of the tumors lacked differentiated structures (Fig. 1C) but contained a high number of Ki67+ cells; these cells constituted an outwardly expanding front of highly proliferative cells (Fig. 1 D and F, arrows). Some cells at the tumor front stained with p21/WAF1 antibodies [Fig. 1 G and I, arrows, and supporting information (SI) Fig. S1], but these cells were far fewer than Ki67+ cells (Fig. 1F). Strong CD44 staining was detected predominantly at the tumor periphery and became progressively less closer to the tumor core (Fig. 1 J–L, arrows). Staining of Cytokeratin 14 (K14) and Cytokeratin 18 (K18) was also observed at the tumor front (not shown). Thus, sLA7 cells generate heterogeneous tumors characterized by a periphery defined by an expanding front of Ki67+, CD44+, and p21/WAF1+ cells and a core of differentiated cells lacking p21/WAF1.
Fig. 1.
Staining of a tumor generated by 100,000 sLA7 cells. H&E staining: panorama (arrow, core of tumor) (A), core (B) (Inset: lumen staining of secreted proteins), and periphery (C). Antibody staining for Ki67: panorama (D), core (E), and periphery (F). Staining for p21/WAF1: periphery (arrow, p21/WAF1+ cells) (G), core (H), and periphery (arrow, p21/WAF1+ cells) (I). Staining for CD44: panorama (J), core (K), and periphery (L). Arrows indicate tumor front. (Magnifications: A, X2.5; D, G, and J, X5; H, K, F, and L, X20; B, C, and E, X40; B (Inset), X60.)
Morphologically Different Cell Types from sLA7-Evoked Tumors Have Different Biological Properties.
sLA7 cells can, at the single-cell level, serially and indefinitely sustain sphere formation and have high capacity for 3D organotypic growth (17). Serial regeneration of sphere and tubule formation was demonstrated using an assay in which single cells from a sphere or tubule are able to generate second and later generations of new spheres or new tubules clonally (17). We showed that the intrinsic capacity to sustain sphere formation is an indication that sLA7 cells can clonally maintain the capacity for trilineage differentiation and mammary 3D organotypic growth (17). We therefore used the capacity for sphere sustainability as a criterion, combined with morphological phenotype and marker expression, to characterize cells isolated from sLA7-derived tumors.
Cells from a tumor generated by 105 sLA7 were plated at low density to isolate single-cell–derived colonies. The characteristics of the various colonies were then determined. Three morphologically distinct cell types were distinguishable microscopically. A small number of colonies contained cells with a polygonal morphology similar to that of the parental sLA7 cells (Fig. S2). Single colonies of polygonal cells growing as holoclones [a phenotype associated with self-renewing keratinocytes and prostate carcinoma cells (29, 30)] were isolated and cultured to generate cell lines and tested for the expression of a variety of markers, including CD29, CD49f, CD44, CD133, p21/WAF1, K14, and K18. Expression of E-Cadherin and p63 was also tested because both have been correlated with the maintenance of the undifferentiated state of mammary and skin stem cells (24, 31) and because p63 was found expressed in sLA7 cells and down-regulated in DMSO-differentiated sLA7 cells (I.Z. and R.D., unpublished data). All the cell lines derived from the polygonal cells, like the parental sLA7, expressed CD29, CD49f, CD44, CD133, p21/WAF1, E-Cadherin, and p63, and they lacked keratin 14 and 18 expression (not shown). Cells from each cell line could also serially generate and sustain sphere formation (for at least 10 passages) and tubule formation ex vivo (for at least 5 passages) and had trilineage differentiation potential (not shown).
We chose one polygonal cell line that met all the previous criteria for further studies. Like sLA7, this cell line (sLA7-derivative clone 16, Fig. S2A), in addition to CD29, CD49f, and CD133 (all not shown), expressed E-Cadherin (Fig. S2B), p21/WAF1 (Fig. S2C), p63 (Fig. S2D), and CD44 (Fig. S2E), and it had an extensive capacity to proliferate in vitro (30 passages) and, at the single-cell level, to serially generate and sustain spheres (Fig. S2F), tubules (Fig. S2G), cysts (not shown), and domes (Fig. S2H). Cysts and domes recapitulate some aspects of alveologenesis in vitro (15). Like sLA7, this cell line lacks expression of keratins 14 (Fig. S2C) and 18 (not shown), as determined by immunohistochemistry and confirmed by Western blot analysis (not shown). Because this cell line had many of the characteristics of sLA7 cells, we designated it as LA7 stem-like (LA7SL) to distinguish it from the sLA7 line.
We also observed colonies composed of two other morphologically distinct cell types: mesenchyme-like and epithelial elongated cells. Mesenchyme-like colonies (designated as LA7ML, Fig. S2I) had a limited proliferative capacity ex vivo (three to five passages) and could not form tubules, cysts, or domes (not shown). When cultured in nonadherent conditions, LA7ML formed disorganized aggregates instead of spheres (Fig. S2J). LA7ML cells (analyzed at passage two to three) expressed CD29, CD49f, CD133, and CD44, and lacked p21/WAF1, p63, E-Cadherin, K14, and K18 expression (all not shown).
Elongated epithelial colonies (designated as LA7E) derived from single cells were individually collected and cultured for 2 passages. Although only one culture, LA7E12 (clone 12), is shown as an example (Fig. S2K), all cultures tested expressed CD29, CD49f, and CD133 (all not shown); K14 (Fig. S2L); CD44 (Fig. S2N); and E-Cadherin (Fig. S2O), and they lacked p21/WAF1 (Fig. S2 L and M), K18 (Fig. S2M), and p63 (not shown) expression. Like LA7SL cells, LA7E cultures showed extensive capacity for ex vivo proliferation (for at least 30 passages) and could form spheres (Fig. S2P) and tubules (Fig. S2Q). Unlike LA7SL cells, however, LA7E cultures could not sustain sphere or tubule formation for more than 1–3 passages.
To assess the tumorigenicity of the morphologically distinct cell types, we carried out transplantation studies using 100 cells obtained from combined cultures showing the same properties of LA7E or LA7ML cells, respectively, and 100 cells derived from a single LA7SL holoclone. Injection of 100 LA7ML cells resulted in no palpable tumors out of 12 fat pads at day 90 (not shown). Injection of LA7SL or LA7E at day 90 resulted in five and four tumors, respectively (ranging in size from 100–300 mm3), out of 6 fat pads injected for each case (not shown).
To assess the tumor-renewing capacity of LA7SL and LA7E cells, we performed serial transplantation studies using 100 LA7 polygonal or 100 LA7 epithelial elongated cells isolated from a tumor generated from 100 parental sLA7 cells (Figs. S3, S4A, and S5). The tumorigenicity of the LA7ML cells was not extensively evaluated because, as indicated previously, these cells exhibited only limited expansion capacity ex vivo. At each tumor generation, LA7 polygonal and LA7 epithelial elongated cells were isolated by morphology and then characterized for marker expression, sphere and 3D organotypic growth sustainability, and trilineage differentiation potential. A unique pair of primers that amplify different size fragments for mouse (300 bp) and rat (1100 bp) genomic DNA was used to confirm by PCR that LA7SL, LA7E, and LA7ML cells were rat cells free of mouse cells (Fig. S4B).
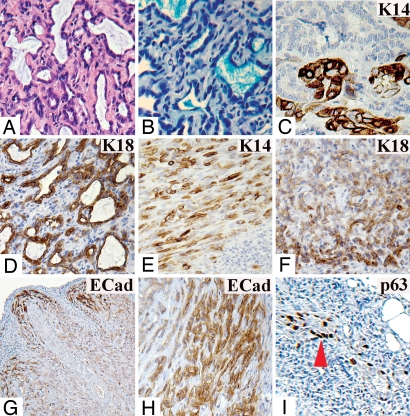
Serial transplantation of the polygonal cells was performed as follows: 100 LA7SL1 cell injection (LA7SL1 cells were isolated from a single holoclone obtained from a first-generation tumor seeded by injecting 100 sLA7 cells; Figs. S3, S4A, and S5) resulted in a second-generation tumor from which LA7SL2 cells were isolated and selected. Then, 100 LA7SL2 cells were used to obtain third-generation tumors, and 100 LA7SL3 cells selected from a third-generation tumor were used to make the fourth-generation tumor; 100 LA7SL4 cells, selected from a fourth-generation tumor gave rise to fifth-generation tumors (Figs. S4A and S5). At each serial transplantation, a holoclone of LA7SL type was isolated and selected using the criteria previously described. sLA7 (parental) or LA7SL1 (first generation) cell injection resulted in four and five tumors out of six fat pads for each cell type (Figs. S3, S4A, and S5). LA7SL2, LA7SL3, or LA7SL4 resulted in five, four, and five tumors, respectively, out of six fat pads injected in each case. All tumors generated from injection of LA7SL-derived cells were structurally identical to the tumors generated by sLA7 cells, exhibiting a heterogeneous core with functional tubules (Fig. 2 A and B) and K14- and K18-expressing cells detected at the tumor core and periphery (Fig. 2. C–F, respectively). E-Cadherin+ cells were localized predominantly at the tumor periphery of all LA7SL-generated tumors, as shown for LA7SL1- (Fig. 2G) and sLA7- (Fig. 2H) generated tumors. Furthermore, p21/WAF1 (not shown) and p63 staining (shown for the sLA7-generated tumor in Fig. 2I) were localized exclusively to the expanding tumor periphery.
Fig. 2.
Staining of a tumor generated by LA7SL1. (A) H&E staining, core. (B) Alcian blue staining of tubule-secreted proteins. Antibody staining for: K14, core (C); K18, core (D); K14, periphery (E); K18, periphery (F); and E-Cadherin, panorama (G). (H) E-Cadherin antibody staining of a tumor generated by 100 sLA7 cells (periphery). (I) p63 antibody staining of tumor generated by 105 cells periphery (arrow, p63+ cells). (Magnifications: G, X5; F and I, X10; A, D, E, and H, X20; B and C, X40.)
Because polygonal cells gave rise to tumors (for at least five generations) that apparently maintain the same differentiative capacity (as assessed by morphology and marker expression) and from which polygonal cells with the same properties can be reisolated, we conclude that they are CSCs. Moreover, these cells appear to be enriched at the edges of the tumor, where they may drive tumor expansion.
Epithelial Elongated Cells Have Tumor-Initiating but Not Tumor Self-Renewal Capacity.
Second-generation tumors were initiated using 100 LA7E1 cells obtained from epithelial elongated cells isolated and selected (as described for LA7E) from a first-generation tumor seeded with 100 sLA7 cells (Figs. S3, S4A, and S5). We carried out serial transplantations of the epithelial elongated cells using an analogous approach to that described previously for LA7SL-derived cells. Injection of LA7E1 (second generation) or LA7E2 (third generation) resulted in four and three tumors, respectively, out of 6 fat pads injected in each case (Figs. S3, S4A, and S5). Injection of LA7E3 (fourth generation) resulted in no palpable tumors out of 12 fat pads injected at day 90. Therefore, 100 LA7E1 cells could give rise to tumors for two generations but thereafter lost their tumorigenic capacity.
All the LA7E1-derived cultures expressed CD29, CD49f, CD44, CD133, K14, and E-Cadherin but lacked p21/WAF1, p63, and K18 expression and could initiate but not sustain sphere or tubule formation for more than one to three passages (not shown).
In contrast to LA7E1, all LA7E2 and LA7E3 colonies, in addition to lacking expression of p21/WAF, p63, and K18, lacked expression of E-Cadherin and K14 and had no capacity to initiate sphere or tubule formation (not shown).
Serially Isolated and Transplanted Epithelial Elongated Cells Show Progressive Loss of Tumor Heterogeneity.
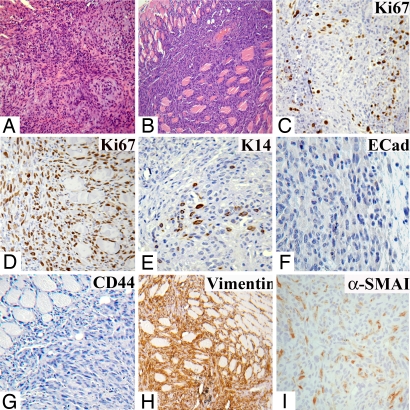
Second-generation tumors derived from LA7E1 cells had central and peripheral areas that, compared with tumors generated by sLA7 (or LA7SL) cells, were more homogeneous (Fig. 3 A and B). This loss of tumor heterogeneity is in agreement with the ex vivo results showing that although LA7E1 cells have an extensive capacity to proliferate, they do not have sphere or 3D organotypic growth-regenerating properties.
Fig. 3.
Staining of a tumor generated by LA7E1 cells. H&E staining: core (A) and periphery (B). Antibody staining for: Ki67, core (C); Ki67, periphery (D); K14, core (E); E-Cadherin, periphery (F); CD44, periphery (G); vimentin, periphery (H); and α-SMAI, periphery (I). (Magnifications: A, B, and H, X10; C–G, and I, X20.)
In contrast to tumors generated by sLA7 or LA7SL tumors, in LA7E1-derived tumors, Ki67+ cells were found at the tumor core and periphery (Fig. 3 C and D); staining for K14 was confined to the tumor core (Fig. 3E), and staining for p63 was not detected (not shown). Also, in contrast to sLA7- or LA7SL-generated tumors in which strong staining for E-Cadherin (Fig. 2 G and H) and CD44 (Fig. 1 J and L) was detected at the tumor periphery, in the LA7E1-generated tumors, staining for E-Cadherin (Fig. 3F) and CD44 (Fig. 3G) was not detected at the periphery but was confined to the core (not shown).
To better characterize second-generation tumors derived from LA7E1 cells, we carried out immunostaining for two additional markers, vimentin and α-smooth actin muscle I (α-SMAI), which are often associated with loss of expression of K14 in committed transitional cells (32). Notably, there was a marked increase in the number of vimentin-expressing cells in second-generation LA7E1 tumors (Fig. 3H) compared with second-generation tumors derived from sLA7 cells (not shown). Moreover, α-SMAI, which was not significantly detected in sLA7-derived tumors (not shown), was observed at the periphery of the second generation of LA7E1 tumors (Fig. 3I).
The third-generation tumors had central (Fig. S6A) and peripheral (not shown) areas characterized by a complete loss of heterogeneity, with no structural complexity or differentiated structures. Such tumors showed high and homogeneous Ki67 staining throughout (Fig. S6B, core [periphery not shown]), and in contrast to second-generation tumors obtained by LA7E1, K14 (Fig. S6C), E-Cadherin (not shown), and CD44 (Fig. S6D) staining was not observed. The high number of cells expressing Ki67 throughout the tumor may correlate with the higher slopes of the tumor growth curve observed in the third-generation tumors as compared with the second-generation tumors (see Fig. S3). Also, compared with LA7E1-derived tumors, increased numbers of vimentin- (Fig. S6E) and α-SMAI– (Fig. S6F) expressing cells were observed.
Thus, although LA7E1 cells retained substantial ex vivo proliferative capacity, their ability to self-renew, in addition to their differentiative potential, was much more restricted than that of LA7SL cells. Hence, we suggest that LA7E1 cells are lineage-committed progenitor cells with significant but not indefinite cell proliferation and tumorigenic capacity (as opposed to CSCs).
p63, a Key Regulatory Gene in the Switch Between sLA7 and LA7E1.
Our data demonstrate that the LA7E1 early progenitor cells have many functional properties similar to sLA7 CSCs, including the ability to generate mammospheres and tubules for one to three passages. Our data also show that the LA7E1 cells are indistinguishable from the sLA7 CSCs in terms of the expression of the stem cell markers CD44 and CD133, because sLA7, LA7E1, LA7E2, and LA7E3 all express CD133 (Fig. S4A); although LA7E2 and LA7E3 do not express CD44 mRNA (Fig. S7A), all or nearly all sLA7 and LA7E1 cells express CD44 protein at the cell surface (Fig. S7B).
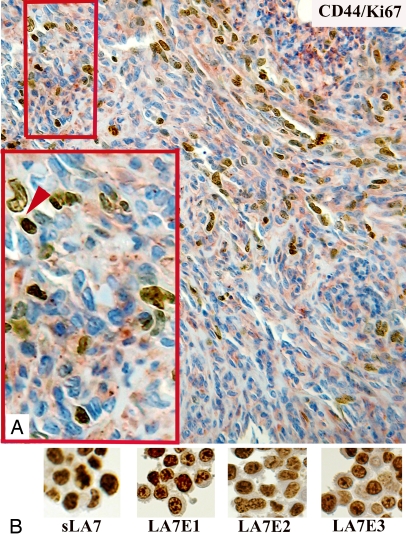
Therefore, because we have previously shown that differentiating or differentiated cells derived from sLA7CSCs, although expressing CD44 and CD133, have limited tumor initiation capacity (17), we can conclude that CD44 and CD133 expression is not just restricted to cells with tumor-initiating potential and that not all tumor-initiating cells express CD44 (e.g., LA7E2). In agreement with this conclusion, we show that in tumors generated by sLA7, not all CD44+ cells are Ki67+ (Fig. 4A, arrow) and that not all Ki67+ cells with tumorigenic potential (e.g., LA7E2, Fig. 4B) express CD44 (Fig. S7A). From these observations, we can conclude that CD44 and CD133 are markers that cannot be used to distinguish sLA7CSCs from progenitor cells with tumorigenic potential.
Fig. 4.
Ki67 and CD44 double-staining of a tumor generated by sLA7 cells. (A) Antibody staining for Ki67 (nuclear, brown), CD44 (red), and Hoechst nuclear dye 33342 (blue). (Magnifications: X20; Inset, X40.) (B) Antibody staining for Ki67 (nuclear, brown) on sLA7, LA7E1, LA7E2, and LA7E3 cells. (Magnification: X40.)
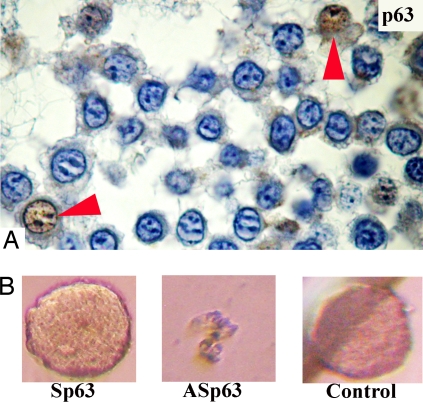
In contrast to CD44 and CD133, p63 protein can be used to distinguish LA7E1 cells from sLA7 cells (Fig. S8A). Because sLA7 cells have the capacity for indefinite self-renewal and tumor regeneration, we hypothesized that p63 is essential for sLA7 cells to indefinitely regenerate mammospheres. To investigate whether p63 is involved in the transition of sLA7 to early progenitor cells, we down-regulated p63 in sLA7 cells using an antisense oligo approach. sLA7 cells were cultured in nonadherent conditions in the presence of p63 antisense oligos for 4 days and monitored for sphere formation potential. Fig. S8B shows that p63, expressed by sLA7 and sLA7-generated mammospheres, is down-regulated in sLA7 cells treated with antisense p63 and in differentiating/differentiated cells derived from DMSO-induced sLA7. In sLA7-generated mammospheres, p63 was found expressed in only a few cells (Fig. 5A, arrows). It was observed that unlike sLA7 cells, p63 antisense oligo-treated sLA7 cells could generate spheres only for one to three generations (Fig. 5B). Loss of indefinite sphere formation capacity suggests that loss of stem cell renewal potential is a consequence of the down-regulation of p63 protein. Sphere formation inhibition was not attributable to an antiproliferative effect, because we determined that p63 sense or p63 antisense oligos do not affect the cell proliferation capacity of the sLA7 cells (Fig. S8C).
Fig. 5.
(A) p63 immunostaining of a sphere generated by a single sLA7 cell. p63 protein is expressed in self-renewing sLA7 mammospheres (arrows, p63+ cells). Hoechst nuclear dye 33342 (blue). (B) Down-regulation of p63 in sLA7 results in loss of indefinite sphere-regeneration potential. sLA7 cells were cultured with no sense oligos p63 (Sp63), antisense p63 (ASp63), or oligos (control) for 96 h in nonadherent conditions.
Down-Regulation of p63 Results in the Loss of Stem Cell Self-Renewal and in the Generation of LA7E1 Cells.
Transient knockdown of p63 protein also results in the inability of sLA7 to self-renew and to generate holoclones; p63 antisense oligo-treated sLA7 cells cultured in non-differentiating conditions gain the ability to generate LA7E1 cells, as shown in Fig. S9A, and are induced to differentiate, as shown by the formation of domes (Fig. S9B).
Discussion
Previously, we showed that sLA7 cells propagated ex vivo under standard culture conditions retain the capacity at the single-cell level to generate and sustain sphere formation indefinitely (17). Furthermore, sLA7-generated spheres have trilineage differentiation potential as well as significant capacity for ex vivo 3D mammary organotypic growth (17). Here, we have shown that sLA7-generated tumors contain a hierarchy of cells with different capacities to generate self-renewing spheres and tubules serially.
We have further characterized this unique cell system by isolating three distinct types of cells from sLA7-generated tumors that differ in morphology, immunophenotype, and their ability to sustain sphere and tumor formation. Mesenchyme-like cells have limited ex vivo proliferative capacity and lack sphere-forming ability and tumorigenicity. PCR analysis confirms that the LA7ML cells are derived from sLA7 rather than reactive host stromal cells (Fig. S4B). Presumably, they reflect cells that have undergone an irreversible epithelial-mesenchymal transition. Although such cells may help to support the tumor mass (e.g., by directly providing trophic factors or promoting angiogenesis), they cannot drive further tumor expansion (33).
Polygonal (LA7SL) and elongated epithelial (LA7E) cells, like the sLA7 CSCs from which they derive, express the normal mammary stem cell markers CD29 and CD49f and the putative CSC markers CD133 and CD44. LA7SL cells, like sLA7 CSCs, are characterized by expression of p21/WAF1 and p63, both of which are involved in stem cell maintenance, generate heterogeneous adenocarcinomas similar to the tumors from which they were originally derived, and can serially regenerate such tumors for five successive generations. In addition to displaying morphological fidelity, LA7SL-derived tumors continue to display the same immunophenotype as parental sLA7-derived tumors (Figs. 2 and S4A). Furthermore, LA7SL cells isolated at each tumor generation on the basis of their polygonal morphology retain the capacity to generate spheres, show 3D organotypic growth, and differentiate into all cell lineages of the mammary gland.
The first-generation LA7E-type cells (LA7E1), also isolated from sLA7-generated tumors, are characterized by expression of K14, CD44, and E-Cadherin and lack of p63 and p21/WAF1 expression. These cells generate tumors with far less complex differentiated structures; lack p63 expression; and lose expression of K14, CD44, or E-Cadherin. Tumor heterogeneity and, ultimately, tumorigenicity itself decline on serial transplantation of LA7E-type tumor cells. Furthermore, LA7E1 cells can only generate spheres or give rise to 3D organotypic growth for one to three passages. Because LA7E1 cells have high proliferative potential, we performed cell-limiting dilution transplantation studies and determined that at least 10 cells are required to generate tumors (data not shown). In contrast to this, it was previously determined that 1 sLA7 cell is sufficient to generate a tumor (17), in agreement with the observation that sLA7, but not LA7E1, cells have the capacity for self-renewal.
Taken together, our data suggest that sLA7 cells, when maintained ex vivo under undifferentiating culture conditions, expand as essentially pure cultures of CSCs. When placed in the appropriate environment (niche), such as sphere-forming culture conditions or the mammary fat pad, these cells display substantial differentiative capacity while retaining their self-renewal potential, both of which are hallmarks of CSCs. In addition to giving rise to additional CSCs (LA7SL-type cells), they generate cells that retain substantial proliferative ability (LA7E-type cells) but that have more restrictive differentiative capacity and no self-renewal capacity and generate cells that appear to differentiate terminally.
Our findings have several important implications. Although LA7E-type cells cannot infinitely self-renew, they still have substantial proliferative capacity and tumor-initiating potential. It has been suggested that it might be possible to cure most tumors simply by ablating CSCs (27). If the proliferative capacity of most “tumor progenitors” resembles that of LA7E cells, it seems likely that most tumors presenting clinically with a critical number of tumor progenitor cells might be lethal even if all CSCs were killed. We also show that early progenitor cells like LA7E1 are indistinguishable from LA7 CSCs in terms of stem cell markers CD29, CD49f, CD44, and CD133. In addition, early progenitor cells have the capacity, although limited, to generate mammospheres, tubuli, and CD44+ and CD44− cells. Our results provide an alternative explanation as to why different types of breast tumor–initiating cells (with different cell surface markers) are observed in tumors that should theoretically be derived from a single CSC. We suggest that tumor heterogeneity may be partially attributable to the currently unrecognized contribution of tumor-initiating progenitor cells to tumor development.
Our research demonstrates that p63 is a key regulator in the maintenance of “stemness” in sLA7 CSCs, in the ability of CSCs to regenerate mammospheres indefinitely, and in the transition of CSCs to early progenitors. In agreement with our results, p63 has recently been shown to be involved in the maintenance of the skin stem cell pool of epidermal keratinocytes, and its loss is associated with induction of differentiation (31). Finally, our data suggest that, at least for this cell system, serial sphere-forming capacity may represent a valid surrogate for CSC self-renewal.
We show that although LA7E1 cells lack indefinite self-renewal and sphere-regenerating capacity, they still have tumor-initiating potential. We suggest that sphere sustainability is a property exclusive of cell-renewing stem cells and that the ability of LA7E1 to form tumors is attributable to their inherent extensive capacity for cell proliferation.
Materials and Methods
Cells, Media, and 3D Cultures.
LA7/3F12ms cells were recently subcloned from the LA7AA10 cell line (9, 17). LA7/3F12ms, LA7SL, LA7E, and LA7ML cells were cultured in DMEM as described. 3D cultures were performed using rat tail collagen as described (17).
Cell Transplantation in NOD-SCID Mice.
As described (17), 100,000 or 100 cells were injected into the fat pad of NOD-SCID mice.
Immunohistochemistry.
Cells obtained from tumors or sections from each tumor biopsy were processed for immunohistochemistry as described (17). The primary mouse monoclonal antibodies used are listed in SI Experimental Procedures.
Negative controls were run in parallel with preimmune rabbit serum. Counterstaining was performed with hematoxylin (17).
Supplementary Material
Acknowledgments.
We thank Drs. Marcos J. Arauzo-Bravo, Jürgen Horst, Werner Böcker, Horst Bürger, and Gaetano Finocchiaro for helpful discussions. We also thank Loredana Ansalone and Bernice Walker for extraordinary secretarial support, Drs. L. Emionite and F. Delucchi for tissue preparation, and the staff of the Department of Pathology of Leno BS for their excellent technical support. This work was supported by the Italy-U.S.A. Project Grant N.527 B-B7, Ministero Istruzione Universita e Ricerca-Fondo Investimenti Ricerca di Base Internazionale (FIRB) Grant RBIP0695BB_003, FIRB Grant RBIN04CBSM_000, Telethon Grant GG004247, Net2Drug Grant 037590, and the Network Operativo per la Biomedicine di Eccellenza in Lombardia grant funded by Fondazione CARIPLO.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0808978105/DCSupplemental.
References
- 1.Reya T, Morrison S-J, Clarke M-F, Weissman I-L. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 2.Dick J-E. Breast cancer stem cells revealed. Proc Natl Acad Sci USA. 2003;100:3547–3549. doi: 10.1073/pnas.0830967100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dontu G, Liu S, Wicha MS. Stem cells in mammary development and carcinogenesis: Implications for prevention and treatment. Stem Cell Review. 2005;1:207–213. doi: 10.1385/SCR:1:3:207. [DOI] [PubMed] [Google Scholar]
- 4.Ponti D, et al. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–5511. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 5.Al-Hajj M, Wicha M-S, Benito-Hernandez A, Morrison S-J, Clarke M-F. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Brien C-A, Pollett A, Gallinger S, Dick J-E. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 7.Collins A-T, Berry P-A, Hyde C, Stower M-J, Maitland N-J. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 8.Li C, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 9.Dulbecco R, Bologna M, Unger M. Differentiation of a rat mammary cell line in vitro. Proc Natl Acad Sci USA. 1979;76:1256–1260. doi: 10.1073/pnas.76.3.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dulbecco R, Bologna M, Unger M. Control of differentiation of a mammary cell line by lipids. Proc Natl Acad Sci USA. 1980;77:1551–1555. doi: 10.1073/pnas.77.3.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zucchi I, Montagna C, Susani L, Vezzoni P, Dulbecco R. The rat gene homologous to the human gene 9–27 is involved in the development of the mammary gland. Proc Natl Acad Sci USA. 1998;95:1079–1084. doi: 10.1073/pnas.95.3.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zucchi I, et al. Genetic dissection of dome formation in a mammary cell line: Identification of two genes with opposing action. Proc Natl Acad Sci USA. 1999;96:13766–13770. doi: 10.1073/pnas.96.24.13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zucchi I, et al. Proteomic dissection of dome formation in a mammary cell line: Role of tropomyosin-5b and maspin. Proc Natl Acad Sci USA. 2001;98:5608–5613. doi: 10.1073/pnas.091101898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zucchi I, Dulbecco R. Proteomic dissection of dome formation in a mammary cell line. J Mammary Gland Biol Neoplasia. 2002;7:373–384. doi: 10.1023/a:1024081914634. [DOI] [PubMed] [Google Scholar]
- 15.Zucchi I, et al. Dome formation in cell cultures as expression of an early stage of lactogenic differentiation of the mammary gland. Proc Natl Acad Sci USA. 2002;99:8660–8665. doi: 10.1073/pnas.132259399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zucchi I, et al. Association of rat8 with Fyn protein kinase via lipid rafts is required for rat mammary cell differentiation in vitro. Proc Natl Acad Sci USA. 2004;101:1880–1885. doi: 10.1073/pnas.0307292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zucchi I, et al. The properties of a mammary gland cancer stem cell. Proc Natl Acad Sci USA. 2007;104:10476–10481. doi: 10.1073/pnas.0703071104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stingl J, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 19.Shackleton M, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 20.Ricci-Vitiani L, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 21.Richardson G-D, et al. CD133, a novel marker for human prostatic epithelial stem cells. J Cell Sci. 2004;117:3539–3545. doi: 10.1242/jcs.01222. [DOI] [PubMed] [Google Scholar]
- 22.Hermann P-C, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Lee A, et al. Isolation of neural stem cells from the postnatal cerebellum. Nat Neurosci. 2005;8:723–729. doi: 10.1038/nn1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woodward W-A, Chen M-S, Behbod F, Rosen J-M. On mammary stem cells. J Cell Sci. 2005;118:3585–3594. doi: 10.1242/jcs.02532. [DOI] [PubMed] [Google Scholar]
- 25.Cheng T, et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- 26.Kippin T-E, Martens D-J, van der Kooy D. p21 loss compromises the relative quiescence of forebrain stem cell proliferation leading to exhaustion of their proliferation capacity. Genes Dev. 2005;19:756–767. doi: 10.1101/gad.1272305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dalerba P, Cho R-W, Clarke M-F. Cancer stem cells: Models and concepts. Annu Rev Med. 2007;58:267–284. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- 28.Gerdes J, et al. Immunobiochemical and molecular biologic characterization of the cell proliferation-associated nuclear antigen that is defined by monoclonal antibody Ki-67. Am J Pathol. 1991;138:867–873. [PMC free article] [PubMed] [Google Scholar]
- 29.Rochat A, Kobayashi K, Barrandon Y. Location of stem cells of human hair follicles by clonal analysis. Cell. 1994;76:1063–1073. doi: 10.1016/0092-8674(94)90383-2. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Chen X, Calhoun-Davis T, Claypool K, Tang D-G. PC3 human prostate carcinoma cell holoclones contain self-renewing tumor-initiating cells. Cancer Res. 2008;68:1820–1825. doi: 10.1158/0008-5472.CAN-07-5878. [DOI] [PubMed] [Google Scholar]
- 31.Blanpain C, Fuchs E. p63: Revving up epithelial stem-cell potential. Nat Cell Biol. 2007;9:731–733. doi: 10.1038/ncb0707-731. [DOI] [PubMed] [Google Scholar]
- 32.Boeker W, Buerger H, Herbst H, Decker T. In: Preneoplasia of the Breast A New Conceptual Approach to Proliferative Breast Disease. Boeker W, editor. Munich: Elsevier Saunders; 2006. pp. 356–359. [Google Scholar]
- 33.Orimo A, Weinberg R-A. Stromal fibroblasts in cancer: A novel tumor-promoting cell type. Cell Cycle. 2006;5:1597–1601. doi: 10.4161/cc.5.15.3112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.