Abstract
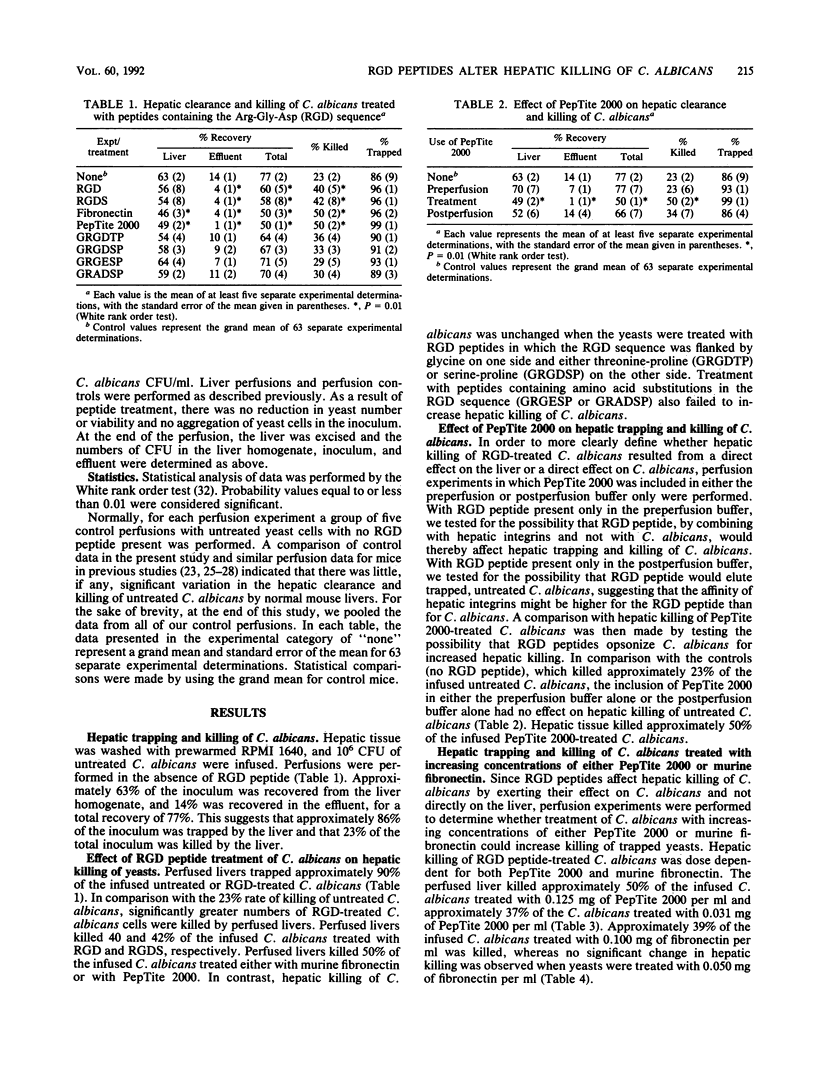
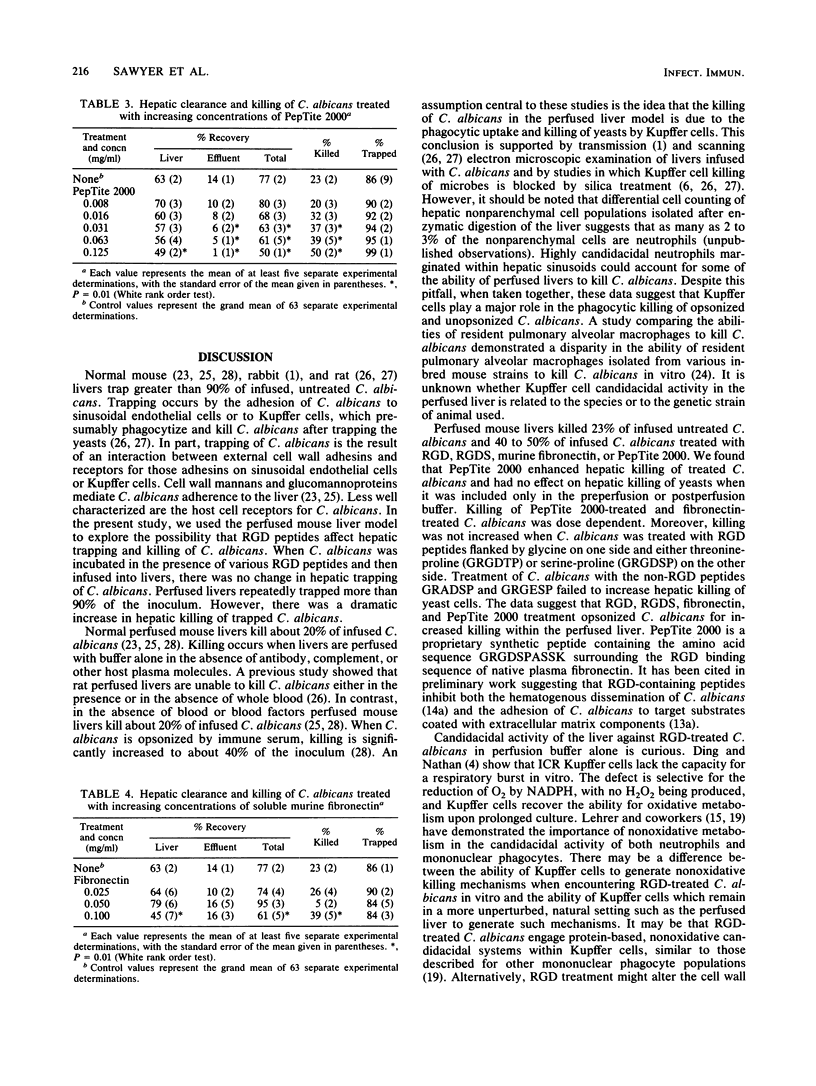
The isolated perfused mouse liver model was used to study the effect of Arg-Gly-Asp (RGD)-containing peptides on hepatic trapping and killing of Candida albicans. After extensive washing, 10(6) C. albicans CFU were infused into mouse livers. At the time of recovery, 63% +/- 2% (mean +/- standard error of the mean) of the infused C. albicans CFU were recovered from the liver and 14% +/- 1% were recovered from the effluent for a total recovery of 77% +/- 2%. This indicates that 86% +/- 9% of the original inoculum was trapped by the liver and that 23% +/- 2% was killed within the liver. Prior to their infusion into livers, 10(7) CFU of C. albicans were incubated at 37 degrees C for 30 min in the presence of various RGD peptides (0.1 mg/ml). Repeatedly, more than 90% of the infused RGD-treated C. albicans was trapped by the perfused liver. In comparison with the 23% killing rate observed in control livers, perfused livers killed approximately 40 to 50% of the infused C. albicans treated either with fibronectin, PepTite 2000, RGD, or RGDS. Hepatic killing of C. albicans treated with PepTite 2000 or fibronectin was dose dependent. Treatment of C. albicans with GRGDTP, GRGDSP, GRADSP, or GRGESP did not alter the ability of the perfused liver to kill C. albicans, suggesting that a degree of specificity for RGD peptides is associated with an increased ability of liver to kill RGD-treated C. albicans. Together, the data suggest that RGD peptides bind to a receptor on the surface of C. albicans, thereby increasing hepatic, and presumably Kupffer cell, killing of C. albicans. Natural or synthetic RGD peptides may serve as opsonins promoting C. albicans killing by Kupffer cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baine W. B., Koenig M. G., Goodman J. S. Clearance of Candida albicans from the bloodstream of rabbits. Infect Immun. 1974 Dec;10(6):1420–1425. doi: 10.1128/iai.10.6.1420-1425.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouali A., Robert R., Tronchin G., Senet J. M. Binding of human fibrinogen to Candida albicans in vitro: a preliminary study. J Med Vet Mycol. 1986 Aug;24(4):345–348. doi: 10.1080/02681218680000511. [DOI] [PubMed] [Google Scholar]
- Bouchara J. P., Tronchin G., Annaix V., Robert R., Senet J. M. Laminin receptors on Candida albicans germ tubes. Infect Immun. 1990 Jan;58(1):48–54. doi: 10.1128/iai.58.1.48-54.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding A., Nathan C. Analysis of the nonfunctional respiratory burst in murine Kupffer cells. J Exp Med. 1988 Mar 1;167(3):1154–1170. doi: 10.1084/jem.167.3.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. E., Jr, Gaither T. A., O'Shea J. J., Rotrosen D., Lawley T. J., Wright S. A., Frank M. M., Green I. Expression of specific binding sites on Candida with functional and antigenic characteristics of human complement receptors. J Immunol. 1986 Dec 1;137(11):3577–3583. [PubMed] [Google Scholar]
- Friedman R. L., Moon R. J. Role of Kupffer cells, complement, and specific antibody in the bactericidal activities of perfused livers. Infect Immun. 1980 Jul;29(1):152–157. doi: 10.1128/iai.29.1.152-157.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg M. H., Loftus J. C., Plow E. F. Cytoadhesins, integrins, and platelets. Thromb Haemost. 1988 Feb 25;59(1):1–6. [PubMed] [Google Scholar]
- Gustafson K. S., Vercellotti G. M., Bendel C. M., Hostetter M. K. Molecular mimicry in Candida albicans. Role of an integrin analogue in adhesion of the yeast to human endothelium. J Clin Invest. 1991 Jun;87(6):1896–1902. doi: 10.1172/JCI115214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidenreich F., Dierich M. P. Candida albicans and Candida stellatoidea, in contrast to other Candida species, bind iC3b and C3d but not C3b. Infect Immun. 1985 Nov;50(2):598–600. doi: 10.1128/iai.50.2.598-600.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries M. J., Olden K., Yamada K. M. A synthetic peptide from fibronectin inhibits experimental metastasis of murine melanoma cells. Science. 1986 Jul 25;233(4762):467–470. doi: 10.1126/science.3726541. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Kalo A., Segal E., Sahar E., Dayan D. Interaction of Candida albicans with genital mucosal surfaces: involvement of fibronectin in adherence. J Infect Dis. 1988 Jun;157(6):1253–1256. doi: 10.1093/infdis/157.6.1253. [DOI] [PubMed] [Google Scholar]
- Klotz S. A., Smith R. L. A fibronectin receptor on Candida albicans mediates adherence of the fungus to extracellular matrix. J Infect Dis. 1991 Mar;163(3):604–610. doi: 10.1093/infdis/163.3.604. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Ladra K. M., Hake R. B. Nonoxidative fungicidal mechanisms of mammalian granulocytes: demonstration of components with candidacidal activity in human, rabbit, and guinea pig leukocytes. Infect Immun. 1975 Jun;11(6):1226–1234. doi: 10.1128/iai.11.6.1226-1234.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisch P. A., Calderone R. A. Adherence of Candida albicans to a fibrin-platelet matrix formed in vitro. Infect Immun. 1980 Feb;27(2):650–656. doi: 10.1128/iai.27.2.650-656.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcantonio E. E., Hynes R. O. Antibodies to the conserved cytoplasmic domain of the integrin beta 1 subunit react with proteins in vertebrates, invertebrates, and fungi. J Cell Biol. 1988 May;106(5):1765–1772. doi: 10.1083/jcb.106.5.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon R. J., Vrable R. A., Broka J. A. In situ separation of bacterial trapping and killing functions of the perfused liver. Infect Immun. 1975 Aug;12(2):411–418. doi: 10.1128/iai.12.2.411-418.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson-Delafield J., Martinez R. J., Lehrer R. I. Microbicidal cationic proteins in rabbit alveolar macrophages: a potential host defense mechanism. Infect Immun. 1980 Oct;30(1):180–192. doi: 10.1128/iai.30.1.180-192.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984 May 3;309(5963):30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Fibronectin and its receptors. Annu Rev Biochem. 1988;57:375–413. doi: 10.1146/annurev.bi.57.070188.002111. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Sawyer R. T. Experimental pulmonary candidiasis. Mycopathologia. 1990 Feb;109(2):99–109. doi: 10.1007/BF00436790. [DOI] [PubMed] [Google Scholar]
- Sawyer R. T., Horst M. N., Garner R. E., Hudson J., Jenkins P. R., Richardson A. L. Altered hepatic clearance and killing of Candida albicans in the isolated perfused mouse liver model. Infect Immun. 1990 Sep;58(9):2869–2874. doi: 10.1128/iai.58.9.2869-2874.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer R. T., Moon R. J., Beneke E. S. Trapping and killing of Candida albicans by Corynebacterium parvum-activated livers. Infect Immun. 1981 May;32(2):945–950. doi: 10.1128/iai.32.2.945-950.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer R. T. The effect of monosaccharides on in situ hepatic trapping of Candida albicans. Mycopathologia. 1988 Nov;104(2):81–85. doi: 10.1007/BF00436931. [DOI] [PubMed] [Google Scholar]
- Schwocho L. R., Moon R. J. Clearance and killing of Candida albicans in the perfused mouse liver. Mycopathologia. 1981 Dec 11;76(3):175–183. doi: 10.1007/BF00437198. [DOI] [PubMed] [Google Scholar]
- Tronchin G., Bouchara J. P., Robert R. Dynamic changes of the cell wall surface of Candida albicans associated with germination and adherence. Eur J Cell Biol. 1989 Dec;50(2):285–290. [PubMed] [Google Scholar]
- Van de Water L., Destree A. T., Hynes R. O. Fibronectin binds to some bacteria but does not promote their uptake by phagocytic cells. Science. 1983 Apr 8;220(4593):201–204. doi: 10.1126/science.6338594. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Reddy P. A., Jong M. T., Erickson B. W. C3bi receptor (complement receptor type 3) recognizes a region of complement protein C3 containing the sequence Arg-Gly-Asp. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1965–1968. doi: 10.1073/pnas.84.7.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]


