Abstract
There are currently 6 unconjugated antibodies and 3 immunoconjugates approved for use in the Unites States in a variety of cancers, with a considerable number of new agents in clinical testing and preclinical development. Unconjugated antibodies alone can be effective, but more often, antibodies need to be combined with chemotherapy, which enhances the efficacy of the standard treatment. Immunoconjugates tend to be more effective than their unconjugated counterparts, but their increased toxicity often restricts when and how they are used. In order to improve efficacy, a number of immunoconjugates are being examined in settings where the disease is more easily accessible, such as leukemias, or within compartments that allow easier and more direct access to the tumor, such as in the peritoneal cavity or brain, or both locally and systemically, in adjuvant situations, where the disease burden has been reduced by some other means, and with the main goal of these treatments being to kill residual disease.
Keywords: Antibody-drug conjugates, antibody-toxin conjugates, immunotherapy, pretargeting, radioimmunotherapy
1. Introduction
Cancer is characterized by an unregulated cell growth, but the trait that sets it apart from other diseases is the ability of the tumor cells to invade and metastasize [1–4]. If detected early, before it has spread outside the natural tissue boundaries (e.g., stage 1), a tumor usually can be removed surgically without requiring additional intervention, giving the patient an excellent prognosis. However, once the cancer has escaped these natural boundaries, surgery alone will not suffice. A determination of the extent that the cancer has spread is essential for selecting a treatment strategy. A wide range of imaging modalities (e.g., CT, MRI, US, 18F-FDG) are used to probe the body for evidence of disease, but these methods only have the ability to detect tumors of about 1.0 cm in diameter, which already contain nearly 1 billion cells. Also, they often lack the specificity needed to differentiate cancer from other pathology. The most common systemic treatment for disseminated disease is chemotherapy, but over the past 10–15 years, a growing number of biological agents have made a significant impact on cancer treatment, not only as therapeutics (e.g., BCG, interleukin-2, interferon-alpha, rituximab, trastuzumab, and other antibodies), but also to help the body counteract some of the harmful side-effects of chemotherapy (e.g., G-CSF, erythropoietin). This article will focus on one group of therapeutic biologicals, antibodies and immunoconjugates (Figure 1 and Table 1), and will evaluate how they may be used to treat more accessible cancers.
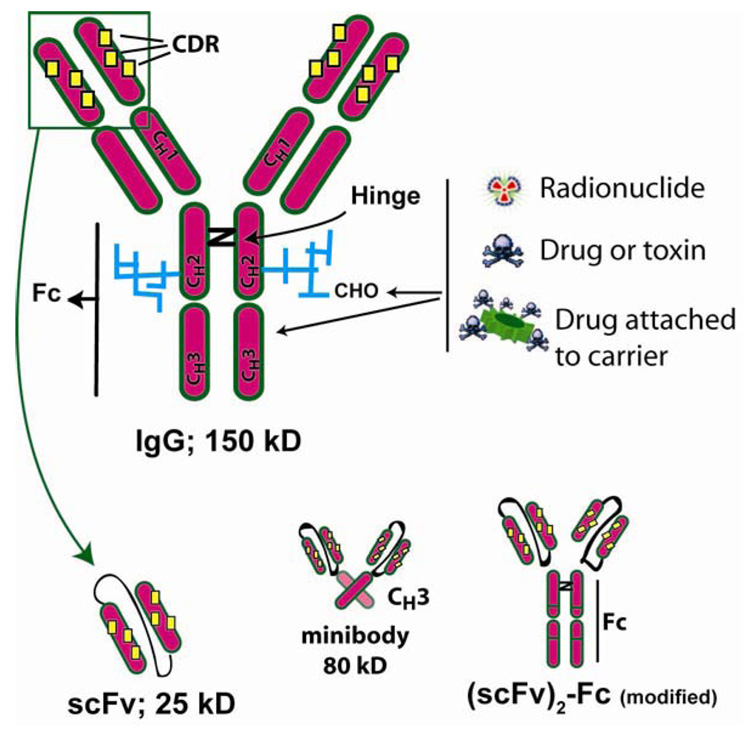
Figure 1. Schematic representation of IgG and several molecularly engineered fragments.
Antigen binding is based on the unique 3-dimensional orientation of the CDRs (complementarity determining regions) interacting with the framework regions within the VH and VL regions of the molecule, while effector activity (CDC and ADCC) are defined in the Fc-portion of the molecule. The molecule is glycosylated (CHO), which can affect the antibody’s blood clearance and function. Immunoconjugates can be prepared by chemically attaching a radionuclide, drug or toxin to the IgG’s amino acids or the carbohydrate side chains. Drugs have often been preloaded on a carrier before this attachment to increase the substitution level. The smallest antigen-binding fragment is a single chain (scFv), which essentially represents the VL and VH chains (collectively known as the Fv) that are tethered together with a 15–18 amino acid linker. The scFv is monovalent, but will form more complex multivalent molecules (diabodies, tribodies, etc.) as the length of the linker is shortened. Single chains are also used in recombinant antibody-toxin constructs, and have been joined to CH3 to make minibodies or to a genetically modified Fc fragment to provide a divalent binding fragment with altered pharmacokinetic properties (scFv2-Fc-modified) [73].
Table 1.
More commonly studied antibody-conjugated therapeutic radionuclides in patients.
| Energy (MeV) βmax |
Penetration (mm in soft tissue) |
Physical half-life |
|
|---|---|---|---|
| Beta-emitters | |||
| 131I | 0.61 | 2.3 | 8.0 days |
| 90Y | 2.27 | 11.3 | 64.1 hr |
| 177Lu | 0.50 | 1.8 | 6.7 days |
| Alpha-emitters | |||
| 213Bi | 8.3 | 0.08 | 46 min |
2. Antibodies and their immunoconjugates as therapeutics
2.1 Unconjugated antibodies
As an integral part of our immune system, antibodies are capable of killing cells by complement-dependent cytotoxicity (CDC) and antibody-dependent cellular cytotoxicity (ADCC). In each of these mechanisms, the antibody marks a cell so that other agents (i.e., proteins of the complement cascade or immune cells, respectively) can deliver the cytotoxic effects (Figure 2). In the early 1980’s, studies showed that anti-tumor antibodies could elicit cell killing through these traditional mechanisms [5–8], which led to several clinical trials using murine monoclonal antibodies developed against melanoma, gastrointestinal cancers, leukemia, and lymphoma [9–15]. At this same time, other investigations were advancing our understanding of how antibodies could be used therapeutically. Antibodies to growth factors, such as the transferrin receptor and to the epithelial growth factor receptor (EGFR), were found to have an anti-proliferative effect on cells growing in culture in the absence of complement or effector cells [16, 17]. These early findings provoked a whole new dimension of antibody therapy, and directly contributed to the development the anti-EGFR antibody, cetuximab. Whereas cetuximab’s mechanism of action involved signaling effects on the tyrosine kinase pathway, it also includes ADCC, whereas the anti-VEGF (vascular endothelial growth factor) antibody, bevacizumab, affects tumor growth by binding to VEGF, a growth factor produced by tumors to initiate new blood vessel formation, thereby inhibiting this essential function [18–20]. Since bevacizumab, unlike cetuximab, does not bind directly to tumor cells, ADCC and CMC are not required for its activity. Other antibodies currently approved for clinical use, trastuzumab and rituximab, affect signaling pathways, but also bind directly to the tumor cells, where ADCC and CMC contribute to their anti-tumor activity [21–23].
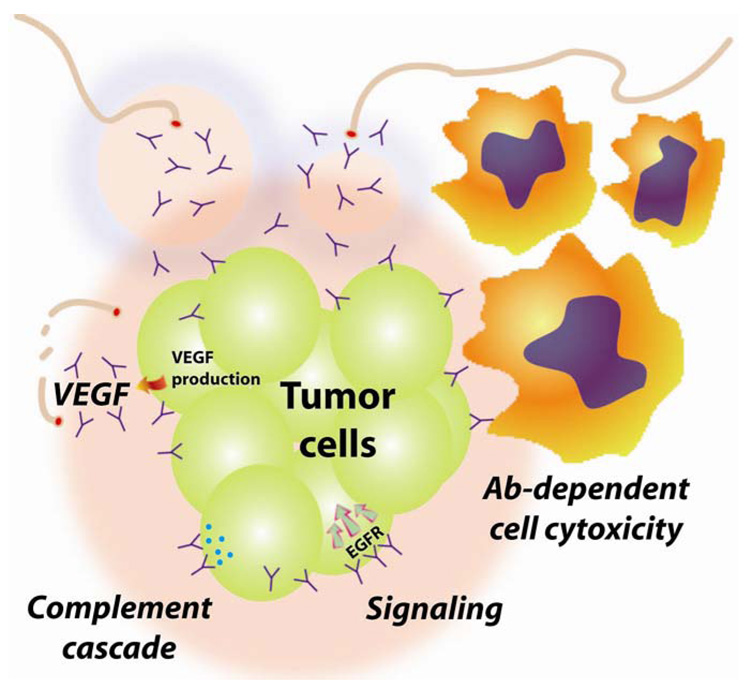
Figure 2. Mechanisms of action for unconjugated antibodies.
Traditional mechanisms of action include antibody-dependent cell cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC). Both require the Fc-portion of the IgG to activate effector cell binding or complement proteins that can lead to the death of the cells. However, antibodies that bind to growth factor receptors on the surface of the tumor cell (such as EGFR) can block critical functions and send signals that will lead to the cell’s death. Alternatively, antibodies can bind to substances produced by tumors, such as VEGF, that are essential for stimulating new blood vessel formation, thereby inhibiting new tumor growth.
With such multiple capabilities for disrupting cellular functions and survival, unconjugated antibodies are attractive therapeutics, but overall, they are not very potent, so that most unconjugated antibodies are commonly used in combination with drugs. Rituximab is perhaps the most active of the unconjugated antibodies, with an overall objective response rate of 50%, with only ~10% being complete responses in patients with relapsed non-Hodgkin’s lymphoma, when given alone [24, 25]. Extending the number of treatments or retreating at the time of progression can be of further benefit in follicular NHL [26, 27], but in other forms of NHL, rituximab is best used in combination with chemotherapy [28–30]. For this reason, antibodies are being conjugated to other cytotoxic compounds to enhance their potency.
Antibodies are highly versatile targeting proteins. The past practice of using murine monoclonal antibodies has been replaced with chimerized, and more often humanized, or fully human antibodies prepared in transgenic mice [31, 32]. Molecular engineering enables these proteins to be constructed in a number of ways, from modifying the size and pharmacokinetic properties of the construct, to altering the valency of antigen binding, and even altering effector activity. Recombinant fusion proteins with biological response modifiers or toxins have shown promising activity [33, 34], and bispecific constructs capable of binding a tumor antigen while also binding another agent (e.g., anti-CD3 for binding T-effector cells, or haptens for binding specialized hapten-peptides) are being explored [35–42].
2.2. Antibody conjugates
In animal models, the unconjugated antibody is most often much less effective therapeutically than the corresponding antibody conjugate. Similar results have been seen clinically. For example, the anti-CD33 antibody, lintuzumab, was ineffective when added to an induction chemotherapy regimen for the treatment of acute myeloid leukemia, but is effective as a stand-alone calicheamicin immunoconjugate (gemtuzumab ozogamicin) [43, 44]. The anti-CD20 radioconjugates, tositumomab and ibritumomab tiuxetan, both have been shown to induce a higher rate of complete responses than their corresponding unconjugated anti-CD20 IgG [45, 46]. Thus, conjugates are prepared to enhance the activity of their unconjugated form, but the added potency usually means increased toxicity.
Toxicity is most often the result of an unintended uptake in a non-targeted tissue, such as hepatotoxicity that frequently limits antibody-toxin conjugates because these constructs, like many macromolecules, are naturally cleared from the blood by the liver. Hepatic toxicity has frequently limited the use of toxin immunoconjugates [47]. Most plant toxins are composed of 2 parts, one responsible for cytotoxicity and another component responsible for binding to cells, which was responsible for severe toxicity in the very early work with immunotoxin conjugates [48, 49]. Even with the cell-binding component removed, toxin conjugates continued to cause severe hepatic toxicity. More recently, a 3-amino acid motif associated with hepatic binding has been identified for ricin A [50]. A new recombinant ricin-antibody construct had similar activity with less hepatic toxicity in SCID mice [51]. Being able to produce antibody-toxin conjugates by recombinant engineering provides a manufacturing advantage over drug conjugates that are prepared chemically. However, most toxins are foreign proteins that are immunogenic, which limits their use [52–54]. The potential exception may be the RNases [55].
Conjugation of drugs to antibodies can change the toxicity profile. A good example is the BR96 (anti-Lewis Y)-doxorubicin conjugate that was limited not by cardiotoxicity from doxorubicin, but by gastrointestinal toxicity because the antibody also bound to the normal gastrointestinal epithelial cells [56, 57]. The hematopoietic specificity of the anti-CD33 gemtuzumab ozogamicin causes severe hematologic toxicity, which is dose-limiting, but severe hepatic toxicities also are associated with gemtuzumab ozogamicin, which is likely caused by calicheamicin delivered to the liver [58]. Hematologic toxicity is dose-limiting for IgG-based radioconjugates because they are cleared too slowly from the blood, thereby continually exposing the radiosensitive bone marrow. While this limitation allows sufficient radiation delivery for successful therapy of NHL, radiation-absorbed doses to tumors rarely exceed 1500–2000 cGy, an insufficient dose for effective therapy of solid tumors [59]. Antibody fragments clear faster from the blood and improve tumor/blood ratios, but because a smaller fraction of the administered dose localizes in the tumor, the total dose delivered to the tumor may not be increased substantially. In addition, many radionuclides of therapeutic interest when conjugated directly to antibody constructs that are <60 kD will become trapped in the kidneys, resulting in unfavorable tumor/kidney ratios.
In contrast to direct conjugation of an antibody with a radionuclide, pretargeting techniques have been developed that separate the antibody and radionuclide targeting steps (Figure 3). In these procedures, a specialized antibody conjugate or construct that has the ability to bind to a tumor antigen as well as another compound is administered. After allowing this construct to bind and clear from the blood, the radionuclide attached to a small compound is administered. This compound is capable of binding to the other portion of the antibody conjugate pre-localized in the tumor. This small molecule rapidly clears from the blood, distributing within the extravascular space, and quickly being eliminated, preferably by urinary excretion and with minimal retention by the kidneys [60]. These procedures are able to deliver a higher radiation dose to tumor than with directly radiolabeled antibodies, improving efficacy, but with much less hematologic toxicity. Still other pretargeting methods also have been developed [112].
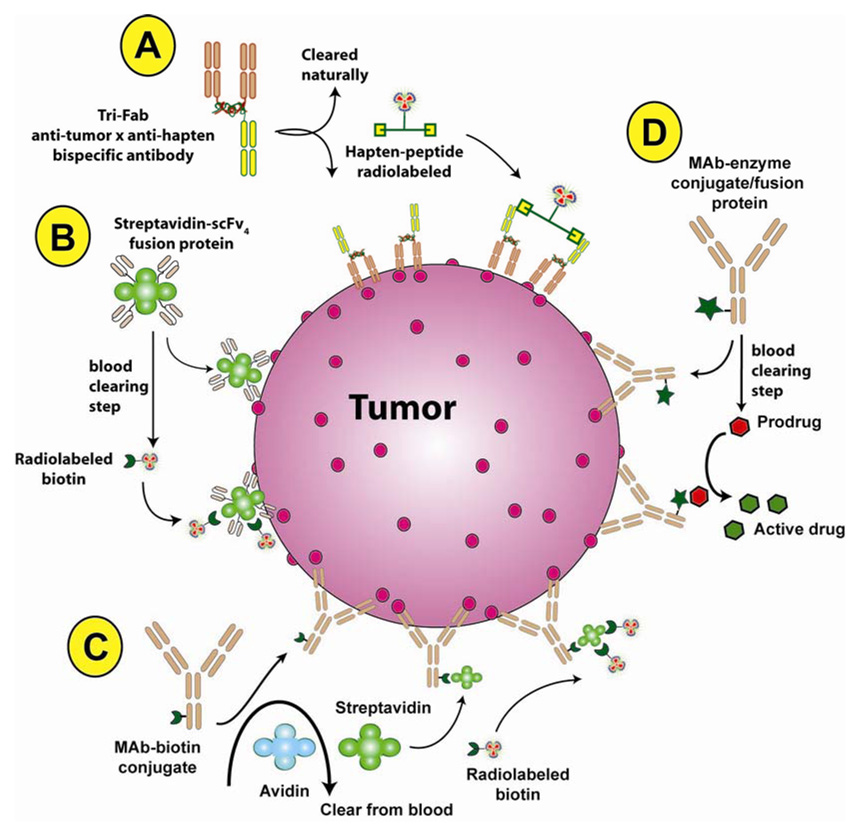
Figure 3. Examples of pretargeting methods used in cancer treatment.
These multi-step processes most often have been used to localize a radionuclide (A–C), but locally activated drug delivery has also been investigated [60, 81]. (A) Bispecific antibodies, such as a trivalent Fab’ construct [39], that can bind to a tumor antigen and a hapten is administered first. Once it localized in the tumor and has cleared from the blood sufficiently, a radiolabeled divalent hapten-peptide is given, which quickly binds to the anti-hapten binding arm of the pretargeted bispecific antibody and clears from the body by urinary excretion. Other pretargeting approaches, such as those shown in B and C, make use of the bacterial protein, streptavidin that has an extremely high binding affinity for biotin. (B) A scFv-streptavidin fusion protein is administered, but due to a slow blood clearance, a separate clearing step is included to remove the construct from the blood where it is metabolized in the liver. Once the excess construct is removed from the blood, radiolabeled biotin is given that will co-localize on the scFv-streptavidin pretargeted to the tumor, with the majority being cleared rapidly from the body by urinary excretion. (C) Another method first injects a biotin-IgG conjugate, which is subsequently cleared from the blood after it localizes in the tumor using avidin (avidin is glycosylated and is readily removed by the liver). A second chase, this time using streptavidin is given, and streptavidin will bind to the pretargeted biotin-IgG. The streptavidin clears from the blood within a day, and then the radiolabeled biotin is given. This technique is possible due to the multivalency of streptavidin that has 4 biotin-binding sites. (D) Antibody-enzyme conjugates can be pretargeted to tumors and then removed from the blood with a clearing agent. The targeted enzymes are specifically able to interact with a prodrug that is subsequently given, with the release of an active drug within the tumor.
Unconjugated antibodies and antibody conjugates prepared with drugs or toxins must bind physically to each tumor cell to have a cytotoxic effect, whereas radioconjugates have the distinct advantage of being able to kill cells located some distance from where the antibody binds, based on type of decay (i.e., alpha or beta) and its energy. Furthermore, drug and toxin conjugates must deliver a critical concentration of their payload inside the cell, sometimes to very specific compartments, for the effect to occur. In this regard, selecting a target on the cell surface that enables conjugate internalization and subsequent processing would seem to be a prerequisite for these agents. The more efficient the internalization process, the more likely the payload will be delivered at the concentration required to kill the cell. For example, although there are relatively few copies of CD74 (invariant chain, Ii) on the surface of cells, this antigen is readily shuttled from the membrane to the cytoplasm and then quickly recycled back to the membrane, making it accessible again for transporting antibody conjugates inside the cell [61]. CD74 expression on a variety of hematopoietic cells makes it an excellent candidate for the delivery of antibody conjugates [62–66], but studies also have shown this antigen is important for cell proliferation and survival [67], and thus unconjugated anti-CD74 antibody has therapeutic activity [68]. Targets that are internalized less efficiently can be used if they are more highly expressed, and it may be necessary to use a more toxic compound. Many of the antibody-drug conjugates that were first investigated used standard chemotherapy agents, but these were often not potent enough, and therefore antibodies coupled to plant toxins, with the theoretical capability of killing cells with very few copies delivered to the cell, were developed [34, 69–71]. So called ultra-toxic drugs, such as calicheamicin, maytansinoids, and auristatins coupled to antibodies, have showed improved activity, which has rekindled interest in drug immunoconjugates [44, 72, 73].
While there is clearly a desire to endow therapeutics with specific binding properties (e.g., antibody, peptide, lectin, etc.), the relatively high endocytic activity of some tumor cells can result in sufficient internalization of even non-targeted conjugates that can kill the cells [74]. For example, the anti-CD33-calicheamicin conjugate, gemtuzumab ozogamicin, is potent in CD33-positive as well as CD33-negative cell lines simply because leukemic cells have a high endocytic activity [75]. There are other situations where a conjugate can be effective without being internalized, as long as its payload is released selectively once localized in tumors. A variety of cleavable linkers have been described [76–81]. Gemtuzumab ozogamicin has a hydrolysable linker that allows the drug to be released, but this property may not always be desirable. For example, the failure in ovarian cancer of a similarly prepared calicheamicin-antibody conjugate was thought to be due to the instability of this bond, but clearly other factors could have contributed [82]. Another technique involves the delivery of an antibody-enzyme conjugate. The tumor-targeted enzyme will cleave a prodrug, allowing the active drug to be locally released within the tumor [83, 84]. Additionally, there is an increasing awareness that non-targeted cells in the immediate vicinity of the tumor cells can be killed non-specifically by the bystander effect [85]. Because the extent that this effect can significantly add to a response is not known, the objective must remain one of delivering specifically as much targeted therapeutic to as many cancer cells as possible.
3. What is “more accessible” cancer?
The vast majority of tumors exist in the form of a solid mass, except leukemia and other hematopoietic tumors, which predominantly are blood/bone marrow-borne diseases. Leukemia is the most accessible of all tumors, because the vascular compartment and the bone marrow are openly accessible to intravenous therapy. However, this presentation also means that the leukemic cells could be in essentially every tissue of the body; therefore, successful delivery of therapeutics can be challenging when the target cells are integrated in the various organs.
The most formidable challenge for the delivery of therapeutics is in solid tumors. Since a solid tumor exists outside the vascular compartment, agents must be able to traverse these channels, enter the interstitial space, and then travel to the cancer cells to exert their activity directly on the tumor. When a tumor forms, as a small cluster of cells, it can garnish nutrients and oxygen from the local environment, but it must induce the proliferation of blood vessels if it is to continue to growth. Tumors can produce the necessary signals to stimulate vessel formation, but the vascular channels are poorly organized and there is no lymphatic bed to assist fluidic movement [86]. However imperfect these blood vessels are, they are essential for the continued growth of the tumor. As tumors progress in size, new vessels form at the circumference, leaving the interior portions to become hypoxic and acidic. Despite being more tolerant than normal cells to these harsh conditions, eventually the cells die, forming necrotic regions within the internal regions.
Tumor physiology creates an interesting delivery paradox. For a variety of reasons, these vessels are leakier than blood vessels found in normal tissues, allowing macromolecules to escape easily [87, 88]. From a delivery viewpoint, this is ideal, but the hastily formed vascular architecture creates an elevated interstitial fluid pressure within the tumor that impedes convectional flow. Thus, while molecules can easily leave the tumor’s blood vessels, they are unable to move efficiently away from the blood vessels. Larger molecules are more dependent on convectional flow than smaller ones, and therefore their movement within a tumor is more restricted.
Regardless of size, therapeutics designed to interact specifically with substances found on tumor cells or within their interstitial space face another impediment, known as the binding site barrier [89, 90]. Once a targeted agent leaves the tumor vasculature and binds to its ligand, its movement within the tumor will be impeded. The higher the binding affinity, the more likely the agent will be localized in the perivascular space, penetrating only a few cells outside the blood vessel [91]. With antibodies, this penetration zone can be expanded by simply increasing the dose [92–96]. As more and more antibody traverses the blood vessel, antigen in the immediate area is bound, allowing the additional unbound protein to move further away from the blood vessel. However, not all agents, particularly antibody conjugates, can benefit from this approach. Dose-limiting toxicity of the immunoconjugate can limit the amount given. Additional unconjugated product can be pre- or co-infused with the immunoconjugate to block some of the antigen in the tumor bed and allow deeper penetration of the immunoconjugate from the vasculature, but this essentially dilutes the moles of active agent in the tumor, which could have a negative impact on efficacy [96, 97]. If there is a large antigen sink in normal tissues, administration of an unconjugated antibody may be required to alleviate excessive uptake of the conjugated antibody in the tissue (e.g., several hundred milligrams of unconjugated anti-CD20 antibody are administered prior to the anti-CD20 radioconjugates to reduce binding to a large B-cell sink primarily in the spleen [98, 99]). Thus, the targeted delivery of molecules, particularly macromolecules, to solid tumor masses is difficult.
The challenge posed by accessibility is particularly difficult for agents that must be delivered to individual tumor cells to be active, and amplified further for agents that also must be internalized. Radioconjugates were among the first immunoconjugates advanced based on the radioactive decay particle’s ability to penetrate from 0.05 mm to as much as 10 mm from where it is deposited (bystander or cross-fire effect), reducing the need for the radioconjugate to target individual cells in order to have a cytotoxic effect [59] (Table 2). Indeed, a high concentration of radioactivity targeted to tumor cells in the perivascular space could not only kill the tumor cells, but conceivably could destroy the vasculature in the vicinity, which might cause other collateral damage within the tumor. However, as with all therapeutics, a critical concentration must be achieved for the desired effect, and therefore the more accessible, the more likely that this critical concentration can be achieved before normal tissue tolerance levels are exceeded. Antigens that are shed or released from within cells when they die often can be detected in the serum, but they also can have a sizeable presence in the interstitial space of the tumor, which can block an antibody from binding to the cell surface. Although binding-site barriers and unfavorable fluid movement within tumors can adversely impact antibody delivery, antibodies targeting intracellular antigens are able to reach necrotic areas of the tumor, where antigen access is no longer impeded by the membrane of intact cells [100]. Thus, these barriers are not impenetrable, but they do place certain limitations on accessibility. For these reasons, there is considerable interest in the selective targeting of the tumor’s vasculature. This represents a more accessible target than one that resides on the tumor cell. However, agents that are designed only to destroy or, as in the case of bevacizumab, disrupt tumor vessel formation have not been sufficiently active by themselves to produce significant clinical benefit, but instead need to be combined with chemotherapy [20]. Immunoconjugates made to target tumor blood vessels and then release a cytotoxic payload or deliver a penetrating radionuclide also would likely have a limited impact, unless sufficient concentrations could be locally delivered. There are also formulation options for proteins that could improve their ability to reduce the impact that some of these natural barriers present [101].
Table 2.
Antibodies and immunoconjugates approved in the US for human use in treating cancer.
| Generic name | Tradename (specificity) |
Approved Use |
|---|---|---|
| Unconjugated antibodies | ||
| Alemtuzumab |
Campath (humanized anti-CD52) |
second-line treatment of chronic B-lymphocytic leukemia who failed fludarabine. |
| Bevacizumab |
Avastin (humanized anti-VEGF) |
combined with intravenous 5-FU-based chemotherapy for first or second line therapy of colorectal cancers. |
| combined with carboplatin and paclitaxel for first line unresectable, locally advanced, recurrent or metastatic non-squamous, non-small cell lung cancer | ||
| Cetuximab |
Erbitux (chimeric anti-EGFR IgG) |
combined with irinotecan for metastatic colon cancer or alone in patients unable to tolerate irinotecan. |
| combined with radiation therapy in head and neck cancer (locally or regionally advanced) or as a single agent in patients with recurrent, or metastatic squamous carcinoma of head and neck who failed platinum-based therapy. | ||
| Panitumumab |
Vectibix (human anti-EGFR IgG) |
second-line in patients who failed FOLFOX or FOLFIRI chemotherapy regimens |
| Rituximab |
Rituxan (chimeric anti-CD20 IgG) |
relapsed low-grade or follicular non-Hodgkin’s lymphoma (NHL) |
| first-line treatment follicular NHL in combination with CVP chemotherapy | ||
| first-line treatment of diffuse large B-cell NHL in combination with CHOP or other anthracyclinesbased chemotherapy; | ||
| low-grade NHL with stable disease or who achieve a partial or complete response following CVP chemotherapy | ||
| Trastuzumab |
Herceptin (humanized anti-HER2 IgG) |
single agent in patients with metastatic breast cancer that overexpress HER2 and who have received one or more chemotherapy regimens; |
| combined with doxorubicin, cyclophosphamide, and paclitaxel in an adjuvant setting for nodepositive breast cancer; | ||
| combined with paclitaxel in patients with metastatic breast who have not received treatment for their metastatic disease. | ||
| Radiolabeled antibodies | ||
| Ibritumomab tiuxetan |
Zevalin (murine anti-CD20 IgG-DTPA conjugate; radiolabeled with 90Y or 111In; rituximab pre-dose) |
treatment of relapsed or refractory low-grade, follicular or transformed B-cell NHL. |
| Tositumomab |
Bexxar (murine anti-CD20 IgG; radiolabeled with 131I; tositumomab pre-dose) |
treatment of patients with refractory, low-grade, follicular, or transformed NHL, including patients with rituximab-refractory NHL |
| Antibody-drug conjugate | ||
| gemtuzumab ozogamicin |
Mylotarg (humanized anti-CD33 IgGcolecheamicin |
acute myeloid leukemia in first relapse and patients ≥60 yr old who are not candidates for other cytotoxic therapy. |
When a solid tumor is localized in a fairly confined region of the body, it is considered a more accessible presentation. In this situation, the therapeutic could be delivered locally, either by direct injection into the lesion, into established vascular (or lymphatic) channels of the tissue where the tumor resides, such as hepatic arterial injection to treat hepatic metastases, or within discrete compartments (e.g., intraperitoneal, intrathecal, intracranial) [102–109]. These methods are designed to take advantage of a first-pass effect [110–112], but in each case, the agent must still contend with the barriers existing within each tumor mass for successful delivery. Intralesional injections potentially can mitigate some of these internal barrier issues, but this depends on the size and volume of the mass, and perhaps the rate at which the product is injected. If the injected volume is small in relation to the tumor, the agent could still have a limited capacity to percolate into other regions, and therefore multiple placements would be preferred. These techniques are designed primarily to control the local spread of the disease, and do not address the likelihood that the cancer has already metastasized to other locations in the body, either regional or distant. Nevertheless, the main goal in these approaches is to allow the therapeutic to be in more direct contact with the tumor, raising the chances that it will localize at higher concentrations than if diluted by intravenous injection. In some of these approaches, the therapeutic does escape from these compartments and circulates throughout the body, albeit usually in concentrations too low to optimize efficacy against tumors located elsewhere in the body.
Minimal residual disease, where the tumors are smaller and with less significant barriers than larger tumors, or in an adjuvant setting, where presumably there are only small clusters of cells hidden in the body, could also be considered as more accessible presentations of cancer. Thus, we will focus our discussion on the application of antibodies and immunoconjugates in leukemia, in compartmental settings, such as in peritoneal disease and within the brain cavity, as well as in minimal disease.
4. Antibody-targeted therapy of leukemia/lymphoma
Antibodies have made significant strides in the treatment of hematological malignancies. Rituximab, a chimeric anti-CD20 IgG, is the most successful therapeutic antibody. It was initially approved for use in follicular and transformed (low-grade) non-Hodgkin’s lymphoma (NHL) alone and in then combination with chemotherapy in aggressive NHL, but now it is being examined for activity in other B-cell lymphomas, as well as against non-malignant conditions where B-cells contribute to the disease, such as a number of autoimmune diseases [25, 30, 113–118]. Rituximab’s commercial success can be credited in a large part for the increased interest in antibody therapy for both solid and hematopoietic cancers, unconjugated as well as conjugated. Other antibodies, such as epratuzumab (humanized anti-CD22) and galiximab (chimeric anti-CD80), alone and in combination with rituximab, are also showing promising results in early clinical trials [119–124]. Apolizumab, a human anti-HLA-DR, had been studied in patients with non-Hodgkin’s lymphoma, but relatively low doses (≤ 1.5 mg/m2) resulted in grade 3 and 4 hematological toxicity, and therefore this antibody’s toxicity was too severe for continued human use [125].
Ideally, the target should exist on the tumor cells and not on normal cells, but in the case of the hematological malignancies, all of the antibody-targeted therapies also affect antigen-positive normal lymphoid cells to varying degrees. For example, CD20 and CD22 are on tumor and normal B-cells, but are restricted to more differentiated B-cells, sparing the progenitor B-cells. Using these targets should restrict toxicity to only the B-cells, while sparing myeloid lineage cells. CD80 and HLA-DR are found on a number of other differentiated immune cells, and therefore there likely is more collateral damage to other non-malignant immune cells. In acute myelocytic leukemia (AML), antibodies to CD33, CD45, and CD66 have been examined as unconjugated and conjugated products [126–130]. Anti-CD33 and anti-CD66 antibodies target tumor blast cells, as well as normal monocytes/myeloid lineage cells, but are not present on stem cells, while CD45 is also present on stem cells. Because of its reactivity with stem cells, radioconjugates of anti-CD45 antibodies are being used to aid in the elimination of stem cells in patients undergoing transplant procedures [131–134]. In chronic lymphocytic leukemia (CLL), unconjugated anti-CD52 humanized antibody, alemtuzumab (Campath-1H), is active against a wide range of immune cells, causing severe lymphopenia that can result in an increased risk of opportunistic infections. The anti-IL2 receptor (CD25) antibody, daclizumab, expressed primarily on T-cells and currently approved for the treatment of renal allograft rejection, also can be found on B-cells and monocytes/macrophages [135, 136]. Anti-CD25 antibodies are being examined in a number of situations requiring immunosuppressive therapy, but also in cancer against T-cell lymphoma and CLL [137]. Zanolimumab, a fully human anti-CD4 IgG, has shown encouraging objective responses in refractory cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome) [138].
As easily accessible as leukemias are, it is surprising that targeted agents have not been more effective. CD33 expression is very low on most myeloid leukemias, except acute promyelocytic leukemia, where conjugates appear to be more active. Thus, a reduced expression of a target will limit the amount of therapeutic product delivered [126, 139]. In the case of CD33, protein doses of only 5 mg/m2 were able to saturate antigen sites in patients with extensive disease [140]. However, analysis of cells taken from patients undergoing multiple cycles of gemtuzumab ozogamicin have indicated that the conjugate is rapidly internalized and re-expressed, allowing new sites for the conjugate to bind [141]. Radiolabeled anti-CD33 antibodies can reduce the number of circulating blast cells significantly in patients with advanced disease, but without complete responses. However, by minimizing disease burden with cytarabine prior to radioantibody treatment, complete responses were observed [142]. Thus, extensive disease, even if it is easily accessible, is difficult to treat with targeted therapies (or for that matter, non-targeted therapies). Even though CD33 expression is low, the sheer mass of cells in patients with extensive AML can be overwhelming for targeted therapies, particularly when the administered dose is limited by toxicity. For this reason, antibody conjugates will more likely contribute significantly to the clinical outcome when used in a minimal disease setting. Unconjugated antibodies, such as rituximab, can be administered repeatedly without serious complications, but rituximab alone is unable to eradicate all disease in more than 90% of patients. Cells can also become resistant to rituximab, further complicating its use as a monotherapy [23, 143]. Combining rituximab with chemotherapy is more effective than rituximab given alone [144], which has been found for most therapeutic antibodies [40]. Combining antibodies with different specificities has been proposed as a means of targeting a larger fraction of the cells, to target other regions of the tumor (e.g., stroma), or because of additivity/synergistic interactions [121, 123, 145, 146]. Cocktails of antibodies may not improve efficacy if the cocktail requires reduction in the dosage to suboptimal levels of an individual antibody that targets one population of cells. As long as the dose of each agent is at an effective level, then the combination may be more effective. This effect was illustrated recently in a pretargeting procedure that examined individual antibodies and the combination of anti-CD20, CD22, and HLA-DR antibodies [147]. Targeting was best when a single antibody against the more highly expressed antigen in a given cell line was used for pretargeting radionuclides as compared to all 3 antibodies. This study suggests that cancers should be screened in advance to determine the highest target expression, using the antibody to this target for individualized treatment. Other options include sequential administration of multiple agents, especially when there are synergistic or additive benefits, or fractionation, which may aid in delivering an agent at a critical time in the cell cycle to optimize effects, or to reduce toxicity, particularly for drug or isotopic immunoconjugates. These latter approaches can be affected adversely if initial treatments disrupt blood vessels or other means to access the tumor cells for the subsequent doses. Indeed, all of these options do not necessarily solve the problem of targeting tumor cells that are sequestered in less accessible sites.
Since lymphomas typically present as solid masses, the same barriers inhibiting targeted therapeutics would apply. In the case of rituximab therapy, where the amount of protein administered is typically 375 mg/m2 (~600 to 700 mg) weekly for a minimum of 4 weeks, this amount of protein could conceivably assist the antibody in penetrating more uniformly in the tumor. However, since CD20 is also expressed on normal B-cells found in the blood, spleen, lymph nodes, and marrow, there is a substantial antigen sink that needs to be overcome. Experience with radiolabeled anti-CD20 antibodies, ibrituzumab tiuxetan and tositumomab, has indicated that a pre-infusion of ~300 to 400 mg of the unlabeled antibody is sufficient to reduce uptake in these sites to allow for better targeting of the radiolabeled antibody. Thus, with nearly 2.5 to 3.5 grams of antibody administered over 4 weeks, the antigen sink would be overcome, leaving a considerable amount of antibody available to overwhelm the binding site barrier. However, there are other barriers within the body that cannot be “broken” by merely increasing the protein dose. Rituximab is not as active in extranodal disease, such as in mucosa-associated lymphoid tissue (MALT lymphoma), or when lymphoma is involved in the leptomeningeal cavity, because full antibodies do not penetrate the blood-brain barrier unless this barrier is compromised [148, 149]. In the latter case, direct intrathecal injection of just 25 mg of rituximab was shown to be active [149]. Thus, in situations where natural anatomic barriers prevent access, a more local/regional therapy needs to be taken.
5. Intracavitary targeting
Intraperitoneal (IP) spread frequently occurs in ovarian, colonic, pancreatic, and gastric cancers. Under normal conditions, fluids flow freely and quickly through the peritoneum via blood capillaries and lymphatic channels below the diaphragm. Most often, peritoneal tumors present as multiple nodules studding the cavity’s surfaces. As the tumor progresses, the lymphatic channels can become blocked, causing a buildup of fluids (ascites) in the peritoneal cavity. This fluid can become inundated with small clusters of tumor cells that are shed from the tumor bed lining this cavity. Adhesions can form loculated areas of ascites, essentially sealing off various compartments. Thus, intraperitoneal presentation can pose a number of challenging conditions, and for this reason, regional IP therapy is best approached after cytoreductive treatment to reduce tumor burden. This is particularly important for selective therapeutics that can otherwise become overwhelmed by the amount of disease.
The pharmacokinetic advantage for IP therapy has been established for some time [150], and this advantage is particularly enhanced for macromolecules that rely on convectional flow for their fluidic movement, since they will be retained longer in this chamber than smaller molecules. A slow clearance allows a selective targeting agent ample time to localize to the target. However, while antibodies introduced into this compartment may have easy access to the tumor nodules spread throughout the cavity, this does not mean that there is full and open access to all tumor cells. Rather than accessing the tumor from the inside by percolating out of the tumor blood vessels, the antibody in the peritoneal cavity bathes the outside of the tumor and needs to be absorbed within in order to access all the cells. In vitro studies have highlighted the difficulty in antibody penetration within tumor spheroids, and in vivo studies have emphasized the importance of the tumor’s microenvironment in restricting antibody movement into tumors [93, 151–154].
The restricted penetration of antibodies raises questions concerning which route of administration might be best for antibody therapy. This issue was evaluated in a clinical study performed in colorectal cancer patients who were co-administered radiolabeled ant-TAG.72 IgG (125I/131I, respectively) either IP or intravenously (iv), and 6–8 days later, they underwent surgery, with uptake measured in the excised tumor and normal tissues. Of the 55 lesions removed, 35 had a 2-fold higher uptake of the IP-administered 125I-IgG, 13 lesions had 2-fold higher uptake of the iv-administered 131I-IgG, and 7 were similar. More detailed pathological examination revealed that true peritoneal implants had a higher uptake with the IP injection, while lesions that were characterized as hematogenous-borne or lymph node metastases, or local recurrences, had higher uptake with the iv-injected antibody [155]. Several patients in this study were co-injected with a radiolabeled non-targeted IgG. In these patients, it took 2 days before peak values (~30% of the injected dose) were found in the plasma [155]. The kinetics of the specific radiolabeled anti-TAG.72 IgG in the plasma was similar to that of the irrelevant IgG, but peak values were only about 10% of the injected dose, indicating that a portion of the dose was retained in the cavity [155]. Because the concentration of the radiolabeled antibody in the blood was substantially lower with the IP route than by intravenous injection, the projected radiation dose to the red marrow was reduced. A similar study was performed using a radiolabeled anti-folate-receptor antibody. It also reported a lower blood concentration of the antibody after the IP injection, but it was found that tumors surgically removed 2 days after the iv injections had 2-fold higher uptake than the IP-administered antibody, and after 6 days, uptake was the same in both groups [156]. Thus, both studies concluded that the main advantage of the IP route of administration was the lowering of activity in the blood, which for radioconjugates is important, since this will lead to less severe hematological toxicity (i.e., allowing higher doses to potentially be given).
Although preclinical testing of antibody-drug and toxin conjugates has been conducted in IP models, no clinical studies with these agents injected into the peritoneal cavity have been reported to date. Perhaps because of penetration issues, clinical studies have instead focused on the use of radiolabeled antibodies. With non-antibody radionuclide IP therapy, clinical studies have found no survival advantage as compared to standard of care [157, 158]. Responses have been reported in a number of Phase I/II trials using several antibodies radiolabeled with different radionuclides administered by IP or intravenous routes [159–165]. However, only one agent, a 90Y-labeled anti-MUC1 IgG, was tested intraperitoneally in a randomized Phase-III clinical trial [166]. Patients were enrolled after responding to primary platinum-based therapy, and upon second-look surgery, had no evidence of disease. The patients were randomized to receive a single injection of 18 mCi/m2 of the 90Y-murine anti-MUC1 IgG plus standard care or standard care only (no active therapy). After a median follow-up of 3.5 years, no difference was found in the overall survival or time to relapse. In a follow-up assessment of this study, Oei et al. [162] found that significantly more of the patients given the specific targeted therapy IP recurred outside the peritoneal cavity, and that the time to IP recurrence was also significantly longer than in the control arm. This analysis provides some indication that IP-administered radiolabeled antibodies are effective in controlling local lesions, but illustrates the problem in a cavitary treatment when in fact the disease likely spread elsewhere. A recent Phase III clinical trial found that administering combination chemotherapy by both IP and iv routes was more efficacious than just the IP route in ovarian cancer, emphasizing the importance of treating systemically as well as locally [167]. Additional preclinical studies have indicated that antibody conjugates prepared with lower energy beta emitters and alpha emitters would be more suitable to the treatment of microscopic disease that might be present after cytoreductive surgery/effective primary chemotherapy than the 90Y-conjugate employed in the Phase III trial [168–173]. It still seems likely that locoregional IP therapy with antibody conjugates could be more effective with careful planning of the appropriate construct/conjugate, and if efforts were undertaken to combine localized targeted therapies with some form of systemic therapy, especially when occult metastataic disease is likely to be present. It is noteworthy that a different method for targeting radionuclides, known as pretargeting, has been reported to be more effective than directly radiolabeled antibodies, with significantly less hematologic toxicity [60]. Thus, combinations of systemic pretargeted radionuclides with locally administered, directly radiolabeled, IgG might be possible for enhanced responses to extraperitoneal and peritoneal disease.
Depending on their location, brain cancers also can be accessible to localized treatment. Primary brain cancers seldom metastasize outside the central nervous system (CNS), reducing the need for a systemic treatment approach, but heightening the need for effective directed therapy to the primary mass and the eradication microscopic disease wherever it might be present. The first option for primary brain cancers is to remove the mass surgically, which leaves a cavity in the space occupied previously by the tumor. Localized radiation is typically administered afterward to kill any residual cancer, but the cavity also serves as a reservoir for the introduction of therapeutics. Metastatic cancers to the brain occur more frequently than primary brain cancers, and are most often treated with external beam radiation. However, stereotactic radiosurgery has become an option for those with evidence of solitary metastases [102, 174–179]. Intravenous antibody therapy with trastuzumab suggests that the blood-brain barrier impedes movement of these antibodies, since there is a relatively high incidence of solitary breast cancer metastasis to the brain in these patients [180]. Thus, for cancers residing in the brain or within the CNS, intracranial or intrathecal routes of administration of targeted macromolecules will be required.
As with regionalized IP treatment, most of the clinical studies for intracranial treatment of primary brain cancers have used radiolabeled antibodies [181]. Radiolabeled anti-tenascin antibodies are the most studied and more advanced in clinical testing [175, 176, 182–190]. Two Phase-II studies with a single injection of the 131I-murine 81C6 in the surgically-created resection cavity of patients with either newly diagnosed [183] or recurrent malignant gliomas [184] have been promising. Of the 32 newly diagnosed patients that received a full dose (120 mCi) of 131I-m81C6, most also received additional external-beam radiation therapy (29 patients) and systemic chemotherapy (30 patients). In the recurrent population, patients first had a gross resection of the primary tumor to create the cavity where the antibody was administered; within 4 weeks of this treatment, they were also allowed to receive adjuvant chemotherapy. The Phase-II efficacy results are highly encouraging, with evidence that the overall survival increased over currently approved therapy regimens. These 2 experiences are good examples of how regionally targeted therapy should likely be applied, even in cancers that do not spread outside the central nervous system. Early clinical studies have also shown responses when the radiolabeled anti-tenascin antibody was administered intrathecally [191], providing evidence that disease that might spread within this cavity could be treated successfully with this approach.
Eligibility for the intracranial trials mentioned above required an assessment of the cavity to show no leakage, essentially ensuring optimal retention within the cavity. This raises a question whether the radionuclide’s attachment to the antibody is necessary or whether any contained radioactive source could prove beneficial. Traditional brachytherapy has not been beneficial in this situation [192], but initial reports of success have appeared with a brachytherapy method that traps the radionuclide inside a balloon that expands to conform with regional boundaries of the cavity [193, 194]. Nontargeted 90Y or 32P colloidal or microsphere therapy has been given intratumorally or locally for a variety of solid tumors and in brain cancer with some success [195–200]. This form of non-targeted therapy is effective in reducing the size of cystic craniopharyngiomas [199–203], which appears to be related to destruction of the epithelial cells that line the cyst wall, replaced with a collagen layer that inhibits fluidic movement [204, 205]. However, these studies also concluded that these methods would not be effective in treating solid tumors. Preclinical studies with the radiolabeled anti-tenascin antibody showed significantly-enhanced therapeutic benefit over an irrelevant antibody in rats bearing intracranial tumors, but the therapeutic was administered intravenously [206]. Initial studies in patients demonstrated the specificity of the murine anti-tenascin antibody as compared to an irrelevant antibody after intracarotid artery injection, with the specific targeting measuring 1.5 to 4-fold higher than the irrelevant antibody (e.g., first-pass) [207]. While the value of using a specific radioantibody for targeting cancer has been demonstrated repeatedly preclinically and clinically, none of these studies specifically addresses the uniqueness of the intracranial cavity. Impressively, dosimetry studies showed that the half-life for the anti-tenascin antibody in the cranial cavity is the same as the physical half-life of 131I (~8 days) [208], but it is still unclear whether this extended retention time was related to the antibody’s ability to bind and hold the radioactivity to the tumor cells, or simply the patency of the cavity. Radioactivity bound to a specific antibody would undoubtedly be deposited on tumor lining the resection margin, providing an important local boost in the radiation dose that could enhance overall survival, whereas radioactivity attached to a non-binding compound might tend to be distributed uniformly in the cavity. Radiological/pathological studies have indicated that viable tumor primarily resides along the lining of the cranial cavity [209], and therefore focal concentrations of radiation in these regions should provide some advantage.
Several other antibodies have been examined clinically for the treatment of primary brain cancers, but these studies involved systemic delivery. An unconjugated anti-EGFR antibody, mAb 425, was ineffective when given systemically, but as an 125I-labeled conjugate given systemically with surgery, external radiation therapy, and chemotherapy, it appeared to have some benefit [210]. Intravenously administered bevacizumab is being used successfully to treat radiation necrosis in the brain [211]. Several clinical studies have reported promising preliminary data with systemically-administered cetuximab as a monotherapy or in combination with radiation and chemotherapy [212, 213]. Intravenously administered anti-tenacin IgG used for pretargeting 90Y-biotin also has been examined in several Phase I/II trials [174, 175, 188]. Imaging studies confirmed the localization of the radiolabeled biotin following the pretargeted localization of the anti-tenascin antibody, and several patients had objective responses with reasonably good durations.
6. Systemic therapy of minimal/micrometastatic cancer
Once an antibody or antibody conjugate is proven to be effective in treating established disease relapsing after initial therapy, inevitably efforts will be undertaken to determine if its use can be expanded to a more naive treatment setting (i.e., front-line vs. second- or third-line), or as an adjuvant/neoadjuvant therapy. For unconjugated antibodies that have minimal long-term risk, use in an adjuvant setting is attractive. For example, randomized studies have shown that trastuzumab (administered every 3 weeks for 1–2 years) added to an adjuvant treatment (given concomitantly or after completion of chemotherapy) improved disease-free survival [214, 215]. Other antibodies, such as cetuximab, panitumumab, and bevacizumab, are being explored in adjuvant settings [216–218]. The anti-CD20 radioconjugates, tositumomab and ibritumomab tiuxetan, originally approved as second-/third-line monotherapies for follicular NHL, are being examined in a front-line setting or in combination with other agents [219–224].
With the lack of Phase-II trials indicating appropriate efficacy for radioconjugates administered systemically in solid tumors, most efforts have been focusing on intracavity applications. However, our early studies in animal models with radioconjugates emphasized that smaller tumors were more likely to be eradicated than larger tumor [225]. In addition, the presence of a large tumor mass could adversely influence the successful treatment of co-existing micrometastatic disease, emphasizing the importance of debulking [226]. Furthermore, in an animal model of microdisseminated human colon cancer in the lungs, specific targeting with radiolabeled antibody IgG could cure a substantial portion of the animals as long as treatment was given before the tumor was too advanced [227, 228]. These studies clearly emphasize that radioimmunotherapy is more likely to have its greatest impact in situations where there is minimal disease. Thus, while the results of many Phase-I/II radioimmunotherapy trials in patients with advance solid tumors have been disappointing [59], efforts to examine systemically-administered antibody conjugates in more appropriate settings should be undertaken.
Indeed, administration of an 131I-humanized anti-CEA humanized antibody (labetuzumab) as an adjuvant treatment for colorectal cancer patients after salvage resection of liver metastases has been studied [229, 230]. After a minimum 5-year follow-up, post-surgical treatment with the radioconjugate nearly doubled the median overall survival time for these patients as compared to a contemporaneous control group not given radioimmunotherapy [229]. Radioconjugates are also showing promise in medullary thyroid cancer. Investigators found a survival advantage for a subset of patients given a bispecific antibody, pretargeted radionuclide therapy, who had a more rapidly progressing serum calcitonin level [231]. It was suggested that this advantage could be attributed to the eradication of micrometastatic bone marrow involvement.
There also have been a number of studies in animal models indicating that chemotherapeutic agents can be given with radioimmunoconjugates to enhance the radioconjugate’s efficacy [232–237]. In these circumstances, the therapeutic agent may be given at doses that are not designed to optimize its activity, but just enough to be a radiosensitizer. Given the poor response rate with radioconjugates in established solid tumors, the gain seen in preclinical models in this setting may not translate well to improved clinical results, unless used in minimal disease or intracavity settings, where a reasonable baseline response with the radioconjugate alone is observed. Several clinical studies have combined directly radiolabeled antibodies with chemotherapy in advanced cancer, but these trials still failed to produce substantive responses [238–241].
A different approach would be to add antibody conjugates to treatments that already have proven efficacy, but with a need for further improvement. Gold et al. showed that a relatively small, fractionated dose of a radioimmunoconjugate could be added to a full human equivalent dose of gemcitabine (~1000 mg/m2) that was given in 3 cycles of 3-weekly injections and improved therapeutic outcome in animals bearing a human pancreatic cancer cell line [242]. A significant obstacle for this approach is that radioconjugates frequently share a similar dose-limiting hematological toxicity with the chemotherapy. In the previous preclinical study, animals did not experience the same severe hematological toxicity associated with a full course of gemcitabine as is found in patients given this drug. To the extent that a similar procedure can be duplicated in patients will require clinical testing. However, pretargeting procedures can deliver similar radiation doses to tumors as a directly radiolabeled antibody, but with substantially less radiation dose to the red marrow, thereby reducing hematological toxicity [40, 60]. Thus, these methods would be more amenable to combinations with chemotherapeutic agents less limited by myelosuppression, which is the major side effect of radioimmunotherapy.
7. Concluding remarks
Antibodies and immunoconjugates are just beginning to have an impact on the treatment of not only cancer, but many other diseases [40]. However, as presented in this overview, even in situations where the cancer is considered to be more accessible, complete, long-lasting responses are difficult to achieve, and in most instances, the tumor burden must be substantially reduced for meaningful responses to occur. In this regard, antibodies are finding a niche in cancer treatment, but more often they are being used in combination with other agents. There are examples where immunoconjugates have been shown to be more effective than their respective unconjugated antibody, but immunoconjugates have additional side effects that can influence their acceptance and adoption. Biologics are also inherently more expensive than traditional chemotherapeutic agents, and with the need to be repeatedly administered, substantial costs are added to treatment regimens. Thus, while there continues to be considerable interest and activity in developing antibodies and immunoconjugates for therapy, it will be increasing important to select appropriate clinical settings where these agents can have the greatest impact on disease progression and survival.
Acknowledgments
Drs. Sharkey and Goldenberg are supported in part by USPHS grants P01 CA10395, R01 CA107088 and R01 CA115755 from the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fidler IJ. Origin of cancer metastases and its implications for therapy. Isr J Med Sci. 1988;24:456–463. [PubMed] [Google Scholar]
- 2.Langley RR, Fidler IJ. Tumor cell-organ microenvironment interactions in the pathogenesis of cancer metastasis. Endocr Rev. 2007;28:297–321. doi: 10.1210/er.2006-0027. [DOI] [PubMed] [Google Scholar]
- 3.Fisher ER, Fisher B. Recent observations on concepts of metastasis. Arch Pathol. 1967;83:321–324. [PubMed] [Google Scholar]
- 4.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 5.Herlyn D, Koprowski H. IgG2a monoclonal antibodies inhibit human tumor growth through interaction with effector cells. Proc Natl Acad Sci U S A. 1982;79:4761–4765. doi: 10.1073/pnas.79.15.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herlyn DM, Steplewski Z, Herlyn MF, Koprowski H. Inhibition of growth of colorectal carcinoma in nude mice by monoclonal antibody. Cancer Res. 1980;40:717–721. [PubMed] [Google Scholar]
- 7.Herlyn D, Herlyn M, Steplewski Z, Koprowski H. Monoclonal antibodies in cell-mediated cytotoxicity against human melanoma and colorectal carcinoma. Eur J Immunol. 1979;9:657–659. doi: 10.1002/eji.1830090817. [DOI] [PubMed] [Google Scholar]
- 8.Hellstrom I, Brankovan V, Hellstrom KE. Strong antitumor activities of IgG3 antibodies to a human melanoma-associated ganglioside. Proc Natl Acad Sci U S A. 1985;82:1499–1502. doi: 10.1073/pnas.82.5.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sears HF, Herlyn D, Steplewski Z, Koprowski H. Phase II clinical trial of a murine monoclonal antibody cytotoxic for gastrointestinal adenocarcinoma. Cancer Res. 1985;45:5910–5913. [PubMed] [Google Scholar]
- 10.Lobuglio AF, Saleh M, Peterson L, Wheeler R, Carrano R, Huster W, Khazaeli MB. Phase I clinical trial of CO17-1A monoclonal antibody. Hybridoma. 1986;5 Suppl 1:S117–S123. [PubMed] [Google Scholar]
- 11.Frodin JE, Biberfeld P, Christensson B, Philstedt P, Sundelius S, Sylven M, Wahren B, Koprowski H, Mellstedt H. Treatment of patients with metastasizing colorectal carcinoma with mouse monoclonal antibodies (Moab 17-1A): a progress report. Hybridoma. 1986;5 Suppl 1:S151–S161. [PubMed] [Google Scholar]
- 12.Miller RA, Levy R. Response of cutaneous T cell lymphoma to therapy with hybridoma monoclonal antibody. Lancet. 1981;2:226–230. doi: 10.1016/s0140-6736(81)90475-x. [DOI] [PubMed] [Google Scholar]
- 13.Goodman GE, Beaumier P, Hellstrom I, Fernyhough B, Hellstrom KE. Pilot trial of murine monoclonal antibodies in patients with advanced melanoma. J Clin Oncol. 1985;3:340–352. doi: 10.1200/JCO.1985.3.3.340. [DOI] [PubMed] [Google Scholar]
- 14.Dillman RO, Shawler DL, Dillman JB, Royston I. Therapy of chronic lymphocytic leukemia and cutaneous T-cell lymphoma with T101 monoclonal antibody. J Clin Oncol. 1984;2:881–891. doi: 10.1200/JCO.1984.2.8.881. [DOI] [PubMed] [Google Scholar]
- 15.Ritz J, Schlossman SF. Utilization of monoclonal antibodies in the treatment of leukemia and lymphoma. Blood. 1982;59:1–11. [PubMed] [Google Scholar]
- 16.Trowbridge IS, Domingo DL. Anti-transferrin receptor monoclonal antibody and toxin-antibody conjugates affect growth of human tumour cells. Nature. 1981;294:171–173. doi: 10.1038/294171a0. [DOI] [PubMed] [Google Scholar]
- 17.Masui H, Kawamoto T, Sato JD, Wolf B, Sato G, Mendelsohn J. Growth inhibition of human tumor cells in athymic mice by anti-epidermal growth factor receptor monoclonal antibodies. Cancer Res. 1984;44:1002–1007. [PubMed] [Google Scholar]
- 18.Cilley JC, Barfi K, Benson AB, 3rd, Mulcahy MF. Bevacizumab in the treatment of colorectal cancer. Expert Opin Biol Ther. 2007;7:739–749. doi: 10.1517/14712598.7.5.739. [DOI] [PubMed] [Google Scholar]
- 19.Giantonio BJ, Catalano PJ, Meropol NJ, O'dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB., 3rd Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 20.Panares RL, Garcia AA. Bevacizumab in the management of solid tumors. Expert Rev Anticancer Ther. 2007;7:433–445. doi: 10.1586/14737140.7.4.433. [DOI] [PubMed] [Google Scholar]
- 21.Maloney DG. Preclinical and phase I and II trials of rituximab. Semin Oncol. 1999;26:74–78. [PubMed] [Google Scholar]
- 22.Valabrega G, Montemurro F, Aglietta M. Trastuzumab: mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann Oncol. 2007 doi: 10.1093/annonc/mdl475. [DOI] [PubMed] [Google Scholar]
- 23.Bonavida B. Rituximab-induced inhibition of antiapoptotic cell survival pathways: implications in chemo/immunoresistance, rituximab unresponsiveness, prognostic and novel therapeutic interventions. Oncogene. 2007;26:3629–3636. doi: 10.1038/sj.onc.1210365. [DOI] [PubMed] [Google Scholar]
- 24.Grillo-Lopez AJ, Dallaire BK, Mcclure A, Weaver R, Varns C, Wei A, Allen R, Lee D, Shen D, Leonard J, Multani P, White CA. Monoclonal antibodies: a new era in the treatment of non-Hodgkin's lymphoma. Curr Pharm Biotechnol. 2001;2:301–311. doi: 10.2174/1389201013378563. [DOI] [PubMed] [Google Scholar]
- 25.Coiffier B. Treatment of non-Hodgkin's lymphoma: a look over the past decade. Clin Lymphoma Myeloma. 2006;7 Suppl 1:S7–S13. doi: 10.3816/clm.2006.s.002. [DOI] [PubMed] [Google Scholar]
- 26.Hainsworth JD, Litchy S, Shaffer DW, Lackey VL, Grimaldi M, Greco FA. Maximizing therapeutic benefit of rituximab: maintenance therapy versus re-treatment at progression in patients with indolent non-Hodgkin's lymphoma--a randomized phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol. 2005;23:1088–1095. doi: 10.1200/JCO.2005.12.191. [DOI] [PubMed] [Google Scholar]
- 27.Collins-Burow B, Santos ES. Rituximab and its role as maintenance therapy in non-Hodgkin lymphoma. Expert Rev Anticancer Ther. 2007;7:257–273. doi: 10.1586/14737140.7.3.257. [DOI] [PubMed] [Google Scholar]
- 28.Thieblemont C, Coiffier B. Lymphoma in older patients. J Clin Oncol. 2007;25:1916–1923. doi: 10.1200/JCO.2006.10.5957. [DOI] [PubMed] [Google Scholar]
- 29.Marcus R. Use of rituximab in patients with follicular lymphoma. Clin Oncol (R Coll Radiol) 2007;19:38–49. doi: 10.1016/j.clon.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 30.Marcus R, Hagenbeek A. The therapeutic use of rituximab in non-Hodgkin's lymphoma. Eur J Haematol Suppl. 2007:5–14. doi: 10.1111/j.1600-0609.2006.00789.x. [DOI] [PubMed] [Google Scholar]
- 31.Presta LG. Engineering of therapeutic antibodies to minimize immunogenicity and optimize function. Adv Drug Deliv Rev. 2006;58:640–656. doi: 10.1016/j.addr.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 32.Daugherty AL, Mrsny RJ. Formulation and delivery issues for monoclonal antibody therapeutics. Adv Drug Deliv Rev. 2006;58:686–706. doi: 10.1016/j.addr.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 33.Helguera G, Penichet ML. Antibody-cytokine fusion proteins for the therapy of cancer. Methods Mol Med. 2005;109:347–374. doi: 10.1385/1-59259-862-5:347. [DOI] [PubMed] [Google Scholar]
- 34.Pastan I, Hassan R, Fitzgerald DJ, Kreitman RJ. Immunotoxin treatment of cancer. Annu Rev Med. 2007;58:221–237. doi: 10.1146/annurev.med.58.070605.115320. [DOI] [PubMed] [Google Scholar]
- 35.Kiewe P, Hasmuller S, Kahlert S, Heinrigs M, Rack B, Marme A, Korfel A, Jager M, Lindhofer H, Sommer H, Thiel E, Untch M. Phase I trial of the trifunctional anti-HER2 x anti-CD3 antibody ertumaxomab in metastatic breast cancer. Clin Cancer Res. 2006;12:3085–3091. doi: 10.1158/1078-0432.CCR-05-2436. [DOI] [PubMed] [Google Scholar]
- 36.Brischwein K, Schlereth B, Guller B, Steiger C, Wolf A, Lutterbuese R, Offner S, Locher M, Urbig T, Raum T, Kleindienst P, Wimberger P, Kimmig R, Fichtner I, Kufer P, Hofmeister R, Da Silva AJ, Baeuerle PA. MT110: a novel bispecific single-chain antibody construct with high efficacy in eradicating established tumors. Mol Immunol. 2006;43:1129–1143. doi: 10.1016/j.molimm.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 37.Manzke O, Tesch H, Borchmann P, Wolf J, Lackner K, Gossmann A, Diehl V, Bohlen H. Locoregional treatment of low-grade B-cell lymphoma with CD3×CD19 bispecific antibodies and CD28 costimulation. I. Clinical phase I evaluation. Int J Cancer. 2001;91:508–515. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1068>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 38.De Gast GC, Van Houten AA, Haagen IA, Klein S, De Weger RA, Van Dijk A, Phillips J, Clark M, Bast BJ. Clinical experience with CD3 × CD19 bispecific antibodies in patients with B cell malignancies. J Hematother. 1995;4:433–437. doi: 10.1089/scd.1.1995.4.433. [DOI] [PubMed] [Google Scholar]
- 39.Rossi EA, Goldenberg DM, Cardillo TM, Mcbride WJ, Sharkey RM, Chang CH. Stably tethered multifunctional structures of defined composition made by the dock and lock method for use in cancer targeting. Proc Natl Acad Sci U S A. 2006;103:6841–6846. doi: 10.1073/pnas.0600982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharkey RM, Goldenberg DM. Targeted therapy of cancer: new prospects for antibodies and immunoconjugates. CA Cancer J Clin. 2006;56:226–243. doi: 10.3322/canjclin.56.4.226. [DOI] [PubMed] [Google Scholar]
- 41.Goldenberg DM, Sharkey RM. Novel radiolabeled antibody conjugates. Oncogene. 2007;26:3734–3744. doi: 10.1038/sj.onc.1210373. [DOI] [PubMed] [Google Scholar]
- 42.Beckman RA, Weiner LM, Davis HM. Antibody constructs in cancer therapy: protein engineering strategies to improve exposure in solid tumors. Cancer. 2007;109:170–179. doi: 10.1002/cncr.22402. [DOI] [PubMed] [Google Scholar]
- 43.Feldman EJ, Brandwein J, Stone R, Kalaycio M, Moore J, O'connor J, Wedel N, Roboz GJ, Miller C, Chopra R, Jurcic JC, Brown R, Ehmann WC, Schulman P, Frankel SR, De Angelo D, Scheinberg D. Phase III randomized multicenter study of a humanized anti-CD33 monoclonal antibody, lintuzumab, in combination with chemotherapy, versus chemotherapy alone in patients with refractory or first-relapsed acute myeloid leukemia. J Clin Oncol. 2005;23:4110–4116. doi: 10.1200/JCO.2005.09.133. [DOI] [PubMed] [Google Scholar]
- 44.Lambert JM. Drug-conjugated monoclonal antibodies for the treatment of cancer. Curr Opin Pharmacol. 2005;5:543–549. doi: 10.1016/j.coph.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 45.Davis TA, Kaminski MS, Leonard JP, Hsu FJ, Wilkinson M, Zelenetz A, Wahl RL, Kroll S, Coleman M, Goris M, Levy R, Knox SJ. The radioisotope contributes significantly to the activity of radioimmunotherapy. Clin Cancer Res. 2004;10:7792–7798. doi: 10.1158/1078-0432.CCR-04-0756. [DOI] [PubMed] [Google Scholar]
- 46.Witzig TE, Gordon LI, Cabanillas F, Czuczman MS, Emmanouilides C, Joyce R, Pohlman BL, Bartlett NL, Wiseman GA, Padre N, Grillo-Lopez AJ, Multani P, White CA. Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin's lymphoma. J Clin Oncol. 2002;20:2453–2463. doi: 10.1200/JCO.2002.11.076. [DOI] [PubMed] [Google Scholar]
- 47.Vitetta ES. Immunotoxins and vascular leak syndrome. Cancer J. 2000;6 Suppl 3:S218–S224. [PubMed] [Google Scholar]
- 48.Cattel L, Delprino L, Brusa P, Dosio F, Comoglio PM, Prat M. Comparison of blocked and non-blocked ricin-antibody immunotoxins against human gastric carcinoma and colorectal adenocarcinoma cell lines. Cancer Immunol Immunother. 1988;27:233–240. doi: 10.1007/BF00205445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brusa P, Dosio F, Pietribiasi F, Delprino L, Feraiorni P, Mariani M, Bussolati G, Cattel L. Antitumour activity of a sterically blocked ricin immunotoxin on a human colorectal adenocarcinoma grafted subcutaneously in nude mice. Cancer Immunol Immunother. 1992;35:373–380. doi: 10.1007/BF01789015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baluna R, Coleman E, Jones C, Ghetie V, Vitetta ES. The effect of a monoclonal antibody coupled to ricin A chain-derived peptides on endothelial cells in vitro: insights into toxin-mediated vascular damage. Exp Cell Res. 2000;258:417–424. doi: 10.1006/excr.2000.4954. [DOI] [PubMed] [Google Scholar]
- 51.Smallshaw JE, Ghetie V, Rizo J, Fulmer JR, Trahan LL, Ghetie MA, Vitetta ES. Genetic engineering of an immunotoxin to eliminate pulmonary vascular leak in mice. Nat Biotechnol. 2003;21:387–391. doi: 10.1038/nbt800. [DOI] [PubMed] [Google Scholar]
- 52.Messmer D, Kipps TJ. Treatment of solid tumors with immunotoxins. Breast Cancer Res. 2005;7:184–186. doi: 10.1186/bcr1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kreitman RJ. Toxin-labeled monoclonal antibodies. Curr Pharm Biotechnol. 2001;2:313–325. doi: 10.2174/1389201013378635. [DOI] [PubMed] [Google Scholar]
- 54.Schellekens H. Immunogenicity of therapeutic proteins: clinical implications and future prospects. Clin Ther. 2002;24:1720–1740. doi: 10.1016/s0149-2918(02)80075-3. [DOI] [PubMed] [Google Scholar]
- 55.Newton DL, Ryback SM. Antibody targeted therapeutics for lymphoma: new focus on the CD22 antigen and RNA. Expert Opin Biol Ther. 2001;1:995–1003. doi: 10.1517/14712598.1.6.995. [DOI] [PubMed] [Google Scholar]
- 56.Hellstrom I, Garrigues HJ, Garrigues U, Hellstrom KE. Highly tumor-reactive, internalizing, mouse monoclonal antibodies to Le(y)-related cell surface antigens. Cancer Res. 1990;50:2183–2190. [PubMed] [Google Scholar]
- 57.Tolcher AW, Sugarman S, Gelmon KA, Cohen R, Saleh M, Isaacs C, Young L, Healey D, Onetto N, Slichenmyer W. Randomized phase II study of BR96-doxorubicin conjugate in patients with metastatic breast cancer. J Clin Oncol. 1999;17:478–484. doi: 10.1200/JCO.1999.17.2.478. [DOI] [PubMed] [Google Scholar]
- 58.Ravjvanshi P, Shulman HM, Sievers EL, Mcdonald GB. Hepatic sinusoidal obstruction after gemtuzumab ozogamicin (Mylotarg) therapy. Blood. 2002;99:2310–2314. doi: 10.1182/blood.v99.7.2310. [DOI] [PubMed] [Google Scholar]
- 59.Sharkey RM, Goldenberg DM. Perspectives on cancer therapy with radiolabeled monoclonal antibodies. J Nucl Med. 2005;46 Suppl 1:115S–127S. [PubMed] [Google Scholar]
- 60.Goldenberg DM, Sharkey RM, Paganelli G, Barbet J, Chatal JF. Antibody pretargeting advances cancer radioimmunodetection and radioimmunotherapy. J Clin Oncol. 2006;24:823–834. doi: 10.1200/JCO.2005.03.8471. [DOI] [PubMed] [Google Scholar]
- 61.Hansen HJ, Ong GL, Diril H, Valdez A, Roche PA, Griffiths GL, Goldenberg DM, Mattes MJ. Internalization and catabolism of radiolabelled antibodies to the MHC class-II invariant chain by B-cell lymphomas. Biochem J. 1996;320(Pt 1):293–300. doi: 10.1042/bj3200293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chang CH, Sapra P, Vanama SS, Hansen HJ, Horak ID, Goldenberg DM. Effective therapy of human lymphoma xenografts with a novel recombinant ribonuclease/anti-CD74 humanized IgG4 antibody immunotoxin. Blood. 2005;106:4308–4314. doi: 10.1182/blood-2005-03-1033. [DOI] [PubMed] [Google Scholar]
- 63.Sapra P, Stein R, Pickett J, Qu Z, Govindan SV, Cardillo TM, Hansen HJ, Horak ID, Griffiths GL, Goldenberg DM. Anti-CD74 antibody-doxorubicin conjugate, IMMU-110, in a human multiple myeloma xenograft and in monkeys. Clin Cancer Res. 2005;11:5257–5264. doi: 10.1158/1078-0432.CCR-05-0204. [DOI] [PubMed] [Google Scholar]
- 64.Michel RB, Rosario AV, Andrews PM, Goldenberg DM, Mattes MJ. Therapy of small subcutaneous B-lymphoma xenografts with antibodies conjugated to radionuclides emitting low-energy electrons. Clin Cancer Res. 2005;11:777–786. [PubMed] [Google Scholar]
- 65.Griffiths GL, Mattes MJ, Stein R, Govindan SV, Horak ID, Hansen HJ, Goldenberg DM. Cure of SCID mice bearing human B-lymphoma xenografts by an anti-CD74 antibody-anthracycline drug conjugate. Clin Cancer Res. 2003;9:6567–6571. [PubMed] [Google Scholar]
- 66.Ong GL, Elsamra SE, Goldenberg DM, Mattes MJ. Single-cell cytotoxicity with radiolabeled antibodies. Clin Cancer Res. 2001;7:192–201. [PubMed] [Google Scholar]
- 67.Starlets D, Gore Y, Binsky I, Haran M, Harpaz N, Shvidel L, Becker-Herman S, Berrebi A, Shachar I. Cell-surface CD74 initiates a signaling cascade leading to cell proliferation and survival. Blood. 2006;107:4807–4816. doi: 10.1182/blood-2005-11-4334. [DOI] [PubMed] [Google Scholar]
- 68.Stein R, Qu Z, Cardillo TM, Chen S, Rosario A, Horak ID, Hansen HJ, Goldenberg DM. Antiproliferative activity of a humanized anti-CD74 monoclonal antibody, hLL1, on B-cell malignancies. Blood. 2004;104:3705–3711. doi: 10.1182/blood-2004-03-0890. [DOI] [PubMed] [Google Scholar]
- 69.Ghose T, Blair AH. Antibody-linked cytotoxic agents in the treatment of cancer: current status and future prospects. J Natl Cancer Inst. 1978;61:657–676. [PubMed] [Google Scholar]
- 70.Ghose T, Blair AH. The design of cytotoxic-agent-antibody conjugates. Crit Rev Ther Drug Carrier Syst. 1987;3:263–359. [PubMed] [Google Scholar]
- 71.Kreitman RJ, Pastan I. Accumulation of a recombinant immunotoxin in a tumor in vivo: fewer than 1000 molecules per cell are sufficient for complete responses. Cancer Res. 1998;58:968–975. [PubMed] [Google Scholar]
- 72.Schrama D, Reisfeld RA, Becker JC. Antibody targeted drugs as cancer therapeutics. Nat Rev Drug Discov. 2006;5:147–159. doi: 10.1038/nrd1957. [DOI] [PubMed] [Google Scholar]
- 73.Wu AM, Senter PD. Arming antibodies: prospects and challenges for immunoconjugates. Nat Biotechnol. 2005;23:1137–1146. doi: 10.1038/nbt1141. [DOI] [PubMed] [Google Scholar]
- 74.Arnon R. Site-directed tumor chemotherapy. Compr Ther. 1978;4:68–73. [PubMed] [Google Scholar]
- 75.Jedema I, Barge RM, Van Der Velden VH, Nijmeijer BA, Van Dongen JJ, Willemze R, Falkenburg JH. Internalization and cell cycle-dependent killing of leukemic cells by Gemtuzumab Ozogamicin: rationale for efficacy in CD33-negative malignancies with endocytic capacity. Leukemia. 2004;18:316–325. doi: 10.1038/sj.leu.2403205. [DOI] [PubMed] [Google Scholar]
- 76.Dubowchik GM, Firestone RA, Padilla L, Willner D, Hofstead SJ, Mosure K, Knipe JO, Lasch SJ, Trail PA. Cathepsin B-labile dipeptide linkers for lysosomal release of doxorubicin from internalizing immunoconjugates: model studies of enzymatic drug release and antigen-specific in vitro anticancer activity. Bioconjug Chem. 2002;13:855–869. doi: 10.1021/bc025536j. [DOI] [PubMed] [Google Scholar]
- 77.Suzawa T, Nagamura S, Saito H, Ohta S, Hanai N, Kanazawa J, Okabe M, Yamasaki M. Enhanced tumor cell selectivity of adriamycin-monoclonal antibody conjugate via a poly(ethylene glycol)-based cleavable linker. J Control Release. 2002;79:229–242. doi: 10.1016/s0168-3659(01)00554-5. [DOI] [PubMed] [Google Scholar]
- 78.Mueller BM, Wrasidlo WA, Reisfeld RA. Antibody conjugates with morpholinodoxorubicin and acid-cleavable linkers. Bioconjug Chem. 1990;1:325–330. doi: 10.1021/bc00005a005. [DOI] [PubMed] [Google Scholar]
- 79.Govindan SV, Griffiths GL, Hansen HJ, Horak ID, Goldenberg DM. Cancer therapy with radiolabeled and drug/toxin-conjugated antibodies. Technol Cancer Res Treat. 2005;4:375–391. doi: 10.1177/153303460500400406. [DOI] [PubMed] [Google Scholar]
- 80.Kovtun YV, Audette CA, Ye Y, Xie H, Ruberti MF, Phinney SJ, Leece BA, Chittenden T, Blattler WA, Goldmacher VS. Antibody-drug conjugates designed to eradicate tumors with homogeneous and heterogeneous expression of the target antigen. Cancer Res. 2006;66:3214–3221. doi: 10.1158/0008-5472.CAN-05-3973. [DOI] [PubMed] [Google Scholar]
- 81.Denny WA. Tumor-activated prodrugs--a new approach to cancer therapy. Cancer Invest. 2004;22:604–619. doi: 10.1081/cnv-200027148. [DOI] [PubMed] [Google Scholar]
- 82.Chan SY, Gordon AN, Coleman RE, Hall JB, Berger MS, Sherman ML, Eten CB, Finkler NJ. A phase 2 study of the cytotoxic immunoconjugate CMB-401 (hCTM01-calicheamicin) in patients with platinum-sensitive recurrent epithelial ovarian carcinoma. Cancer Immunol Immunother. 2003;52:243–248. doi: 10.1007/s00262-002-0343-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mayer A, Francis RJ, Sharma SK, Tolner B, Springer CJ, Martin J, Boxer GM, Bell J, Green AJ, Hartley JA, Cruickshank C, Wren J, Chester KA, Begent RH. A phase I study of single administration of antibody-directed enzyme prodrug therapy with the recombinant anti-carcinoembryonic antigen antibody-enzyme fusion protein MFECP1 and a bis-iodo phenol mustard prodrug. Clin Cancer Res. 2006;12:6509–6516. doi: 10.1158/1078-0432.CCR-06-0769. [DOI] [PubMed] [Google Scholar]
- 84.Sharma SK, Bagshawe KD, Begent RH. Advances in antibody-directed enzyme prodrug therapy. Curr Opin Investig Drugs. 2005;6:611–615. [PubMed] [Google Scholar]
- 85.Schwulst SJ, Davis CG, Coopersmith CM, Hotchkiss RS. Adoptive transfer of dying cells causes bystander-induced apoptosis. Biochem Biophys Res Commun. 2007;353:780–785. doi: 10.1016/j.bbrc.2006.12.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cairns R, Papandreou I, Denko N. Overcoming physiologic barriers to cancer treatment by molecularly targeting the tumor microenvironment. Mol Cancer Res. 2006;4:61–70. doi: 10.1158/1541-7786.MCR-06-0002. [DOI] [PubMed] [Google Scholar]
- 87.Goldenberg DM, Preston DF, Primus FJ, Hansen HJ. Photoscan localization of GW-39 tumors in hamsters using radiolabeled anticarcinoembryonic antigen immunoglobulin G. Cancer Res. 1974;34:1–9. [PubMed] [Google Scholar]
- 88.Duran-Reynals F. Studies on the localization of dyes and foreign proteins in normal and malignant tissues. Am. J. Cancer. 1939;35:98–107. [Google Scholar]
- 89.Fujimori K, Covell DG, Fletcher JE, Weinstein JN. A modeling analysis of monoclonal antibody percolation through tumors: a binding-site barrier. J Nucl Med. 1990;31:1191–1198. [PubMed] [Google Scholar]
- 90.Baxter LT, Zhu H, Mackensen DG, Jain RK. Physiologically based pharmacokinetic model for specific and nonspecific monoclonal antibodies and fragments in normal tissues and human tumor xenografts in nude mice. Cancer Res. 1994;54:1517–1528. [PubMed] [Google Scholar]
- 91.Adams GP, Schier R, Mccall AM, Simmons HH, Horak EM, Alpaugh RK, Marks JD, Weiner LM. High affinity restricts the localization and tumor penetration of single-chain fv antibody molecules. Cancer Res. 2001;61:4750–4755. [PubMed] [Google Scholar]
- 92.Blumenthal RD, Fand I, Sharkey RM, Boerman OC, Kashi R, Goldenberg DM. The effect of antibody protein dose on the uniformity of tumor distribution of radioantibodies: an autoradiographic study. Cancer Immunol Immunother. 1991;33:351–358. doi: 10.1007/BF01741594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Graff CP, Wittrup KD. Theoretical analysis of antibody targeting of tumor spheroids: importance of dosage for penetration, and affinity for retention. Cancer Res. 2003;63:1288–1296. [PubMed] [Google Scholar]
- 94.Van Osdol W, Fujimori K, Weinstein JN. An analysis of monoclonal antibody distribution in microscopic tumor nodules: consequences of a "binding site barrier". Cancer Res. 1991;51:4776–4784. [PubMed] [Google Scholar]
- 95.Baxter LT, Jain RK. Transport of fluid and macromolecules in tumors. III. Role of binding and metabolism. Microvasc Res. 1991;41:5–23. doi: 10.1016/0026-2862(91)90003-t. [DOI] [PubMed] [Google Scholar]
- 96.Sato N, Saga T, Sakahara H, Yao Z, Nakamoto Y, Zhang M, Kuroki M, Matsuoka Y, Iida Y, Konishi J. Intratumoral distribution of radiolabeled antibody and radioimmunotherapy in experimental liver metastases model of nude mouse. J Nucl Med. 1999;40:685–692. [PubMed] [Google Scholar]
- 97.Boerman OC, Sharkey RM, Wong GY, Blumenthal RD, Aninipot RL, Goldenberg DM. Influence of antibody protein dose on therapeutic efficacy of radioiodinated antibodies in nude mice bearing GW-39 human tumor. Cancer Immunol Immunother. 1992;35:127–134. doi: 10.1007/BF01741860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Knox SJ, Goris ML, Trisler K, Negrin R, Davis T, Liles TM, Grillo-Lopez A, Chinn P, Varns C, Ning SC, Fowler S, Deb N, Becker M, Marquez C, Levy R. Yttrium-90-labeled anti-CD20 monoclonal antibody therapy of recurrent B-cell lymphoma. Clin Cancer Res. 1996;2:457–470. [PubMed] [Google Scholar]
- 99.Kaminski MS, Zasadny KR, Francis IR, Milik AW, Ross CW, Moon SD, Crawford SM, Burgess JM, Petry NA, Butchko GM, et al. Radioimmunotherapy of B-cell lymphoma with [131I]anti-B1 (anti-CD20) antibody. N Engl J Med. 1993;329:459–465. doi: 10.1056/NEJM199308123290703. [DOI] [PubMed] [Google Scholar]
- 100.Khawli LA, Biela B, Hu P, Epstein AL. Comparison of recombinant derivatives of chimeric TNT-3 antibody for the radioimaging of solid tumors. Hybrid Hybridomics. 2003;22:1–9. doi: 10.1089/153685903321538026. [DOI] [PubMed] [Google Scholar]
- 101.Daugherty AL, Mrsny RJ. Emerging technologies that overcome biological barriers for therapeutic protein delivery. Expert Opin Biol Ther. 2003;3:1071–1081. doi: 10.1517/14712598.3.7.1071. [DOI] [PubMed] [Google Scholar]
- 102.Ferguson S, Lesniak MS. Convection enhanced drug delivery of novel therapeutic agents to malignant brain tumors. Curr Drug Deliv. 2007;4:169–180. doi: 10.2174/156720107780362302. [DOI] [PubMed] [Google Scholar]
- 103.Guo J, Zhu J, Sheng X, Wang X, Qu L, Han Y, Liu Y, Zhang H, Huo L, Zhang S, Lin B, Yang Z. Intratumoral injection of dendritic cells in combination with local hyperthermia induces systemic antitumor effect in patients with advanced melanoma. Int J Cancer. 2007;120:2418–2425. doi: 10.1002/ijc.22551. [DOI] [PubMed] [Google Scholar]
- 104.Vukelja SJ, Anthony SP, Arseneau JC, Berman BS, Cunningham CC, Nemunaitis JJ, Samlowski WE, Fowers KD. Phase 1 study of escalating-dose OncoGel (ReGel/paclitaxel) depot injection, a controlled-release formulation of paclitaxel, for local management of superficial solid tumor lesions. Anticancer Drugs. 2007;18:283–289. doi: 10.1097/CAD.0b013e328011a51d. [DOI] [PubMed] [Google Scholar]
- 105.Seror O, N'kontchou G, Tin Tin Htar M, Durand-Zaleski I, Trinchet JC, Sellier N, Beaugrand M. Ethanol versus radiofrequency ablation for the treatment of small hepatocellular carcinoma in patients with cirrhosis: a retrospective study of efficacy and cost. Gastroenterol Clin Biol. 2006;30:1265–1273. doi: 10.1016/s0399-8320(06)73534-5. [DOI] [PubMed] [Google Scholar]
- 106.Matthes K, Mino-Kenudson M, Sahani DV, Holalkere N, Fowers KD, Rathi R, Brugge WR. EUS-guided injection of paclitaxel (OncoGel) provides therapeutic drug concentrations in the porcine pancreas (with video) Gastrointest Endosc. 2007;65:448–453. doi: 10.1016/j.gie.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 107.Gonzalez R, Hutchins L, Nemunaitis J, Atkins M, Schwarzenberger PO. Phase 2 trial of Allovectin-7 in advanced metastatic melanoma. Melanoma Res. 2006;16:521–526. doi: 10.1097/01.cmr.0000232299.44902.41. [DOI] [PubMed] [Google Scholar]
- 108.Kerl K, Prins C, Saurat JH, French LE. Intralesional and intravenous treatment of cutaneous B-cell lymphomas with the monoclonal anti-CD20 antibody rituximab: report and follow-up of eight cases. Br J Dermatol. 2006;155:1197–1200. doi: 10.1111/j.1365-2133.2006.07523.x. [DOI] [PubMed] [Google Scholar]
- 109.Ter Horst M, Verwijnen SM, Brouwer E, Hoeben RC, De Jong M, De Leeuw BH, Sillevis Smitt PA. Locoregional delivery of adenoviral vectors. J Nucl Med. 2006;47:1483–1489. [PubMed] [Google Scholar]
- 110.Lorenz M, Muller HH. Randomized, multicenter trial of fluorouracil plus leucovorin administered either via hepatic arterial or intravenous infusion versus fluorodeoxyuridine administered via hepatic arterial infusion in patients with nonresectable liver metastases from colorectal carcinoma. J Clin Oncol. 2000;18:243–254. doi: 10.1200/JCO.2000.18.2.243. [DOI] [PubMed] [Google Scholar]
- 111.Ducreux M, Ychou M, Laplanche A, Gamelin E, Lasser P, Husseini F, Quenet F, Viret F, Jacob JH, Boige V, Elias D, Delperro JR, Luboinski M. Hepatic arterial oxaliplatin infusion plus intravenous chemotherapy in colorectal cancer with inoperable hepatic metastases: a trial of the gastrointestinal group of the Federation Nationale des Centres de Lutte Contre le Cancer. J Clin Oncol. 2005;23:4881–4887. doi: 10.1200/JCO.2005.05.120. [DOI] [PubMed] [Google Scholar]
- 112.Kemeny N, Daly J, Reichman B, Geller N, Botet J, Oderman P. Intrahepatic or systemic infusion of fluorodeoxyuridine in patients with liver metastases from colorectal carcinoma. A randomized trial. Ann Intern Med. 1987;107:459–465. doi: 10.7326/0003-4819-107-4-459. [DOI] [PubMed] [Google Scholar]
- 113.Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. Monoclonal antibody and intravenous immunoglobulin therapy for rheumatic diseases: rationale and mechanisms of action. Nat Clin Pract Rheumatol. 2007;3:262–272. doi: 10.1038/ncprheum0481. [DOI] [PubMed] [Google Scholar]
- 114.Kaczmarek I, Deutsch MA, Sadoni S, Brenner P, Schmauss D, Daebritz SH, Weiss M, Meiser BM, Reichart B. Successful management of antibody-mediated cardiac allograft rejection with combined immunoadsorption and anti-CD20 monoclonal antibody treatment: case report and literature review. J Heart Lung Transplant. 2007;26:511–515. doi: 10.1016/j.healun.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 115.Sperr WR, Lechner K, Pabinger I. Rituximab for the treatment of acquired antibodies to factor VIII. Haematologica. 2007;92:66–71. doi: 10.3324/haematol.10553. [DOI] [PubMed] [Google Scholar]
- 116.Eisenberg R. Targeting B cells in SLE: the experience with rituximab treatment (anti-CD20) Endocr Metab Immune Disord Drug Targets. 2006;6:345–350. doi: 10.2174/187153006779025757. [DOI] [PubMed] [Google Scholar]
- 117.Srock S, Schriever F, Neubauer A, Herold M, Huhn D. Long-term treatment with rituximab is feasible in selected patients with B-CLL: Response-adjusted low-dose maintenance treatment with rituximab in patients with relapsed B-CLL, who achieved a partial or minimal response to prior rituximab therapy. Leuk Lymphoma. 2007;48:905–911. doi: 10.1080/10428190701225874. [DOI] [PubMed] [Google Scholar]
- 118.Kay NE, Geyer SM, Call TG, Shanafelt TD, Zent CS, Jelinek DF, Tschumper R, Bone ND, Dewald GW, Lin TS, Heerema NA, Smith L, Grever MR, Byrd JC. Combination chemoimmunotherapy with pentostatin, cyclophosphamide, and rituximab shows significant clinical activity with low accompanying toxicity in previously untreated B chronic lymphocytic leukemia. Blood. 2007;109:405–411. doi: 10.1182/blood-2006-07-033274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cheson BD. Monoclonal antibody therapy for B-cell malignancies. Semin Oncol. 2006;33:S2–S14. doi: 10.1053/j.seminoncol.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 120.Czuczman MS, Thall A, Witzig TE, Vose JM, Younes A, Emmanouilides C, Miller TP, Moore JO, Leonard JP, Gordon LI, Sweetenham J, Alkuzweny B, Finucane DM, Leigh BR. Phase I/II study of galiximab, an anti-CD80 antibody, for relapsed or refractory follicular lymphoma. J Clin Oncol. 2005;23:4390–4398. doi: 10.1200/JCO.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 121.Goldenberg DM. Epratuzumab in the therapy of oncological and immunological diseases. Expert Rev Anticancer Ther. 2006;6:1341–1353. doi: 10.1586/14737140.6.10.1341. [DOI] [PubMed] [Google Scholar]
- 122.Leonard J, Friedberg J, Younes A, Fisher D, Gordon L, Moore J, Czuczman M, Miller T, Stiff P, Cheson B, Forero-Torres A, Chieffo N, Mckinney B, Finucane D, Molina A. A phase I/II study of galiximab (an anti-CD80 monoclonal antibody) in combination with rituximab for relapsed or refractory, follicular lymphoma. Ann Oncol. 2007 doi: 10.1093/annonc/mdm114. [DOI] [PubMed] [Google Scholar]
- 123.Leonard JP, Coleman M, Ketas J, Ashe M, Fiore JM, Furman RR, Niesvizky R, Shore T, Chadburn A, Horne H, Kovacs J, Ding CL, Wegener WA, Horak ID, Goldenberg DM. Combination antibody therapy with epratuzumab and rituximab in relapsed or refractory non-Hodgkin's lymphoma. J Clin Oncol. 2005;23:5044–5051. doi: 10.1200/JCO.2005.13.821. [DOI] [PubMed] [Google Scholar]
- 124.Strauss SJ, Morschhauser F, Rech J, Repp R, Solal-Celigny P, Zinzani PL, Engert A, Coiffier B, Hoelzer DF, Wegener WA, Teoh NK, Goldenberg DM, Lister TA. Multicenter phase II trial of immunotherapy with the humanized anti-CD22 antibody, epratuzumab, in combination with rituximab, in refractory or recurrent non-Hodgkin's lymphoma. J Clin Oncol. 2006;24:3880–3886. doi: 10.1200/JCO.2006.05.6291. [DOI] [PubMed] [Google Scholar]
- 125.Rech J, Repp R, Rech D, Stockmeyer B, Dechant M, Niedobitek G, Gramatzki M, Valerius T. A humanized HLA-DR antibody (hu1D10, apolizumab) in combination with granulocyte colony-stimulating factor (filgrastim) for the treatment of non-Hodgkin's lymphoma: a pilot study. Leuk Lymphoma. 2006;47:2147–2154. doi: 10.1080/10428190600757944. [DOI] [PubMed] [Google Scholar]
- 126.Abutalib SA, Tallman MS. Monoclonal antibodies for the treatment of acute myeloid leukemia. Curr Pharm Biotechnol. 2006;7:343–369. doi: 10.2174/138920106778521578. [DOI] [PubMed] [Google Scholar]
- 127.Sievers EL. Targeted therapy of acute myeloid leukemia with monoclonal antibodies and immunoconjugates. Cancer Chemother Pharmacol. 2000;46 Suppl:S18–S22. doi: 10.1007/pl00014043. [DOI] [PubMed] [Google Scholar]
- 128.Scheinberg DA, Jurcic JG, Maslak P. Antibody-drug conjugates in acute myeloid leukemia. Nat Clin Pract Oncol. 2006;3:238–239. doi: 10.1038/ncponc0478. [DOI] [PubMed] [Google Scholar]
- 129.Zenz T, Glatting G, Schlenk RF, Buchmann I, Dohner H, Reske SN, Bunjes D. Targeted marrow irradiation with radioactively labeled anti-CD66 monoclonal antibody prior to allogeneic stem cell transplantation for patients with leukemia: results of a phase I-II study. Haematologica. 2006;91:285–286. [PubMed] [Google Scholar]
- 130.Bunjes D. 188Re-labeled anti-CD66 monoclonal antibody in stem cell transplantation for patients with high-risk acute myeloid leukemia. Leuk Lymphoma. 2002;43:2125–2131. doi: 10.1080/1042819021000033015. [DOI] [PubMed] [Google Scholar]
- 131.Glatting G, Muller M, Koop B, Hohl K, Friesen C, Neumaier B, Berrie E, Bird P, Hale G, Blumstein NM, Waldmann H, Bunjes D, Reske SN. Anti-CD45 monoclonal antibody YAML568: A promising radioimmunoconjugate for targeted therapy of acute leukemia. J Nucl Med. 2006;47:1335–1341. [PubMed] [Google Scholar]
- 132.Bethge WA, Wilbur DS, Sandmaier BM. Radioimmunotherapy as non-myeloablative conditioning for allogeneic marrow transplantation. Leuk Lymphoma. 2006;47:1205–1214. doi: 10.1080/00423110500485822. [DOI] [PubMed] [Google Scholar]
- 133.Pagel JM, Appelbaum FR, Eary JF, Rajendran J, Fisher DR, Gooley T, Ruffner K, Nemecek E, Sickle E, Durack L, Carreras J, Horowitz MM, Press OW, Gopal AK, Martin PJ, Bernstein ID, Matthews DC. 131I-anti-CD45 antibody plus busulfan and cyclophosphamide before allogeneic hematopoietic cell transplantation for treatment of acute myeloid leukemia in first remission. Blood. 2006;107:2184–2191. doi: 10.1182/blood-2005-06-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Matthews DC, Appelbaum FR, Eary JF, Fisher DR, Durack LD, Hui TE, Martin PJ, Mitchell D, Press OW, Storb R, Bernstein ID. Phase I study of 131I-anti-CD45 antibody plus cyclophosphamide and total body irradiation for advanced acute leukemia and myelodysplastic syndrome. Blood. 1999;94:1237–1247. [PubMed] [Google Scholar]
- 135.Waldmann TA. Daclizumab (anti-Tac, Zenapax) in the treatment of leukemia/lymphoma. Oncogene. 2007;26:3699–3703. doi: 10.1038/sj.onc.1210368. [DOI] [PubMed] [Google Scholar]
- 136.Waldmann TA. Anti-Tac (daclizumab, Zenapax) in the treatment of leukemia, autoimmune diseases, and in the prevention of allograft rejection: a 25-year personal odyssey. J Clin Immunol. 2007;27:1–18. doi: 10.1007/s10875-006-9060-0. [DOI] [PubMed] [Google Scholar]
- 137.Kreitman RJ, Wilson WH, White JD, Stetler-Stevenson M, Jaffe ES, Giardina S, Waldmann TA, Pastan I. Phase I trial of recombinant immunotoxin anti-Tac(Fv)-PE38 (LMB-2) in patients with hematologic malignancies. J Clin Oncol. 2000;18:1622–1636. doi: 10.1200/JCO.2000.18.8.1622. [DOI] [PubMed] [Google Scholar]
- 138.Kim YH, Duvic M, Obitz E, Gniadecki R, Iversen L, Osterborg A, Whittaker S, Illidge TM, Schwarz T, Kaufmann R, Cooper K, Knudsen KM, Lisby S, Baadsgaard O, Knox SJ. Clinical efficacy of zanolimumab (HuMax-CD4): two phase 2 studies in refractory cutaneous T-cell lymphoma. Blood. 2007;109:4655–4662. doi: 10.1182/blood-2006-12-062877. [DOI] [PubMed] [Google Scholar]
- 139.Maslak PG, Jurcic JG, Scheinberg DA. Monoclonal antibody therapy of APL. Curr Top Microbiol Immunol. 2007;313:205–219. doi: 10.1007/978-3-540-34594-7_11. [DOI] [PubMed] [Google Scholar]
- 140.Scheinberg DA, Lovett D, Divgi CR, Graham MC, Berman E, Pentlow K, Feirt N, Finn RD, Clarkson BD, Gee TS, et al. A phase I trial of monoclonal antibody M195 in acute myelogenous leukemia: specific bone marrow targeting and internalization of radionuclide. J Clin Oncol. 1991;9:478–490. doi: 10.1200/JCO.1991.9.3.478. [DOI] [PubMed] [Google Scholar]
- 141.Van Der Velden VH, Te Marvelde JG, Hoogeveen PG, Bernstein ID, Houtsmuller AB, Berger MS, Van Dongen JJ. Targeting of the CD33-calicheamicin immunoconjugate Mylotarg (CMA-676) in acute myeloid leukemia: in vivo and in vitro saturation and internalization by leukemic and normal myeloid cells. Blood. 2001;97:3197–3204. doi: 10.1182/blood.v97.10.3197. [DOI] [PubMed] [Google Scholar]
- 142.Mulford DA, Scheinberg DA, Jurcic JG. The promise of targeted {alpha}-particle therapy. J Nucl Med. 2005;46 Suppl 1:199S–204S. [PubMed] [Google Scholar]
- 143.Jazirehi AR, Vega MI, Bonavida B. Development of rituximab-resistant lymphoma clones with altered cell signaling and cross-resistance to chemotherapy. Cancer Res. 2007;67:1270–1281. doi: 10.1158/0008-5472.CAN-06-2184. [DOI] [PubMed] [Google Scholar]
- 144.Czuczman MS. Combination chemotherapy and rituximab. Anticancer Drugs. 2001;12 Suppl 2:S15–S19. [PubMed] [Google Scholar]
- 145.Del Poeta G, Ilaria Del Principe M, Buccisano F, Maurillo L, Niscola P, Venditti A, Amadori S. Role of immunochemotherapy in the treatment of chronic lymphocytic leukemia. Expert Rev Anticancer Ther. 2006;6:1787–1800. doi: 10.1586/14737140.6.12.1787. [DOI] [PubMed] [Google Scholar]
- 146.Scott AM, Wiseman G, Welt S, Adjei A, Lee FT, Hopkins W, Divgi CR, Hanson LH, Mitchell P, Gansen DN, Larson SM, Ingle JN, Hoffman EW, Tanswell P, Ritter G, Cohen LS, Bette P, Arvay L, Amelsberg A, Vlock D, Rettig WJ, Old LJ. A Phase I dose-escalation study of sibrotuzumab in patients with advanced or metastatic fibroblast activation protein-positive cancer. Clin Cancer Res. 2003;9:1639–1647. [PubMed] [Google Scholar]
- 147.Pantelias A, Pagel JM, Hedin N, Saganic L, Wilbur S, Hamlin DK, Wilbur DS, Lin Y, Stone D, Axworthy D, Gopal AK, Press OW. Comparative biodistributions of pretargeted radioimmunoconjugates targeting CD20, CD22, and DR molecules on human B-cell lymphomas. Blood. 2007;109:4980–4987. doi: 10.1182/blood-2006-11-056895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Raderer M, Jager G, Brugger S, Puspok A, Fiebiger W, Drach J, Wotherspoon A, Chott A. Rituximab for treatment of advanced extranodal marginal zone B cell lymphoma of the mucosa-associated lymphoid tissue lymphoma. Oncology. 2003;65:306–310. doi: 10.1159/000074641. [DOI] [PubMed] [Google Scholar]
- 149.Rubenstein JL, Fridlyand J, Abrey L, Shen A, Karch J, Wang E, Issa S, Damon L, Prados M, Mcdermott M, O'Brien J, Haqq C, Shuman M. Phase I study of intraventricular administration of rituximab in patients with recurrent CNS and intraocular lymphoma. J Clin Oncol. 2007;25:1350–1356. doi: 10.1200/JCO.2006.09.7311. [DOI] [PubMed] [Google Scholar]
- 150.Dedrick RL, Myers CE, Bungay PM, Devita VT., Jr. Pharmacokinetic rationale for peritoneal drug administration in the treatment of ovarian cancer. Cancer Treat Rep. 1978;62:1–11. [PubMed] [Google Scholar]
- 151.Choi J, Credit K, Henderson K, Deverkadra R, He Z, Wiig H, Vanpelt H, Flessner MF. Intraperitoneal immunotherapy for metastatic ovarian carcinoma: Resistance of intratumoral collagen to antibody penetration. Clin Cancer Res. 2006;12:1906–1912. doi: 10.1158/1078-0432.CCR-05-2141. [DOI] [PubMed] [Google Scholar]
- 152.Davies Cde L, Berk DA, Pluen A, Jain RK. Comparison of IgG diffusion and extracellular matrix composition in rhabdomyosarcomas grown in mice versus in vitro as spheroids reveals the role of host stromal cells. Br J Cancer. 2002;86:1639–1644. doi: 10.1038/sj.bjc.6600270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wenning LA, Murphy RM. Coupled cellular trafficking and diffusional limitations in delivery of immunotoxins to multicell tumor spheroids. Biotechnol Bioeng. 1999;62:562–575. [PubMed] [Google Scholar]
- 154.Hjelstuen MH, Rasch-Halvorsen K, Bruland O, De LDC. Uptake, penetration, and binding of monoclonal antibodies with increasing affinity in human osteosarcoma multicell spheroids. Anticancer Res. 1998;18:3153–3161. [PubMed] [Google Scholar]
- 155.Colcher D, Esteban J, Carrasquillo JA, Sugarbaker P, Reynolds JC, Bryant G, Larson SM, Schlom J. Complementation of intracavitary and intravenous administration of a monoclonal antibody (B72.3) in patients with carcinoma. Cancer Res. 1987;47:4218–4224. [PubMed] [Google Scholar]
- 156.Van Zanten-Przybysz I, Molthoff CF, Roos JC, Verheijen RH, Van Hof A, Buist MR, Prinssen HM, Den Hollander W, Kenemans P. Influence of the route of administration on targeting of ovarian cancer with the chimeric monoclonal antibody MOv18: i.v. vs. i.p. Int J Cancer. 2001;92:106–114. [PubMed] [Google Scholar]
- 157.Varia MA, Stehman FB, Bundy BN, Benda JA, Clarke-Pearson DL, Alvarez RD, Long HJ. Intraperitoneal radioactive phosphorus (32P) versus observation after negative second-look laparotomy for stage III ovarian carcinoma: a randomized trial of the Gynecologic Oncology Group. J Clin Oncol. 2003;21:2849–2855. doi: 10.1200/JCO.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 158.Young RC, Brady MF, Nieberg RK, Long HJ, Mayer AR, Lentz SS, Hurteau J, Alberts DS. Adjuvant treatment for early ovarian cancer: a randomized phase III trial of intraperitoneal 32P or intravenous cyclophosphamide and cisplatin--a gynecologic oncology group study. J Clin Oncol. 2003;21:4350–4355. doi: 10.1200/JCO.2003.02.154. [DOI] [PubMed] [Google Scholar]
- 159.Grana C, Bartolomei M, Handkiewicz D, Rocca P, Bodei L, Colombo N, Chinol M, Mangioni C, Malavasi F, Paganelli G. Radioimmunotherapy in advanced ovarian cancer: is there a role for pre-targeting with 90Y-biotin? Gynecol Oncol. 2004;93:691–698. doi: 10.1016/j.ygyno.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 160.Alvarez RD, Partridge EE, Khazaeli MB, Plott G, Austin M, Kilgore L, Russell CD, Liu T, Grizzle WE, Schlom J, Lobuglio AF, Meredith RF. Intraperitoneal radioimmunotherapy of ovarian cancer with 177Lu-CC49: a phase I/II study. Gynecol Oncol. 1997;65:94–101. doi: 10.1006/gyno.1996.4577. [DOI] [PubMed] [Google Scholar]
- 161.Mahe MA, Fumoleau P, Fabbro M, Guastalla JP, Faurous P, Chauvot P, Chetanoud L, Classe JM, Rouanet P, Chatal JF. A phase II study of intraperitoneal radioimmunotherapy with iodine-131-labeled monoclonal antibody OC-125 in patients with residual ovarian carcinoma. Clin Cancer Res. 1999;5:3249s–3253s. [PubMed] [Google Scholar]
- 162.Oei AL, Verheijen RH, Seiden MV, Benigno BB, Lopes A, Soper JT, Epenetos AA, Massuger LF. Decreased intraperitoneal disease recurrence in epithelial ovarian cancer patients receiving intraperitoneal consolidation treatment with yttrium-90-labeled murine HMFG1 without improvement in overall survival. Int J Cancer. 2007;120:2710–2714. doi: 10.1002/ijc.22663. [DOI] [PubMed] [Google Scholar]
- 163.Alvarez RD, Huh WK, Khazaeli MB, Meredith RF, Partridge EE, Kilgore LC, Grizzle WE, Shen S, Austin JM, Barnes MN, Carey D, Schlom J, Lobuglio AF. A Phase I study of combined modality 90Yttrium-CC49 intraperitoneal radioimmunotherapy for ovarian cancer. Clin Cancer Res. 2002;8:2806–2811. [PubMed] [Google Scholar]
- 164.Juweid M, Sharkey RM, Alavi A, Swayne LC, Herskovic T, Hanley D, Rubin AD, Pereira M, Goldenberg DM. Regression of advanced refractory ovarian cancer treated with iodine-131-labeled anti-CEA monoclonal antibody. J Nucl Med. 1997;38:257–260. [PubMed] [Google Scholar]
- 165.Juweid M, Swayne LC, Sharkey RM, Dunn R, Rubin AD, Herskovic T, Goldenberg DM. Prospects of radioimmunotherapy in epithelial ovarian cancer: results with iodine-131-labeled murine and humanized MN-14 anti-carcinoembryonic antigen monoclonal antibodies. Gynecol Oncol. 1997;67:259–271. doi: 10.1006/gyno.1997.4870. [DOI] [PubMed] [Google Scholar]
- 166.Verheijen RH, Massuger LF, Benigno BB, Epenetos AA, Lopes A, Soper JT, Markowska J, Vyzula R, Jobling T, Stamp G, Spiegel G, Thurston D, Falke T, Lambert J, Seiden MV. Phase III trial of intraperitoneal therapy with yttrium-90-labeled HMFG1 murine monoclonal antibody in patients with epithelial ovarian cancer after a surgically defined complete remission. J Clin Oncol. 2006;24:571–578. doi: 10.1200/JCO.2005.02.5973. [DOI] [PubMed] [Google Scholar]
- 167.Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, Copeland LJ, Walker JL, Burger RA. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 168.Koppe MJ, Bleichrodt RP, Soede AC, Verhofstad AA, Goldenberg DM, Oyen WJ, Boerman OC. Biodistribution and therapeutic efficacy of 125/131I-, 186Re-,88/90Y-, or 177Lu-labeled monoclonal antibody MN-14 to carcinoembryonic antigen in mice with small peritoneal metastases of colorectal origin. J Nucl Med. 2004;45:1224–1232. [PubMed] [Google Scholar]
- 169.Koppe MJ, Hendriks T, Boerman OC, Oyen WJ, Bleichrodt RP. Radioimmunotherapy is an effective adjuvant treatment after cytoreductive surgery of experimental colonic peritoneal carcinomatosis. J Nucl Med. 2006;47:1867–1874. [PubMed] [Google Scholar]
- 170.Aarts F, Koppe MJ, Hendriks T, Van Eerd JE, Oyen WJ, Boerman OC, Bleichrodt RP. Timing of adjuvant radioimmunotherapy after cytoreductive surgery in experimental peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol. 2007;14:533–540. doi: 10.1245/s10434-006-9247-x. [DOI] [PubMed] [Google Scholar]
- 171.Elgqvist J, Andersson H, Back T, Hultborn R, Jensen H, Karlsson B, Lindegren S, Palm S, Warnhammar E, Jacobsson L. Therapeutic efficacy and tumor dose estimations in radioimmunotherapy of intraperitoneally growing OVCAR-3 cells in nude mice with 211At-labeled monoclonal antibody MX35. J Nucl Med. 2005;46:1907–1915. [PubMed] [Google Scholar]
- 172.Milenic DE, Garmestani K, Brady ED, Albert PS, Abdulla A, Flynn J, Brechbiel MW. Potentiation of high-LET radiation by gemcitabine: targeting HER2 with trastuzumab to treat disseminated peritoneal disease. Clin Cancer Res. 2007;13:1926–1935. doi: 10.1158/1078-0432.CCR-06-2300. [DOI] [PubMed] [Google Scholar]
- 173.Janssen ML, Pels W, Massuger LF, Oyen WJ, Boonstra H, Corstens FH, Boerman OC. Intraperitoneal radioimmunotherapy in an ovarian carcinoma mouse model: Effect of the radionuclide. Int J Gynecol Cancer. 2003;13:607–613. doi: 10.1046/j.1525-1438.2003.13013.x. [DOI] [PubMed] [Google Scholar]
- 174.Bartolomei M, Mazzetta C, Handkiewicz-Junak D, Bodei L, Rocca P, Grana C, Maira G, Sturiale C, Villa G, Paganelli G. Combined treatment of glioblastoma patients with locoregional pre-targeted 90Y-biotin radioimmunotherapy and temozolomide. Q J Nucl Med Mol Imaging. 2004;48:220–228. [PubMed] [Google Scholar]
- 175.Paganelli G, Bartolomei M, Ferrari M, Cremonesi M, Broggi G, Maira G, Sturiale C, Grana C, Prisco G, Gatti M, Caliceti P, Chinol M. Pre-targeted locoregional radioimmunotherapy with 90Y-biotin in glioma patients: phase I study and preliminary therapeutic results. Cancer Biother Radiopharm. 2001;16:227–235. doi: 10.1089/10849780152389410. [DOI] [PubMed] [Google Scholar]
- 176.Reardon DA, Zalutsky MR, Bigner DD. Antitenascin-C monoclonal antibody radioimmunotherapy for malignant glioma patients. Expert Rev Anticancer Ther. 2007;7:675–687. doi: 10.1586/14737140.7.5.675. [DOI] [PubMed] [Google Scholar]
- 177.Rao RD, Brown PD, Buckner JC. Innovation in the management of brain metastases. Oncology. 2007;21:473–481. [PubMed] [Google Scholar]
- 178.Muldoon LL, Soussain C, Jahnke K, Johanson C, Siegal T, Smith QR, Hall WA, Hynynen K, Senter PD, Peereboom DM, Neuwelt EA. Chemotherapy delivery issues in central nervous system malignancy: a reality check. J Clin Oncol. 2007;25:2295–2305. doi: 10.1200/JCO.2006.09.9861. [DOI] [PubMed] [Google Scholar]
- 179.Penas-Prado M, Gilbert MR. Molecularly targeted therapies for malignant gliomas: advances and challenges. Expert Rev Anticancer Ther. 2007;7:641–661. doi: 10.1586/14737140.7.5.641. [DOI] [PubMed] [Google Scholar]
- 180.Yau T, Swanton C, Chua S, Sue A, Walsh G, Rostom A, Johnston SR, O'Brien ME, Smith IE. Incidence, pattern and timing of brain metastases among patients with advanced breast cancer treated with trastuzumab. Acta Oncol. 2006;45:196–201. doi: 10.1080/02841860500486630. [DOI] [PubMed] [Google Scholar]
- 181.Gerber DE, Laterra J. Emerging monoclonal antibody therapies for malignant gliomas. Expert Opin Investig Drugs. 2007;16:477–494. doi: 10.1517/13543784.16.4.477. [DOI] [PubMed] [Google Scholar]
- 182.McLendon RE, Akabani G, Friedman HS, Reardon DA, Cleveland L, Cokgor I, Herndon JE, 2nd, Wikstrand C, Boulton ST, Friedman AH, Bigner DD, Zalutsky MR. Tumor resection cavity administered iodine-131-labeled antitenascin 81C6 radioimmunotherapy in patients with malignant glioma: neuropathology aspects. Nucl Med Biol. 2007;34:405–413. doi: 10.1016/j.nucmedbio.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Reardon DA, Akabani G, Coleman RE, Friedman AH, Friedman HS, Herndon JE, 2nd, Cokgor I, McLendon RE, Pegram CN, Provenzale JM, Quinn JA, Rich JN, Regalado LV, Sampson JH, Shafman TD, Wikstrand CJ, Wong TZ, Zhao XG, Zalutsky MR, Bigner DD. Phase II trial of murine 131I-labeled antitenascin monoclonal antibody 81C6 administered into surgically created resection cavities of patients with newly diagnosed malignant gliomas. J Clin Oncol. 2002;20:1389–1397. doi: 10.1200/JCO.2002.20.5.1389. [DOI] [PubMed] [Google Scholar]
- 184.Reardon DA, Akabani G, Coleman RE, Friedman AH, Friedman HS, Herndon JE, 2nd, McLendon RE, Pegram CN, Provenzale JM, Quinn JA, Rich JN, Vredenburgh JJ, Desjardins A, Gururangan S, Badruddoja M, Dowell JM, Wong TZ, Zhao XG, Zalutsky MR, Bigner DD. Salvage radioimmunotherapy with murine iodine-131-labeled antitenascin monoclonal antibody 81C6 for patients with recurrent primary and metastatic malignant brain tumors: phase II study results. J Clin Oncol. 2006;24:115–122. doi: 10.1200/JCO.2005.03.4082. [DOI] [PubMed] [Google Scholar]
- 185.Reardon DA, Quinn JA, Akabani G, Coleman RE, Friedman AH, Friedman HS, Herndon JE, 2nd, McLendon RE, Pegram CN, Provenzale JM, Dowell JM, Rich JN, Vredenburgh JJ, Desjardins A, Sampson JH, Gururangan S, Wong TZ, Badruddoja MA, Zhao XG, Bigner DD, Zalutsky MR. Novel human IgG2b/murine chimeric antitenascin monoclonal antibody construct radiolabeled with 131I and administered into the surgically created resection cavity of patients with malignant glioma: phase I trial results. J Nucl Med. 2006;47:912–918. [PubMed] [Google Scholar]
- 186.Riva P, Arista A, Franceschi G, Frattarelli M, Sturiale C, Riva N, Casi M, Rossitti R. Local treatment of malignant gliomas by direct infusion of specific monoclonal antibodies labeled with 131I: comparison of the results obtained in recurrent and newly diagnosed tumors. Cancer Res. 1995;55:5952s–5956s. [PubMed] [Google Scholar]
- 187.Akabani G, Reardon DA, Coleman RE, Wong TZ, Metzler SD, Bowsher JE, Barboriak DP, Provenzale JM, Greer KL, Delong D, Friedman HS, Friedman AH, Zhao XG, Pegram CN, McLendon RE, Bigner DD, Zalutsky MR. Dosimetry and radiographic analysis of 131I-labeled anti-tenascin 81C6 murine monoclonal antibody in newly diagnosed patients with malignant gliomas: a phase II study. J Nucl Med. 2005;46:1042–1051. [PubMed] [Google Scholar]
- 188.Grana C, Chinol M, Robertson C, Mazzetta C, Bartolomei M, De Cicco C, Fiorenza M, Gatti M, Caliceti P, Paganelli G. Pretargeted adjuvant radioimmunotherapy with yttrium-90-biotin in malignant glioma patients: a pilot study. Br J Cancer. 2002;86:207–212. doi: 10.1038/sj.bjc.6600047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Paganelli G, Grana C, Chinol M, Cremonesi M, De Cicco C, De Braud F, Robertson C, Zurrida S, Casadio C, Zoboli S, Siccardi AG, Veronesi U. Antibody-guided three-step therapy for high grade glioma with yttrium-90 biotin. Eur J Nucl Med. 1999;26:348–357. doi: 10.1007/s002590050397. [DOI] [PubMed] [Google Scholar]
- 190.Sampson JH, Akabani G, Friedman AH, Bigner D, Kunwar S, Berger MS, Bankiewicz KS. Comparison of intratumoral bolus injection and convection-enhanced delivery of radiolabeled antitenascin monoclonal antibodies. Neurosurg Focus. 2006;20:E14. doi: 10.3171/foc.2006.20.4.9. [DOI] [PubMed] [Google Scholar]
- 191.Brown MT, Coleman RE, Friedman AH, Friedman HS, McLendon RE, Reiman R, Felsberg GJ, Tien RD, Bigner SH, Zalutsky MR, Zhao XG, Wikstrand CJ, Pegram CN, Herndon JE, 2nd, Vick NA, Paleologos N, Fredericks RK, Schold SC, Jr., Bigner DD. Intrathecal 131I-labeled antitenascin monoclonal antibody 81C6 treatment of patients with leptomeningeal neoplasms or primary brain tumor resection cavities with subarachnoid communication: phase I trial results. Clin Cancer Res. 1996;2:963–972. [PubMed] [Google Scholar]
- 192.Vitaz TW, Warnke PC, Tabar V, Gutin PH. Brachytherapy for brain tumors. J Neurooncol. 2005;73:71–86. doi: 10.1007/s11060-004-2352-4. [DOI] [PubMed] [Google Scholar]
- 193.Gabayan AJ, Green SB, Sanan A, Jenrette J, Schultz C, Papagikos M, Tatter SP, Patel A, Amin P, Lustig R, Bastin KT, Watson G, Burri S, Stea B. GliaSite brachytherapy for treatment of recurrent malignant gliomas: a retrospective multi-institutional analysis. Neurosurgery. 2006;58:701–709. doi: 10.1227/01.NEU.0000194836.07848.69. discussion 701–709. [DOI] [PubMed] [Google Scholar]
- 194.Welsh J, Sanan A, Gabayan AJ, Green SB, Lustig R, Burri S, Kwong E, Stea B. GliaSite brachytherapy boost as part of initial treatment of glioblastoma multiforme: a retrospective multi-institutional pilot study. Int J Radiat Oncol Biol Phys. 2007;68:159–165. doi: 10.1016/j.ijrobp.2006.11.053. [DOI] [PubMed] [Google Scholar]
- 195.Geschwind JF, Salem R, Carr BI, Soulen MC, Thurston KG, Goin KA, Van Buskirk M, Roberts CA, Goin JE. Yttrium-90 microspheres for the treatment of hepatocellular carcinoma. Gastroenterology. 2004;127:S194–S205. doi: 10.1053/j.gastro.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 196.Goh AS, Chung AY, Lo RH, Lau TN, Yu SW, Chng M, Satchithanantham S, Loong SL, Ng DC, Lim BC, Connor S, Chow PK. A novel approach to brachytherapy in hepatocellular carcinoma using a phosphorous32 (32P) brachytherapy delivery device--a first-in-man study. Int J Radiat Oncol Biol Phys. 2007;67:786–792. doi: 10.1016/j.ijrobp.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 197.Order SE, Siegel JA, Lustig RA, Principato R, Zeiger LS, Johnson E, Zhang H, Lang P, Pilchik NB, Metz J, et al. A new method for delivering radioactive cytotoxic agents in solid cancers. Int J Radiat Oncol Biol Phys. 1994;30:715–720. doi: 10.1016/0360-3016(92)90960-p. [DOI] [PubMed] [Google Scholar]
- 198.Nguyen H, Ghanem G, Morandini R, Verbist A, Larsimont D, Fallais C, Fruhling J, Van Houtte P. Tumor type and vascularity: important variables in infusional brachytherapy with colloidal 32P. Int J Radiat Oncol Biol Phys. 1997;39:481–487. doi: 10.1016/s0360-3016(97)00294-0. [DOI] [PubMed] [Google Scholar]
- 199.Order SE, Siegel JA, Principato R, Zeiger LS, Johnson E, Lang P, Lustig R, Kroprowski C, Wallner PE. Preliminary experience of infusional brachytherapy using colloidal 32P. Ann Acad Med Singapore. 1996;25:347–351. [PubMed] [Google Scholar]
- 200.Narayana A, Bhatia S, Souweidane M, Khakoo Y, Zaider M. 32P radioisotope therapy for recurrent pilocytic astrocytoma. Brachytherapy. 2005;4:171–173. doi: 10.1016/j.brachy.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 201.Schefter JK, Allen G, Cmelak AJ, Johnson M, Toms S, Duggan D, Blevins LS. The utility of external beam radiation and intracystic 32P radiation in the treatment of craniopharyngiomas. J Neurooncol. 2002;56:69–78. doi: 10.1023/a:1014467721132. [DOI] [PubMed] [Google Scholar]
- 202.Taasan V, Shapiro B, Taren JA, Beierwaltes WH, Mckeever P, Wahl RL, Carey JE, Petry N, Mallette S. Phosphorus-32 therapy of cystic Grade IV astrocytomas: technique and preliminary application. J Nucl Med. 1985;26:1335–1338. [PubMed] [Google Scholar]
- 203.Hasegawa T, Kondziolka D, Hadjipanayis CG, Lunsford LD. Management of cystic craniopharyngiomas with phosphorus-32 intracavitary irradiation. Neurosurgery. 2004;54:813–820. doi: 10.1227/01.neu.0000114262.30035.af. discussion 820-812. [DOI] [PubMed] [Google Scholar]
- 204.Szeifert GT, Julow J, Slowik F, Balint K, Lanyi F, Pasztor E. Pathological changes in cystic craniopharyngiomas following intracavital 90yttrium treatment. Acta Neurochir (Wien) 1990;102:14–18. doi: 10.1007/BF01402179. [DOI] [PubMed] [Google Scholar]
- 205.Szeifert GT, Balint K, Sipos L, Sarker MH, Czirjak S, Julow J. Pathological findings in cystic craniopharyngiomas after stereotactic intracavitary irradiation with yttrium-90 isotope. Prog Neurol Surg. 2007;20:297–302. doi: 10.1159/000100173. [DOI] [PubMed] [Google Scholar]
- 206.Lee Y, Bullard DE, Humphrey PA, Colapinto EV, Friedman HS, Zalutsky MR, Coleman RE, Bigner DD. Treatment of intracranial human glioma xenografts with 131I-labeled anti-tenascin monoclonal antibody 81C6. Cancer Res. 1988;48:2904–2910. [PubMed] [Google Scholar]
- 207.Zalutsky MR, Moseley RP, Coakham HB, Coleman RE, Bigner DD. Pharmacokinetics and tumor localization of 131I-labeled anti-tenascin monoclonal antibody 81C6 in patients with gliomas and other intracranial malignancies. Cancer Res. 1989;49:2807–2813. [PubMed] [Google Scholar]
- 208.Akabani G, Reist CJ, Cokgor I, Friedman AH, Friedman HS, Coleman RE, Zhao XG, Bigner DD, Zalutsky MR. Dosimetry of 131I-labeled 81C6 monoclonal antibody administered into surgically created resection cavities in patients with malignant brain tumors. J Nucl Med. 1999;40:631–638. [PubMed] [Google Scholar]
- 209.Selker RG, Mendelow H, Walker M, Sheptak PE, Phillips JG. Pathological correlation of CT ring in recurrent, previously treated gliomas. Surg Neurol. 1982;17:251–254. doi: 10.1016/0090-3019(82)90115-x. [DOI] [PubMed] [Google Scholar]
- 210.Quang TS, Brady LW. Radioimmunotherapy as a novel treatment regimen: 125I-labeled monoclonal antibody 425 in the treatment of high-grade brain gliomas. Int J Radiat Oncol Biol Phys. 2004;58:972–975. doi: 10.1016/j.ijrobp.2003.09.096. [DOI] [PubMed] [Google Scholar]
- 211.Gonzalez J, Kumar AJ, Conrad CA, Levin VA. Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys. 2007;67:323–326. doi: 10.1016/j.ijrobp.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 212.Belda-Iniesta C, Carpeno Jde C, Saenz EC, Gutierrez M, Perona R, Baron MG. Long term responses with cetuximab therapy in glioblastoma multiforme. Cancer Biol Ther. 2006;5:912–914. doi: 10.4161/cbt.5.8.3118. [DOI] [PubMed] [Google Scholar]
- 213.Combs SE, Heeger S, Haselmann R, Edler L, Debus J, Schulz-Ertner D. Treatment of primary glioblastoma multiforme with cetuximab, radiotherapy and temozolomide (GERT)--phase I/II trial: study protocol. BMC Cancer. 2006;6:133. doi: 10.1186/1471-2407-6-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr., Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N. Trastuzumab lus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 215.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Lang I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Ruschoff J, Suto T, Greatorex V, Ward C, Straehle C, Mcfadden E, Dolci MS, Gelber RD. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 216.De Gramont A, Tournigand C, Andre T, Larsen AK, Louvet C. Adjuvant therapy for stage II and III colorectal cancer. Semin Oncol. 2007;34:S37–S40. doi: 10.1053/j.seminoncol.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 217.Zhang W, Gordon M, Lenz HJ. Novel approaches to treatment of advanced colorectal cancer with anti-EGFR monoclonal antibodies. Ann Med. 2006;38:545–551. doi: 10.1080/09546630601070812. [DOI] [PubMed] [Google Scholar]
- 218.Caprioni F, Fornarini G. Bevacizumab in the treatment of metastatic colorectal cancer. Future Oncol. 2007;3:141–148. doi: 10.2217/14796694.3.2.141. [DOI] [PubMed] [Google Scholar]
- 219.Press OW, Unger JM, Braziel RM, Maloney DG, Miller TP, Leblanc M, Fisher RI. Phase II trial of CHOP chemotherapy followed by tositumomab/iodine I-131 tositumomab for previously untreated follicular non-Hodgkin's lymphoma: five-year follow-up of Southwest Oncology Group Protocol S9911. J Clin Oncol. 2006;24:4143–4149. doi: 10.1200/JCO.2006.05.8198. [DOI] [PubMed] [Google Scholar]
- 220.Kaminski MS, Radford JA, Gregory SA, Leonard JP, Knox SJ, Kroll S, Wahl RL. Re-treatment with I-131 tositumomab in patients with non-Hodgkin's lymphoma who had previously responded to I-131 tositumomab. J Clin Oncol. 2005;23:7985–7993. doi: 10.1200/JCO.2005.01.0892. [DOI] [PubMed] [Google Scholar]
- 221.Kaminski MS, Tuck M, Estes J, Kolstad A, Ross CW, Zasadny K, Regan D, Kison P, Fisher S, Kroll S, Wahl RL. 131I-tositumomab therapy as initial treatment for follicular lymphoma. N Engl J Med. 2005;352:441–449. doi: 10.1056/NEJMoa041511. [DOI] [PubMed] [Google Scholar]
- 222.Davies AJ. Radioimmunotherapy for B-cell lymphoma: Y90 ibritumomab tiuxetan and I131 tositumomab. Oncogene. 2007;26:3614–3628. doi: 10.1038/sj.onc.1210378. [DOI] [PubMed] [Google Scholar]
- 223.Shimoni A, Zwas ST, Oksman Y, Hardan I, Shem-Tov N, Yerushalmi R, Avigdor A, Ben-Bassat I, Nagler A. Yttrium-90-ibritumomab tiuxetan (Zevalin) combined with high-dose BEAM chemotherapy and autologous stem cell transplantation for chemo-refractory aggressive non-Hodgkin's lymphoma. Exp Hematol. 2007;35:534–540. doi: 10.1016/j.exphem.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 224.Emmanouilides C, Witzig TE, Gordon LI, Vo K, Wiseman GA, Flinn IW, Darif M, Schilder RJ, Molina A. Treatment with yttrium 90 ibritumomab tiuxetan at early relapse is safe and effective in patients with previously treated B-cell non-Hodgkin's lymphoma. Leuk Lymphoma. 2006;47:629–636. doi: 10.1080/10428190500376076. [DOI] [PubMed] [Google Scholar]
- 225.Sharkey RM, Pykett MJ, Siegel JA, Alger EA, Primus FJ, Goldenberg DM. Radioimmunotherapy of the GW-39 human colonic tumor xenograft with 131I-labeled murine monoclonal antibody to carcinoembryonic antigen. Cancer Res. 1987;47:5672–5677. [PubMed] [Google Scholar]
- 226.Boerman OC, Sharkey RM, Blumenthal RD, Aninipot RL, Goldenberg DM. The presence of a concomitant bulky tumor can decrease the uptake and therapeutic efficacy of radiolabeled antibodies in small tumors. Int J Cancer. 1992;51:470–475. doi: 10.1002/ijc.2910510322. [DOI] [PubMed] [Google Scholar]
- 227.Blumenthal RD, Sharkey RM, Haywood L, Natale AM, Wong GY, Siegel JA, Kennel SJ, Goldenberg DM. Targeted therapy of athymic mice bearing GW-39 human colonic cancer micrometastases with 131I-labeled monoclonal antibodies. Cancer Res. 1992;52:6036–6044. [PubMed] [Google Scholar]
- 228.Sharkey RM, Weadock KS, Natale A, Haywood L, Aninipot R, Blumenthal RD, Goldenberg DM. Successful radioimmunotherapy for lung metastasis of human colonic cancer in nude mice. J Natl Cancer Inst. 1991;83:627–632. doi: 10.1093/jnci/83.9.627. [DOI] [PubMed] [Google Scholar]
- 229.Liersch T, Meller J, Bittrich M, Kulle B, Becker H, Goldenberg DM. Update of carcinoembryonic antigen radioimmunotherapy with 131I-labetuzumab after salvage resection of colorectal liver metastases: comparison of outcome to a contemporaneous control group. Ann Surg Oncol. 2007 doi: 10.1245/s10434-006-9328-x. [DOI] [PubMed] [Google Scholar]
- 230.Liersch T, Meller J, Kulle B, Behr TM, Markus P, Langer C, Ghadimi BM, Wegener WA, Kovacs J, Horak ID, Becker H, Goldenberg DM. Phase II trial of carcinoembryonic antigen radioimmunotherapy with 131I-labetuzumab after salvage resection of colorectal metastases in the liver: five-year safety and efficacy results. J Clin Oncol. 2005;23:6763–6770. doi: 10.1200/JCO.2005.18.622. [DOI] [PubMed] [Google Scholar]
- 231.Chatal JF, Campion L, Kraeber-Bodere F, Bardet S, Vuillez JP, Charbonnel B, Rohmer V, Chang CH, Sharkey RM, Goldenberg DM, Barbet J. Survival improvement in patients with medullary thyroid carcinoma who undergo pretargeted anti-carcinoembryonic-antigen radioimmunotherapy: a collaborative study with the French Endocrine Tumor Group. J Clin Oncol. 2006;24:1705–1711. doi: 10.1200/JCO.2005.04.4917. [DOI] [PubMed] [Google Scholar]
- 232.Clarke K, Lee FT, Brechbiel MW, Smyth FE, Old LJ, Scott AM. Therapeutic efficacy of anti-Lewis(y) humanized 3S193 radioimmunotherapy in a breast cancer model: enhanced activity when combined with taxol chemotherapy. Clin Cancer Res. 2000;6:3621–3628. [PubMed] [Google Scholar]
- 233.DeNardo SJ, Kroger LA, Lamborn KR, Miers LA, O'Donnell RT, Kukis DL, Richman CM, DeNardo GL. Importance of temporal relationships in combined modality radioimmunotherapy of breast carcinoma. Cancer. 1997;80:2583–2590. doi: 10.1002/(sici)1097-0142(19971215)80:12+<2583::aid-cncr34>3.3.co;2-8. [DOI] [PubMed] [Google Scholar]
- 234.O'Donnell RT, DeNardo SJ, Miers LA, Kukis DL, Mirick GR, Kroger LA, DeNardo GL. Combined modality radioimmunotherapy with Taxol and 90Y-Lym-1 for Raji lymphoma xenografts. Cancer Biother Radiopharm. 1998;13:351–361. doi: 10.1089/cbr.1998.13.351. [DOI] [PubMed] [Google Scholar]
- 235.O'Donnell RT, DeNardo SJ, Miers LA, Lamborn KR, Kukis DL, DeNardo GL, Meyers FJ. Combined modality radioimmunotherapy for human prostate cancer xenografts with taxanes and 90yttrium-DOTA-peptide-ChL6. Prostate. 2002;50:27–37. doi: 10.1002/pros.10029. [DOI] [PubMed] [Google Scholar]
- 236.Supiot S, Gouard S, Charrier J, Apostolidis C, Chatal JF, Barbet J, Davodeau F, Cherel M. Mechanisms of cell sensitization to alpha radioimmunotherapy by doxorubicin or paclitaxel in multiple myeloma cell lines. Clin Cancer Res. 2005;11:7047s–7052s. doi: 10.1158/1078-0432.CCR-1004-0021. [DOI] [PubMed] [Google Scholar]
- 237.Tschmelitsch J, Barendswaard E, Williams C, Jr., Yao TJ, Cohen AM, Old LJ, Welt S. Enhanced antitumor activity of combination radioimmunotherapy (131I-labeled monoclonal antibody A33) with chemotherapy (fluorouracil) Cancer Res. 1997;57:2181–2186. [PubMed] [Google Scholar]
- 238.Forero A, Meredith RF, Khazaeli MB, Shen S, Grizzle WE, Carey D, Busby E, Lobuglio AF, Robert F. Phase I study of 90Y-CC49 monoclonal antibody therapy in patients with advanced non-small cell lung cancer: effect of chelating agents and paclitaxel co-administration. Cancer Biother Radiopharm. 2005;20:467–478. doi: 10.1089/cbr.2005.20.467. [DOI] [PubMed] [Google Scholar]
- 239.Wong JY, Shibata S, Williams LE, Kwok CS, Liu A, Chu DZ, Yamauchi DM, Wilczynski S, Ikle DN, Wu AM, Yazaki PJ, Shively JE, Doroshow JH, Raubitschek AA. A Phase I trial of 90Y-anti-carcinoembryonic antigen chimeric T84.66 radioimmunotherapy with 5-fluorouracil in patients with metastatic colorectal cancer. Clin Cancer Res. 2003;9:5842–5852. [PubMed] [Google Scholar]
- 240.Richman CM, DeNardo SJ, O'Donnell RT, Yuan A, Shen S, Goldstein DS, Tuscano JM, Wun T, Chew HK, Lara PN, Kukis DL, Natarajan A, Meares CF, Lamborn KR, DeNardo GL. High-dose radioimmunotherapy combined with fixed, low-dose paclitaxel in metastatic prostate and breast cancer by using a MUC-1 monoclonal antibody, m170, linked to indium-111/yttrium-90 via a cathepsin cleavable linker with cyclosporine to prevent human anti-mouse antibody. Clin Cancer Res. 2005;11:5920–5927. doi: 10.1158/1078-0432.CCR-05-0211. [DOI] [PubMed] [Google Scholar]
- 241.Sharkey RM, Hajjar G, Yeldell D, Brenner A, Burton J, Rubin A, Goldenberg DM. A phase I trial combining high-dose 90Y-labeled humanized anti-CEA monoclonal antibody with doxorubicin and peripheral blood stem cell rescue in advanced medullary thyroid cancer. J Nucl Med. 2005;46:620–633. [PubMed] [Google Scholar]
- 242.Gold DV, Schutsky K, Modrak D, Cardillo TM. Low-dose radioimmunotherapy (90Y-PAM4) combined with gemcitabine for the treatment of experimental pancreatic cancer. Clin Cancer Res. 2003;9:3929S–3937S. [PubMed] [Google Scholar]