Abstract
Background
Ozone exposure is known to cause oxidative stress. We investigated the acute effects of ozone (O3) on lung function in the elderly, a suspected risk group. We then investigated whether genetic polymorphisms of antioxidant genes (heme oxygenase-1 [HMOX1] and glutathione S-transferase pi [GSTP1]) modified these associations.
Methods
We studied 1,100 elderly men from the Normative Aging Study whose lung function (forced vital capacity [FVC] and forced expiratory volume in one second [FEV1]) was measured every 3 years from 1995–2005. We genotyped the GSTP1 Ile105Val and Ala114Val polymorphisms and the (GT)n repeat polymorphism in the HMOX1 promoter, classifying repeats as short (n<25) or long (n 25). Ambient O3 was measured continuously at locations in the Greater Boston area. We used mixed linear models, adjusting for known confounders.
Results
A 15 ppb increase in O3 during the previous 48 hours was associated with a 1.25% decrease in FEV1 (95% CI: −1.96%, −0.54%). This estimated effect was worsened with either the presence of a long (GT)n repeat in HMOX1 (−1.38%, 95% CI: −2.11%, −0.65) or the presence of an allele coding for Val105 in GSTP1 (−1.69%, 95% CI: −2.63%, −0.75). A stronger estimated effect of O3 on FEV1 was found in subjects carrying both the GSTP1 105Val variant and the HMOX1 long (GT)n repeat (−1.94%, 95% CI: −2.89%, −0.98%). Similar associations were also found between FVC and ozone exposure.
Conclusions
Our results suggest that ozone has an acute effect on lung function in the elderly, and the effects may be modified by the presence of specific polymorphisms in antioxidant genes.
Keywords: FEV1, FVC, GSTP1, ozone, air pollution
INTRODUCTION
Strong associations have been identified between short-term ambient ozone exposure and increased risk of mortality, with an even larger risk associated with elderly populations.1–4 Other studies have linked ambient ozone concentration to increased respiratory-related emergency room visits and hospital admission in the elderly.5–8 Thus, the elderly should be considered a susceptible population to the effects of ozone.
The basis of the increased susceptibility of the elderly to ozone effects on mortality and morbidity is unknown. However, lung function, as measured by spirometry, has been shown to predict all-cause mortality and cause-specific (such as respiratory and cardiovascular) mortality.9–12 Upon entering the lung, ozone reacts preferentially with antioxidants, while also generating reactive oxygen species (ROS) in secondary reactions with lipids.13 Thus, the intensity of the secondary reactions depends on the presence of sufficient levels of antioxidants to react with the amount of ozone inhaled. One hypothesis for the increased susceptibility of the elderly population is that the antioxidant defenses in the respiratory tract lining fluid may be altered in that population.14
Differences in defenses against oxidative stress have been linked to genetic polymorphisms. Glutathione pathways defend against ROS and their toxic byproducts,15 and the glutathione S-transferase (GST) genes are up-regulated in response to oxidative stress.16 Increased oxidative stress is thought to be part of the pathogenesis of asthma,17,18 and the GSTP1 105 Val/Val genotype has been associated with increases in reported breathing difficulty in asthmatic children in response to ozone exposure,19 and may be a determinant of the severity of oxidative damage in asthmatic children.20 Among the GST genes, the most highly expressed in the lung is GSTP1, making it a good candidate for study in relation to the effect of inhaled pollutants on lung function.
Heme oxygenase functions as an antioxidant through its key involvement in the production of bilirubin which is cytoprotective against oxidative stress.21,22 The (GT)n dinucleotide repeat polymorphism in the promoter region of the gene is thought to influence the inducibility of HMOX1. When analyzed by transient-transfection assay, transcriptional activity was up-regulated in genes with (GT)16 and (GT)20 repeats, but not in those with (GT)29 or (GT)38, suggesting a change in inducibility at repeat length between 20 and 29.23 Also, longer repeats have been associated with having chronic pulmonary emphysema23 and faster decline in lung function,24 suggesting that this gene would also be a good candidate for modifying the effect of ozone on lung function.
Although many studies have examined the acute effects of ozone on younger subpopulations, responsiveness to ozone has been shown to vary with age.25 Research on the acute effects of ozone in older populations is limited. Several controlled human studies looked at the acute effect of 1 to 2 hours of a fixed ozone exposure in a chamber, using only small populations (n < 20) of healthy nonsmokers.26–30 Using these data to extrapolate to the entire elderly population is severely limited by the small number of subjects, the inclusion of only healthy nonsmokers, and the unrealistic exposure conditions.
We hypothesized that increases in ambient ozone concentration would be related to a transient decrease in lung function in the elderly. In this study, we examined the association between short-term ozone exposure and lung function in elderly subjects in the greater Boston area. Because of the suggested influence of antioxidants, we also examined whether that effect was modified by polymorphisms in genes related to defense against oxidative stress.
METHODS
Study Population
We studied subjects from the Normative Aging Study (NAS), a longitudinal study established by the Veterans Administration in 1963. We give here only a brief description of the study since details have been published previously. 31 The study enrolled 2,280 men from the Greater Boston area, ages 21 to 80, who were determined to be free of known chronic medical conditions by an initial health screening. Participants visited the study center every three years to undergo physical examinations and fill out questionnaires. By 1995, the cohort consisted of 1,114 subjects in total. Our analyses included 1,100 of those subjects whose lung function was measured at least once between January 1995 and June 2005. Out of the subjects no longer in the cohort, roughly 45% had passed away, 34% moved out of the area, and 19% dropped out of the study because of loss of interest in the research program including triennial examinations and questionairres.
Study center visits took place in the morning, after an overnight fast and abstinence from smoking. Physical examinations included measurement of height and lung function (forced vital capacity [FVC] and forced expiratory volume in one second [FEV1]). Questionnaires included information on smoking habits and pulmonary disorders (asthma, chronic bronchitis, emphysema) based on the American Thoracic Society-Division of Lung Diseases-1978 questionnaire.32 Descriptive statistics for these data are listed in Table 1.
Table 1.
Descriptive Statistics of Study Population (at first visit). Listed as mean (sd) or number (%).
| Variable | All Subjects n = 1100 | Genotyped Subjects n = 1015 |
|---|---|---|
| Age, yr | 68.9 (7.2) | 68.9 (7.2) |
| Height, m | 1.74 (0.067) | 1.74 (0.066) |
| Ethnic Background, n (%) | ||
| White | 1067 (98.3) | 984 (98.1) |
| Black | 19 (1.7) | 19 (1.9) |
| Smoking Status, n (%) | ||
| Never smoker | 313 (28.5) | 303 (29.9) |
| Former smoker | 715 (65.0) | 642 (63.3) |
| Current smoker | 72 (6.5) | 70 (6.9) |
| Lifetime smoking, pack-years* | 31.5 (24.6) | 31.4 (24.8) |
| Chronic Pulmonary Diseases, n (%) | ||
| Doctor Confirmed Asthma | 65 (5.9) | 63 (6.2) |
| Unconfirmed Asthma | 4 (0.36) | 4 (0.39) |
| Doctor Confirmed Chronic Bronchitis | 70 (6.4) | 67 (6.6) |
| Unconfirmed Chronic Bronchitis | 5 (0.45) | 5 (0.49) |
| Doctor Confirmed Emphysema | 34 (3.1) | 32 (3.2) |
| Unconfirmed Emphysema | 2 (0.18) | 2 (0.20) |
| Forced Vital Capacity (FVC), L | 3.45 (0.69) | 3.44 (0.70) |
| Forced Expiratory Volume in 1 Second (FEV1), L | 2.55 (0.60) | 2.54 (0.61) |
Pack-years for former or current smokers (n = 787)
As a surrogate for the socio-economic status, we give the number of years of education. The highest education level attained by our cohort was as follows: 34% completed high school or less, 53% attended college for 1–4 years, and 13% were educated beyond college.
Lung Function Measurement
Pulmonary function was assessed using spirometric tests performed as previously reported to obtain measures of FVC and FEV1.33 Briefly, spirometry was performed using a water-filled recording spirometer, and values were adjusted by body temperature and pressure. Throughout the 10 years in this study, spirometry was assessed in the standing position with noseclip using a 10-liter water-filled survey recording spirometer and an Eagle II minicomputer (Warren E. Collins, Braintree, MA). The measures of FVC and FEV1 were obtained in accordance with ATS guidelines, which included obtaining a minimum of 3 acceptable spirograms, at least 2 of which were reproducible within 5% for both FEV1 and FVC. In addition, each technician underwent a training protocol prior to taking measurements for this study. Hence, spirometry was performed according to a strict protocol, thus ensuring repeatability and minimizing any possibility of measurement error.
Air Pollution
Ambient O3 was measured continuously at four monitoring sites, with all monitors conforming to US EPA standards. One monitor was located in each of four cities in the Greater Boston area: Boston, Chelsea, Lynn, and Waltham. All four monitors were highly correlated, with the pairwise correlations ranging from 0.74 to 0.95. In our analyses, the exposure measurement used for each participant was an average of the monitors. We calculated average O3 concentrations for periods of 1–5 days prior to the study visit, where the 1-day measure corresponds to the averaged hourly measurements for the 24 hours before the study visit (rather then midnight to midnight), the 2-day measure corresponds to the 48 hours before, etc. The correlation beween the 24 hour averages we used and the highest 8 hour concentration within that period was 0.92.
We obtained meteorological data including temperature and relative humidity from the Boston airport weather station. To control for outdoor weather, we used the apparent temperature, which is defined as a person’s perceived air temperature.34,35 We calculated apparent temperature averages for periods of 1–5 days prior to study visit to correspond with the 1–5 day O3 concentration averages. Descriptive statistics for the two day averages (which are representative of the other averages) of apparent temperature and O3 concentration are listed in Table 2.
Table 2.
Pollution and Weather Variables (for all averages used in analyses).
| Variable | Mean (SD) |
|---|---|
| Apparent Temperature, 2 day mean, °C | 11.4 (9.6) |
| Ozone O3, 2 day mean, ppb | 24.4 (11.0) |
Ozone concentrations had low correlations with particulate matter < 2.5 μm (.29), carbon monoxide (−.21), nitrogen dioxide (−.15), and hence we felt it was reasonable to examine O3 alone without controlling for other pollutants. We have limited data on particle components, but for the days we do have data (about one third), ozone correlations were as expected: O3 was not correlated with elemental carbon (.05), but had a moderate correlation with sulfates (.45).
HMOX1 and GSTP1 Genotyping
We genotyped two single nucleotide polymorphisms (SNPs) on GSTP1: Ile105Val, caused by an A-to-G substitution that changes codon 105 from ATC (Ile) to GTC (Val), and Ala114Val, a C-to-T substitution that changes codon 114 from GCG (Ala) to GTG (Val).36 Both SNPs were genotyped using unlabeled minisequencing reactions and mass spectrometry in Sequenom (San Diego, CA).37
We also genotyped the HMOX1 (GT)n repeat polymorphism. The HMOX1 short tandem repeat (STR) assay was designed as described by Yamada and coworkers.23 Resulting PCR products were analyzed with a laser-based automated DNA sequencer, and the sizes were compared with the human genome sequence (http://genome.ucsc.edu/) to determine the number of repeats.
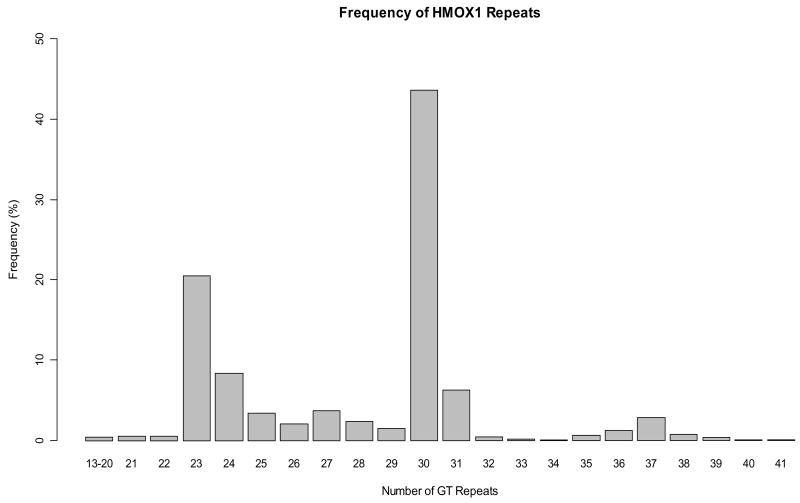
Of the 1100 subjects used in our analysis for the overall effect of ozone, 1015 were successfully genotyped for at least one polymorphism (descriptive statistics for this subset are listed in Table 1A). The (GT)n repeat polymorphism in the HMOX1 promoter was successfully genotyped in 971 subjects (success rate 88%), with allele distribution shown in Figure 1. Repeats were classified as short (S) (n < 25) or long (L) (n ≥ 25). For the polymorphisms of GSTP1, 959 subjects (success rate 87%) were genotyped for GSTP1 Ile105Val, and 959 subjects were genotyped for GSTP1 Ala114Val. Both GSTP1 Ile105Val and Ala114Val were determined to be in Hardy-Weinberg Equilibrium. Our genotyping success rates were slightly lower than average because some blood samples were several years old at the time of genotyping and had deteriorated. The genotype frequency for each polymorphism is given in Table 3.
Figure 1.
Frequency distribution of (GT)n repeats for 971 subjects genotyped (1,942 alleles).
Table 3.
Genetic Analyses Variables.
| Variable | Number (%) |
|---|---|
| HMOX1 (n = 971) | |
| No long repeats (S/S) | 90 (9.3) |
| Long repeats in one allele (S/L) | 413 (42.5) |
| Long repeats in both alleles (L/L) | 468 (48.2) |
| GSTP1 105 (n = 959) | |
| Both alleles code for Isoleucine (Ile/Ile) | 445 (46.4) |
| One allele codes for Valine (Val/Ile) | 420 (43.8) |
| Both alleles code for Valine (Val/Val) | 94 (9.8) |
| GSTP1 114 (n = 959) | |
| Both alleles code for Alanine (Ala/Ala) | 821 (85.6) |
| One allele codes for Valine (Val/Ala) | 130 (13.6) |
| Both alleles code for Valine (Val/Val) | 8 (0.83) |
Statistical Methods
Lung function measurements FVC and FEV1 were log-transformed to increase normality and stabilize variance. We chose the following variables for each visit a priori and included them in our model: age, age squared, height, race, cigarette smoking (former smoker, current smoker, and packyears), chronic lung conditions (asthma, emphysema, and chronic bronchitis), obesity, airways hyper-resposiveness, season, day of the week, year of visit, apparent temperature, and O3 concentration. In this study, we defined airways hyper-responsiveness as a positive response to a methacholine challenge test, which occurred when a subject experienced a 20% decline in FEV1 following inhalation of a methacholine dose of 8.58 μmol or less.
Subjects had their FVC and FEV1 measured on up to four visits, with a mean of 2.2 visits for each subject. We used a mixed model for our regression analyses to account for the repeated measurements on the same subjects. We considered an association between the dependent variable and a covariate to be significant if the covariate had a p-value < 0.05 in the model.
We present the estimated effect of O3 as a percent change in lung function. Because FVC and FEV1 were loge-transformed in our model, the percent changes in FVC and FEV1 were calculated by [exp(ΔO3 × β) −1] × 100%, with 95% confidence intervals [exp(ΔO3 × [β ± 1.96 × SE]) −1] × 100%, where exp(X) is the mathematical constant e (≈ 2.718) raised to the power of X, ΔO3 is the change in ozone concentration, β is the estimated regression coefficient, and SE is the standard error of β. For the change in ozone concentration, we used the increment of 15 ppb, which is approximately equal to the interquartile range (IQR) of the 2-day O3 concentration averages.
RESULTS
All the models used to obtain these results included both white and black subjects, using a dummy variable to account for race. Because less than 2% of subjects were black, we also ran these models including only white subjects and produced equivalent results (data not shown). Thus, we kept both races in the model to increase the power by using more observations.
For both FVC and FEV1 we ran five separate models, estimating the effect of O3 for each averaged measure ranging from 1 to 5 days before the study visit (Table 4). All five moving averages for O3 were significantly associated with a decrease in FVC, and for the 1-day, 2-day and 3-day measures we found a significant association between O3 and decreasing FEV1. The 2-day measure of O3 corresponded to our most significant (smallest p-value) results for both FVC and FEV1, so we used the 2-day measure in the additional analyses examining the interaction of O3 with genetic variables.
Table 4.
Effect of a 15 ppb increase in O3 on FVC and FEV1
| O3 measure | % Change in FVC | 95% Confidence Interval | p-value | % Change in FEV1 | 95% Confidence Interval | p-value |
|---|---|---|---|---|---|---|
| 1 day mean | −0.74 | −1.29, −0.17 | 0.0104 | −0.83 | −1.43, −0.23 | 0.0071 |
| 2 day mean | −1.29 | −1.95, −0.63 | 0.0001 | −1.25 | −1.96, −0.54 | 0.0006 |
| 3 day mean | −1.29 | −2.02, −0.55 | 0.0006 | −1.08 | −1.86, −0.28 | 0.0081 |
| 4 day mean | −1.02 | −1.81, −0.21 | 0.0134 | −0.63 | −1.49, 0.23 | 0.1525 |
| 5 day mean | −1.09 | −1.96, −0.21 | 0.0153 | −0.66 | −1.61, 0.29 | 0.1709 |
In our analyses of the possible genetic modification of the effects of O3, we first looked at the effect of O3 stratified by HMOX1 classification (class I: S/S, class II: S/L or L/L), with results listed in Table 5. The decreases in FVC and FEV1 per 15 ppb increase in O3 were estimated to be larger for class II than class I, although the interaction between HMOX1 classes and ozone was not significant.
Table 5.
Effect of 15 ppb increase in O3 by gene.
| Gene Combinations | % Change in FVC | 95% Confidence Interval | % Change in FEV1 | 95% Confidence Interval | |
|---|---|---|---|---|---|
| Overall Effect of O3 | −1.29 | −1.95, −0.63 | −1.25 | −1.96, −0.54 | |
| Effect by Gene | |||||
| HMOX1 | |||||
| S/S | −0.84 | −2.50, 0.84 | −1.14 | −2.87, 0.61 | |
| S/L, L/L | −1.47 | −2.17, −0.77 | −1.38 | −2.11, −0.65 | |
| GSTP1 Ile105Val | |||||
| I. Ile/Ile | −1.00 | −1.82, −0.17 | −0.74 | −1.62, 0.16 | |
| II. Ile/Val, Val/Val | −1.50 | −2.37, −0.63 | −1.69 | −2.63, −0.75 | |
| GSTP1 | |||||
| Ile105Val | Ala114Val | ||||
| I. Ile/Ile | Ala/Ala | −0.99 | −1.81, −0.16 | −0.74 | −1.63, 0.16 |
| IIa. Ile/Val, Val/Val | Ala/Ala | −1.43 | −2.39, −0.45 | −1.53 | −2.56, −0.48 |
| IIb. Ile/Val, Val/Val | Ala/Val, Val/Val | −1.67 | −3.26, −0.05 | −2.14 | −3.85, −0.40 |
We examined the effect of O3 modified by two SNPs in GSTP1, Ile105Val and Ala114Val (Table 5). For Ile105Val, class II subjects (Ile/Val or Val/Val) were estimated to have a larger decrease in both FVC and FEV1 than class I (Ile/Ile) subjects per 15ppb increase in O3. The interaction between the Ile105Val classes and ozone was not significant for FVC, but was marginally significant for FEV1 (p-value = 0.089). We considered grouping Ile/Val and Val/Val separately, but those classes were estimated to have very similar effect modification in this grouping scenario. In addition, combining those classes allowed easier interpretation of intereactions and eliminated the issue of the high variability in the Val/Val group due to its small sample size. To examine the effect of Ala114Val, we further divided the subjects in class II into those with no 114Val alleles (class IIa) and those with at least one 114Val allele (class IIb). For a 15ppb increase in O3, the estimated declines in FVC and FEV1 were smallest for class I subjects and greatest for class IIb subjects (Table 5). These results suggested a trend where the effects increased in magnitude across classes I through IIb, so we performed a linear trend test. This trend was not significant for FVC, but was marginally significant for FEV1 (p-value = 0.074).
We also investigated the combined effect of HMOX1 with GSTP1 Ile105Val by separating the cohort into four classes (class I: S/S with Ile/Ile, class II: S/S with Ile/Val or Val/Val, class III: S/L or L/L with Ile/Ile, class IV: S/L or L/L with Ile/Val or Val/Val). Results for the effect of O3 with this class separation are listed in Table 6. We wanted to test for a trend where the effects increased in magnitude across classes I through IV. Because we had no prior hypothesis as to whether HMOX1 or GSTP1 Ile105Val would result in a greater effect modification for ozone, we defined our trend variable as the interaction of a (1,2) variable for HMOX1 with a (1,2) variable for GSTP1 Ile105Val. Our resulting nonlinear trend was (1,2,4) where the classes II and III as defined above were both coded 2. The trend was significant for FEV1 (p-value = 0.045), but not significant for FVC. We also directly tested for an interaction of these four classes with ozone and found that the interaction was not significant, which suggests that the effect of the interaction of each gene with ozone is additive. This approach has less power than the trend test, but is a more formal test of three way interaction. Thus, a greater risk is estimated for those with both genetic mutations compared to those with only one, but our results suggest that the additional risk may come from these genes acting independently, although the trend test suggests an interaction may be present for FEV1.
Table 6.
Effect of 15 ppb increase in O3 by gene combinations
| Gene Combinations | % Change in FVC | 95% Confidence Interval | % Change in FEV1 | 95% Confidence Interval | |
|---|---|---|---|---|---|
| HMOX1 | GSTP1 Ile105Val | ||||
| I. S/S | Ile/Ile | −1.06 | −3.57, 1.50 | −2.00 | −4.59, 0.67 |
| II. S/S | Ile/Val, Val/Val | 0.69 | −2.00, 3.46 | 0.34 | −2.47, 3.23 |
| III. S/L, L/L | Ile/Ile | −1.04 | −1.90, −0.17 | −0.69 | −1.59, 0.21 |
| IV. S/L, L/L | Ile/Val, Val/Val | −1.79 | −2.70, −0.87 | −1.94 | −2.89, −0.98 |
DISCUSSION
In this elderly cohort, we found that lung function was negatively affected by increases in ambient ozone concentration. These results could help explain the association of ambient ozone with increased morbidity and mortality in elderly populations. As suggested by Kelly et al. in 2003, the lung function of many elderly is already greatly reduced from the natural effects of aging, so any additional decrease could push them past a critical threshold.14 Although the estimated percent change in lung function we identified is small, this change could represent a vital functional difference.
Our results also suggest that the presence of certain polymorphisms of antioxidant genes may modify the effect of ozone on lung function. For FEV1, there is evidence of a graded response, and marginal significance of an interaction. For FVC, there is some evidence of a graded response.
Few epidemiological studies have looked at lung function response to ozone in the elderly. A study evaluating the effects of ambient ozone on 530 nonsmoking hikers, ages 18–64 found significant associations between O3 exposure and declines in FVC and FEV1.38 When divided into four age categories, no difference in responsiveness to O3 was found, suggesting that the associations found for the entire cohort are representative of the oldest age group (ages 48–64) for this sample population. However, other studies have demonstrated that ozone responsiveness differs with age,25 so that assumption is questionable. The generalization of these findings to all elderly is limited given that subjects were all nonsmokers healthy enough to hike, with the oldest subject only 64 years old.
Höppe et al. examined the effect of ozone on lung function in four suspected risk groups: children, asthmatics, athletes, and elderly.39 Overall, this study found no effect of ozone exposure on lung function in the group of 41 elderly subjects (ages 69–95). These null findings may be due to its small sample size and to its examination of associations with exposures measured over shorter time intervals (3 hours compared to the minimum 24 hours used in our study). For a population study in which individuals maintain their everyday activities, a longer average may be more appropriate since the effect of exposure could take longer to build, especially considering the low ventilation rate of the elderly. While some chamber studies found associations with shorter exposures comparable to those in Höppe et al., this could be explained by the exercise requirements in the chamber studies which increased the ventilation rate and sped up the effect of exposure. In addition, some of the null findings of epidemiological studies may come from a difficulty in measuring the exact exposure to ozone because the elderly typically spend little time outside.
Of the chamber studies of elderly in which acute effects were reported, magnitudes range from 3 to 7% decreases in FVC and 3 to 11% decreases FEV1 in response to .45 ppm O3 for 2 hours, with varying exercise requirements.28,29 The exposure concentrations, conditions, and durations differed from those in our study, but overall, the magnitudes of our results seem reasonable compared to these estimates. The significant associations we found for both FVC and FEV1 across several time intervals considered together with past findings suggest that ozone does indeed have an acute effect on lung function in the elderly. Key factors in the previous findings of no association were probably the lack of power from small sample sizes and short durations of exposure.
Our study has two key advantages over prior research on ozone in the elderly: the large number of observations and the longitudinal design where each subject acts as his own control to account for intrasubject variability. Hence, in addition to using variables to account for the factors known to affect lung function, including age, height, smoking history and respiratory illnesses, our longitudinal design allows us to control for unmeasured intrasubject variability by using a random intercept for each person. The only longitudinal epidemiological study mentioned previously was by Höppe et al., but the measures were taken over the course of one summer, whereas our measurements spanned up to ten years.
Research comparing the ozone responsiveness in adults of different age groups has consistently found that the percent change in FEV1 from baseline following ozone exposure decreases with age.25 However, given the strong evidence linking short-term ambient ozone exposure with increased risk of mortality in elderly populations1–4 and respiratory-related emergency room visits and hospital admission,5–8 the elderly has been shown to be a susceptible population to the effects of short-term ozone exposure. Our research provides an additional link between ozone exposure and lung function decline in this population, and in combination with other research could help explain the relationship between ozone and other health outcomes in elderly populations.
The results of our genetic analyses demonstrate that polymorphisms of antioxidant genes may be associated with different responses to ozone exposure. Although effect estimates were not significant in all genotype classes, when considered together there is evidence for modification of the response to ozone depending on these antioxidant genes. Also, many of the nonsignificant effects were seen in classes with a smaller frequency of people sharing that genotype, suggesting that additional data for the less frequent genotypes could produce significant results for these categories.
It has been suggested that in searching for genes involved in susceptibility for complex diseases, models should account for gene-gene interactions to produce more replicable significant findings.40 Our interaction model estimated a more severe effect of O3 in the joint category of HMOX1 long repeats with GSTP1 105Val than in either of those categories analyzed separately, which implies that combinations of antioxidant genotypes are an important factor in determining susceptibility. The number of subjects in each class probably contributed to the significance of the effect estimates since classes I and II contain substantially fewer subjects that classes III and IV.
We also considered within-gene interaction in our analysis of GSTP1, examining Ala114Val as a modifier of Ile105Val polymorphisms rather than as having an independent effect. We chose this method of analysis because there is evidence of strong linkage disequilibrium between the two loci,41 and it has been suggested that the Ala114Val mutation may augment the effect of the Ile105Val polymorphism.42 Our results supported this hypothesis that Ala114Val modifies the effect of Ile105Val. A fourth rare haplotype exists for GSTP1 (105Ile/114Val),43 which was not observed in our cohort, so our analysis includes only the three more common groups. We would have liked to expand our model of the interaction between HMOX1 and GSTP1 by including the Ala114Val polymorphism in addition to Ile105Val. However, our data were not well-suited for such a model because several of the genetic combinations were too uncommon in our dataset to accurately compare the effect sizes across classes. A future study examining this interaction would be of great interest considering our findings for the interaction between HMOX1 and GSTP1 Ile105Val and for the modification of the effect of Ile105Val by Ala114Val.
Previous research has found distinct differences in individual susceptibility to the effects of ozone on lung function that are highly reproducible, and thus seem to result from differences in intrinsic responsiveness to O3.44,45 Our findings offer antioxidant genotypes as a possible intrinsic factor that could explain this interindividual difference in responsiveness. Additionally, our results showing a modifying effect of antioxidant genes implicate ROS as a mechanism for the negative effects seen in lung function and suggest the importance of antioxidant defenses. Further investigation into the status of antioxidant defenses in the elderly may reveal whether their susceptibility is determined by a difference in ability to defend against ROS.
A key limitation to our study is that the effect modification by the antioxidant genes was modest, typically 30–80% higher in the more susceptible groups. While this may still identify an important susceptibility factor, the differences in responsiveness in chamber studies was larger, suggesting additional factors are likely important.
Our study is limited by the study population consisting of only males. Our results cannot easily be extrapolated from elderly men to elderly women because of inconclusive research on the differences between men and women lung function response to ozone. Some studies examining ozone exposure and decreasing lung function have found that women may be more responsive than men,46,47 while others have reported no gender differences.48,49 A more recent study divided men and women into two age groups; among young subjects (ages 18–35) the study found women more responsive to O3 than men, while for middle-aged subjects (ages 36–60) men were more responsive than women.25 Future studies need to include sufficient numbers of men and women from different age groups to allow the determination of the effects of O3 on gender and age.
Another limitation of the study is the lack of individual exposure data since we used averages collected from several outdoor monitors, with no requirements for subjects spending time outside. Other factors may increase the susceptibility to the effects of ozone, such as outdoor physical activity. For example, the study of Korrick et al. mentioned above found that hiking was a modifier of the effect of ozone on lung function.38 While the lack of activity pattern data in our study is a concern, we have no reason to believe this could upwardly bias our effect, and the likely direction of the bias is downward.50 Another concern is that small studies of personal exposure have demonstrated moderate to low associations between ambient ozone levels and personal ozone exposure and some correlation with personal exposure to other products of photochemistry, including particulates, with effects varying by season.51,52 Additional research is needed to address the association of personal exposure to ozone and other pollutants with pulmonary responses in large longitudinal studies. Finally, since all participants were recruited in a single metropolitan area, we were unable to use this cohort to examine the long term effects of ozone since long term ambient ozone averages are the same for each subject.
This study provides evidence of an acute effect of ozone on lung function in the elderly. The evidence of a graded response to ozone by polymorphisms of antioxidant genes suggests that the negative effects of ozone exposure on lung function are related to oxidative stress, but also involve other factors that still must be identified to explain more of the intersubject variability in responsiveness to ozone.
Acknowledgments
We wish to thank Elaine R. Dibbs, Shelly Amberg and Jordan Awerbach for their invaluable contributions to the VA Normative Aging Study (NAS). This work was supported by the NIEHS grant ES0002 and the US EPA grants R827353 and R832416. The VA NAS is supported by the Cooperative Studies Program/Epidemiology Research and Information Center of the U.S. Department of Veterans Affairs and is a component of the Massachusetts Veterans Epidemiology Research and Information Center, Boston, Massachusetts.
Footnotes
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in Occupational and Environmental Medicine editions and any other BMJPG products to exploit all subsidiary rights, as set out in our licence (http://oem.bmjjournals.com/misc/ifora/licenceform.shtml).
References
- 1.Bell ML, Dominici F, Samet JM. A meta-analysis of time-series studies of ozone and mortality with comparison to the national morbidity, mortality, and air pollution study. Epidemiology. 2005;16:436–45. doi: 10.1097/01.ede.0000165817.40152.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gouveia N, Fletcher T. Time series analysis of air pollution and mortality: effects by cause, age and socioeconomic status. J Epidemiol Community Health. 2000;54:750–5. doi: 10.1136/jech.54.10.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levy JI, Chemerynski SM, Sarnat JA. Ozone exposure and mortality: an empiric bayes metaregression analysis. Epidemiology. 2005;16:458–68. doi: 10.1097/01.ede.0000165820.08301.b3. [DOI] [PubMed] [Google Scholar]
- 4.Simpson RW, Williams G, Petroeschevsky A, et al. Associations between outdoor air pollution and daily mortality in Brisbane, Australia. Arch Environ Health. 1997;52:442–54. doi: 10.1080/00039899709602223. [DOI] [PubMed] [Google Scholar]
- 5.Delfino RJ, Murphy-Moulton AM, Burnett RT, et al. Effects of air pollution on emergency room visits for respiratory illnesses in Montreal, Quebec. Am J Respir Crit Care Med. 1997;155:568–76. doi: 10.1164/ajrccm.155.2.9032196. [DOI] [PubMed] [Google Scholar]
- 6.Moolgavkar SH, Luebeck EG, Anderson EL. Air pollution and hospital admissions for respiratory causes in Minneapolis-St. Paul and Birmingham Epidemiology. 1997;8:364–70. doi: 10.1097/00001648-199707000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz J, Spix C, Touloumi G, et al. The APHEA project. Methodological issues in studies of air pollution and daily counts of deaths or hospital admissions. Short term effects of air pollution on health: a European approach using epidemiological time series data. In: St Leger S, editor. J Epidemiol Commun Health. Vol. 50. 1996. pp. S3–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Q, Chen Y, Shi Y, et al. Association between ozone and respiratory admissions among children and the elderly in Vancouver, Canada. Inhalation Toxicol. 2003;15:1297–308. doi: 10.1080/08958370390241768. [DOI] [PubMed] [Google Scholar]
- 9.Beaty TH, Cohen BH, Newill CA, et al. Impaired pulmonary function as a risk factor for mortality. Am J Epidemiol. 1982;116:102–13. doi: 10.1093/oxfordjournals.aje.a113385. [DOI] [PubMed] [Google Scholar]
- 10.Hole DJ, Watt GC, Davey-Smith G, et al. Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. BMJ. 1996;313:711–5. doi: 10.1136/bmj.313.7059.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schunemann HJ, Dorn J, Grant BJ, et al. Pulmonary function is a long-term predictor of mortality in the general population: 29-year follow-up of the Buffalo Health Study. Chest. 2000;118:656–64. doi: 10.1378/chest.118.3.656. [DOI] [PubMed] [Google Scholar]
- 12.Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest. 2005;127:1952–9. doi: 10.1378/chest.127.6.1952. [DOI] [PubMed] [Google Scholar]
- 13.Mudway IS, Kelly FJ. Ozone and the lung: a sensitive issue. Mol Aspects Med. 2000;21:1–48. doi: 10.1016/s0098-2997(00)00003-0. [DOI] [PubMed] [Google Scholar]
- 14.Kelly FJ, Dunster C, Mudway IS. Air pollution and the elderly: oxidant/antioxidant issues worth consideration. Eur Respir J Suppl. 2003;21:70s–5s. doi: 10.1183/09031936.03.00402903. [DOI] [PubMed] [Google Scholar]
- 15.Hayes JD, McLellan LI. Glutathione and glutathione-dependent enzymes represent a coordinately regulated defence against oxidative stress. Free Radic Res. 1999;31:273–300. doi: 10.1080/10715769900300851. [DOI] [PubMed] [Google Scholar]
- 16.Nebert DW, Vasiliou V. Analysis of the glutathione S-transferase (GST) gene family. Hum Genomics. 2004;1:460–4. doi: 10.1186/1479-7364-1-6-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacNee W. Oxidative stress and lung inflammation in airways disease. Eur J Pharmacol. 2001;429:195–207. doi: 10.1016/s0014-2999(01)01320-6. [DOI] [PubMed] [Google Scholar]
- 18.Mak JC, Leung HC, Ho SP, et al. Systemic oxidative and antioxidative status in Chinese patients with asthma. J Allergy Clin Immunol. 2004;114:260–4. doi: 10.1016/j.jaci.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 19.Romieu I, Ramirez-Aguilar M, Sienra-Monge JJ, et al. GSTM1 and GSTP1 and respiratory health in asthmatic children exposed to ozone. Eur Respir J. 2006;28:953–9. doi: 10.1183/09031936.06.00114905. [DOI] [PubMed] [Google Scholar]
- 20.Ercan H, Birben E, Dizdar EA, et al. Oxidative stress and genetic and epidemiologic determinants of oxidant injury in childhood asthma. J Allergy Clin Immunol. 2006;118:1097–104. doi: 10.1016/j.jaci.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Clark JE, Foresti R, Green CJ, et al. Dynamics of haem oxygenase-1 expression and bilirubin production in cellular protection against oxidative stress. Biochem J. 2000;348:615–9. [PMC free article] [PubMed] [Google Scholar]
- 22.Maines MD, Gibbs PE. 30 some years of heme oxygenase: from a “molecular wrecking ball” to a “mesmerizing” trigger of cellular events. Biochem Biophys Res Commun. 2005;338:568–77. doi: 10.1016/j.bbrc.2005.08.121. [DOI] [PubMed] [Google Scholar]
- 23.Yamada N, Yamaya M, Okinaga S, et al. Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with susceptibility to emphysema. Am J Hum Genet. 2000;66:187–95. doi: 10.1086/302729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guenegou A, Leynaert B, Benessiano J, et al. Association of lung function decline with the heme oxygenase-1 gene promoter microsatellite polymorphism in a general population sample. Results from the European Community Respiratory Health Survey (ECRHS), France. J Med Genet. 2006;43:e43. doi: 10.1136/jmg.2005.039743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hazucha MJ, Folinsbee LJ, Bromberg PA. Distribution and reproducibility of spirometric response to ozone by gender and age. J Appl Physiol. 2003;95:1917–25. doi: 10.1152/japplphysiol.00490.2003. [DOI] [PubMed] [Google Scholar]
- 26.Bedi JF, Horvath SM, Drechsler-Parks DM. Reproducibility of the pulmonary function response of older men and women to a 2- hour ozone exposure. JAPCA. 1988;38:1016–9. doi: 10.1080/08940630.1988.10466442. [DOI] [PubMed] [Google Scholar]
- 27.Bedi JF, Horvath SM, Drechsler-Parks DM. Adaptation by older individuals repeatedly exposed to 0.45 ppm ozone for two hours. JAPCA. 1989;39:194–9. doi: 10.1080/08940630.1989.10466521. [DOI] [PubMed] [Google Scholar]
- 28.Drechsler-Parks DM, Bedi JF, Horvath SM. Pulmonary function responses of older men and women to ozone exposure. Exp Gerontol. 1987;22:91–101. doi: 10.1016/0531-5565(87)90044-1. [DOI] [PubMed] [Google Scholar]
- 29.Drechsler-Parks DM, Horvath SM, Bedi JF. The “effective dose” concept in older adults exposed to ozone. Exp Gerontol. 1990;25:107–15. doi: 10.1016/0531-5565(90)90041-y. [DOI] [PubMed] [Google Scholar]
- 30.Reisenauer CS, Koenig JQ, McManus MS, et al. Pulmonary response to ozone exposures in healthy individuals aged 55 years or greater. JAPCA. 1988;38:51–5. doi: 10.1080/08940630.1988.10466353. [DOI] [PubMed] [Google Scholar]
- 31.Bell B, Rose C, Damon A. The Normative Aging Study: an interdisciplinary and longitudinal study of health and aging. Aging Hum Dev. 1972;3:4–17. [Google Scholar]
- 32.Ferris BG. Epidemiology Standardization Project (American Thoracic Society) Am Rev Respir Dis. 1978;118:1–120. [PubMed] [Google Scholar]
- 33.Sparrow D, O'Connor G, Colton T, et al. The relationship of nonspecific bronchial responsiveness to the occurrence of respiratory symptoms and decreased levels of pulmonary function: the Normative Aging Study. Am Rev Respir Dis. 1987;135:1255–60. doi: 10.1164/arrd.1987.135.6.1255. [DOI] [PubMed] [Google Scholar]
- 34.Kalkstein LS, Valimont KM. An evaluation of summer discomfort in the United States using a relative climatological index. Bull Am Meteorol Soc. 1986;67:842–8. [Google Scholar]
- 35.O'Neill MS, Zanobetti A, Schwartz J. Modifiers of the temperature and mortality association in seven US cities. Am J Epidemiol. 2003;157:1074–82. doi: 10.1093/aje/kwg096. [DOI] [PubMed] [Google Scholar]
- 36.Ali-Osman F, Akande O, Antoun G, et al. Molecular cloning, characterization, and expression in Escherichia coli of full-length cDNAs of three human glutathione S-transferase Pi gene variants: evidence for differential catalytic activity of the encoded proteins. J Biol Chem. 1997;272:10004–100012. doi: 10.1074/jbc.272.15.10004. [DOI] [PubMed] [Google Scholar]
- 37.Sun X, Ding H, Hung K, et al. A new MALDI-TOF based mini-sequencing assay for genotyping of SNPs. Nucleic Acids Res. 2000;28:E68. doi: 10.1093/nar/28.12.e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Korrick SA, Neas LM, Dockery DW, et al. Effects of ozone and other pollutants on the pulmonary function of adult hikers. Environ Health Perspect. 1998;106:93–9. doi: 10.1289/ehp.9810693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Höppe P, Peters A, Rabe G, et al. Environmental ozone effects in different population subgroups. Int J Hyg Environ Health. 2003;206:505–16. doi: 10.1078/1438-4639-00250. [DOI] [PubMed] [Google Scholar]
- 40.Millstein J, Conti DV, Gilliland FD, et al. A Testing Framework for Identifying Susceptibility Genes in the Presence of Epistasis. Am J Hum Genet. 2006;78:15–27. doi: 10.1086/498850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harris MJ, Coggan M, Langton L, et al. Polymorphism of the Pi class glutathione S-transferase in normal populations and cancer patients. Pharmacogenetics. 1998;8:27–31. doi: 10.1097/00008571-199802000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Hayes JD, Strange RC. Glutathione S-Transferase Polymorphisms and Their Biological Consequences. Pharmacology. 2000;61:154–66. doi: 10.1159/000028396. [DOI] [PubMed] [Google Scholar]
- 43.Watson MA, Stewart RK, Smith GBJ, et al. Human glutathione S-transferase P1 polymorphisms: relationship to lung tissue enzyme activity and population frequency distribution. Carcinogenesis. 1998;19:275–80. doi: 10.1093/carcin/19.2.275. [DOI] [PubMed] [Google Scholar]
- 44.McDonnell WF, Horstman DH, Abdul-Salaam S, et al. Reproducibility of individual responses to ozone exposure. Am Rev Respir Dis. 1985;131:36–40. doi: 10.1164/arrd.1985.131.S5.S36. [DOI] [PubMed] [Google Scholar]
- 45.Weinmann GG, Bowes SM, Gerbase MW, et al. Response to acute ozone exposure in healthy men. Results of a screening procedure. Am J Respir Crit Care Med. 1995;151:33–40. doi: 10.1164/ajrccm.151.1.7812569. [DOI] [PubMed] [Google Scholar]
- 46.Lauritzen SK, Adams WC. Ozone inhalation effects consequent to continuous exercise in females: comparison to males. J Appl Physiol. 1985;59:1601–6. doi: 10.1152/jappl.1985.59.5.1601. [DOI] [PubMed] [Google Scholar]
- 47.Messineo TD, Adams WC. Ozone inhalation effects in females varying widely in lung size: comparison with males. J Appl Physiol. 1990;69:96–103. doi: 10.1152/jappl.1990.69.1.96. [DOI] [PubMed] [Google Scholar]
- 48.Adams WC, Brooks KA, Schelegle ES. Effects of NO2, alone and in combination with O3 on young men and women. J Appl Physiol. 1987;62:1698–704. doi: 10.1152/jappl.1987.62.4.1698. [DOI] [PubMed] [Google Scholar]
- 49.Weinmann GG, Weidenbach GM, Foster WM, et al. Evidence for ozone-induced small-airway dysfunction: lack of menstrual-cycle and gender effects. Am J Respir Crit Care Med. 1995;152:988–96. doi: 10.1164/ajrccm.152.3.7663815. [DOI] [PubMed] [Google Scholar]
- 50.Zeger SL, Thomas D, Dominici F, et al. Exposure measurement error in time-series studies of air pollution: concepts and consequences. Environ Health Perspect. 2000;108:419–26. doi: 10.1289/ehp.00108419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee K, Parkhurst WJ, Xue J, et al. Outdoor/indoor/personal ozone exposures of children in Nashville, Tennessee. J Air Waste Manag Assoc. 2004;54:352–9. doi: 10.1080/10473289.2004.10470904. [DOI] [PubMed] [Google Scholar]
- 52.Sarnat JA, Brown KW, Schwartz J, et al. Ambient gas concentrations and personal particulate matter exposures: implications for studying the health effects of particles. Epidemiology. 2005;16:385–95. doi: 10.1097/01.ede.0000155505.04775.33. [DOI] [PubMed] [Google Scholar]