Abstract
In this work we have uncovered a role for Wnt signaling as an important regulator of stem cell self-renewal in the developing brain. We identified Wnt-responsive cells in the subventricular zone of the developing E14.5 mouse brain. Responding cell populations were enriched for self-renewing stem cells in primary culture, suggesting that Wnt signaling is a hallmark of self-renewing activity in vivo. We also tested whether Wnt signals directly influence neural stem cells. Using inhibitors of the Wnt pathway, we found that Wnt signaling is required for the efficient cloning and expansion of single-cell derived populations that are able to generate new stem cells as well as neurons, astrocytes, and oligodendrocytes. The addition of exogenous Wnt3a protein enhances clonal outgrowth, demonstrating not only a critical role for the Wnt pathway for the regulation of neurogenesis but also its use for the expansion of neural stem cells in cell culture and in tissue engineering.
During the development of the nervous system, primitive neurectodermal stem cells act as a source for the specialized neurons, astrocytes, and oligodendrocytes that make up the functioning brain. Several studies have suggested that these precursor cells are able to self-renew, a hallmark of stem cells, and that renewal maintains a reservoir of stem cells throughout life (1). In the embryo, signals provided by the microenvironment regulate the maintenance, proliferation, and neuronal fate commitment of the local stem cell populations. These signals and the microenvironment constitute a niche in which stem cells are present and compete for limiting concentrations of growth factors, thereby maintaining a balance between self-renewal and differentiation of the cells. Factors that regulate renewing versus differentiating cell divisions strongly influence the stem cell pool size.
While much effort has been devoted to understanding the development of the central nervous system in both the embryonic and adult settings, the identity of the signals regulating stem cell activity and neurogenesis is largely unknown. Identifying these factors may increase opportunities to regulate neurogenesis and gliogenesis in vivo for therapy, as well as to grow and expand neural stem cells in culture, a prerequisite for tissue engineering.
Wnt signaling and Wnt proteins are important for the maintenance of stem cells of various lineages. The classic example is in the digestive tract, where in the crypt of the colon the loss of transcription factor TCF4 leads to depletion of stem cells (2, 3). The Wnt pathway has also been implicated as a self-renewal signal in the hematopoietic system (4, 5). Alternatively, loss of the tumor suppressor APC or gain of β-catenin activity leads to deregulated self-renewal and cancer (6, 7).
In the nervous system, the anatomical phenotypes of mouse Wnt mutants suggest that Wnts are involved in regulating neural stem and progenitor cell activity. Loss of Wnt1 results in malformation of most of the midbrain and some rostral metencephalon (8), and Wnt3a mutant mice exhibit underdevelopment of the hippocampus because of lack of proliferation (9). Recent work demonstrating enhanced neurogenesis in vivo via exogenous expression of Wnt3a via lentiviral vectors strengthens the model that the Wnt signaling pathway is a major regulator of adult stem cell activity and fate in the hippocampus (10). A β-catenin gain-of function study by Chenn and Walsh shows that continuous Wnt signaling results in marked and generalized hypercellularity of the brain (11).
While these studies have indicated an important role for Wnt signaling in the control over stem cells, they bring up a number of important questions. Where are the Wnt responsive cells located relative to the known neurogenic zones? Is Wnt responsiveness a hallmark of neural stem cells that enables prospective enrichment for self-renewal? What is the direct effect of Wnt signals on neural stem cells: is it mitogenic or does Wnt control the symmetry of fate in two daughter cells (e.g., self-renewal)? Are Wnt proteins by themselves sufficient to act as a signal for single stem cells in isolation or does Wnt act through indirect mechanisms? Herein we address some of these key questions about the role of Wnt signaling on the fate decision of neural stem cells both in vitro and in vivo, and use purified soluble Wnts as tools to expand and manipulate neural stem cells in culture.
Results and Discussion
The Axin2-LacZ Reporter Visualizes Wnt Signaling in the Developing CNS.
Axin2 is a negative feedback regulator of the Wnt pathway and is expressed in response to Wnt signaling (12). Insertion of a β-galactosidase gene into the Axin2 locus (Axin2-LacZ) provides a useful tool for visualizing cells that are actively responding to Wnt in vivo. The LacZ insert mimics the expression pattern of Axin2 but does not lead to a detectable phenotype in the heterozygous state (13). The pattern of endogenous Wnt pathway activation in the developing mammalian CNS has not been previously reported. Thus, we isolated embryos from heterozygous Axin2-LacZ mice at embryonic day 14.5 (E14.5) and stained tissues with an anti-β-galactosidase antibody.
Wnt-responsive LacZ-positive cells were found scattered throughout the cortex and white matter tracks, consistent with known Wnt signaling in differentiated cells. In addition, a small subpopulation of cells (1–5%) in the subventricular zone (SVZ) expressed the reporter gene (Fig. 1). Morphologically, Axin-2 expressing cells in the SVZ resembled radial glial cells with bipolar morphology and end feet contacting the ventricular and pial surfaces (see Fig. 1 C). Cells with radial glial morphology within the SVZ have been proposed as the central nervous system stem cells (1).
Fig. 1.
A small subpopulation of cells (1–5%) in the ventricular and subventricular zone of E14.5 Axin2-LacZ embryos show expression of the Wnt reporter. (A) Low magnification view (10×) of the lateral ventricle of the reporter mouse (Scale bar, 100 μm); (B) DAPI marking the nuclei of cells lining the ventricle (40× magnification; scale bar, 20 μm); (C) The marker for the activation of the reporter as demonstrated by the FITC staining (green); (D) Overlay of nuclei and reporter shows the population of cells that are Wnt-responsive.
Wnt Proteins and Neural Colony Formation.
An ex vivo approach was used to directly determine if and how Wnt signaling influences neural stem cells. Primary cultures of neural stem and progenitor cells isolated from the fetal SVZ include a heterogenous mixture of cell types, ranging from the most immature self-renewing neural stem cell to fully committed neuronal and glial progenitor cells. In cell culture, the most stringent test for the presence of stem cells is to ask whether single cells can expand into colonies and whether these colonies include progeny that retain the ability to form new clonally derived colonies, each of which can produce neurons, astrocytes, and oligodendrocytes. The formation of secondary or tertiary colonies indicates that self-renewing stem cells were produced in the first (and second) colony and that these individual cells were multipotent, with the capacity to generate progeny that differentiate into neurons, astrocytes, and oligodendrocytes.
A limitation of such colony-forming assays is the inability to distinguish between colonies derived from a single cell versus aggregation of multiple cells. To avoid this caveat, we used mixtures of cells isolated from the forebrains of E14.5 mice from a β-actin GFP (where GFP is expressed in every cell) and a non-GFP mouse of the same background strain to determine the plating density required to produce spheres of clonal origin. As expected, mixed colonies became less common as cell density was decreased. At cell densities greater than 2 cells/μl within a well of a 96-well plate (200 cells per well), we found that 85% of the colonies contained either green or white cells and ≈15% contained mixed phenotype. At cell densities equal to or less than 2 cell/μl (1–200 cells per well), fewer than 5% of the colonies were mixed, suggesting that the majority of colonies formed under these conditions were clonally derived (Fig. 2).
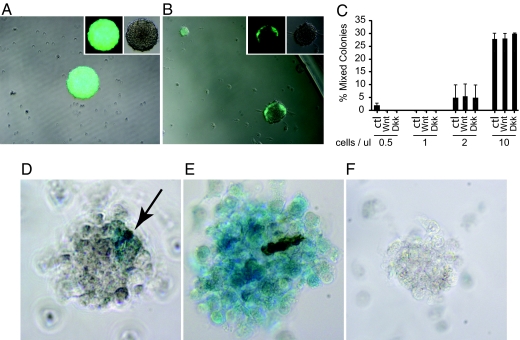
Fig. 2.
Cell mixing experiments were used to determine cell densities at which clonal colony formation occurs. (A) By mixing equal numbers of cells from an E14.5 β-actin GFP and a non-GFP mouse, we ascertained that at cell densities of 2 cells/μl or less, the colonies formed are predominantly a single phenotype resulting from clonal expansion of a single cells. (B) At higher cell densities (≥5 cells/μl) the colonies are formed from cells of each phenotype and represent oligoclonal rather than monoclonal populations. (C) Analysis of the colonies from cell mixing experiments at various densities. The bars represent the percent of mixed colonies present at each cell density. Treatment with Wnt or Dkk did not alter incidence of mixed colonies. At cell densities equal to or less than 2 cells/μl, the colonies generated are less than 10% mixed. Axin2-LacZ reporter illustrates endogenous Wnt signaling within newly formed clones. (D) Clonal growth of stem cells isolated from the E14.5 fetal mouse brain show that Wnt pathway is activated in a small subset of cells (blue cells indicated by arrow). (E) The addition of purified Wnt protein results in more cells responding to the Wnt signal. (F) Addition of purified Dkk, a Wnt inhibitor, abolishes the intrinsic Wnt signal leading to an absence of detectable Lac-Z-positive cells.
Endogenous Wnt Signaling Is Present in Clonally Derived Colonies.
Cells were isolated from Axin2 reporter mice and plated at clonal density (0.5–1 cells/μl) and the resulting colonies evaluated for expression of the Wnt reporter. Using quantitative PCR, we detected the RNA transcript of several members of the Wnt family (data not shown). Even without the addition of exogenous Wnt, cells within the colony expressed the Axin2 reporter, suggesting that Wnt signaling was active within the clonally derived colony (see Fig. 2 D), and that upon the formation of a multicellular colony some cells commit to producing Wnts while others gain the ability to respond to the signal. Addition of exogenous Wnt resulted in many more cells responding to the Wnt signal (see Fig. 2 E), while inhibition of the pathway with Dkk abolished the Axin2-reporter expression, suggesting complete inhibition of Wnt signaling within these colonies (see Fig. 2 F).
Wnt Protein Increases Stem Cell Cloning Efficiency in Vitro.
To further explore the effects of exogenous Wnt protein on colony formation, unsorted primary cells were isolated from the forebrain of E14.5 mice and plated at three cellular densities ranging from limiting dilutions, where many wells failed to generate colonies (0.2 cell/μl or 20 cells per well in a 96-well plate), to higher concentrations (500 cells per well), where a significant number of wells contained at least one colony. Wnt and Dkk proteins were added to determine the effect of elevated or reduced Wnt signaling on efficiency of colony formation. Adding Wnt protein (1 ng/μl) increased the number of colonies at all cell densities, while the Dkk protein resulted in fewer colonies at all cell densities relative to the control condition (Fig. 3A). The addition of soluble Wnt protein resulted in an average twofold increase in colony initiation efficiency, suggesting increased survival or increased recruitment into cell cycle for clonogenic cells.
Fig. 3.
Limiting dilution indicates a role for Wnt signaling in clonal establishment of colonies. (A) A limiting dilution analysis of the affect of Wnt and Dkk on primary isolates of neural stem cells shows that Wnt treatment increased the efficiency of colony formation at all cell densities. Dkk inhibited the production of colonies at all cell densities (*, P < 0.01, n = three independent experiments, student's t-test). (B) Linear regression analysis of the data shown in (A) was used to estimate the relative efficiency of colony formation (number of colonies formed relative to the number of total cells plated). Addition of Wnt protein leads to a twofold increase in colony forming ability (Wnt3a), while Dkk results in a fivefold depletion in the ability to form primary colonies. Relative abundance of stem cells in populations is enriched for in vivo Wnt-activated cells. (C) Flow cytometry shows that 5–20% of cells isolated from the E14.5 fetal brain express the Wnt reporter gene Axin2-LacZ. The top panel represents the control staining in which nonreporter mice were treated with the LacZ staining agent. The bottom panel shows representative preparation in which 9% of cells in the preparation were Wnt-responsive. (D) Cells were sorted and plated into 96-well plates and colony-forming efficiency (number of primary colonies per cell plated) was measured in each population under control (vehicle, V)-, Wnt (W)-, or Dkk (D)-treated conditions. Primary colonies were dissociated and replated to measure efficiency of secondary colony formation. (E) Treatment with Wnt or Dkk itself showed highly significant effects on secondary colony formation, with a roughly 10-fold increase or decrease, respectively, in colony formation. Colony size and morphology is altered by Wnt treatment. (F) Colonies formed in control conditions were of variable sizes, containing both smaller and large colonies. Wnt-treated cells generated colonies that were smaller and more homogenous than colonies formed under control conditions. Dkk-treated cells generated fewer colonies, which were similar in size to the larger colonies in the control condition. (G) Estimation of colony diameter shows significant differences in colony size between control- and Wnt-treated cells in primary and secondary colonies (**, P < 0.0001). The size difference between vehicle and Dkk-treated colonies were not statistically significant for either primary or secondary colonies (P = 0.2, 0.7 respectively). (H) Cells that expressed the Axin2-LacZ reporter in vivo also showed an intrinsic difference in colony size, with smaller colonies generated by Axin2+ cells (P < 0.0001). The total cell population showed an intermediate mean colony size.
Using linear regression to estimate the abundance of clonogenic cells in the total population (Fig. 3 B), we found that under control conditions, roughly one colony was formed for every 395 cells plated (a frequency of 0.003 clones per plated cell, R2 = 0.99). When Wnt was added to the medium, the incidence increased to 0.005 or one colony in 205 cells (R2 = 0.99), and when endogenous Wnt signaling was blocked by the addition of Dkk1c, colony frequency dropped to 0.001 or roughly one colony per 1,650 cells (R2 = 0.98). This indicates that endogenous Wnt signaling is necessary for efficient colony formation and that exogenously added Wnt can further increase cloning efficiency.
Axin2-Expression in Vivo Is Predictive of Self-Renewing Properties in Vitro.
To determine if Axin2-expressing Wnt responsive cells in vivo represented self-renewing stem cells, cells were isolated from E14.5 Axin2-LacZ mice and enriched by flow cytometry for the presence or absence of expression of the Axin2-LacZ reporter (14). Roughly 9% of the total cell population from the E14.5 brain expressed the Axin2-LacZ reporter (Fig. 3 C). Sorted LacZ-positive cells, LacZ-negative cells, or cells passed through the sorter without segregation (unsorted) were plated at densities that ensured predominant clonal colony formation (0.5–2 cells/μl). Cultures were maintained for 10 to 14 days and the number of primary colonies formed was scored. Primary colonies were then dissociated and replated into identical conditions to assay potential change in the abundance of colony forming cells per total cell population. There was no significant effect of in vivo Axin2-expression on the frequency of colony-forming cells in primary culture (f = 0.6, P = 0.6 for effect of Axin2-phenotype over the combined experiments by MANOVA, with no significant interaction between phenotype and Wnt or Dkk treatment, f = 1.3, P = 0.3). Furthermore, the addition of Wnt had only slight effects on primary colony formation for each Axin2 phenotype, with an increase approaching significance only in unsorted primary colony cultures (P = 0.06, t-test, n = 3). Otherwise, Wnt effects did not reach significance for any of the sorted primary cultures, regardless of Axin2 phenotypes (P > 0.2, t-test. n = 3) (Fig. 3 D). Upon secondary plating, however, Axin2+ cells had an increased ability to generate colonies when plated in the presence of Wnt protein relative to Axin2− cells plated in the presence of Wnt (P = 0.09). Even in the absence of exogenous Wnt protein, Axin2+ cells show increased secondary colony formation relative to Axin2− cells (P = 0.09).
Contrasting the specific effects of Wnt or Dkk on colony formation in primary versus secondary cultures suggests that the generation of colonies in the presence of Wnt encourages the accumulation of Wnt-responsive neural stem cells (Fig. 3 E). The efficiency of secondary colony formation (colonies per cell plated) in vehicle- versus Wnt-treated cultures is substantially increased when primary cultures were treated with Wnt and substantially decreased when primary cultures were treated with Dkk. Primary cultures generate colonies roughly two times more efficiently in the presence of Wnt (see Fig. 3 A and E), while secondary colonies from these Wnt-treated cultures show nearly 10-fold increases in colony formation in the presence of Wnt versus vehicle (P = 0.03, n = 3, t-test), suggesting a nearly 5-fold increase in the abundance of Wnt-responsive neural stem cells. Dkk-treated cultures showed nearly 10-fold depletion of colony-forming cells (P < 0.01, n = 3, t-test), suggesting that Wnt signaling is both necessary for the maintenance of neural stem cells and sufficient for the selective increase in the fraction of neural stem cells present in the resulting cell populations.
Wnt Treatment Results in the Generation of Morphologically Unique Primary Clones.
Wnt treatment also influenced the size of colonies formed in primary culture. Contrary to expectation, Wnt-treated cells generated primary and secondary colonies that were morphologically smaller and more homogenous than the colonies treated with vehicle or Dkk (Fig. 3 F–H). The average size of Wnt-treated colonies was 177 +/− 15 μm (sem, n = 3), nearly half the size of the average vehicle-treated colony. The Dkk colonies were not significantly different from vehicle-treated colonies, with an average size of 300 +/− 25 μm (sem, n = 3) (see Fig. 3 F–G). Vehicle-treated colonies had a variable range of sizes, containing both small and large colonies. Cells that were already expressing Axin2 in vivo (e.g., those sorted for Axin2-LacZ expression) also formed small colonies, even under vehicle conditions (see Fig. 3 H), suggesting that Wnt pathway activation, either in vivo before plating or by virtue of the addition of exogenous Wnt, limited colony growth in the 10 to 14 days after plating. Colony size and number was consistently lower in cells passed through the sorter versus those plated directly, presumably because of the physiological and shear stresses of the sorting process itself.
Colonies Formed in the Presence of Wnt Contain Cells that Differentiate into Neurons, Astrocytes, or Oligodendrocytes.
To confirm that colonies generated under Wnt conditions retained the ability to generate both neuronal and glial progeny, cells were isolated from the E14.5 forebrain, harvested, and cultured at clonal density (1 cell/μl) for 10 to 14 days in the presence of Wnt. BrdU was added at a concentration of 5 μmoles per liter overnight to label dividing cells. Colonies were then transferred to laminin-coated chamber slides and cultured for an additional 1 to 2 weeks in differentiation medium. Cells were fixed and immunostained to detect markers for neurons (doublecortin), oligodendrocytes (NG2), and astrocytes (glial fibrillary acidic protein, GFAP), as well as nuclei labeled with BrdU. Cells treated at clonal density with the Wnt protein were indeed capable of generating neurons, astrocytes, and oligodendrocytes, and each was labeled with BrdU, confirming that the differentiated cells were derived from proliferative progenitor cells in vitro rather than from cells that had already differentiated in vivo (Fig. 4).
Fig. 4.
Generation of neurons and glia from colonies formed at clonal density. (A and B) Colonies were generated from E14.5 forebrain at clonal density or high density in the presence or absence of exogenous Wnt protein. BrdU was added to the cultures overnight to label dividing cells. Colonies were then plated into differentiation conditions on laminin-coated chamber slides. After 1 to 2 weeks, the differentiated cells were fixed and stained to detect neurons, oligodendrocytes, and astrocytes. Clonal-density colonies yielded cells capable of generating astrocytes (GFAP, green in A), neurons (doublecortin, white in A) and oligodendrocytes (NG2, green in B). BrdU incorporation (red) is detected in the nuclei (blue) of the differentiated neurons and glia (arrows for each inset), indicating that the differentiated cells were derived from proliferative stem cells that were dividing in vitro, and not simply contaminating postmitotic cells from the fetal brain.
The Axin2-LacZ mouse strain is a line in which the Wnt target gene Axin2 is mutated by insertion of LacZ. Axin2 is a negative-feedback regulator of Wnt signaling and, like negative feedback regulators in other pathways (Patched), Axin2 expression is a faithful readout of the Wnt signal in numerous tissues. Using this reporter in the heterozygous state, we show that there is a small subpopulation of Wnt-responsive cells lining the neurogenic zone (SVZ) in the developing (E14.5) mouse brain. The location of the Wnt-responsive cells correlates well with established neurogenic zones (1). We further note that these cells at the SVZ have the morphology of radial glia, with foot processes lining the ventricle. A great deal of literature supports the role of the radial glia cell as the stem cell population in the central nervous system (1).
Wnt Signaling in Vivo.
The presence of Wnt signaling within the SVZ suggests a role for Wnt in neural development, and in this work we have shown that exogenous Wnt protein promotes the clonal expansion of stem cell populations that can generate neurons, astrocytes, and oligodendrocytes. Moreover, using a Wnt inhibitor, we established that Wnt is required for self-renewal and expansion of stem-like cells in primary culture. The Axin2 reporter system shows that stem cell colonies generated in vitro spontaneously activate endogenous Wnt signaling and that Dkk can abrogate this activation. The addition of Wnt protein resulted in more cells responding to the Wnt signal. The duration of Wnt treatment was too short to allow for specific expansion of Wnt-responsive cells, thus suggesting that many cells are capable of responding to a Wnt signal but that few cells are presented with the signal. It is well known that primary neural stem cell cultures are heterogeneous, containing both stem cells and differentiated cells. In this respect, neural stem cell colonies and neurospheres can be viewed as self-organizing structures, with multiple cell types sustaining each other by intercellular signaling. We show here that Wnt protein is important to maintain stem cells, the self-renewing subpopulation of progenitor cells that make up these complex structures.
The Role of Wnt Protein in Neural Stem Cell Cultures.
Our experimental approach was based on neural stem cell colonies initiated from single cells. Under conditions that favor the generation of clonal colonies, we find that the addition of Wnt protein caused an increase in the number of colonies detected 10 to 14 days later. This could be because of: (i) increased survival of neural stem cells and more efficient colony initiation; (ii) proliferative amplification of neural stem cells to increase their abundance; or (iii) decreased intercellular adhesion of cells that are dividing within the newly forming colony and seeding of secondary colonies. The fact that stem cells cultured in the presence of Wnt increase the abundance of Wnt-responsive stem cells in secondary colony cultures by nearly 10-fold (see Fig. 3 E) indicates that Wnt does indeed stimulate an increase in the abundance of neural stem cells through self-renewing divisions.
Studies of hematopoesis suggest that a hierarchy may exist between self renewal and proliferative amplification, with the most primitive subset of stem cells cycling slowly but with the greatest self-renewal capacity and the more differentiated progenitors having more proliferative activity but less self-renewal capacity (15, 16). It should be noted in the present studies, Wnt by itself is not sufficient for amplifying self-renewal in neural stem cells because FGF and EGF are also required in the cell-culture assays. Wnt blockade with Dkk decreased the relative abundance of neural stem cells by roughly 10-fold; however, the colonies formed under Dkk conditions were just as large as those generated in control conditions, suggesting that inhibition of Wnt affects self-renewing divisions but does not hinder transiently amplifying divisions of the more differentiated progenitors.
Observing Wnt reporter-positive cells in vivo, we asked whether these cells are enriched for stem cells by FACS isolation and by culturing them in the presence of the factors. In primary colony assay, we were not able to detect differences in the capacity of Axin2 expressing versus nonexpressing cells. Axin2+ cells grown in the presence of Wnt protein and replated in the presence of Wnt protein did exhibit increased self-renewal, suggesting that Wnt protein can be used to enrich self-renewing stem cells in culture. In the absence of Wnt protein, Axin2+ cells show increased secondary colony formation relative to the Axin2− counterparts, again suggesting that the Axin2+ cells contain stem-like activity. We did note that these Wnt-responsive cells formed small colonies, consistent with the effects of exogenously added Wnt on the population as a whole.
Wnt proteins have diverse effects on different stem cell populations; while they inhibit neural differentiation and maintain pluripotency in embryonic stem cells (ESCs) (7, 22), they can also promote differentiation in other progenitor populations (17). Experiments using a TCF-dependent reporter gene in differentiating cortical neurons during development suggests that canonical Wnt signaling is involved in the differentiation process (18), and our observations with the Axin2 reporter mouse show that mature neurons in the adult mouse brain remain Axin2-positive, suggesting a continuing role for Wnt in adult neural function [supporting information (SI) Fig. S1]. Wnt3a has been reported to promote differentiation into the neural and astrocyte lineage by inhibiting neural stem cell maintenance (19). Additionally, Wnt7a signaling has been shown to induce differentiation in neural stem cells of the neocortex (20), reducing the neural progenitor cell pool at late developmental stages (E13.5), whereas neural stem cells at earlier developmental stages (E10.5) are not affected by the Wnt treatment. Conversely, a great body of literature supports the role of the Wnt pathway in neural stem cell maintenance and expansion. The Wnt1-knockout mouse exhibits agenesis of the midbrain, suggesting a Wnt signal is necessary for the maintenance of stem cell populations giving rise to the midbrain (21). Chenn and Walsh used a transgenic mouse expressing a stabilized β-catenin in neural precursors. These mice develop enlarged brains with increased cerebral cortical surface area and folds resembling sulci and gyri of higher mammals. Brains from transgenic animals have enlarged lateral ventricles lined with neuroepithelial precursor cells, reflecting an expansion of the precursor population (11). A recent article from Lie et al. implicates Wnt3a in promoting neurogenesis in the adult hippocampus (10).
The observation that the pathway is active in radial cells of the ventricular zone at E14.5, the ability of the factor to result in expansion of self-renewing cells in vitro, and the observation that blockage of the pathway results in depletion of neural stem cells in culture all point to the importance of Wnt in regulating self-renewal in central nervous system stem cells. Interestingly, although Wnt-treated neural stem cells formed a greater number of colonies with greater replating efficiency, the absolute number of cells produced was lower than vehicle or Dkk-treated cells (data not shown), suggesting that Wnt3a acts at the level of self-renewal rather than as a mitogen. The ability to manipulate these cells in culture with essential signaling molecules allows for a strategy to both dissect niche effects and selectively manipulate cells in culture to produce selective cell profiles useful in cell transplantation and other therapies.
Materials and Methods
All research was approved by the Stanford University committee on animal research.
Isolation of CNS Stem Cells.
In all cases, tissue was removed from E14.5 forebrains of the Axin2-LacZ reporter mice (13), and suspended after dissociation in culture medium: Neurobasal-A, penicillin/streptomycin (BioWhittaker), and 250 U/ml DNase I (DNAse1, Sigma D-4527), 2.5 U/ml papain, and 1 U/ml dispase II (Worthington Chemicals). After centrifuging at 500 × g, the cells were triturated with pipettes of various calibers, filtered through nylon screen (40-μm filter) (BD Falcon), counted by hemocytometer, and plated.
Cell Culture.
Nonadherent cultures of CNS stem cells were performed by plating cells on ultra nonadherent 96-well plates (Corning Incorporated). In all cases the culture medium was based on a Neurobasal-A medium. The medium was supplemented with 20-ng/ml recombinant human bFGF (R&D Systems), 20-ng/ml recombinant mouse EGF (R&D Systems), 2% B27 without vitamin-A supplement (Gibco), 60-μg/ml N-acetylcysteine (Sigma), nonessential amino acids (Gibco), and penicillin/streptomycin (Biowhittaker). All cultures were maintained at 37°C in 5% CO2/balance air.
Assay for Neural Stem Cell Colony Formation.
Cells derived from forebrain cultures as described above or sorted from the forebrains of reporter mice were plated into 96-well plates at various cell densities (0.1–5 cell/μl) to evaluate directly the clonal frequency of precursors that initiate colonies. Each well contained 100 μl of the above mentioned media. EGF, bFGF, B27, and vehicle, Wnt, or Dkk (final concentration of 1 ng/μl) were replenished every other day to their original concentrations by adding the appropriate concentrations of EGF, FGF, and B27 in a small volume to the well. Halfway through the experiment, half of the media was carefully removed from each well and replenished with fresh media, with the growth factors adjusted to the proper concentrations. Plates were scored for neurosphere growth blinded to each condition using phase-contrast microscopy at 10 to 14 days. Linear regression analysis of the proportion of negative wells at each cell concentration was used to determine the frequency of colony formation (22, 23).
Cell Mixing Experiments.
Cells were isolated from the forebrain of E14.5 mice embryos from β-actin GFP:C57/BL6 and non-GFP:C57/BL6 mice as above. These cells were plated at various densities in a 96-well plate containing 100 μl of media as described above. Cells were mixed at equal quantities to produce cell concentrations of 50 or 0.5 cell/μl (25 GFP+, 25 GFP−), 100 or 1 cell/μl (50 GFP+, 50 GFP−), 200 or 2 cells/μl (100 GFP+, 100 GFP−) and 1,000 or 10 cells/μl (500 GFP+, 500 GFP−) cells/μl. After 10 to 14 days the colonies were analyzed for the presence of GFP using phase-contrast/fluorescent and confocal microscopy.
Differentiation Conditions.
Neural stem cells were harvested either clonally at a density of 1,000 cell/ml or in bulk at 100,000 cells/ml, and cultured with differentiation medium [N-acetylcsteine, brain-derived neurotrophic factor (10 ng/ml) glial-derived neurotrophic factor (10 ng/ml), EFG and FGF (2 ng/ml)] on laminin-coated chamber slides. After 1 to 2 weeks, chamber slides were fixed with 4% paraformaldehyde in PBS and stained to detect differentiation into neurons, oligodendrocytes, and astrocytes, and retention of any progenitors with mAbs against doublecortin (1:800; Chemicon), NG2 (1:500, Chemicon), glial fibrillary acidic protein (1:500;Chemicon) and musashi (1:800, Chemicon). An anti β-galactosidase antibody was used to detect the activation of the Wnt pathway (1:500–1,000, Promega). In all cases, cells were counterstained for 10 min at room temperature with 10 mg/ml DAPI (Sigma D-8417) to visualize nuclei. For the BrdU labeling experiments, 5 μmol/liter final concentration of BrdU was added to the cells overnight. It was subsequently washed out and the cells were allowed to grow for 10 to 14 days in the differentiation media above. BrdU immunostaining was accomplished by antigen retrieval by HCl treatment, followed by using an antibody against BrdU (1:500; Chemicon) and a fluorescent secondary.
Protein Purification.
Wnt3a Purification-Wnt3a protein was purified from 6.5 L of media conditioned by mouse L-cells, stably over-expressing mouse Wnt3a created in the laboratory as previously described (5).
Dkk Purification-Dkk1c protein was purified from 1 L of media conditioned by 293 cells stably over-expressing mouse Dkk1c protein as described in (24).
x-Gal Staining.
CNS colonies were stained by incubating them with x-Gal staining reagent overnight at 37 °C before fixation in 4% paraformaldehyde for 20 min at 4°C.
Embryonic Tissue Preparation.
E14.5 animals from Wnt-reporter mice were fixed in 4% paraformaldehyde for 5 days followed by embedding in cryoprotectant (1× PBS with 25% glycerin and 25% ethylene glycol at pH 6.7 and stored at −20°C). They were subsequently sectioned and stained with the same antibody concentrations as described above.
Cell Sorting.
Cells were incubated with a fluorescent marker against β-Galactosidase (FACS Blue lacZ β-Galactosidase Detection kit from Marker Gene Technologies, Inc.) at a dilution of 1:50 and incubated at 37°C for 30 min before sorting on a Beckman Aria FACS-sorter. Background levels of staining were determined by exposing neural stem cells from nonreporter mice of the same strain to the staining reagent. All cells were double sorted to greater than 90% purity. Low cell yields and high variability in cell survival following sorting limited the accuracy of this assay.
Supplementary Material
Acknowledgments.
M.Y.S.K. is a fellow of Paul & Daisy Soros, the Howard Hughes Medical Institute (HHMI), the Hanbery Society, and Stanford University Medical Scholar's Program. S.C. is a Giannini Foundation Fellow. S.B. is a fellow of HHMI. R.N. is an investigator of the HHMI. I.W. is the V&DK Ludwig Professor. This work was supported by grants from the HHMI, the National Institutes of Health, and California Institute of Regenerative Medicine (RC1–00133–1).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0808616105/DCSupplemental.
References
- 1.Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev. 2001;2:287–293. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- 2.Korinek V, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 3.van de Wetering M, et al. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111:241–250. doi: 10.1016/s0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 4.Reya T, et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 5.Willert K, et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 6.Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000;103:311–320. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 7.Jamieson CH, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. New Engl J Med. 2004;351:657–667. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- 8.McMahon AP, Joyner AL, Bradley A, McMahon JA. The midbrain-hindbrain phenotype of Wnt-1-/Wnt-1- mice results from stepwise deletion of engrailed-expressing cells by 9.5 days postcoitum. Cell. 1992;69:581–595. doi: 10.1016/0092-8674(92)90222-x. [DOI] [PubMed] [Google Scholar]
- 9.Lee SM, Tole S, Grove E, McMahon AP. Development. Vol. 127. Cambridge, UK: 2000. A local Wnt-3a signal is required for development of the mammalian hippocampus; pp. 457–467. [DOI] [PubMed] [Google Scholar]
- 10.Lie DC, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 11.Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- 12.Jho EH, et al. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soshnikova N, et al. Genetic interaction between Wnt/beta-catenin and BMP receptor signaling during formation of the AER and the dorsal-ventral axis in the limb. Genes Dev. 2003;17:1963–1968. doi: 10.1101/gad.263003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parks DR, Herzenberg LA. Fluorescence-activated cell sorting: theory, experimental optimization, and applications in lymphoid cell biology. Methods Enzymol. 1984;108:197–241. doi: 10.1016/s0076-6879(84)08086-1. [DOI] [PubMed] [Google Scholar]
- 15.Cheshier SH, Morrison SJ, Liao X, Weissman IL. In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc Natl Acad Sci USA. 1999;96:3120–3125. doi: 10.1073/pnas.96.6.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrison SJ, Weissman IL. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994;1:661–673. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 17.Kleber M, Sommer L. Wnt signaling and the regulation of stem cell function. Curr Opin Cell Biol. 2004;16:681–687. doi: 10.1016/j.ceb.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Maretto S, et al. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci USA. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muroyama Y, Kondoh H, Takada S. Wnt proteins promote neuronal differentiation in neural stem cell culture. Biochem Biophys Res Commun. 2004;313:915–921. doi: 10.1016/j.bbrc.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 20.Hirabayashi Y, et al. Development. Vol. 131. Cambridge, UK: 2004. The Wnt/beta-catenin pathway directs neuronal differentiation of cortical neural precursor cells; pp. 2791–2801. [DOI] [PubMed] [Google Scholar]
- 21.Brault V, et al. Development. Vol. 128. Cambridge, UK: 2001. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development; pp. 1253–1264. [DOI] [PubMed] [Google Scholar]
- 22.Uchida N, et al. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci USA. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henry C, Marbrook J, Vann DC, Kodlin D, Wofsy C. In: Selected Methods in Cell Immunol. Mishell BB, Shiigi SM, editors. San Francisco: W.J. Freeman and Company; 1980. pp. 138–152. [Google Scholar]
- 24.Fedi P, et al. Isolation and biochemical characterization of the human Dkk-1 homologue, a novel inhibitor of mammalian Wnt signaling. J Biol Chem. 1999;274:19465–19472. doi: 10.1074/jbc.274.27.19465. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.